Abstract
This article reviews recent significant advances in the design of nanofiber scaffolds for orthopedic tissue repair and regeneration. It begins with a brief introduction on the limitations of current approaches for orthopedic tissue repair and regeneration. It then illustrates that rationally designed scaffolds made up of electrospun nanofibers could be a promising solution to overcome the problems that current approaches encounter. The article also discusses the intriguing properties of electrospun nanofibers, including control of composition, structures, orders, alignments and mechanical properties, use as carriers for topical drug and/or gene sustained delivery, and serving as substrates for the regulation of cell behaviors, which could benefit musculoskeletal tissue repair and regeneration. It further highlights a few of the many recent applications of electrospun nanofiber scaffolds in repairing and regenerating various orthopedic tissues. Finally, the article concludes with perspectives on the challenges and future directions for better design, fabrication and utilization of nanofiber scaffolds for orthopedic tissue engineering.
Keywords: design, electrospinning, nanofiber scaffold, orthopedic tissue, regeneration, repair, tissue engineering
Injuries frequently occur in the musculoskeletal system, accounting for 60–67% of all unintentional injuries in the USA per annum [1]. It has been reported that more than 34 million musculoskeletal-related surgeries are performed each year in the USA [2]. Clinically, the main options available for the surgical treatment of musculoskeletal injuries include: transplantation of autografts/allografts and utilization of synthetic substitutes composed of metals, ceramics and/or polymers. However, each strategy suffers from a number of limitations. For example, the benefits of autografts are counterbalanced by function loss at the donor sites, scar tissue formation, structural differences between donor and recipient grafts preventing successful regeneration, and the shortage of graft material for extensive repair. Patients receiving transplantation of allografts are at risk of immune rejection and transmitting infectious diseases. Synthetic substitutes, such as metal transplants, mainly serve as a replacement for damaged tissues or organs rather than as a platform for repair and regeneration of tissue defects, and they are often associated with issues such as poor integration with surrounding tissue and infection [3,4].
To overcome the limitations associated with these approaches, regenerative medicine has emerged as a promising strategy for developing functional tissue constructs to repair, regenerate and restore damaged musculoskeletal tissues or organs [5]. Three general strategies have been adopted for the creation of tissue constructs: to use isolated cells or cell substitutes; to use acellular biomaterials/scaffolds that are capable of inducing tissue regeneration in vivo; and to use a combination of cells and materials typically in the form of scaffolds [6,7]. It is critical to design and fabricate a suitable scaffold for use in specific tissue regeneration, as it directly comes into contact with cells, and provides structural support and guidance for subsequent tissue development [8]. Towards this end, more and more attention has been paid to the design of scaffolds for guiding cell behaviors and tissue regeneration. One effective approach to obtain scaffolds is to harvest the remaining extracellular matrix (ECM) from a decellularized donor organ. The harvested ECM seeded with patient-specific cells could create functional tissue constructs. However, the availability of donor organs remains a stringent limitation. This limitation has encouraged researchers to develop tissue constructs using synthetically derived biomaterials/scaffolds.
The design of scaffolds should be based on knowledge learned from native tissues, such as their anatomic structures, compositions and functions. Generally, the ECM is composed of a complex meshwork of proteins and proteoglycans. Collagen is one of the major components of the ECM, existing in many different types depending on its tissue of origin, and often forming nanofibers. Integrating nanofiber features is particularly important for recapitulating the ECM architecture in the design and fabrication of scaffolds that host multiple cell types, and precisely define cell–cell and cell–matrix interactions in a 3D environment. Thus, nanofiber materials play a paramount role in tissue repair and regeneration. The development of nanotechnology allows for the fabrication of nanofiber scaffolds that are characterized by a nanoscale diameter, high surface area:volume ratio and high porosity. The interconnected porous structure of nanofiber scaffolds provides a large surface area for cell attachment and sufficient space for nutrient and waste exchange. Until now, several approaches have been developed for fabricating nanofiber scaffolds, such as temperature-induced phase separation, molecular self-assembly, template synthesis, drawing and electrospinning. Among these, electrospinning is a cost-effective approach for producing polymeric fibers from a variety of polymer melts and solutions. This is a simple, robust and versatile technique that is capable of producing fibers with nanoscale diameters [9]. Studies have shown that electrospun nanofiber scaffolds hold great promise for musculoskeletal tissue repair and regeneration [10–12].
The aim of this article is to provide a review of recent studies that are related to the use of electrospun nanofiber scaffolds for orthopedic tissue repair and regeneration. We first describe how electrospun nanofibers can be designed to meet the specific requirements for repairing and regenerating musculoskeletal tissues. We also highlight the recent applications of electrospun nanofibers in repairing and regenerating a number of musculoskeletal tissues, including bone, tendon, cartilage, meniscus, intervertebral disc (IVD) and the tendon-to-bone insertion site. We conclude this article with a perspective on the challenges and future directions in designing better nanofiber scaffolds for orthopedic tissue engineering.
Why electrospun nanofibers?
Control of diameter, composition, structure, alignment, order & porosity
Electrospinning is a versatile technique capable of generating continuous nanofibers. The diameter of electrospun fibers, typically ranging from several nanometers to micrometers, can be readily controlled by adjusting the operating parameters (i.e., feeding rate, applied voltage, and the distance between the nozzle and collector) and electrospinning solution properties (i.e., molecular weight, concentration, conductivity, dielectric constant and surface tension) [13]. The composition of fibers can be easily tailored by making using different polymers, composite materials or encapsulations during electrospinning. Currently, over 100 types of polymers, including naturally occurring matrix proteins and synthetic polymers, have been used to produce nanofibers using the electrospinning technique. In addition, the composition can be further modified by post-treatment (i.e., surface modifcation and thermal treatment). The materials for use in the fabrication of nanofiber tissue regeneration listed in Table 1 have been approved by the US FDA and applied for various purposes in clinics [14]. Various secondary structures (i.e., porous, hollow, core-sheath and multicompartments) can also be achieved using phase separation or specially designed configurations of spinnerets. In addition, many shapes of fibers (i.e., beaded and belt-shaped fibers) can be generated by controlling the concentration of solution and molecular weight of polymers. Alignment (i.e., uniaxially aligned, radially aligned or wavy) of electrospun fibers can be readily obtained by utilizing mechanical force, or manipulation of the electrical field or magnetic field [15–17]. The order of fibers or 3D architecture can be controlled by layer-by-layer stacking, 3D weaving and template deposition. For example, Figure 1A shows an electrospinning setup in our laboratory that is routinely used to fabricate nanofibers with a core–sheath structure. By using specially designed collectors, various nanofiber assemblies/scaffolds can be generated, including random fiber mat, uniaxially aligned fiber mat, random-to-aligned fiber mat, radially aligned fiber mat and fiber yarns/bundles (Figure 1B–G). One limiting factor of conventional electrospinning is that electrospun nanofiber mats consist entirely of tightly packed nanofibers layers with low porosity, which limits oxygen and nutrient diffusion, further hindering cell penetration. However, recent advances in fabrication allow the modulation of electrospun nanofiber scaffolds pore size by increasing fiber diameter or selectively removing sacrificial fibers [18–21].
Table 1.
Scaffold materials in clinical use.
