Abstract
Adult stem cells are responsible for maintaining the balance between cell proliferation and differentiation within self-renewing tissues. The molecular and cellular mechanisms mediating such balance are poorly understood. The production of reactive oxygen species (ROS) has emerged as an important mediator of stem cell homeostasis in various systems. Our recent work demonstrates that Rac1-dependent ROS production mediates intestinal stem cell (ISC) proliferation in mouse models of colorectal cancer (CRC). Here, we use the adult Drosophila midgut and the mouse small intestine to directly address the role of Rac1 in ISC proliferation and tissue regeneration in response to damage. Our results demonstrate that Rac1 is necessary and sufficient to drive ISC proliferation and regeneration in an ROS-dependent manner. Our data point to an evolutionarily conserved role of Rac1 in intestinal homeostasis and highlight the value of combining work in the mammalian and Drosophila intestine as paradigms to study stem cell biology.
Keywords: Rac1, intestinal stem cells, Drosophila, mouse, ROS, regeneration
Introduction
Constraining the production of intracellular ROS is critical to maintain homeostatic balance of stem cell-based tissues across species.1-3 Uncontrolled ROS production leads to stem cell hyperproliferation, misdiferentiation, and loss of regenerative capacity in various systems.4-6 Consistently, elevated ROS levels are often associated with cancer and aging.7 However, the cellular and molecular mechanisms leading to ROS production in hyperplastic conditions remain largely unknown.
The intestinal epithelium is constantly replenished by pluripotent ISCs. The remarkable self-renewing capacity of the intestine seems to impact in its high propensity for malignant transformation.8 ROS are emerging as conserved mediators of intestinal proliferation. Our recent work in the vertebrate intestine shows that ROS is elevated within the proliferative compartment of the intestinal epithelium in a mouse model of CRC driven by loss of the tumor suppressor adenomatous polyposis coli (Apc).9 Treatment with N-acetyl-cysteine (NAC) strongly suppressed intestinal hyperproliferation and stem cell upregulation in Apc-deficient mice.9 Modulation of the redox balance in the adult intestine of the fruit fly Drosophila melanogaster directly impacts ISC proliferation. Flies fed with the ROS-inducing compound paraquat or deficient for the ROS-detoxifying enzyme catalase display significant increases in ISC proliferation, leading to intestinal hyperplasia6,10,11. On the other hand, treating flies with NAC is sufficient to limit ISC proliferation.12
The cellular and molecular regulators of ROS production within stem cells remain largely unexplored. We recently reported that the small GTPase RAC1, which modulates multiple cellular processes and pathways, including ROS production,13 mediates ROS increase and intestinal proliferation in Apc-deficient mice.9 However, the functional role of RAC1 within stem cells remains to be addressed. Here we use the posterior adult Drosophila midgut (Fig. 1A) and the mouse small intestine to directly address the role of Rac1 in ISCs by means of gain and loss of function experiments. Our results demonstrate that Rac1 activation in ISCs is necessary and sufficient to drive ISC proliferation and damage-induced intestinal regeneration in an ROS-dependent manner.
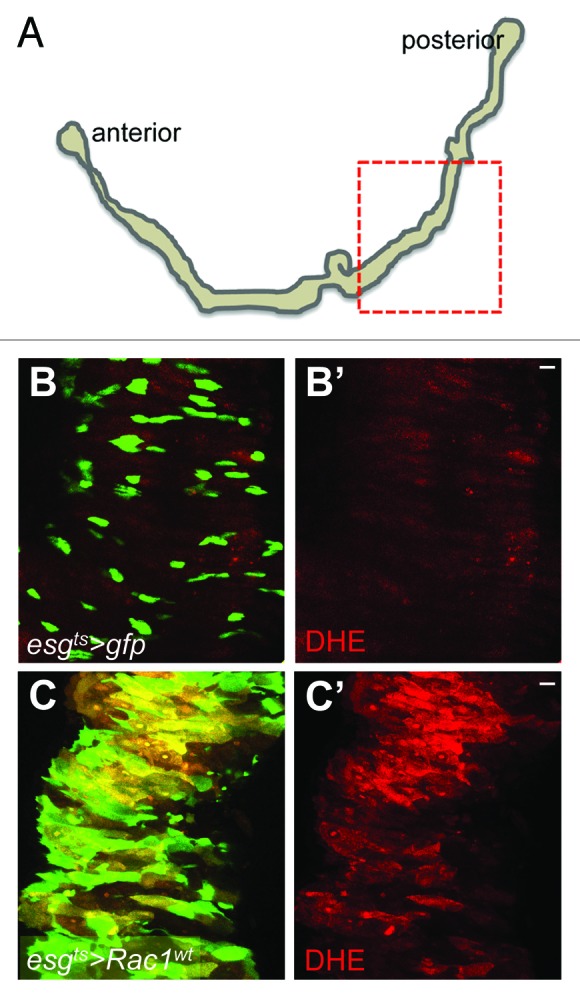
Figure 1. Rac1 overexpression in ISCs drives ROS production in the adult Drosophila midgut. (A) Tracing of an adult Drosophila gastrointestinal tract. The dotted box highlights the region of the posterior midgut, which was used for our studies. (B–C') Posterior midguts of animals incubated at 29 °C during 2 d to induce the expression of gfp only (B and B') or gfp and Rac1wt (C and C') under the control of the intestinal stem cell (ISC)/enteroblast (EB) diver escargot-gal4 (esgts > gfp and esgts > Rac1wt, respectively). Midguts were dissected and stained with DHE (red) to detect ROS production and anti-GFP (green) to label esg+ve cells. Scale bars: 20 μm.
