Abstract
The transforming growth factor β (TGF-β) pathway acts as a double-edged sword in tumorigenesis. By constraining epithelial cell growth, TGF-β is a potent tumor suppressor. However, TGF-β also acts as a key player in the induction of epithelial-to-mesenchymal transition (EMT), thereby enhancing invasiveness and metastasis. Furthermore, TGF-β signaling has recently been correlated with resistance against both targeted and conventional anticancer agents. Here, we present data demonstrating a role for TGF-β in chemotherapy resistance in colorectal cancer (CRC). We discuss these results in the context of recent findings indicating TGF-β signaling as an emerging player in cancer drug resistance.
Keywords: EMT, TGF-β, chemotherapy, drug resistance
Introduction
The TGF-β superfamily consists of a plethora of factors, which can be divided into subgroups of TGF-βs, activins, inhibins, anti-Müllerian hormone (AMH), nodal, and bone morphogenic proteins (BMPs).1 TGF-β1, -β2, and -β3 ligands activate downstream signaling pathways by binding with high affinity to type 2 TGF-β receptors (TGF-βR2), which dimerize with and phosphorylate the type 1 TGF-β receptor (TGF-βR1). The latter can propagate the signal via the canonical SMAD pathway and the non-canonical SMAD-independent pathways, which include, among others, p38 mitogen-activated protein kinases (MAPK), phosphoinositide 3-kinase (PI3K)-AKT, Rho GTPases, nuclear factor-κB (NF-κB), and JUN N-terminal kinase (JNK).2 Upon phosphorylation, the receptor-SMADs form a complex with SMAD4 and translocate to the nucleus to regulate transcription. The precise function of TGF-β signaling is highly cell type- and context-dependent, since the SMAD complex needs to interact with a combination of co-factors to exert its function.3
TGF-β signaling has important roles in many cellular processes, including cell cycle regulation, cell migration, apoptosis, and immune modulation. TGF-β acts as a tumor suppressor by inhibiting cell cycle progression via multiple mechanisms. TGF-β signaling suppresses MYC transcriptional activity, resulting in downregulation of cyclin expression and upregulation of the cyclin-dependent kinase inhibitors (CDKi) p21Cip1 and p15INK4b. Furthermore, the SMAD complex directly enhances expression of the CDK inhibitors p15INK4b, p21Cip1, and p57Kip2 1. Other SMAD targets, such as plasminogen activator inhibitor-1 (PAI-1), have also been found to play a crucial role in the anti-proliferative effect of TGF-β4. Besides exerting cytostatic effects, TGF-β signaling also contributes to the induction of apoptosis via the upregulation of death-associated protein kinase DAPK, the pro-apoptotic BH3-domain protein BIM, and the death receptor FAS.5
TGF-β not only exerts its tumor-suppressive role in a cell-autonomous manner, but also through the extracellular matrix. TGF-β suppresses the production of mitogens by fibroblasts, thereby inhibiting tumor cell proliferation.6 Furthermore, TGF-β enhances the levels of CD4+CD25+ regulatory T cells (Tregs) and simultaneously suppresses the activity of effector cells of both the innate and adaptive immune system.7 This combined suppressive effect on inflammation can induce a state of immune tolerance in the intestinal environment and can result in tumorigenesis when disturbed.8 However, TGF-β signaling might also induce a misbalance in immune suppression, possibly resulting in immune evasion by tumor cells.
The tumor-suppressive role of TGF-β is often lost in cancer, as evidenced by mutations in TGF-β receptors or the SMAD genes, particularly in colorectal cancer.9,10 Furthermore, mutations downstream of TGF-β signaling, such as homozygous loss of CDKN2B in glioblastoma, specifically abrogate the tumor-suppressive aspects of TGF-β while retaining its pro-tumorigenic capacity.11 Once the fine balance of TGF-β signaling is disrupted, TGF-β might turn into a strong enhancer of tumorigenesis during later stages of tumorigenesis.
TGF-β is known to be a potent mediator of epithelial-to-mesenchymal transition (EMT), a process crucial in the process of tumor cell dissemination. TGF-β induces the expression of the transcription factors SNAIL1/2, SLUG, TWIST, ZEB1/2, and TCF3.12 These factors inhibit E-cadherin expression and upregulate mesenchymal markers such as N-cadherin, vimentin, and the secretion of matrix metalloproteases (MMPs). Furthermore, TGF-β also directly affects tight junction stability by activating PAR6. Phosphorylation of PAR6 by TGF-βRII results in association of PAR6 with the E3 ubiquitin ligase SMURF1, which subsequently targets the cell-adherence regulator RHOA for degradation.13 The combined effect of TGF-β and other EMT drivers results in the loss of cell–cell junctions, cell polarity, and adherence while inducing enhanced motility. Both TGF-β signaling and EMT are also associated with the formation of cancer stem cells (CSCs), which are thought to play a key role in driving tumorigenesis by sustaining tumor growth.14
Mesenchymal phenotypes have been correlated with poor prognosis in colon cancer and some other types of cancer.15-19 A partial explanation can be found in the increased metastatic capacity associated with EMT. In addition, recent reports have linked TGF-β signaling and EMT to drug resistance, which might also contribute to the poor prognosis of patients having more mesenchymal tumors.15,20-23 Here, we will discuss the recently described role of TGF-β signaling in resistance to multiple cancer drugs. We provide data indicating that the TGF-β signaling cascade is associated with chemotherapy resistance in colorectal cancer and reflect on the possible implications for treating these tumors.