| Polymer | Melting point (°C) | Processing method | Approximate degradation time (months) | Area of application and references | Products with regulatory approval |
|---|---|---|---|---|---|
| Poly(lactide) | 60–65 | Extrusion, injection moulding, compression moulding, solvent casting | 6–12 | Orthopedic surgery, and oral and maxillofacial surgery | Fix Sorb® (Takiron Co., Ltd, Osaka, Japan; screws, nails, pins), Neofix® (Nicca USA Inc., CA, USA; screws, nails, pins), Bio-Tenodesis® interference screw (Arthrex, FL, USA), Bio-Corkscrew® suture anchor (Arthrex), SmartScrew® (Conmed Linvatec, NY, USA), SmartNail® (Conmed Linvatec), SmartTack® (Conmed Linvatec), SmartPin® (Conmed Linvatec), BioScrew® (Conmed Linvatec), Bio-statak® (Zimmer, IN, USA; suture anchor), prostatic stent, suture anchor, bone cement plug |
| Poly(glycolide) | 225–230 | Extrusion, injection moulding, compression moulding, solvent casting | >24 | Orthopedic surgery, general surgery and suture | Biofix® screws (BD Biosciences, NJ, USA), Dexon™ sutures and mesh (Covidien, Dublin, Ireland), Bondek® suture (Teleflex, NC, USA), Valtrac™ anastomosis ring and prostatic stent (Covidien) |
| Polycaprolactone | ∼60 | Extrusion, injection moulding, compression moulding, solvent casting | >24 | Drug delivery, sutures | Capronor® (Research Triangle Institute, NC, USA), Monocryl® suture (Ethicon, NJ, USA) |
| Poly(d,l-lactide-co-glycolide) Poly(d,l-lactide-co-glycolide) 85:15 Poly(d,l-lactide-co-glycolide) 82:18 Poly(d,l-lactide-co-glycolide) 10:90 |
Amorphous | Extrusion, injection moulding, compression moulding, solvent casting | 5–6 | Sutures, drug delivery, oral and maxillofacial surgery, general surgery, and periodontal surgery | Polysorb™ sutures (Covidien), Biologically Quiet™ (Instrument Makar Inc., MI, USA), interference screw and staple (Instrument Makar Inc.; lactide:glycolide ratio 85:15), Lactosorb® (Biomet, IN, USA; screw, plates, mesh, surgical clip, pins, anchor), vicryl suture (Ethicon), vicryl mesh (Ethicon) |
| Poly(llactide-co-d,l-lactide) 98:2 Poly(llactide-co-d,l-lactide) 50:50 Poly(llactide-co-d,l-lactide) 70:30 Poly(llactide-co-d,l-lactide-co-llactide) |
Amorphous | Extrusion, injection moulding, compression moulding, solvent casting | 12–16 | Orthopedic surgery, oral and maxillofacial surgery, BD Biosciences (no clinical approval) | Phusis line® interference screw (Phusis materiaux bioresorbables, Le Versound, France), Sysorb® screw (Sulzer, Winterthur, Switzerland; l-lactide:d,l-lactide ratio 50:50), Resor Pin® (Geistlich biomaterial, Wolhusen, Switzerland; l-lactide:d,l-lactide ratio 70:30), Macrosorb® system (MacroPore Inc., CA, USA; screws, plates, mesh, nails, pins; l-lactide:d,l-lactide ratio 70:30), Protego FX screw no hits and no net (MacroPore Inc.), Polypin® (Zimmer), Leadfix® (Zimmer), 3D OPLA® (open-cell polylactic acid) scaffold (BioMed Diagnostics, Inc., OR, USA) |
| Polydioxanone | 58–63 | Extrusion, injection moulding, compression moulding, solvent casting | >24 | Orthopedic surgery, general surgery and sutures | Ethipin (Ethicon), Orthosorb® suture mesh foils (Biomet Inc., IN, USA), bone cement plug (Biomet Inc.) |
| Poly(d,l-lactide-co-caprolactone) 65:35 | Amorphous | Dip coating from chloroform | 24 | Nerve regeneration | Neurolac® (Polyganics, Groningen, The Netherlands) |
| Polycaprolactone-based composite containing dimethacylate monomers | 60 | – | – | Oral surgery | Resilon™ root canal filling (Resilon Research LLC, CT, USA) |
| Polycaprolactone-based polyurethane | Amorphous | – | – | Tissue reinforcement, torn tendon replacement patch and interpositional spacer in osteoarthritis | Artelon® Sportmesh™ (Artimplant, Västra Frölunda, Sweden), Artelon® CMC Spacer Arthro (Artimplant) |
| Lactide-co-caprolactone | – | – | – | To prevent adhesion | Mesofol® surgical sheet (Biomet Inc.) |
Reproduced with permission from [14].
Figure 1. Setup and collectors for electrospinning in the laboratory.
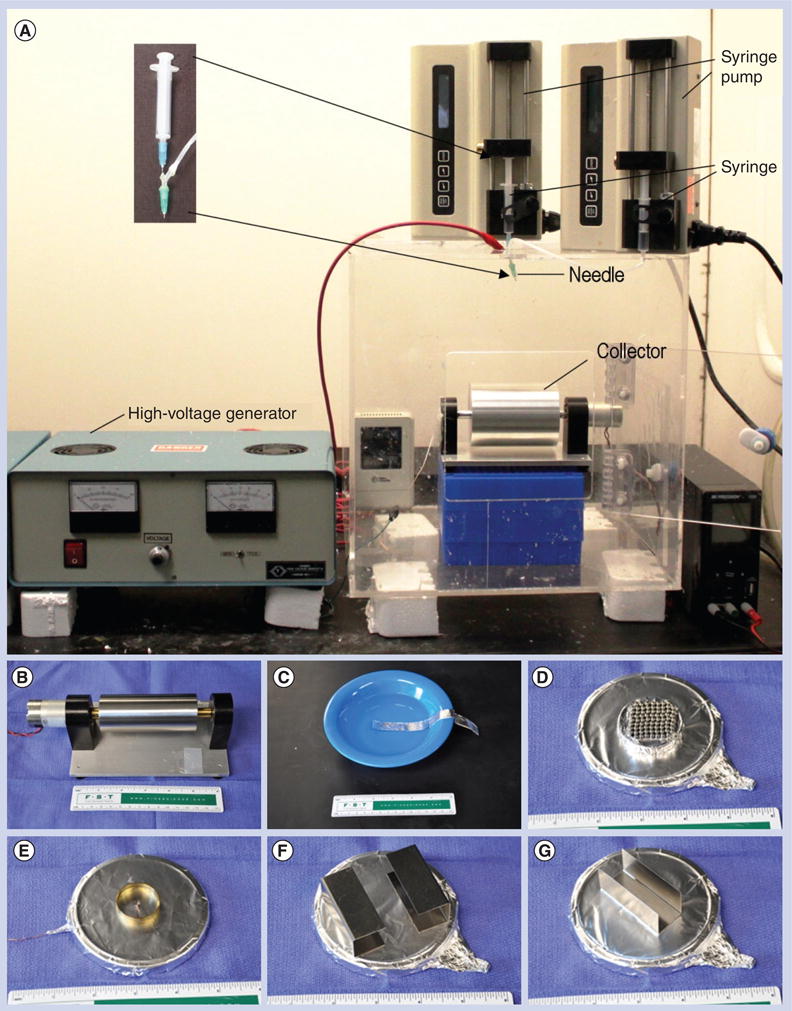
(A) A typical setup for coaxial electrospinning containing three major components: a high voltage generator, a coaxial spinneret (customized spinneret; inset) and a collector (a rotating metal drum). Nanofiber assemblies with different degrees of alignment can be achieved using the rotating drum as a collector through the control of rotating speed. (B–G) Examples of customized collectors that are commonly used in the laboratory for the fabrication of different assemblies of nanofibers including uniaxially aligned, fiber bundles or yarns, arrayed microwell, radially aligned and random to aligned.
Control of mechanical properties
The mechanical property of electrospun nanofibers may play an important role in musculo-skeletal tissue regeneration, as previous studies have demonstrated that mechanical properties (i.e., elastic modulus) can greatly influence cellular behaviors [22–24]. An important biological function of most musculoskeletal tissues is load bearing. Therefore, the purpose of controlling the mechanical properties of electrospun nanofibers lies in regulating cell response and matching the mechanical property of musculoskeletal tissues. Fortunately, the mechanical properties of electrospun nanofibers can be tailored by post-treatment (i.e., thermal treatment), and by varying the composition (i.e., different types of polymers, polymer blend, surface modification and encapsulation), operating conditions (i.e., humidity and rotating mandrel speed), size of individual nanofibers, and their alignment and density of the nanofibers. For example, thermal treatment (annealing) was successfully applied to manipulate the mechanical properties of electrospun nanofibers [25,26]. Thermal treatment to electrospun nanofibers often leads to thermal fiber bonding or an increase in the degree of crystallinity. Thermal fiber bonding can result in a significant increase of tensile strength and elongation of poly(ε-caprolactone) (PCL) fibers. The increase in the degree of crystallinity could cause higher Young's modulus, and ultimate stress and smaller strain [27]. It was reported that scaffolds made of poly(l-lactide-co-gly-colide) (PLGA) nanofibers can be ten-times stiffer than scaffolds made of PCL nanofibers. Various surface coatings (i.e., polydopamine and calcium phosphate) were also used to enhance the mechanical properties of electrospun nanofibers [28–30]. Separate studies have shown that the encapsulation of different types of substances (i.e., NaCl, bovine serum albumin, retinoic acid, nanoparticles, nanotubes and nanowires) can modulate the mechanical property of electrospun nanofibers [31–36]. It is also known that a reduction in the size of the fibers could improve the structure and orientation of the material, and reduce the size and quantity of defects, usually leading to higher modulus and strength [37–41]. Reducing fiber diameters from approximately 5 μm to 200 nm was demonstrated to increase both the strength and stiffness of fiber scaffolds. The strength of fibers is normally increased at the expense of the nanofibers strain at failure and toughness. However, one recent study demonstrated that reduction of the polyacrylonitrile fiber's diameter from 2.8 μm to approximately 100 nm could result in simultaneous increases in elastic modulus, true strength and toughness of the fiber [42]. Humidity during electrospinning can be tuned to tailor the mechanical properties of nanofibers, as humidity may affect the diameter and porosity of the obtained fibers [43]. Improved rotating speed of a mandrel has been shown to induce a highly ordered molecular structure in an electrospun poly(l-lactide) (PLLA) fiber, which consequently led to higher tensile modulus and strength [44]. In addition, recent studies showed higher modulus and ultimate stress in the aligned PLGA nanofiber scaffolds relative to their random counterparts. Toughness (the area under the stress–strain curve) was also significantly higher in the aligned scaffolds compared with the random scaffolds (aligned: 142.5 ± 97.9 MPa; random: 52.7 ± 24.2 MPa) [45–47].
Electrospun nanofibers as carriers for topical drug/gene/cell delivery
Signaling molecules (i.e., drugs, proteins, growth factors and DNA), one of the basic elements for regenerative medicine, can be incorporated into electrospun nanofibers for topically sustained delivery at a controlled rate [48,49]. The sustained exposure to signaling molecules could result in enhanced function of cells, and thus promote tissue regeneration. The sustained release of bioactive proteins or genes from nanofibers has shown great potential for applications in musculoskeletal tissue regeneration. Sahoo et al. demonstrated that bFGF could be successfully incorporated into electrospun nanofibers in the form of random dispersion, and released in a bioactive form over a 1-week period. The released bioactive bFGF activated tyrosine phosphorylation signaling within seeded bone marrow stem cells [50]. Fu et al. encapsulated BMP-2 in 3D PLGA/hydroxylapatite composite fiber scaffolds by electrospinning for bone regeneration [51]. The sustained release property was confirmed in vivo by measuring the BMP-2 concentration in serum for 6 weeks after implantation of the scaffolds. Furthermore, it was demonstrated that the bioactivity of BMP-2 released from the PLGA/hydroxylapatite composite fiber scaffolds was well preserved, which could contribute to the improvement in new bone formation and healing of segmental defects in vivo. The same group also examined BMP-2 plasmid encapsulation inside PLGA/hydroxylapatite composite nanofibers and its release from the scaffolds [52]. The results revealed that the bioactivity of the BMP-2 plasmid released from the scaffolds was well maintained, which eventually helped improve the healing of bone segmental defects in vivo.