Results and Discussion
Rac1 overexpression in ISCs drives ROS production in the adult Drosophila midgut
The epithelium of the posterior adult Drosophila midgut is replenished by ISCs.14,15 Each ISC proliferates to give rise to an uncommitted enteroblast (EB), which will differentiate into either an enterocyte (EC) or an enteroendocrine cell (ee). ISCs are the only proliferative cells within the adult fly posterior midgut.
Our recent work shows that deletion of Rac1 suppresses intestinal hyperproliferation and ROS production in Apc-deficient mice.9 We therefore first asked whether Rac1 is sufficient to drive ROS production within ISCs in the Drosophila midgut. We used the UAS/Gal4 system16 to specifically overexpress Drosophila Rac1 in ISCs/EBs (progenitor cells) using the temperature-controlled escargot-gal4, UAS-gfp; tubulin-gal80ts driver (esgts > gfp).14 Overexpression of Rac1 resulted in a dramatic expansion of the esg > gfp cell population and increased ROS production in the midgut (Fig. 1B–C'). These results suggest that Rac1 overexpression in progenitor cells is sufficient to drive ROS production within the intestinal epithelium.
Rac1 overexpression leads to ROS-dependent ISC hyperproliferation in the adult Drosophila midgut
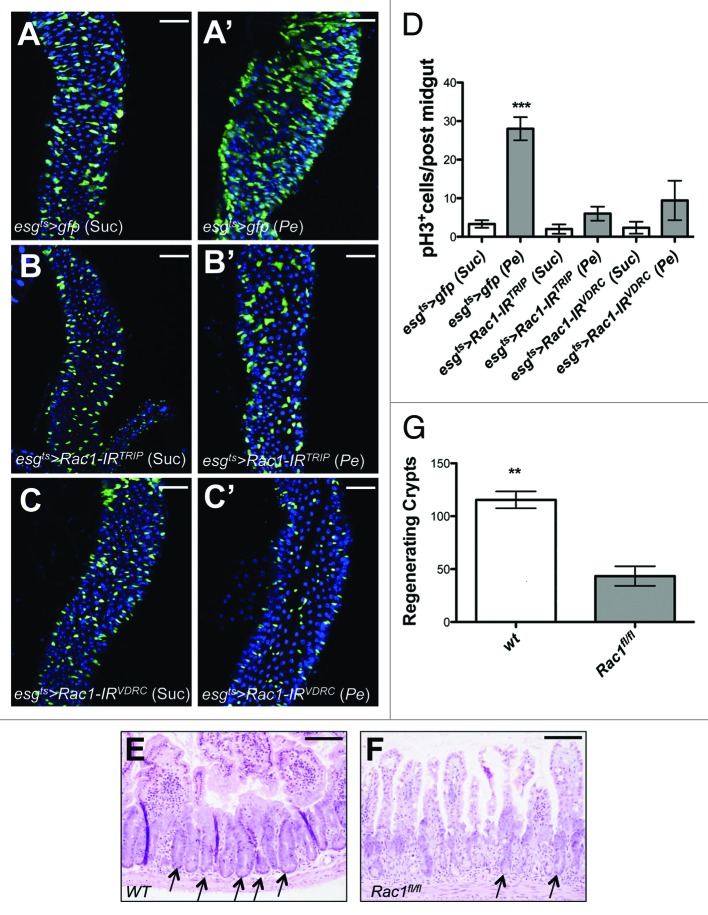
The epithelium of the adult posterior Drosophila midgut has a remarkable regenerative capacity. Damage induced by agents such as bacterial infection, Bleomycin, or dextran sodium sulfate (DSS) treatment leads to activation of ISC proliferation to regenerate the damaged intestinal epithelium.12,17-19 Previous work demonstrated that ROS production is essential for damaged-induced ISC proliferation in the fly midgut.12 We therefore asked whether ROS upregulation was important for the phenotype resulting from Rac1 overexpression in the midgut. Consistent, with the previous report12 preventing ROS production by NAC impaired ISC proliferation in posterior midguts from flies infected with the pathogenic bacteria Pseudomonas entomophila (Pe) (Fig. 2A–C' and F). Importantly, NAC treatment strongly suppressed ISC hyperproliferation in Rac1-overexpressing midguts (Fig. 2D–E' and F). These results suggest that ROS production is essential for Rac1-dependent ISC hyperproliferation in the intestine.