TGF-β-Induced Drug Resistance against Targeted Cancer Therapies
The effects of targeted therapies in cancer are often diminished by the emergence of resistance. Drug resistance can occur through development of secondary mutations in the target itself, e.g., T790M gatekeeper mutation in EGFR, via activating mutations in components of the signaling pathway downstream of the drug target, or via upregulation of parallel signaling pathways (reviewed in ref. 24). Many of these mechanisms are still unidentified. Using a large-scale RNAi screen, we recently identified loss of the MEDIATOR complex protein MED12 as a resistance mechanism against multiple tyrosine kinase inhibitors (TKIs).15 MED12 was found to interfere with maturation of TGF-βRII in the Golgi, thereby preventing its expression on the cell surface. Consequently, suppression of MED12 expression led to activation of TGB-β signaling.15 Either loss of MED12, overexpression of TGF-βRII, or treatment with recombinant TGF-β was sufficient to induce TKI resistance in multiple cancer types. TGF-β signaling induced activation of MEK/ERK signaling and thereby restored the reduced MAPK pathway activation by TKIs. Although TGF-β signaling in untreated cells was unfavorable in many cell types as a result of growth-inhibitory effects, it became beneficial when combined with TKIs. As expected, treatment with the TGF-β receptor inhibitor LY2157299 restored sensitivity to TKIs in MED12KD cells. Interestingly, a MED12KD expression signature displayed significant overlap with a previously described EMT signature. This signature was predictive for MEK inhibitor response in heterogeneous panel of 152 cancer cell lines. Furthermore, we found that a gene expression profile of the tumor of a NSCLC patient that developed gefitinib resistance demonstrated significant overlap with the MED12KD signature.15 Other studies in NSCLC have also described a correlation between EMT and acquired resistance against EGFR inhibitors.20,22,25-27
The emergence of TGF-β signaling in acquired resistance against TKIs is not limited to NSCLC. EMT has also been described as a resistance mechanism against EGFR inhibition in pancreatic cancer and head and neck cancer.28,29 Furthermore, TGF-β signaling was found to decrease sensitivity of the dual IGF-I/IR inhibitor OSI-906 in hepatocellular carcinoma.30 Another study by Oliveras-Ferraros et al. described EMT as a mechanism for trastuzumab resistance in HER2-positive breast cancer.23 Finally, in colon cancer, a subgroup of tumors having a mesenchymal phenotype were resistant to cetuximab treatment.16 Taken together, TGF-β signaling proves to be an important resistance mechanism against multiple targeted agents in a number of cancer types.
TGF-β Pathway Activation is Associated with Chemotherapy Resistance
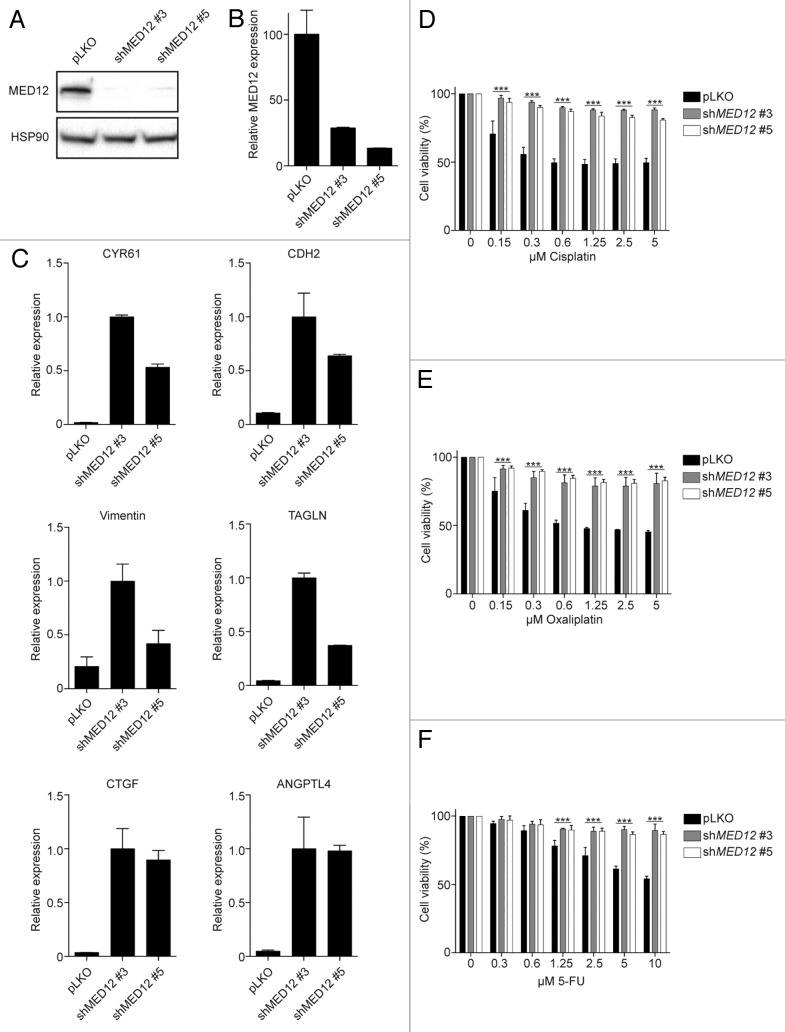
In our previous work, we demonstrated that loss of MED12 not only resulted in TKI resistance, but also induced resistance against 5-FU and cisplatin in lung cancer cell lines.15 To determine whether TGF-β treatment also induces resistance against chemotherapeutic agents in colon cancer, we studied the effects of MED12 suppression in SKCO-1 CRC cells. Utilizing 2 independent shRNAs, we knocked down MED12 (Fig. 1A and B), which resulted in strong upregulation of a panel of downstream TGF-β target genes (Fig. 1C). Subsequently, control and MED12-knockdown cells were treated with increasing concentrations of cisplatin, oxaliplatin, and 5-FU, and cell viability was determined. Interestingly, upregulation of TGF-β signaling by MED12 knockdown resulted in resistance against these drugs (Fig. 1D–F). Similar results were obtained when parental SKCO-1 cells were treated with recombinant TGF-β (data not shown). These data suggest that TGF-β signaling protects against chemotherapy-induced cell death. This is supported by our recent findings, where we demonstrated that CRC patients with a MED12KD signature did not benefit from chemotherapy treatment.15
Figure 1. MED12 knockdown-induced TGF-β signaling confers chemotherapy resistance. (A and B) Knockdown of MED12 was assessed on protein and RNA level (C) MED12 knockdown results in enhanced expression of a panel of TGF-β target genes (D–F) Cell viability assay of SKCO-1 cells treated with cisplatin, oxaliplatin, or 5-FU. Cell viability was measured by the conversion of resazurin into resorufin after 72 h drug treatment (n = 3).