Electrospun fibers with topically sustained release of antibiotics have desirable utility in repairing and regenerating musculoskeletal tissues, particularly in the prevention of postsurgical infections. Zeng et al. encapsulated rifampin in PLLA fibers and examined its release in vitro [53]. It was found that the release of rifampin in the presence of proteinase nearly followed zero-order kinetics due to the gradual degradation of the PLLA fibers. Kim et al. demonstrated the successful encapsulation and sustained release of a hydrophilic antibiotic drug, cefoxitin sodium [54]. In separate studies, Shi et al. dispersed amoxicillin-loaded nano-hydroxyapatite or laponite particles into PLGA nanofibers to form hybrid nanofibers, which presented a sustained release profile and noncompromised activity to inhibit the growth of a model bacterium, Staphylococcus aureus [55–57]. However, several pathogenic bacteria are developing antibiotic resistance. Silver nanoparticles/ions are powerful antimicrobial agents against multidrug-resistant bacteria. Hong et al. prepared poly(vinyl alcohol) (PVA)/silver fibers by electrospinning a PVA/AgNO3 aqueous solution, and examined the release of silver ions from fibers with and with-out UV and heat treatment [58]. It was found that with no-UV treatment electrospun PVA/AgNO3 fibers had a faster and more regular release ability of silver ions than the UV-treated ones. In another study, Shi et al. examined the silver-release profiles from silver/polyacrylonitrile hybrid nanofibers over 10 days, showing that the silver-release rate and cumulative-release amount were sufficient to exhibit sustained antibacterial activity [59].
Electrospun nanofibers as substrates for regulating cell behaviors
It is known that the control of cell–material interactions is critical for tissue regeneration. Therefore, many materials have been developed for regulating cell behaviors. Among them, owing to the ease of control of composition, size, order, alignment, mechanical properties and sustained release properties, electrospun nanofiber materials used as a physiologically relevant substrate have been investigated for regulating the responses of cells, including morphologies, proliferation, differentiation, ECM production and gene expression. Here, we highlight studies on the use of electrospun nanofibers for regulating the behaviors of cells seen in musculoskeletal tissues and stem cells related to musculoskeletal tissue repair and regeneration.
It has been demonstrated that the nanotopographic cues rendered by electrospun nanofiber scaffolds can influence the morphology of tendon fibroblasts. Xie et al. seeded rat tendon fibroblast cells on aligned-to-random PLGA nanofiber scaffolds and found that cells on the random scaffolds showed an irregularly shaped morphology with no preferred orientation, while cells on the aligned scaffolds showed an elongated morphology with a preferred orientation along the direction of the fiber's alignment [45]. The nanofiber composition can also affect cellular response. Theisen et al. found that PLLA nanofibers possessed a growth inhibitory effect on human tendon-derived fibroblasts. However, no meaningful influence on the gene expression of collagen I and III, and decorin was observed, while the expression of collagen X increased during the course of cultivation [60]. By contrast, PLLA/collagen I blend nanofibers had no negative influence on the growth of tendon-derived fibroblasts, but blending PLLA nanofibers with collagen had a positive effect on the gene expression of collagen I, III and X, and decorin. In addition, the diameter of fibers also influences the growth and differentiation of human tendon fibroblasts. Erisken et al. studied the response of human tendon fibroblasts on aligned polymeric fiber scaffolds with different diameters, averaging 320 nm, 680 nm and 1.8 μm, respectively. Specifically, a higher cell number, and total collagen and proteoglycan production were found on the nanofiber scaffolds, while microfibers promoted the expression of phenotypic markers of tendon fibroblasts, such as collagen I, III and V, and tenomodulin [61]. Fiber diameter was also utilized to direct the activation of ERK and p38 kinase in osteoblasts [62]. In another study, Li et al. examined the culture of chondrocytes on PLLA micro- and nanofiber scaffolds [63]. Results indicated that chondrocytes on microfibers had a well-spread morphology and organized cytoskeleton, while chondrocytes on the nanofibers had a rounded morphology and a disorganized actin cytoskeletal structure. Both scaffolds supported chondrocyte proliferation, with a higher rate seen in cultures with nanofiber scaffolds. Moreover, a higher level of sulfated glycosaminoglycan in the nanofiber culture was confirmed by more intense Alcian blue histologic staining. The nanofiber cultures also showed higher immunostaining for collagen types II and IX, aggrecan and cartilage proteoglycan link protein. In separate studies, C2C12 murine myoblasts were examined to respond to different assemblies of electrospun nanofibers. Riboldi et al. were the first to show that C2C12 cells can adhere, proliferate, fuse and form multinucleated cells on electrospun membranes made of random fibers [64]. Subsequently, Huang et al. showed that myotubes on aligned nanofibers were not only highly organized but also significantly longer than those on randomly oriented nanofiber scaffolds, with 30–40 nuclei in each myotube [65]. Several studies have further confirmed this finding [66–68]. Recent studies incorporated electrical cues into nanofiber scaffolds and demonstrated the synergistic effects on the enhancement of myoblast differentiation [69,70]. In a similar study, human skeletal muscle cells were seeded onto the electrospun PCL/collagen nanofiber meshes and cell adhesion, proliferation and organization were examined, demonstrating that unidirectionally oriented nanofibers dramatically induced muscle cell alignment and myotube formation as compared with random nanofibers [71].
Some studies have demonstrated that electrospun nanofiber scaffolds can also regulate the differentiation of stem cells into musculoskeletal phenotypes. Yin et al. examined the differentiation of stem cells on PLLA nanofiber scaffolds, and found that human tendon stem/progenitor cells were spindle shaped and well orientated on the aligned nanofibers [72]. The expression of tendon-specific genes was significantly higher in human tendon stem/progenitor cells growing on aligned nanofibers than those on randomly oriented nanofibers. The randomly oriented fiber scaffolds induced osteogenesis, while the aligned scaffold hindered the process. Other than nanotopographic cues rendered by electrospun nanofibers, the ECM components were readily incorporated into nanofibers for regulating the differentiation of stem cells. Ravichandran et al. developed an electrospun PLLA/poly-benzyl-l-glutamate/collagen nanofiber scaffold coated with nanohydroxyapatite [73]. When cultured with primary rabbit adipose-derived mescenchymal stem cells (ASCs), the ECM components introduced to the nanofibers can regulate the differentiation of ASCs into osteogenic lineage. Cobun et al. fabricated electrospun nanofiber scaffolds composed of PVA and chondroitin sulfate [74]. After goat mesenchymal stem cells (MSCs) derived from bone marrow were cultured on the scaffolds under chondrogenic induction conditions for 42 days, a biological cue caused scaffold-enhanced chondrogenic differentiation of MSCs, which was indicated by increased ECM production and cartilage-specific gene expression, while it simultaneously permitted cell proliferation. In combination with stimulation of GDF-5, James et al. demonstrated the significantly enhanced gene expression of tendon markers (i.e., scleraxis and collagen type I) in primary ASCs when they were cultured on PLGA fiber scaffolds [75].
Nanofiber scaffolds for orthopedic tissue repair & regeneration
Figure 2 illustrates the fiber organizations in various musculoskeletal tissues, including bone, cartilage, tendon, muscle, ligament, tendon-to-bone insertion, meniscus and IVD. These unique anisotropic structures and fibrous architectures cannot be recapitulated by scaffolds fabricated using conventional methods. By contrast, electrospinning can be used to generate nanofiber assemblies for mimicking the special fiber organizations in musculoskeletal tissues. Therefore, electrospun nanofiber scaffolds are capable of mimicking the structural organization of musculoskeletal tissues. As mentioned above, nanofibers could also recapitulate the compositions of musculoskeletal tissues. In the following sections, we mainly illustrate notable examples of the recent advances in nanofiber scaffolds for application in orthopedic tissue repair and regeneration.
Figure 2. The features of collagen nanofiber organizations in different musculoskeletal tissues.