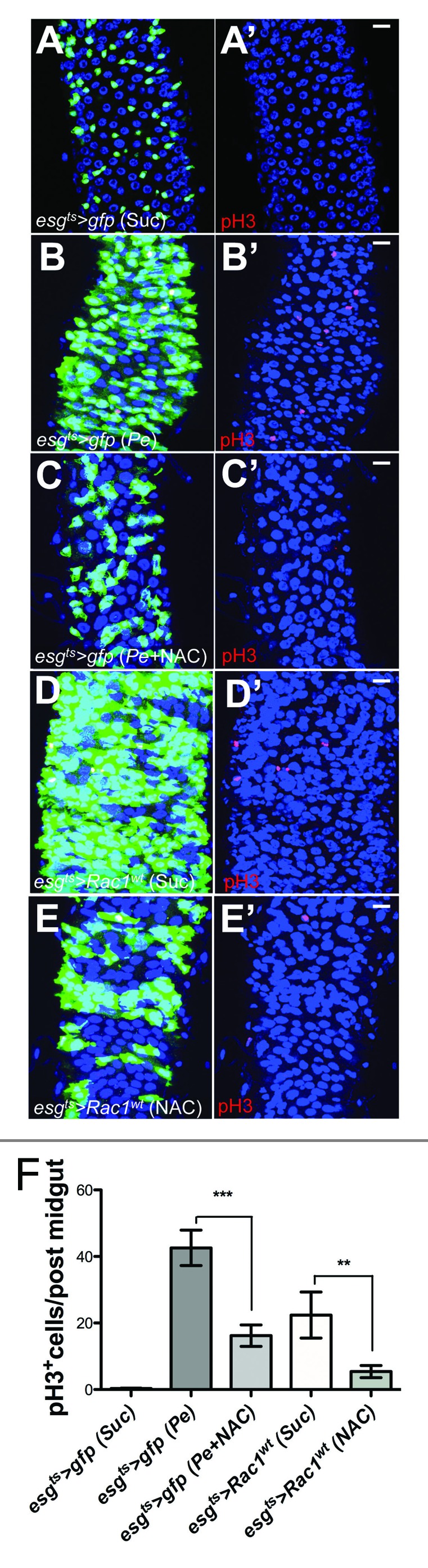
Figure 2. Rac1 overexpression leads to ROS-dependent ISC hyperproliferation in the adult Drosophila midgut. (A–E') Posterior midguts from esgts > gfp and esgts > Rac1wt animals fed with Sucrose (A, A', D, and D'); Pe (B and B'); Pe + NAC (C and C'), or NAC only (E and E'). Midguts were dissected and stained with anti-GFP (green; left panels) to label esg+ve cells and anti-pH3 to visualize proliferating ISCs (red; right panels). DAPI (blue) labels all cell nuclei. (F) pH3 counts of posterior midguts of animals as in (A–E') (***P < 0.0001; ** P < 0.001 one-way ANOVA with Bonferroni multiple comparison test). Scale bars: 20 μm.
Rac1 is required for intestinal regeneration in the Drosophila and mouse intestine
We finally asked whether Rac1 was necessary to drive ISC proliferation in response to damage. This is a question, which also derives from our previous work in the mammalian intestine.9 We used the genetic approach described in Figure 1 to knockdown Rac1 within progenitors cells of the Drosophila midgut by RNA interference (RNAi) (esgts > Rac1-IR). Knockdown of Rac1 by 2 independent RNAi lines (Fig. 3B–C') resulted in almost complete suppression of ISC proliferation in regenerating posterior midguts subject to Pe infection (Fig. 3B–D; compare with Fig. 3A, A', and D). Similar to the Drosophila midgut, the mammalian intestine displays a remarkable regenerative capacity following damage.20 We therefore addressed if the requirement for Rac1 during intestinal regeneration is conserved across these species. We conditionally deleted Rac1 from the mouse intestinal epithelium using the vil-Cre-ERT2 and tested the effect of Rac1 loss on tissue regeneration upon DNA damage (see “Materials and Methods”). Consistent with our results in the fly midgut, Rac1 deletion significantly suppressed regeneration in the mouse intestinal epithelium (Fig. 3E–G).