Other studies have correlated poor prognosis in CRC with a mesenchymal molecular phenotype.16,31 Moreover, Roepman et al. recently classified CRC based on gene expression profiling in 3 subgroups, A-, B-, and C-types, of which the latter demonstrated the least benefit from chemotherapy and the worst prognosis.32 Interestingly, this subtype of tumors also displayed high expression levels of mesenchymal markers.
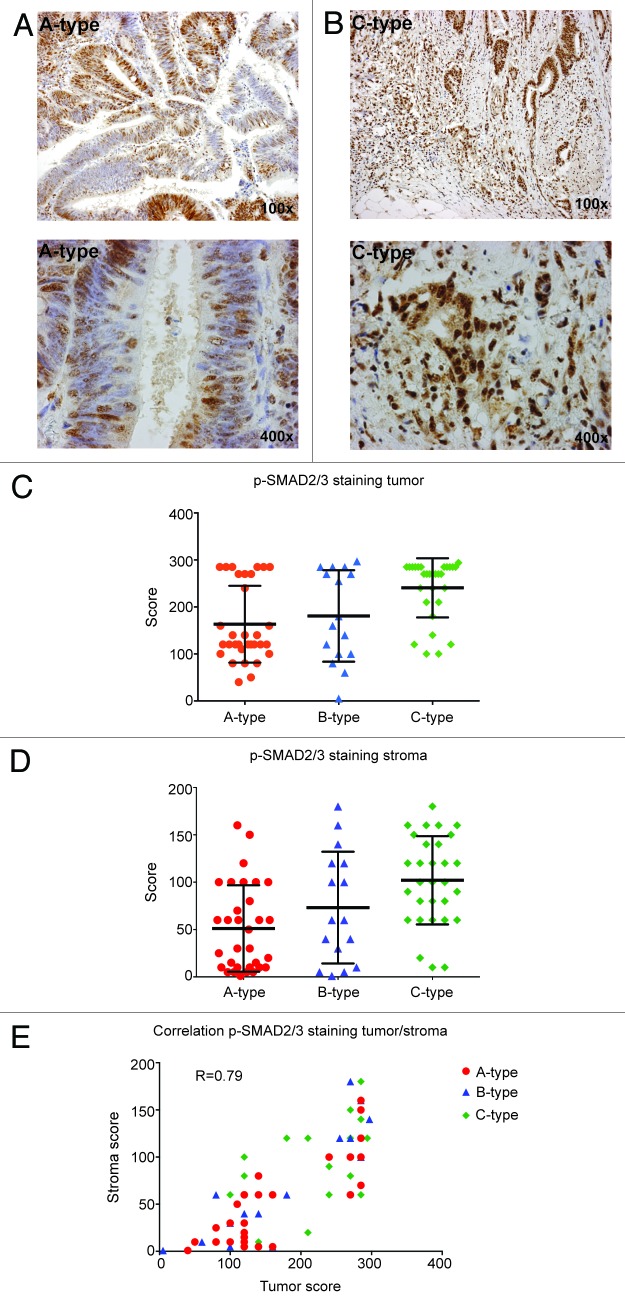
To determine whether TGF-β signaling is elevated in these C-type CRC tumors, we analyzed a panel of 78 patient biopsies for p-SMAD2/3 expression using immunohistochemistry. Type A tumors show wide, well-differentiated ducts with nuclear staining in 60% of the tumor cells. The ducts in type C CRC are more irregular and show EMT, especially at the invading front, which parallels strong nuclear expression in all tumor cells (Fig. 2A–C). The difference between B- and C-type tumors was not significant, which might be due to the low number of B-type tumors in this panel. Notably, the cancer-associated fibroblasts (CAFs) in the ECM of the C-type tumors also displayed a significant increase in nuclear p-SMAD2/3 expression compared with those in the A/B subtypes (Fig. 2D), which correlated with expression in the tumor cells (Fig. 2E). These findings indicate increased TGF-β signaling in C-type CRC, which is in line with other studies demonstrating a correlation between TGF-β signaling and disease outcome.33-35 TGF-β signaling has, furthermore, been described to be a good predictor of CRC metastasis, since a single measurement of plasma TGF-β levels following curative tumor resection is found to be predictive for future liver metastasis.36
Figure 2. C-type CRC tumors display higher p-SMAD2/3 levels in both tumor and stroma. Representative images of p-SMAD2/3 staining in A-type CRC tumors (A) and C-type tumors (B). Quantitative analysis of p-SMAD2/3 staining in tumor tissue (C). A-type vs B-type (ns), A-type vs C-type (***), B-type vs C-type (ns). (D) Quantitative analysis of p-SMAD2/3 staining in CAFs. A-type vs B-type (ns), A-type vs C-type (****), B-type vs C-type (ns). (E) Spearman correlation between p-SMAD2/3 staining in tumor cells and CAFs.