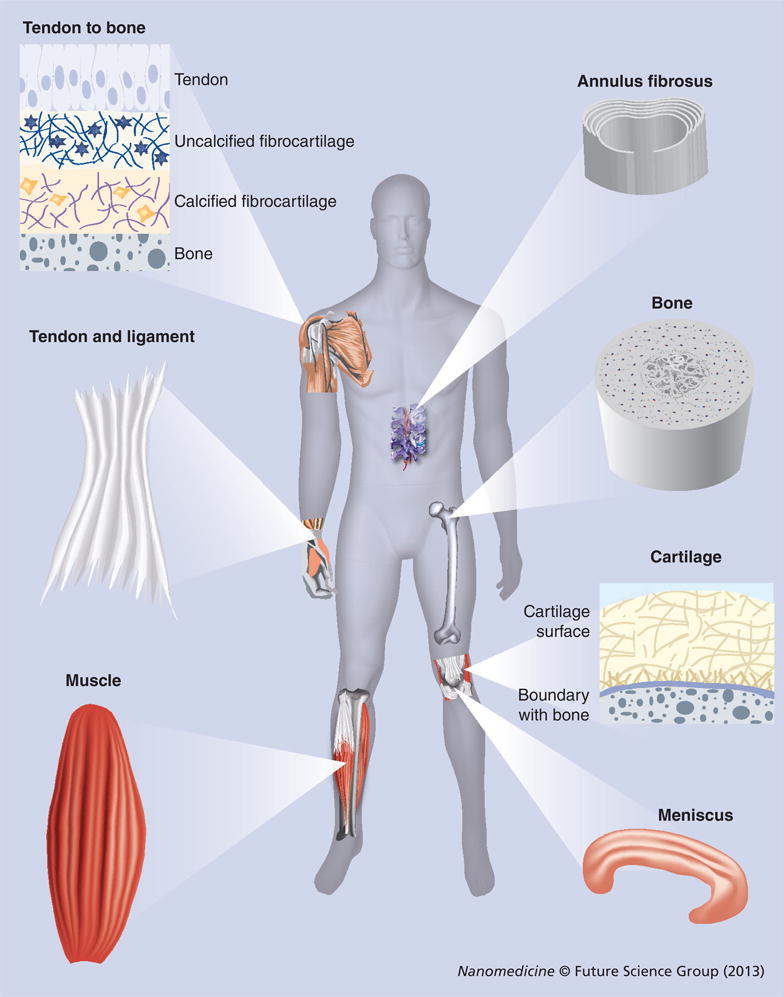
Collagen nanofibers are uniaxially aligned and wavy in tendon and ligament tissues. Collagen fibers have graded organizations (from uniaxially aligned to random) at tendon-to-bone insertion sites and are circumferentially aligned in meniscus and annulus fibrosus. Cartilage contains fine collagen fibers arranged in layered arrays.
Bone
Bone is a dynamic, highly vascularized tissue and its ECM has two major components: an inorganic phase (constituted by hydroxylapatite) that contributes with 65–70% of bone weight; and an organic phase (composed of glycoproteins, proteoglycans, sialoproteins and bone ‘gla’ proteins) that comprises the remaining 25–30% of the total bone weight [76]. Collagen fibers overlap adjacent fibers and hydroxyapatite crystals are arranged in layers within each fiber, resembling overlapping bricks. Therefore, the design of nanofiber scaffolds for bone tissue regeneration is often based on the following criteria: highly porous (easy for vascularization); incorporating hydroxyapatite or collagen (mimicking ECM components and mechanical function); and incorporating growth factors (signaling molecules). Electrospun nanofibers are usually deposited on a conductive collector to form a random, porous fiber mat. Furthermore, the porosity can be enhanced using a variety of approaches [77,78]. Incorporation of hydroxylapatite into nanofiber scaffolds can be readily realized by encapsulation or postsurface coating. Collagen itself can be electrospun into nanofibers. In addition, collagen can be blended with other polymers and electrospun to form hybrid fibers. Furthermore, growth factors can be encapsulated inside nanofibers or adsorbed onto the surface of nanofibers. Owing to these intriguing properties, electrospun nanofiber scaffolds have been examined for bone regeneration both in vitro and in vivo.
Nanofiber scaffolds have been shown to support osteogenic differentiation of progenitor cells and stem cells in vitro [79–82]. A number of studies examined the performance of nanofiber scaffolds with different combinations (i.e., incorporating hydroxyapatite, and incorporating collagen and signaling molecule delivery systems) for repairing bone defects in vivo [83–85]. Rainer et al. developed a hydroxyapatite-functionalized PLLA nanofiber scaffold, with the aim to recapitulate the native histoarchitecture and ECM component of sternal bone tissue [86]. Such a scaffold was tested in a rabbit model of median sternotomy. Computed tomography (CT) follow-up confirmed complete healing in the scaffold-treated group 1 week before the control. Histological analysis indicated the formation of new bone trabeculae among implanted nanofibers having a higher degree of maturity compared with the untreated control group. In a separate study, Kolambkar et al. developed a scaffold in combination with an osteogenic signaling molecule delivery system that consisted of a perforated electrospun PCL nanofiber tube and peptide-modified alginate hydrogel injected inside the tube for the sustained release of growth factors (rhBMP-2) [87]. This scaffold was tested to repair a critically sized, femoral segmental defect in rats. Figure 3A shows that bilateral 8-mm segmental defects were created in the mid-femoral diaphysis of rats. Prior to defect creation, the femora were stabilized by modular fixation plates consisting of a polysulfone plate and two stainless steel plates. Nanofiber tubes were placed around the adjacent bone ends, such that the tube lumen contained the defect and there was an overlap of 2.5 mm with the native bone ends at each end of the tube. The tube lumen was filled with 125-μl pregelled 2% alginate gel with 5-μg rhBMP-2. Quantitative assessments of bone regeneration were provided by longitudinal 2D radiographs (Figure 3B & C), 3D in vivo μ-CT imaging (Figure 3D & E), torsional testing and histological analysis (Figure 3F). The results indicated that this novel scaffold resulted in consistent bony bridging of the challenging bone defects within 12 weeks. Compared with the intact bone, the biomechanical properties of the repaired bone had statistically equivalent maximum torque and stiffness at week 12. In vivo μCT scanning revealed the presence of perforations in scaffold accelerated early bone formation and defect bridging. In another study, Fu et al. used a composite nanofiber scaffold with incorporation of hydroxyapatite and sustained release of BMP-2 to repair tibia bone defects in mice, demonstrating that the bioactivity of BMP-2 released from the scaffolds was well maintained, which improves new bone formation and the healing of segmental defects [88]. However, these scaffolds could not fully imitate the native structure and compositions of bone ECM. Normal bone function is based on the hierarchical structure at its various length scales. Therefore, the nanofiber scaffold designed for bone tissue regeneration should recapitulate the native structure and composition of bone ECM to a greater extent.
Figure 3. Electrospun nanofiber scaffolds for bone regeneration.
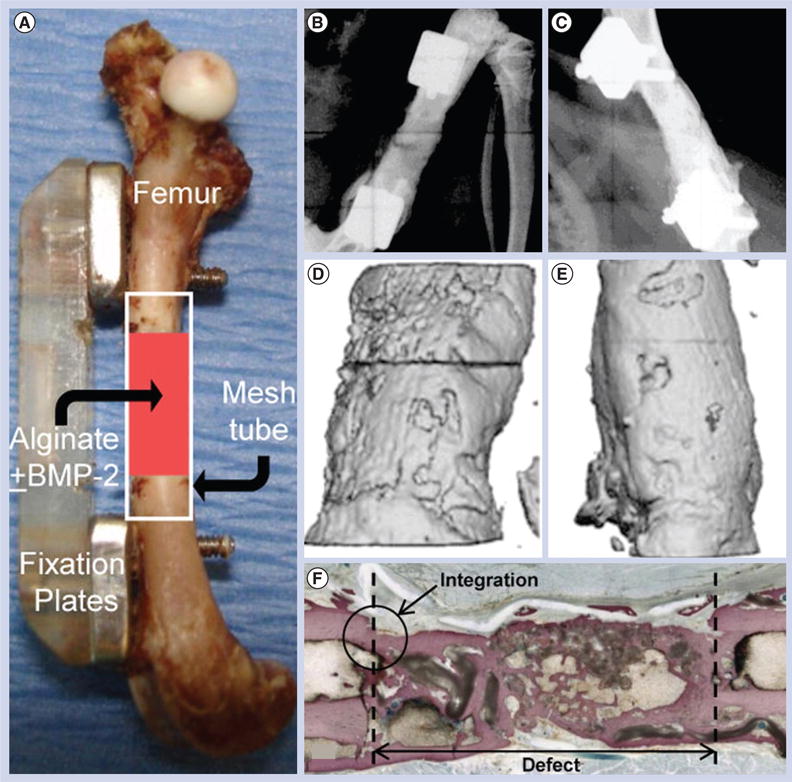
(A) Implantation of a perforated poly(ε-caprolactone) nanofiber mesh tube filled with a peptide-modified alginate hydrogel containing rhBMP-2 for repairing a segmental defect (8 mm) in the mid-femoral diaphysis of a rat. Modular fixation plates are used to stabilize the femur. Representative radiographs indicating the defect exhibited a robust mineraliztion at (B) week 4, while the defect was bridged with densely packed bone at (C) week 12. (D & E) Microcomputed tomography analysis of bone regeneration at 4 and 12 weeks indicating that the defect was filled with newly formed bone. (F) Ground section stained with Sanderson's rapid bone stain at 12 weeks (four-times) indicating the occurrence of extensive bone deposition throughout the defect and good integration between the newly formed bone and the native bone.
Reproduced with permission from [87].
Tendon
Tendons consist of soft and fibrous connective tissue that is composed of densely packed collagen fiber bundles aligned parallel to the longitudinal tendon axis and surrounded by a tendon sheath, also consisting of ECM components [89]. Tendon tissue has a crimped, waveform appearance under microscopic observation. Collagen type I constitutes approximately 60% of the dry mass of the tendon. Owing to the ease of controlling alignment and orders, electrospun nanofiber scaffolds have been investigated in repairing and regenerating tendon tissues. In vitro studies have demonstrated that tendon fibroblasts can adhere and proliferate on electrospun nanofiber scaffolds [45]. Tendon fibroblasts exhibited spindle shape and elongated morphology on aligned nanofiber scaffolds. Nanofiber scaffolds may provide the requisite mechanical properties that may minimize the risk of rerupture associated with the movement of the tendon gap defect following surgical repair. Recently, several studies have been carried out to test the performance of nanofiber scaffolds for tendon repair and regeneration using different animal models [90,91].