Figure 3. Rac1 is required for intestinal regeneration in Drosophila and mice. Posterior midgut from control animals (esgts > gfp;A and A') or animals overexpressing 2 independent Rac1 RNAi lines in ISCs/EBs for 10 d (esgts > Rac1-IR;B–C') followed by Sucrose (A, B, and C) or Pe (A', B', and C') feeding. Midguts were dissected and stained with anti-GFP (green) and DAPI (blue). (D) pH3 counts of posterior midguts as in (A–C') (***P < 0.0001 one-way ANOVA with Bonferroni multiple comparison test). Scale bars: 50 μm. (E and F) Control (E) and Rac1-deficient mouse small intestines (F) subject to intestinal regeneration by DNA damage. Arrows point to regenerating crypts. Scale bars: 100 μm. (G) Quantification of the number of regenerating crypts in animals as in (E and F) (**P = 0.0025 unpaired t-test).
Altogether, our results suggest a central conserved role for the small GTPase RAC1 as a driver of ISC proliferation through the production of ROS. These data highlight RAC1 as key player and potential therapeutic target for conditions linked to oxidative stress such as cancer and aging.
Materials and Methods
Fly stocks
esgts > gfp (Shigeo Hayashi)
UAS-Rac1wt (Bloomington stock: 6293)
UAS-Rac1-IRTRIP (Rac1 RNAi) (Bloomington Stock: 34910)
UAS-Rac1-IRVDRC (VDRC stock: 49246)
Fly maintainace and genetics
Crosses were maintained at 18 °C in standard medium. Only posterior midguts of female flies were analyzed in this study (Fig. 1A). Animals of the desired genotypes were collected within 48–72 h of eclosion, and then switched to 29 °C incubators to activate the expression of the desired transgenes under the control of gal4/gal80ts. Fly food was changed every other day. The phenotype of Rac1 overexpression was analyzed 2 d after transgene activation. The requirement of Rac1 during intestinal regeneration was assessed following 10 d of RNAi overexpression.
Fly Genotypes
yw; escargot-gal4, UAS-gfp/+; tub-gal80ts/+
yw; escargot-gal4, UAS-gfp/UAS-Rac1wt; tub-gal80ts/+
UAS-Dicer2/+; escargot-gal4, UAS-gfp/+; tub-gal80ts/ UAS-Rac1-IRTRIP
UAS-Dicer2/+; escargot-gal4, UAS-gfp/UAS-Rac1-IRVDRC; tub-gal80ts/+
Mouse genotypes
All experiments were performed under the UK Home Office guidelines. Experiments were performed on mice of mixed background (50% C57Bl6J, 50% S129). The alleles used were: vil-Cre-ERT221 and Rac1fl.22
Histology and tissue analysis
Immunofluorescence
Tissues were dissected in PBS and fixed 30–45 min in 4% para-formaldehyde (Polysciences, Inc). After fixation, samples were washed 3 times in PBS + 0.1% TritonX-100 (PBST) and incubated in primary antibodies over night at 4 °C. Samples were then washed as described and subjected to secondary antibody staining for 2 h at room temperature followed by washing and mounting on Vectashield containing DAPI (Vector Laboratories, Inc). Primary and secondary antibodies were incubated in PBST+ 0.5% BSA. Primary antibodies used: chicken anti-GFP 1:4,000 (Abcam); rabbit anti-pH3 S10 and S28 1:100 (Cell Signaling). Secondary antibodies used: Alexa 488 1:200 and Alexa 594 1:100 (Invitrogen).
Confocal images were captured under a Zeiss 710 Confocal microscope using 20X (Fig. 3) and 40X (Figs. 1 and 2) lenses and 1.0 optical zoom. Images were processed with Adobe photoshop CS.
Detection of ROS by DHE staining
DHE staining was done as described in ref. 6. Briefly, fly guts freshly dissected in Schneider medium (HyClone) were incubated in medium containing 30 μM DHE (Invitrogen) for 5 min at room temperature and protected from light. Tissues were then washed, mounted, and immediately imaged.