The question remains whether it is the tumor or the CAF that is responsible for the increased TGF-β production. Calon et al. have discovered a mechanism in CRC, where tumor-produced TGF-β activates TGF-β signaling in the tumor stroma, which, in turn, results in IL11 production by CAFs. This cytokine strongly enhanced metastasis initiation in a GP130/STAT3-dependent manner.35 Inhibition of stromal TGF-β signaling using the TGF-β inhibitor LY2157299 effectively blocked metastasis initiation. These findings indicate that TGF-β not only affects tumor cells directly, but that it is also capable of modulating the stroma to favor tumor progression.
It is intriguing to speculate that chemotherapy treatment itself might induce EMT, or lead to selection of a subset of cancer cells that have undergone EMT prior to drug treatment, resulting in acquired resistance. Yang et al. found that chronic oxaliplatin treatment of CRC cell lines induces an EMT phenotype, resulting in long-term resistance.37 Another study by Creighton et al. demonstrated that residual tumor cell populations in docetaxel-treated breast cancer displayed an increased expression of mesenchymal markers.38 Furthermore, the percentage of CD44+/CD24− cells was enriched upon therapy. These cells have previously been described as CSC-like cells with enhanced invasive and chemotherapy-resistant properties39-41. Moreover, analysis of matched pairs of triple-negative breast cancer biopsies before and after paclitaxel treatments indicated that chemotherapy increased expression of genes associated with TGF-β signaling and CSCs.42
The striking correlation between TGF-β signaling/EMT is not restricted to CRC but has been described for many types of cancer. Therefore, plasma TGF-β levels or SMAD IHC staining might prove to be important biomarkers for disease-specific survival and therapy outcome.
TGF-β Inhibits Chemotherapy-Induced Apoptosis
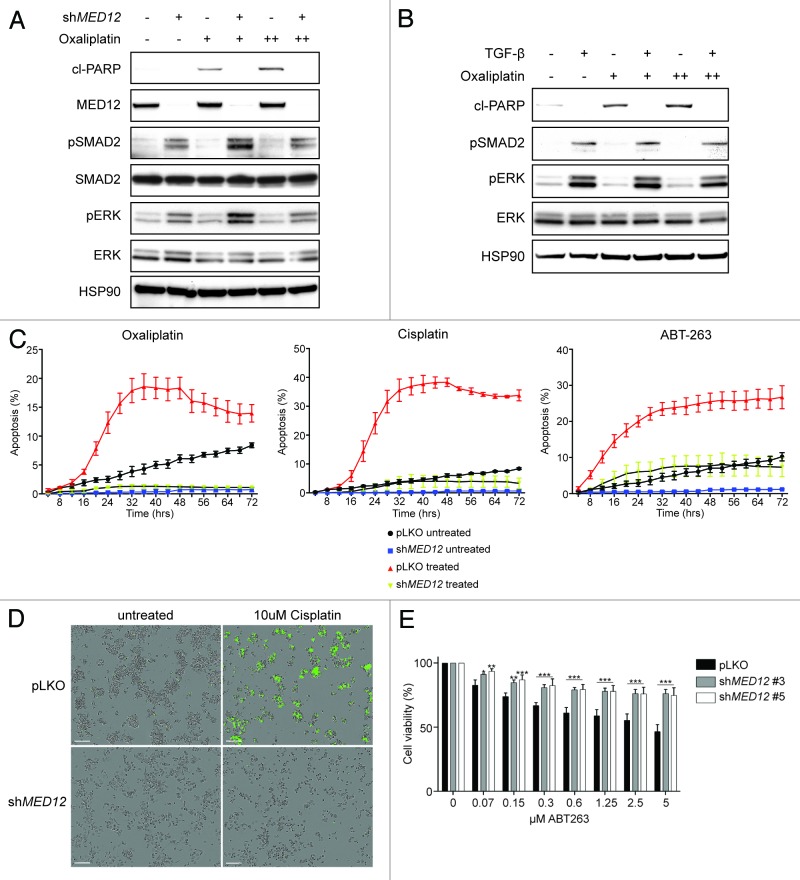
The precise mechanisms by which TGF-β signaling and EMT result in chemotherapy resistance are of high interest. Insights into these mechanisms are important for the development of novel therapeutic approaches. One plausible explanation could be an anti-apoptotic state induced by TGF-β. To investigate the effect of chemotherapeutics on apoptosis in TGF-β-treated or MED12KD CRC cells, we incubated cells for 24 h with oxaliplatin and measured the level of cleaved PARP, an early sign of apoptosis. Both TGF-β treatment and MED12 knockdown induced activation of the TGF-β pathway, as assessed by enhanced p-SMAD2 and p-ERK levels. Strikingly, MED12KD cells or cells pretreated with TGF-β did not undergo apoptosis upon treatment of oxaliplatin (Fig. 3A and B). Time-course analysis of caspase 3/7 activity upon cisplatin/oxaliplatin treatment yielded similar results (Fig. 3C and D). TGF-β signaling furthermore prevented apoptosis induced by the BH3 mimetic ABT-263, suggesting that the impaired induction of apoptosis by TGF-β is independent of its effect on cell proliferation (Fig. 3C). Similarly, MED12 knockdown resulted in resistance against ABT-263 in a short-term cell viability assay (Fig. 3E).