Elements of regenerative medicine consisting of scaffolds, cells and signaling molecules have been combined for tendon injury repair and regeneration. Manning et al. developed PDGF-BB and ASC-loaded nanofiber scaffolds, and tested their performance in repairing tendon defects using a canine model [90]. Figure 4A shows the layered scaffolds that consisted of 11 alternating layers of aligned electrospun PLGA nanofiber mats and a fibrin/heparin-binding delivery system (HBDS). The HBDS allows for the delivery of cells and growth factors in a controlled manner, while the PLGA backbone provides a structure that mimics collagen nanofibrillars and alignment in tendon tissues, and simultaneously enhances the surgical handling properties of the scaffold. Sustained delivery of PDGF-BB was achieved by the HBDS/nanofiber scaffold. The scaffolds were implanted into the sharply transected intrasynovial flexor tendons of adult mongrel dogs. Figure 4B shows the scaffold was grasped by a core structure and secured within the repair site. The dogs were sacrificed at 3 or 9 days postoperatively. The operated tendons were removed by dissection and prepared for histologic analysis at 9 days. Hematoxylin and eosin staining showed no obvious inflammatory response after implantation of the scaffold for 9 days (Figure 4C & D). Only a small number of immune cells infiltrated the scaffold. Histologic studies showed that the HBDS/nanofiber scaffolds were well tolerated and delivery of ASCs was successfully achieved in the in vivo flexor tendon repair setting. In a separate study, James et al. developed a combined treatment of an Achilles tendon gap with tubular electrospun nanofiber scaffolds, ASC seeding and GDF-5 immobilization in rats [91]. However, the prevention of adhesion after repair was not investigated in these studies.
Figure 4. A layer-by-layer scaffold composed of poly(l-lactide-co-glycolide) nanofibers and a heparin/fibrin-based delivery system containing growth factor PDGF-BB along with adipose-derived mesenchymal stem cells for flexor tendon regeneration in dogs.
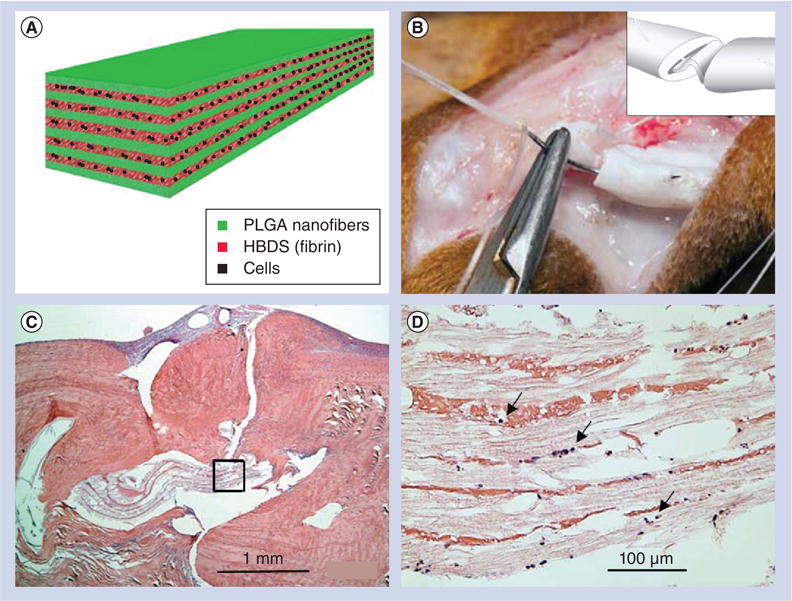
(A) The layer-by-layer structure of scaffolds. (B) The scaffold was grasped by a core suture and secured within the repair site. Inset shows that the scaffold was secured within the repair site. (C) Hematoxylin and eosin staining showing no obvious inflammatory response to the implantation of the scaffold 9 days postoperatively. (D) Magnified region in (C) showing only a small number of immune cells infiltrated the scaffold (arrows).
HBDS: Heparin/fibrin-based delivery system; PLGA: Poly(l-lactide-co-glycolide).
Reproduced with permission from [90].
Prevention of tendon adhesion and rerupture after surgical repair to tendon injury remains a clinical challenge. Ni et al. developed a combinatorial approach by combining standard suture and the photobonded electrospun silk nanofiber wrap, which can provide a stronger, adhesion resistant repair, to a tendon injury in adult female New Zealand white rabbits [92]. Biological cues were also incorporated into nanofiber scaffolds for prevention of tendon adhesion and simultaneous promotion of tendon healing. Liu et al. invented a bilayer sheath membrane, consisting of hyaluronic acid-loaded PCL fiber membranes as the inner layer and PCL fiber membranes as the outer layer, for mimicking the tendon sheath, and tested its performance on preventing peritendinous adhesion in a chicken model [93]. It was found that the outer PCL layer is able to reproduce the antiadhesive role of the outer fibrotic layer to reduce peritendinous adhesions, while the inner hydroxyapatite-loaded PCL layer can mimic the biological function of hydroxyapatite secretion to promote tendon healing and gliding. Recently, the same group encapsulated bFGF-loaded dextran nanoparticles in PLLA nanofiber membranes and examined their capability in tendon healing and preventing adhesion in male Sprague–Dawley rats, demonstrating that such membranes can protect the bioactivity of bFGF in a sustained manner for the promotion of tendon healing and simultaneous adhesion prevention [94].
However, the nanofiber scaffolds designs that are mentioned above failed to recapitulate the wavy structure of collagen fibers in native tendon without loading. Although the wavy-structured nanofiber scaffolds were fabricated, such scaffolds have not been investigated for tendon repair and regeneration in vivo. Here, we highlight the studies on the fabrication of wavy-structured nanofiber scaffolds. Surrao et al. fabricated aligned, wavy poly(l-lactide-co-ε-caprolactone) fibers using a conventional electrospinning setup in conjunction with a rotating wire mandrel [95]. Figure 5A shows a scanning electron micrograph image of nanofiber scaffolds exhibiting aligned and crimpled morphology that were collected with a mandrel at a very high rotating speed. The crimped structure could be preserved even after immersion in phosphate-buffered saline for 4 weeks (Figure 5B). Masson's trichrome staining shows bands of collagen fibers were formed when scaffolds of aligned and random nanofibers were used in vivo at week 6 (Figure 5C & D). Aligned fibers were capable of inducing the alignment of deposited collagen and tendon ECM similar to the native collagen fibrils. It was discovered that the aligned nanofibers, with nano- to micro-meter-sized diameters, underwent crimping after removal from the mandrel. The resulting crimped fibers had similar structural characteristics (amplitude and wavelength) to that of native collagen fibrils. In another study, Liu et al. developed a simple and effective method to fabricate aligned, wavy ester-terminated PLGA and poly(vinyl pyr-rolidone) electrospun nanofibers by introducing an external magnetic field to the collector region [96]. Therefore, future studies should be devoted to testing wavy-structured nanofiber scaffolds in animal models for tendon repair and regeneration.
Figure 5. Fabrication of wavy nanofibers for ligament tissue regeneration.
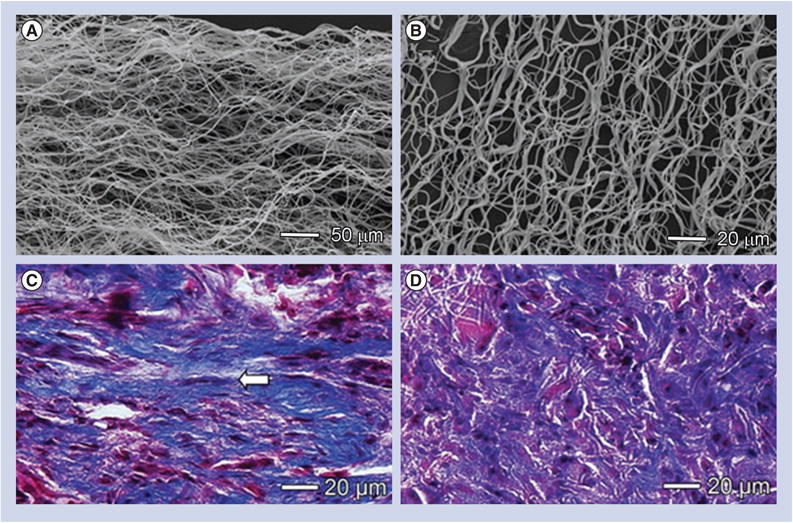
(A) Scanning electron micrograph image showing a scaffold made of aligned but crimped nanofibers and collected with a mandrel at a very high rotation speed. (B) The crimped structure could be preserved after immersion in phosphate-buffered saline for 4 weeks. (C & D) Masson's trichrome staining showing bands of collagen fibers formed after scaffolds of aligned and random nanofibers were implanted in vivo for 6 weeks. Aligned fibers induced the formation of aligned collagen fibers (arrow) similar to the native collagen fibrils.