Tissue analysis of mouse small intestines
Standard histological techniques were used throughout. Tissue was fixed in 4% neutral buffered formalin and stained with hematoxylin and eosin. Slides were analyzed using an Olympus BX51 microscope and images taken with a DP70 camera.
Fly feeding experiments
Pe feeding experiments were done as previously described.23 Briefly, flies were subject to starvation by placing them in empty vials at 29 °C for 2 h prior to feeding them the different agents. Animal were then kept at 29 °C and fed either 5% sucrose (Suc); 10× overnight Pseudomona entomophila culture in 5% sucrose (Pe); NAC (10 mM final concentration in 5% sucrose; Sigma) (NAC); or 10× overnight (Pe) + NAC (10 mM final concentration) (Pe + NAC) on filter-paper discs (Whatman). NAC treatment was done for 2 d, and Pe infection was done for 1 d during the last day of incubation. Media were changed daily. Guts were then dissected and analyzed using immunofluorescence and confocal imaging. esgts > Rac1wt animals were treated with Sucrose or NAC during transgene activation.
Mouse intestinal regeneration
Conditional deletion of Rac1 was induced by single intraperitoneal injections of 80 mg/kg tamoxifen on 2 consecutive days. Intestinal regeneration was induced by irradiating mice with 8 Gy gamma-irradiation the day after the first tamoxifen injection. Mice were sacrificed 72 h post irradiation and the small intestine isolated and flushed with tap water. 10× 1 cm portions of small intestine were bound together with surgical tape and fixed in 4% neutral buffered formalin. Hematoxylin and eosin stained sections were used for analysis. Crypts were scored as regenerating if they contained 6 or more consecutive cells. The average number of regenerating crypts across cross sections of the 10 gut pieces was used for statistical analysis. Three control and four Rac1-deleted mice were used for this experiment.
Quantifications and statistics
We analyzed between 5–10 posterior fly midguts and 3–4 mouse small intestines in each experiment. Quantification of the data was presented in bar graphs created with Graphpad Prism 5. Data represents average values ± SEM. We used one-way ANOVA with Bonferroni multiple comparison test and unpaired t test to calculate statistical significance in our fly and mouse experiments, respectively.
Acknowledgments
We thank our colleagues, the VDRC and Bloomington stock centers and the Developmental Studies Hybridoma Bank for providing fly lines and reagents. MV and OJS are Cancer Research UK investigators. This work was partly funded by a NC3Rs grant to JBC, MV, and OJS. JBC is funded by Marie Curie and EMBO fellowships.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/26031
References
- 1.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 2.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–41. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsatmali M, Walcott EC, Crossin KL. Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res. 2005;1040:137–50. doi: 10.1016/j.brainres.2005.01.087. [DOI] [PubMed] [Google Scholar]
- 4.Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1:140–52. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–12. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8:188–99. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitagishi Y, Matsuda S. Redox regulation of tumor suppressor PTEN in cancer and aging (Review) Int J Mol Med. 2013;31:511–5. doi: 10.3892/ijmm.2013.1235. [Review] [DOI] [PubMed] [Google Scholar]
- 8.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–9. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 9.Myant KB, Cammareri P, McGhee EJ, Ridgway RA, Huels DJ, Cordero JB, Schwitalla S, Kalna G, Ogg EL, Athineos D, et al. ROS production and NF-κB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell. 2013;12:761–73. doi: 10.1016/j.stem.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–55. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi Y-J, Hwang M-S, Park J-S, Bae S-K, Kim Y-S, Yoo M-A. Age-related upregulation of Drosophila caudal gene via NF-kappaB in the adult posterior midgut. Biochim Biophys Acta. 2008;1780:1093–100. doi: 10.1016/j.bbagen.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–44. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellenbroek SI, Collard JG. Rho GTPases: functions and association with cancer. Clin Exp Metastasis. 2007;24:657–72. doi: 10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- 14.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–9. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 15.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–4. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 16.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 17.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–11. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–55. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashton GH, Morton JP, Myant K, Phesse TJ, Ridgway RA, Marsh V, Wilkins JA, Athineos D, Muncan V, Kemp R, et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev Cell. 2010;19:259–69. doi: 10.1016/j.devcel.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–93. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 22.Walmsley MJ, Ooi SK, Reynolds LF, Smith SH, Ruf S, Mathiot A, Vanes L, Williams DA, Cancro MP, Tybulewicz VL. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–62. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- 23.Cordero JB, Stefanatos RK, Scopelliti A, Vidal M, Sansom OJ. Inducible progenitor-derived Wingless regulates adult midgut regeneration in Drosophila. EMBO J. 2012;31:3901–17. doi: 10.1038/emboj.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]