Figure 3. TGF-β signaling prevents chemotherapy induces apoptosis (A) SKCO-1 cells were treated for 24 h with 1 or 2 μM of oxaliplatin and cell lysates were harvested for western blot analysis. MED12 knockdown activates TGF-β signaling resulting in increased pSMAD2 and pERK levels and prevents apoptosis, measured by cleaved PARP (n = 3). Equal results are observed upon TGF-β treatment (B). SKCO-1 cells are treated with 10 μM oxaliplatin, 10 μM Cisplatin, or 5 μM ABT-263 and induction of apoptosis was analyzed in real time by using CellPlayer Caspase 3/7 reagent (n = 3). (D) Representative image of apoptosis induction (green) in control or MED12KD cells upon 32 h cisplatin treatment. (E) Cell viability assay of SKCO-1 cells treated with ABT-263. Cell viability was measured by the conversion of resazurin into resorufin after 72 h drug treatment (n = 3).
Recently, López-Diaz et al. reported that TGF-β1 signaling inhibits p53 both on a transcriptional and translational level.43 Activation of the TGF-β pathway induces formation of a SMAD/E2F4/p107 complex, which is recruited to SMAD-binding regions in the TP53 gene, resulting in transcriptional repression. Furthermore, TGF-β signaling prevents the association of the ribosomal protein RPL26 and elongation factor eEF1A with p53 mRNA, thereby suppressing translation. The reduction of p53 protein levels subsequently protected breast cancer cells against DNA damage-induced apoptosis.43 Low levels of p53 have been correlated with reduced chemotherapeutic response and poor prognosis in breast cancer,44 colorectal cancer,45 chronic lymphoid leukemia,46 and other types of cancer.
Besides a direct effect on p53, TGF-β signaling also indirectly affects p53 activity. The transcription factor TWIST is an important mediator of EMT, and its expression is partially regulated by TGF-β47. TWIST can modulate p53 function, possibly via reducing CDKN2A expression, which, in turn, prevents apoptosis.48 Furthermore, TGF-β can induce expression of the E3 ubiquitin ligase human murine double minute (HDM2), resulting in p53 degradation.49
TGF-β might also protect cells from apoptosis in a p53-independent manner. In bladder cancer, a mesenchymal subgroup associated with poor prognosis expresses high levels of the TGF-β target SIP1, which has been found to protect these tumors from DNA damage-induced apoptosis.19 TGF-β signaling was furthermore found to protect against ionizing radiation-induced DNA damage by enhancing the kinase activity of ATM.50 Besides enhancing DNA repair, TGF-β can also prevent apoptosis by induction of the anti-apoptotic gene BCL2 via TWIST.51 Furthermore, autocrine TGF-β production in breast cancer cells prevents apoptosis by repressing BIM protein levels.52 Interestingly, we also found that TGF-β treatment in SKCO-1 cells resulted in an increase of phosphorylated BIM and decreased levels of total BIM, probably linked to activation of p90-RSK (data not shown). However, whether BIM plays an important role in TGF-β-mediated protection of apoptosis remains unclear, since it has been reported that TGF-β is also capable of upregulating BIM expression.53
Taken together, our data and those of others demonstrate a role for TGF-β in chemotherapy resistance via inhibition of apoptosis.
Conclusions and Future Perspectives
Our findings and those by others indicate that TGF-β plays an important role in drug resistance against both targeted and conventional agents. This strongly argues for combinational use of these drugs, with agents suppressing TGF-β signaling. Several studies have already indicated the benefit of combining drugs with TGF-β inhibitors. Recent work by Bhola et al. for instance shows that treatment of triple-negative breast cancer xenografts with paclitaxel induces autocrine TGF-β signaling, CSC formation, and drug resistance.42 Strikingly, combined treatment with the TGF-β inhibitor LY2157299 prevented the recurrence of tumors after paclitaxel treatment, supporting a treatment strategy combining both drugs in these tumors. Similar results might be achieved by combining chemotherapy treatment with LY2157299 in the mesenchymanl subgroup of CRCs or other types of cancer. At the moment, clinical trials are ongoing with combined treatment of chemotherapeutic drugs and LY2157299 in glioblastoma (NCT01582269), hepatocellular carcinoma (HCC) (NCT01246986), and pancreatic cancer (NCT01373164). Furthermore, based on multiple reports indicating TGF-β as a mediator of resistance against targeted agents, it is likely that clinical trials combining targeted therapy with TGF-β inhibitors will soon be underway.
Materials and Methods
Cell culture and lentiviral transduction
SKCO-1 cells were cultured in high glucose Dulbecco modified Eagle medium supplemented with 8% heat-inactivated FBS and 1% penicillin/streptomycin at 37 °C and 5% CO2.