Articular cartilage
Articular cartilage is a type of connective tissue composed of chondrocytes and collagen or yellow elastic fibers, where the fibers and the cells are embedded in a firm gel-like matrix, rich in mucopolysaccharides, exhibiting flexibility and elasticity [97]. The healing of injured cartilage is slow because cartilage has no blood vessels or lymphatics, and the cells' nutrients diffuse through the matrix. Electrospun nanofibers have shown their potential for use in in vivo cartilage regeneration. Toyokawa et al. examined the possible use of two types of electrospun PLGA nanofiber scaffolds, a solid cylindrical type and a cannulated tubular type, for the treatment of a full-thickness articular defect without the addition of exogenous cells in a rabbit model [98]. It was observed that nanofiber scaffolds were absorbed, articular cartilage and subchondral bone were regenerated, and the regenerated cartilage remained present after surgery for 24 weeks. The tubular scaffolds performed better in cartilage tissue regeneration and had higher histology scores than solid ones and the control group, thus, confirming the potential of electrospun nanofiber scaffolds to repair the osteochondral defect without cultured cells and growth factors.
However, nanofiber scaffolds fabricated from the traditional electrospinning procedure hindered cell infiltration due to low porosity. Therefore, there is an imperative need to develop a highly porous nanofiber scaffold that can efficiently entrap cells throughout the entire scaffold and provide the necessary cues to stimulate new tissue development. Cobun et al. developed a low-density, electrospun nanofiber scaffold composed of PVA-methacrylate and a composite of PVA-methacrylate/chondroitin sulfate-methacrylate by making use of an ethanol bath as a collector, and subsequent UV light crosslinking of the methacrylate groups [74]. The chondroitin sulfate that was added to the scaffolds, a compound commonly found in many joint supplements, can serve as a growth trigger. After 42 days of culturing goat MSCs under chondrogenic induction conditions, the scaffolds appeared as discrete cartilage-like constructs, with a shiny appearance similar to hyaline cartilage. Cells proliferated well in the scaffolds and produced collagen with a high ratio of type II:type I collagen. Then, cell-free scaffolds were implanted into damaged cartilage in the knees of rats. Nanofiber scaffolds had significantly more proteoglycan deposition than empty defects but less than native cartilage (Figure 6A–C). Results of immunostaining for type II collagen showed that the scaffolds augmented endogenous articular cartilage regeneration in an osteochondral defect model (Figure 6D–F). The findings in the repaired defects include columnar cell orientation radiating from cell-rich cavities with subchondral bone containing numerous pockets of cellular cavities. Additionally, the early establishment of the multilayer architecture of articular cartilage can be observed, including a gradient of proteoglycan deposition highest near the subchondral bone, and cells oriented perpendicular and parallel to the cartilage surface in the deep and superficial zone, respectively.
Figure 6. Electrospun poly(vinyl alcohol)-methacrylate/chondroitin sulfate nanofiber scaffolds for cartilage repair.
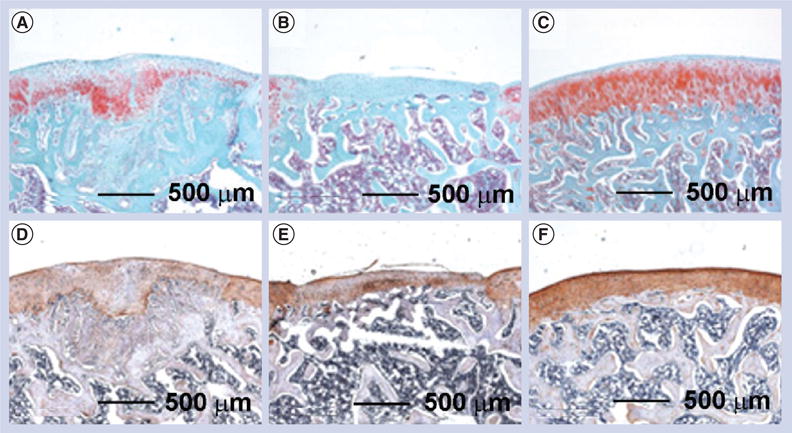
After implantation for 6 weeks in a rat osteochondral defect model, (A–C) safranin-O staining indicated that (A) the fiber implants promoted significant proteoglycan deposition compared with (B) the negative control (without treatment), while (C) native cartilage had the largest amount of proteoglycan deposition. (D–F) Immunohistochemical staining indicated that even (D) chondroitin sulfate fibers induced higher type II collagen production compared with (E) empty defects, but (F) native articular cartilage still contained significantly more type II collagen.
Reproduced with permission from [74].
Electrospun nanofiber scaffolds in combination with stem cells or chondrocytes were also applied to regenerate cartilage. Shafiee et al. implanted MSCs seeded with electrospun PVA/PCL nanofiber scaffolds into the full-thickness articular cartilage defects of rabbits [99]. After 12 weeks, defects were repaired with chondrocyte-like cells displaying rounded morphology with lacunae. In addition, collagen type II expression increased in the neocartilage. The seeding of stem cells improved healing of defects, compared with the untreated control and those that received cell-free scaffolds. In a different study, Xu et al. developed a five-layer tissue construct of 1-mm thickness with alternate layers of rabbit elastic chondrocytes suspended in a fibrin–collagen hydrogel and PCL nanofiber membrane, using a combination of electrospinning and ink-jet printing [100]. The fabricated constructs formed cartilage-like tissues in vivo as demonstrated by the position of type II collagen and glycosaminoglycans.
However, previous studies have been limited to the examination of nanofiber scaffolds made of random nanofibers on articular cartilage regeneration. The anatomic structure of human cartilage is divided into four different zones, which are termed the superficial, middle (or transitional), deep (or radial) and calcified zones, each with varying matrix composition, morphology, and cellular, mechanical and metabolic properties [101]. The organization of the collagen fibrils in the cartilage presents five features: individual fibrils within the trabeculae join to form small fiber bundles that become grouped into larger bundles at the calcified/uncalcified interface; fibrils in the deep and middle zones, which exhibit the surface periodicity characteristic of collagen, are generally oriented toward the articular surface in large bundles approximately 55 μm across; in the superficial zone, fibrils run parallel to the surface; the surface fibrils have random orientation; and the collagen fibrils of the lacunar walls appear to be thinner and more closely packed than those between the lacunae [102]. Therefore, more appropriate nanofiber scaffolds need to be designed with multiple layers to recapitulate different fiber orientations and compositions in the different zones of native cartilage.
Meniscus
The fibrocartilaginous menisci are load-bearing tissues composed of circumferentially aligned collagen fibers, which are critical to the normal functioning of the knee [103]. Failures in the functioning of the meniscus may occur as a result of traumatic injury or degenerative processes. Repaired tears in the vascular periphery heal well, while those in the avascular inner region fail to do so, and, thus, damaged elements are commonly resected via partial meniscectomy. Removal of tissue results in higher cartilage contact stresses, which may predispose patients to osteoarthritic progression [104]. Replacing damaged regions of the meniscus with an engineered tissue construct may restore function and protect against further deleterious changes in the joint.
Tissue engineering strategies based on electrospun nanofibers present a promising alternative that could allow for regeneration of meniscus tissues. However, no literature has been reported on the use of electrospun nanofibers for menisci tissue regeneration in vivo. Here, we reviewed recent advances of in vitro studies on meniscus regeneration using electrospun nanofiber scaffolds. Mauck's group carried out a series of studies on this topic [105–108]. Their initial study confirmed that MSC seeded, aligned PCL nanofiber scaffolds had great potential for meniscus tissue regeneration [105]. With increasing incubation time, a significantly larger increase in mechanical properties was observed for aligned nanofiber scaffolds compared with random counterparts seeded with meniscal fibrochondrocytes or MSCs. Their subsequent study found that the application of dynamic culture, by incubating constructs on an orbital shaker, dramatically improved the infiltration of MSCs into aligned nanofiber scaffolds [106]. Their further study evaluated the effects of changing fiber angle and sample aspect ratio on the shear properties of aligned PCL scaffolds, and they determined how ECM deposited by resident MSCs modulated the measured shear response [107]. Encouraged by these results, the same group successfully developed a novel electrospinning method to produce scaffolds composed of circumferentially aligned nanofibers using a rotated plate as a collector that could mimic the circumferentially aligned collagen fibers in menisci ECM [108]. Figure 7A & B illustrates the meniscus, showing the generalized anatomic macrostructure and a wedge-like cross section displaying a simplified collagen fiber organization, with the majority of fiber bundles in the circumferential direction, with occasional radial ‘tie’ fibers. Figure 7C shows scanning electron micrograph images of different locations of circumferentially aligned nanofiber scaffolds. Fibers were locally oriented in these scaffolds, but their orientation varied considerably according to their position. Figure 7D–F, shows fluorescent microscopy images of juvenile bovine MSCs seeded on circumferentially aligned nanofiber scaffolds, with staining of actin in green and nuclei in blue. Juvenile bovine MSCs that were seeded on these scaffolds resulted in a similar orientation to the direction of fiber alignment. Mechanical analysis of these scaffolds revealed significant interactions between scaffold length and region, with the tensile modulus near the edge of the scaffolds decreasing with increasing scaffold length. These scaffolds, with spatially varying local orientations and mechanics, will need to be further tested in animal models.
Figure 7. Fabrication of circumferentially aligned poly(ε-caprolactone) nanofiber scaffolds for knee meniscus tissue engineering.