PEI transfection of HEK293T cells was used to produce lentiviral supernatant. Lentiviral transduction was performed as described at http://www.broadinstitute.org/rnai/public/resources/protocols. All lentiviral shRNA vectors were retrieved from the arrayed TRC human genome-wide shRNA collection. Control infections were performed with the empty pLKO.1 vector. Cells were selected for successful lentiviral integration using 2 μg/ul puromycin.
Reagents
ABT-263 (S1001), Oxaliplatin (S1224), and cisplatin (S1166) were purchased from Selleckchem. 5-FU was obtained from the pharmacy at The Netherlands Cancer Institute. Recombinant human TGF-β1 (240-B) was purchased from R&D Systems.
Protein lysate preparation and western blot analysis
Cells were lysed using RIPA buffer containing 150 mM NaCl, 50 mM Tris pH 8.0, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS supplemented with protease inhibitors (Complete, Roche) and phosphatase inhibitor cocktails II and III (Sigma). Sample buffer (60 mM Tris pH 6.8, 5% glycerol, 1% SDS, 2% β-mercaptoethanol, 0.02% bromophenol blue) was added, lysates were boiled for 10 min, and equal amounts of sample were subjected to SDS gel electrophoresis followed by western blotting. Primary antibodies against HSP90 (SC-7947), ERK1 (SC-93), ERK2 (SC-154), and pERK (SC-7383) were purchased from Santa Cruz. Antibodies against MEK1/2 (4694), pMEK (9154), PARP (9542), SMAD2 (3103), and pSMAD2 (3101) were from Cell Signaling. Anti-MED12 (A300-774A) was purchased from Bethyl Laboratories. Secondary antibody was obtained from Bio-Rad Laboratories.
Quantitative RT-PCR
The 7500 Fast Real-Time PCR System from Applied Biosystems was used to measure mRNA levels. mRNA expression levels were normalized to expression of GAPDH. The following primer sequences were used in the SYBR Green master mix:
GAPDH_forward 5′-AAGGTGAAGGTCGGAGTCAA-3′;
GAPDH_reverse, AATGAAGGGGTCATTGATGG;
MED12_forward 5′-GCTGGTGCACATAGCCACT-3′;
MED12_reverse, 5′ TACTCCAGCCAGCCTTACCA-3′;
CDH2_forward, 5′-CCACCTTAAAATCTGCAGGC-3′;
CHD2-reverse, 5′- GTGCATGAAGGACAGCCTCT-3′;
Vimentin_forward, 5′- CTTCAGAGAGAGGAAGCCGA-3′;
Vimentin_reverse, 5′- AATCCACTTTGCGTTCAAGG-3′;
ANGPTL4_forward, 5′- GGAACAGCTCCTGGCAATC-3′;
ANGPTL4_reverse, 5′- GCACCTAGACCATGAGGTGG-3′;
TAGLN_forward, 5′- GTCCGAACCCAGACACAAGT-3′;
TAGLN_reverse, 5′- CTCATGCCATAGGAAGGACC-3′;
CTGF_forward, 5′- TACCAATGACAACGCCTCCT-3′;
CTGF_reverse, 5′- TGGAGATTTTGGGAGTACGG-3′;
CYR61_forward, 5′- GCTGGAATGCAACTTCGG-3′;
CYR61_reverse, 5′- CCCGTTTTGGTAGATTCTGG-3′
Cell viability assay
Cells were seeded in 96-well plates. After 24 h, drugs were added to the medium in 2-fold serial dilutions using a HP Direct Digital Dispenser. After 72 h, medium was refreshed with medium containing CellTiter-Blue (Promega). The conversion of resazurin into resorufin was measured by using an EnVision Multilabel Reader.
Kinetic measurement of apoptosis
Cells were grown in 384-well plates and images were taken every 4 h using the Incucyte Kinetic Imaging System (Essen BioScience). Apoptosis was measured in real-time using CellPlayer Caspase 3/7 reagent (Essen BioScience).
Immunohistochemistry
Formalin-fixed, paraffin-embedded (FFPE) sections were used for immunohistochemical staining with anti-pSMAD2/3 using clone sc-11769-R (Santa Cruz). Sections (4-um-thick) were mounted on 3-aminopropylethoxysilane (Sigma) and glutaraldehyde coated slides and dried overnight at 37 °C. After antigen retrieval (citrate, 20 min), staining was performed according to the manufacturer’s protocol followed by incubation with the PowerVision Poly-HRP anti-Rabbit IgG (ImmunoLogic). Sections were counterstained with hematoxylin. Staining was evaluated using the H-score (*, intensity percentage), with intensity ranging from 0–3.
Statistical analysis
Statistical analyses were conducted using GraphPad Prismv4.0a. Cell viability data was analyzed using a 2-way ANOVA with Dunnett multiple comparisons test. Differences in p-SMAD2 staining between subtypes were tested for significance using a Mann-Whitney test. A P value of less than 0.05 was considered statistically significant and is denoted by *. Note: ** P < 0.01, *** P < 0.001, and **** P < 0.0001.