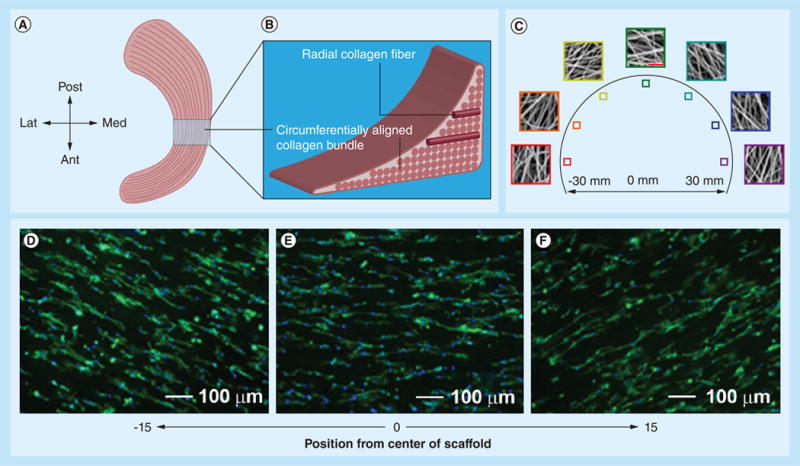
(A) Anatomic macrostructure of meniscus. (B) A wedge-like cross-section displaying a simplified collagen fiber organization, with the majority of fiber bundles in the circumferential direction with occasional radial ‘tie’ fibers. (C) Scanning electron micrograph images showing different locations of samples for circumferentially aligned scaffolds (scale bar: 5 μm). (D–F) Fluorescent microscopy images of actin (green) and nuclei (blue) in juvenile bovine mesenchymal stem cells seeded on the different portions of circumferentially aligned scaffolds.
Ant: Anterior; Lat: Lateral; Med: Medial; Post: Posterior.
Reproduced with permission from [108].
This scaffold designed by Mauck's group can partially mimic the structure of the meniscus by recapitulating its circumferentially aligned collagen fibrous architecture. However, the meniscus is a complex structural tissue, a glossy white, complex wedge-shaped fibrocartilaginous tissue, comprised of cells, specialized ECM molecules, and region-specific innervation and vascularization. The ECM is generated and maintained by meniscal fibrochondrocytes, a heterogeneous cell population sparsely distributed throughout the tissue [109]. Thus, future design of nanofiber scaffolds should be devoted to fully imitating the wedge-shaped, circumferentially aligned collagen fibrous architecture.
Intervertebral disc
The IVD consists of three tissue components; a gel-like nucleus pulposus (NP) surrounded by the annulus fibrosus (AF), which are sandwiched between cartilage end plates and vertebral bodies, functioning as a ligament to hold the vertebrae of the spine together; a shock absorber; and a ‘pivot point’ that allows the spine to bend, rotate and twist [110]. The AF is a collagen-rich fibrous structure of approximately 15–25 concentric sheets of collagen (lamellae) that confines the pressurized NP. The lamellar structure of AF, which is composed of collagen type I and II fibers, helps to maintain the tensile properties of the disc while providing structural support for proteoglycan synthesis. The AF has a multilayered, oriented lamellar structure with concentric layers creating a regular pattern of collagen type I fibers. The collagen fibrils are oriented concentrically, with each subsequent layer oriented 60° toward the spinal column. As the outer AF moves inward and approaches the NP, the orientation of the concentric lamellae gradually changes from angles of 62 to 45°. A variety of solid tissue engineering constructs have been investigated for IVD tissue regeneration. However, one major hurdle in the development of scaffolds for IVD tissue engineering is the inability to mimic the lamellar organization of the AF [111].
In order to address this problem, Nerurkar et al. produced anisotropic nanofiber laminates seeded with MSCs using a layer-by-layer stacking strategy, which could mimic the fibrous architecture of AF tissue to some degree [112]. Figure 8A shows the fabrication of bilamellar-aligned PCL nanofiber scaffolds. In order to replicate the hierarchical structure of the AF, bilamellar tissue constructs were formed first as single lamella tissues from aligned nanofiber scaffolds seeded with MSCs, and then formed into bilayers after 2 weeks of in vitro culture. Electrospun fiber mats, approximately 250 μm thick, can match the natural lamellar thickness of the AF. Rectangular scaffolds (5 × 30 mm) were excised from the nanofiber mat with their long axis rotated 30° from the prevailing fiber direction. Bilayers were oriented with either parallel (+30°/+30°) or opposing (+30°/-30°) fiber alignment relative to the long axis of the scaffold. Sections were collected obliquely across lamellae, stained with picrosirius red, and viewed under polarized light microscopy to visualize collagen organization. When viewed under crossed polarizers, birefringent intensity indicates the direction of alignment. After 10 weeks of in vitro culture, parallel bilayers contained coaligned intralamellar collagen within each lamella (Figure 8B). Opposing bilayers contained intralamellar collagen aligned along two opposing directions (Figure 8C), successfully replicating the gross fiber orientation of native bovine AF (Figure 8D). After an additional 2 weeks of culture, the external supports were removed and laminates remained intact. These scaffolds directed the deposition of organized, collagen-rich ECM that mimicked the angle-ply, multi-lamellar architecture, and achieved mechanical parity with the native tissue. Their further study identified a novel role for interlamellar shearing in reinforcing the tensile response of biological laminates. In addition, the same group also developed a construction algorithm in which electrospun nanofiber scaffolds are coupled with a biocompatible hydrogel to engineer a MSC-based disc replacement. Similarly, Nesti et al. seeded human MSCs into a novel biomaterial amalgam to develop a biphasic construct that consisted of an electrospun nanofiber scaffold enveloping a hyaluronic acid hydrogel center, which could architecturally resemble a native IVD [113]. Such an engineered IVD was composed of both AF- and NP-like components, demonstrating the resemblance of overall gross, histological, biochemical and biosynthetic properties of a native IVD. Other than mimicking the architecture of IVD, growth factors were also incorporated to nanofiber scaffolds for AF repair and regeneration. Vadala et al. fabricated a bioactive nanofiber scaffold that can release TGF-β1 for AF tissue engineering [114]. The sustained release of TGF-β1 from the nanofiber scaffold could significantly enhance GAG and collagen synthesis in the seeded AF cells.
Figure 8. Fabrication of bilamellar-aligned poly(ε-caprolactone) nanofibrous scaffold for annulus fibrosus tissue engineering.
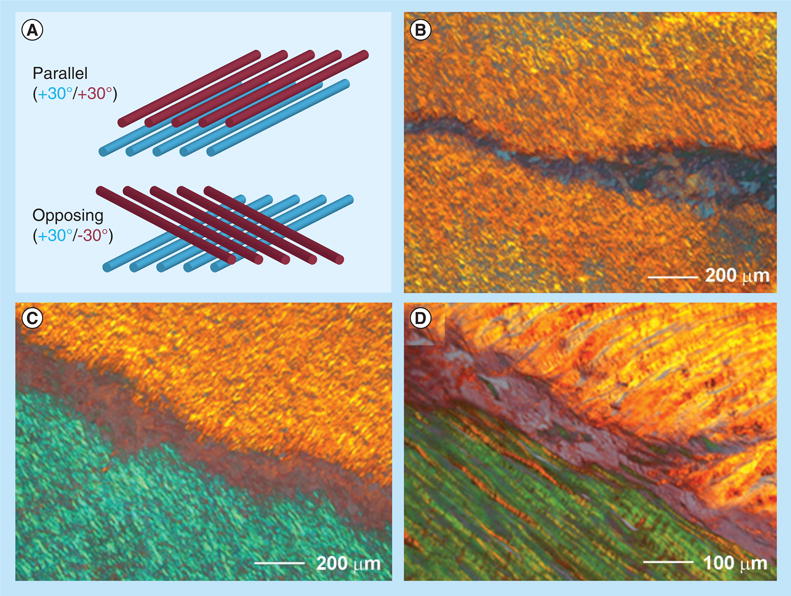
Scaffolds were excised 30° from the prevailing fiber direction of electrospun nanofibrous mats to replicate the oblique collagen orientation within a single lamella of the annulus fibrosus. (A) Bilayers were oriented with either parallel (+30°/+30°) or opposing (+30°/-30°) fiber alignment relative to the long axis of the scaffold. Sections were collected obliquely across lamellae, stained with picrosirius red and viewed under polarized light microscopy to visualize collagen organization. When viewed under crossed polarizers, birefringent intensity indicates the direction of alignment. (B) After 10 weeks of in vitro culture, parallel bilayers contained coaligned intralamellar collagen within each lamella. (C) Opposing bilayers contained intralamellar collagen aligned along two opposing directions, successfully replicating the gross fiber orientation of (D) native bovine annulus fibrosus.
Reproduced with permission from [112].
Although some progress was made towards the development of nanofiber scaffolds for AF regeneration, the studies were limited to in vitro investigations. AF has several concentric lamellae of collagen fibers running at angles of approximately 60–80° to the vertical axis [115]. This multiscale structural hierarchy needs to be captured by nanofiber scaffolds in the future. IVD is comprised of AF, NP and cartilage end plates. Perhaps a better design for scaffolds could consist of a combinatorial approach that can integrate hydrogel systems with electrospun nanofibers to fully recapitulate the native architecture of IVD [116]. More efforts should be devoted to testing developed nanofiber scaffolds for IVD regeneration in vivo.
Tendon-to-bone insertion site
As the rotator cuff tendon inserts into the proximal humerus, there is a gradual transition between the four zones at the direct tendon-to-bone insertion: tendon, nonmineralized fibrocartilage, mineralized fibrocartilage and bone. None of the current repair strategies replicate this normal transitional zone, which leads to stress concentrations that weaken the healed tendon-to-bone insertion site, majorly contributing to the high failure rates observed [117]. The stress concentrations are mainly attributed to the mechanical mismatch between the tendon, a soft tissue with a modulus of 200 MPa, and bone, with a modulus of 20 GPa, one of the biggest mechanical mismatches in nature [118]. There are two features at the tendon-to-bone insertion site: gradual organization in collagen fiber orientation and a linear gradient in mineral content from the tendon to the bone. Therefore, a scaffold presenting these two characteristics would be ideal for reducing the concentrated local stress and repairing the injury at the tendon-to-bone insertion site. Accordingly, considerable efforts should be made to develop nanofiber scaffolds that could mimic both the structure and/or composition of the the tendon-to-bone insertion site [119].