Acknowledgments
We thank all members of the Bernards lab for helpful support and discussions. This work was supported by a grant from the EU FP7 grants RATHER and COLTHERES. S.M.W. is funded by the Dutch Cancer Society (clinical fellowship: 2011-4964).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/26034
References
- 1.Massagué J. TGFbeta in Cancer. Cell. 2008;134:215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kortlever RM, Nijwening JH, Bernards R. Transforming growth factor-beta requires its target plasminogen activator inhibitor-1 for cytostatic activity. J Biol Chem. 2008;283:24308–13. doi: 10.1074/jbc.M803341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heldin CH, Landström M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009;21:166–76. doi: 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 7.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 8.Engle SJ, Ormsby I, Pawlowski S, Boivin GP, Croft J, Balish E, Doetschman T. Elimination of colon cancer in germ-free transforming growth factor beta 1-deficient mice. Cancer Res. 2002;62:6362–6. [PubMed] [Google Scholar]
- 9.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–8. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 10.Fleming NI, Jorissen RN, Mouradov D, Christie M, Sakthianandeswaren A, Palmieri M, Day F, Li S, Tsui C, Lipton L, et al. SMAD2, SMAD3 and SMAD4 mutations in colorectal cancer. Cancer Res. 2013;73:725–35. doi: 10.1158/0008-5472.CAN-12-2706. [DOI] [PubMed] [Google Scholar]
- 11.Jen J, Harper JW, Bigner SH, Bigner DD, Papadopoulos N, Markowitz S, Willson JK, Kinzler KW, Vogelstein B. Deletion of p16 and p15 genes in brain tumors. Cancer Res. 1994;54:6353–8. [PubMed] [Google Scholar]
- 12.Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76–84. doi: 10.1097/CCO.0b013e32835b6371. [DOI] [PubMed] [Google Scholar]
- 13.Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–9. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 14.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang S, Hölzel M, Knijnenburg T, Schlicker A, Roepman P, McDermott U, Garnett M, Grernrum W, Sun C, Prahallad A, et al. MED12 controls the response to multiple cancer drugs through regulation of TGF-β receptor signaling. Cell. 2012;151:937–50. doi: 10.1016/j.cell.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Sousa E Melo F, Wang X, Jansen M, Fessler E, Trinh A, de Rooij LP, de Jong JH, de Boer OJ, van Leersum R, Bijlsma MF, et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. 2013;19:614–8. doi: 10.1038/nm.3174. [DOI] [PubMed] [Google Scholar]
- 17.Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A. 2010;107:15449–54. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003–11. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 19.Sayan AE, Griffiths TR, Pal R, Browne GJ, Ruddick A, Yagci T, Edwards R, Mayer NJ, Qazi H, Goyal S, et al. SIP1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer. Proc Natl Acad Sci U S A. 2009;106:14884–9. doi: 10.1073/pnas.0902042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao Z, Fenoglio S, Gao DC, Camiolo M, Stiles B, Lindsted T, Schlederer M, Johns C, Altorki N, Mittal V, et al. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci U S A. 2010;107:15535–40. doi: 10.1073/pnas.1009472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uramoto H, Shimokawa H, Hanagiri T, Kuwano M, Ono M. Expression of selected gene for acquired drug resistance to EGFR-TKI in lung adenocarcinoma. Lung Cancer. 2011;73:361–5. doi: 10.1016/j.lungcan.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Oliveras-Ferraros C, Corominas-Faja B, Cufí S, Vazquez-Martin A, Martin-Castillo B, Iglesias JM, López-Bonet E, Martin AG, Menendez JA. Epithelial-to-mesenchymal transition (EMT) confers primary resistance to trastuzumab (Herceptin) Cell Cycle. 2012;11:4020–32. doi: 10.4161/cc.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majewski IJ, Bernards R. Taming the dragon: genomic biomarkers to individualize the treatment of cancer. Nat Med. 2011;17:304–12. doi: 10.1038/nm.2311. [DOI] [PubMed] [Google Scholar]
- 25.Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, Shen L, Fan Y, Giri U, Tumula PK, et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res. 2013;19:279–90. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yauch RL, Januario T, Eberhard DA, Cavet G, Zhu W, Fu L, Pham TQ, Soriano R, Stinson J, Seshagiri S, et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res. 2005;11:8686–98. doi: 10.1158/1078-0432.CCR-05-1492. [DOI] [PubMed] [Google Scholar]
- 27.Thomson S, Buck E, Petti F, Griffin G, Brown E, Ramnarine N, Iwata KK, Gibson N, Haley JD. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65:9455–62. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- 28.Buck E, Eyzaguirre A, Barr S, Thompson S, Sennello R, Young D, Iwata KK, Gibson NW, Cagnoni P, Haley JD. Loss of homotypic cell adhesion by epithelial-mesenchymal transition or mutation limits sensitivity to epidermal growth factor receptor inhibition. Mol Cancer Ther. 2007;6:532–41. doi: 10.1158/1535-7163.MCT-06-0462. [DOI] [PubMed] [Google Scholar]
- 29.Frederick BA, Helfrich BA, Coldren CD, Zheng D, Chan D, Bunn PA, Jr., Raben D. Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non-small cell lung carcinoma. Mol Cancer Ther. 2007;6:1683–91. doi: 10.1158/1535-7163.MCT-07-0138. [DOI] [PubMed] [Google Scholar]
- 30.Zhao H, Desai V, Wang J, Epstein DM, Miglarese M, Buck E. Epithelial-mesenchymal transition predicts sensitivity to the dual IGF-1R/IR inhibitor OSI-906 in hepatocellular carcinoma cell lines. Mol Cancer Ther. 2012;11:503–13. doi: 10.1158/1535-7163.MCT-11-0327. [DOI] [PubMed] [Google Scholar]
- 31.Salazar R, Roepman P, Capella G, Moreno V, Simon I, Dreezen C, Lopez-Doriga A, Santos C, Marijnen C, Westerga J, et al. Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J Clin Oncol. 2011;29:17–24. doi: 10.1200/JCO.2010.30.1077. [DOI] [PubMed] [Google Scholar]
- 32.Roepman P, Schlicker A, Tabernero J, Majewski I, Tian S, Moreno V, Snel MH, Chresta CM, Rosenberg R, Nitsche U, et al. Colorectal cancer intrinsic subtypes predict chemotherapy benefit, deficient mismatch repair and epithelial-to-mesenchymal transition. Int J Cancer. 2013 doi: 10.1002/ijc.28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman E, Gold LI, Klimstra D, Zeng ZS, Winawer S, Cohen A. High levels of transforming growth factor beta 1 correlate with disease progression in human colon cancer. Cancer Epidemiol Biomarkers Prev. 1995;4:549–54. [PubMed] [Google Scholar]
- 34.Robson H, Anderson E, James RD, Schofield PF. Transforming growth factor beta 1 expression in human colorectal tumours: an independent prognostic marker in a subgroup of poor prognosis patients. Br J Cancer. 1996;74:753–8. doi: 10.1038/bjc.1996.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Céspedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–84. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsushima H, Ito N, Tamura S, Matsuda Y, Inada M, Yabuuchi I, Imai Y, Nagashima R, Misawa H, Takeda H, et al. Circulating transforming growth factor beta 1 as a predictor of liver metastasis after resection in colorectal cancer. Clin Cancer Res. 2001;7:1258–62. [PubMed] [Google Scholar]
- 37.Yang AD, Fan F, Camp ER, van Buren G, Liu W, Somcio R, Gray MJ, Cheng H, Hoff PM, Ellis LM. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006;12:4147–53. doi: 10.1158/1078-0432.CCR-06-0038. [DOI] [PubMed] [Google Scholar]
- 38.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–5. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R, Jr., Badve S, Nakshatri H. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 41.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhola NE, Balko JM, Dugger TC, Kuba MG, Sánchez V, Sanders M, Stanford J, Cook RS, Arteaga CL. TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest. 2013;123:1348–58. doi: 10.1172/JCI65416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.López-Díaz FJ, Gascard P, Balakrishnan SK, Zhao J, Del Rincon SV, Spruck C, Tlsty TD, Emerson BM. Coordinate transcriptional and translational repression of p53 by TGF-β1 impairs the stress response. Mol Cell. 2013;50:552–64. doi: 10.1016/j.molcel.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller LD, Smeds J, George J, Vega VB, Vergara L, Ploner A, Pawitan Y, Hall P, Klaar S, Liu ET, et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci U S A. 2005;102:13550–5. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benhattar J, Cerottini JP, Saraga E, Metthez G, Givel JC. p53 mutations as a possible predictor of response to chemotherapy in metastatic colorectal carcinomas. Int J Cancer. 1996;69:190–2. doi: 10.1002/(SICI)1097-0215(19960621)69:3<190::AID-IJC7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 46.Oscier DG, Gardiner AC, Mould SJ, Glide S, Davis ZA, Ibbotson RE, Corcoran MM, Chapman RM, Thomas PW, Copplestone JA, et al. Multivariate analysis of prognostic factors in CLL: clinical stage, IGVH gene mutational status, and loss or mutation of the p53 gene are independent prognostic factors. Blood. 2002;100:1177–84. [PubMed] [Google Scholar]
- 47.Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor-beta employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174:175–83. doi: 10.1083/jcb.200512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maestro R, Dei Tos AP, Hamamori Y, Krasnokutsky S, Sartorelli V, Kedes L, Doglioni C, Beach DH, Hannon GJ. Twist is a potential oncogene that inhibits apoptosis. Genes Dev. 1999;13:2207–17. doi: 10.1101/gad.13.17.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Araki S, Eitel JA, Batuello CN, Bijangi-Vishehsaraei K, Xie XJ, Danielpour D, Pollok KE, Boothman DA, Mayo LD. TGF-beta1-induced expression of human Mdm2 correlates with late-stage metastatic breast cancer. J Clin Invest. 2010;120:290–302. doi: 10.1172/JCI39194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirshner J, Jobling MF, Pajares MJ, Ravani SA, Glick AB, Lavin MJ, Koslov S, Shiloh Y, Barcellos-Hoff MH. Inhibition of transforming growth factor-beta1 signaling attenuates ataxia telangiectasia mutated activity in response to genotoxic stress. Cancer Res. 2006;66:10861–9. doi: 10.1158/0008-5472.CAN-06-2565. [DOI] [PubMed] [Google Scholar]
- 51.Pham CG, Bubici C, Zazzeroni F, Knabb JR, Papa S, Kuntzen C, Franzoso G. Upregulation of Twist-1 by NF-kappaB blocks cytotoxicity induced by chemotherapeutic drugs. Mol Cell Biol. 2007;27:3920–35. doi: 10.1128/MCB.01219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoshino Y, Katsuno Y, Ehata S, Miyazono K. Autocrine TGF-β protects breast cancer cells from apoptosis through reduction of BH3-only protein, Bim. J Biochem. 2011;149:55–65. doi: 10.1093/jb/mvq114. [DOI] [PubMed] [Google Scholar]
- 53.Ramesh S, Qi XJ, Wildey GM, Robinson J, Molkentin J, Letterio J, Howe PH. TGF beta-mediated BIM expression and apoptosis are regulated through SMAD3-dependent expression of the MAPK phosphatase MKP2. EMBO Rep. 2008;9:990–7. doi: 10.1038/embor.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]