Here, we highlight the nanofiber scaffolds that have been recently designed for the surgical treatment of rotator cuff injury in various animal models. Figure 9A shows that a nanofiber scaffold comprising random poly(glycolic acid) nanofibers was applied to bridge the gap between the infraspinatus tendon humerus bone in a rabbit [120]. Figure 9B shows that another type of scaffold made of aligned PCL and PCL/poly(ethylene oxide) nanofiber were used to augment supraspinatus in rats [121]. After supraspinatus tendon exposure and detachment, scaffolds were implanted using a simple overlay by suturing along the anterior and posterior borders of the scaffold and supraspinatus tendon. The supraspinatus tendon was then repaired back to greater tuberosity. In order to mimic the composition at the bone site, Moffat et al. developed a biphasic nanofiber scaffold composed of two layers of fiber membranes, and then implanted them into the rat shoulder (Figure 9C) [122]. In this rat shoulder injury and repair model, the supraspinatus tendon was sharply detached at the insertion site, fibrocartilage at the insertion site was removed and the bony footprint was abraded to mimic the clinical scenario. The scaffold was inserted between the cancellous bone and distal end of the detached tendon. The tendon was sutured to bone. None of these scaffolds could recapitulate either the structural organization of collagen fibers or compositions in the millimeter-sized transitional zone at the tendon-to-bone insertion site, which could partly attribute to the poor functional recovery, such as compromised mechanical performance. In order to mimic the composition at the insertion site, Li et al. developed a continuously graded, bonelike calcium phosphate-coated electrospun PLGA nanofiber scaffold [123]. The mineral content gradient resulted in functional consequences, such as a gradient in the stiffness throughout the scaffold. Recently, Xie et al. successfully fabricated an electrospun PCL nanofiber scaffold with gradations in fiber organization [45]. Additionally, Xie et al. controlled biomineralization of PCL nanofibers using polydopamine as a mediator. Based on these studies, Xie et al. have designed a nanofiber scaffold with dual gradations in both fiber organization and mineral content, and demonstrated the feasibility of performing implantation of such a scaffold to the rotator cuff injury site using a modified rat shoulder injury and repair model (Figure 9D) [124]. Specifically, the supraspinatus was detached sharply at its insertion on the greater tuberosity of the humerus. A 0.5-mm drill hole was then made transversely at the base, creating a channel for the scaffold with an exit hole on the proximal lateral humeral metadiaphyseal region. Following suture of the scaffold to the undersurface of the supraspinatus, the tendon–scaffold construct was then secured to the humeral head defect using the 5–0 prolene suture secured at the lateral cortex of the humerus. The fiber scaffold was expected to serve as a graft to provide mechanical stability, and a template to regulate cell activity and improve the healing process. Future studies will be directed toward histological analysis of tissue regeneration and functional recovery tests. In addition, combining stem cell therapy with such scaffolds could be beneficial to the healing of rotator cuff injury, as stem cells could differentiate into multiple cell types at different scaffold portions (i.e., tendon fibroblasts at tendon site, osteoblast at bone site and fibrochondrocytes in between).
Figure 9. State-of-the-art nanofiber scaffolds for repairing rotator cuff injury in vivo.
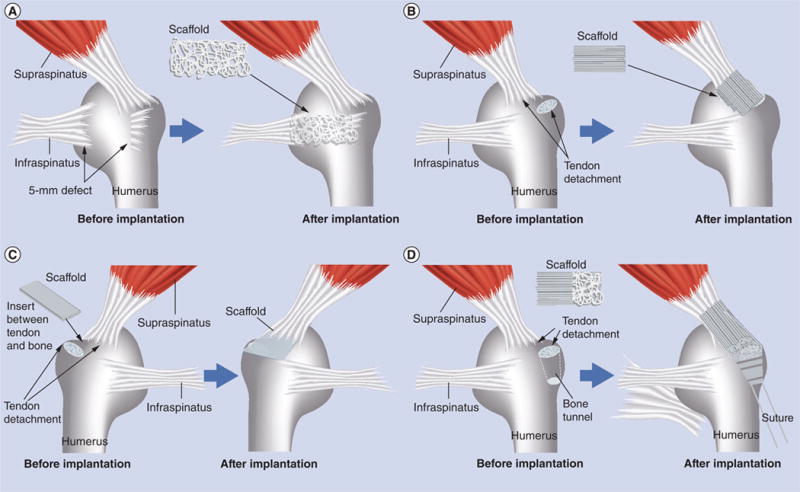
(A) The scaffold (5-mm width and 5-mm length) composed of random poly(l-lactide-co-glycolide) (PLGA) nanofibers was used to bridge the gap between the infraspinatus tendon and humerus in a rabbit rotator cuff injury model. (B) The scaffold composed of aligned poly(ε-caprolactone) nanofiber scaffolds was implanted at the site between the supraspinatus tendon and humerus in a rat rotator cuff injury model. (C) The biphasic nanofiber scaffold composed of an aligned PLGA nanofiber layer and a PLGA–hydroxylapatite composite nanofiber layer was inserted between the bone and the detached tendon for integrative rotator cuff repair in a rat rotator cuff injury model. (D) The poly(ε-caprolactone) nanofiber scaffold with dual gradations in fiber organization and mineral content was used to repair rotator cuff injury in a rat shoulder model by inserting the mineral end into the bone tunnel and suturing the aligned end to the tendon.
Conclusion & future perspective
Studies have demonstrated that electrospun nanofiber scaffolds, in combination with cell therapy and drug delivery, have great potential in orthopedic tissue repair and regeneration. Topographic cues rendered by electrospun nanofibers have shown significant influences on the regulation of cell behaviors, including adhesion, migration, proliferation and differentiation [125]. Although mechanical properties of nanofibers can be modulated in a number of ways, such mechanical cues presented from electrospun nanofibers have not been thoroughly investigated for regulating cell behaviors. Future efforts may be devoted to the design of nanofiber scaffolds with a wide range of mechanical properties (i.e., elastic modulus) that can both tailor cell response and match the mechanical properties of a variety of orthopedic tissues. Orthopedic tissues usually contain multiple components. Most nanofiber scaffolds that have been designed so far only recapitulate the fiber organization of orthopedic tissues to a certain extent. Multiple components related to orthopedic tissues should be added to nanofiber scaffolds to fully imitate their compositions (i.e., encapsulation, surface modification or combining hydrogel systems). In addition, based on our knowledge of developmental biology, multiple signaling molecules should be incorporated into nanofiber scaffolds to be released sequentially at various times for orthopedic tissue regeneration in vivo. Electrospun nanofiber scaffolds developed for orthopedic tissue engineering have mainly focused on fiber mats or tubes. Novel approaches with a combination of electrospinning and other techniques (i.e., 3D weaving) should be developed for the creation of 3D nanofiber scaffolds with controlled porosity and anisotropic properties [126].
The ultimate goal for orthopedic tissue repair and regeneration is to restore the function of damaged orthopedic tissues. Nanofiber scaffolds for repairing and regenerating orthopedic tissue should not only be capable of mimicking their key microstructures, compositions and mechanical properties, but also enable the recovery of functional loss. Recent findings have demonstrated the ability to directly form cell-laden nanofibers in relatively large quantities using cell electrospinning. Such an approach could be useful for constructing 3D functional tissues for repairing and regenerating damaged orthopedic tissues [127,128]. Although electrospun nanofiber scaffolds have been widely examined for orthopedic tissue repair and regeneration, most studies are still in their infancy and are limited to in vitro studies. In order to facilitate the translational research of newly designed electrospun nanofiber scaffolds for repairing orthopedic tissue injury, more in vivo and clinical studies will be needed to comprehensively test their performance. Although there are various preclinical efforts in the field of nanofiber scaffold development for orthopedic tissue regeneration, perhaps very few will reach clinical trial. However, it is expected that the clinical trials will be conducted for some nanofiber scaffolds that are currently in development in laboratories over the next 5–10 years.
Executive summary.
Background
Current approaches for the treatment of orthopedic injury are associated with a number of limitations.
Rationally designed nanofiber scaffolds may hold promise for the repair and regeneration of orthopedic tissues.
Electrospinning is a simple and versatile technique for producing nanofiber scaffolds.
Why electrospun nanofibers?
Studies have demonstrated the control of diameter, composition, structure, alignment and order of electrospun nanofiber scaffolds.
The mechanical properties of electrospun nanofibers can be tailored in different ways, which could be used for regulating cell response and matching the mechanical property of musculoskeletal tissue.
Electrospun nanofibers can be used as carriers for topically sustained drug/gene delivery.
Electrospun nanofibers can be used as substrates for regulating cell behaviors, including morphology, proliferation, migration, differentiation and gene expression.
Nanofiber scaffolds for orthopedic tissue repair & regeneration
Nanofiber scaffolds have been shown to support osteogenic differentiation of progenitor and stem cells in vitro. Nanofiber scaffolds with different combinations (i.e., incorporating hydroxyapatite, and incorporating collagen and signaling molecule delivery systems) have been tested for repairing bone defects in vivo.
A combination of nanofiber scaffolds, cells and signaling molecules is used for tendon injury repair and regeneration. Nanofiber scaffolds are also used for the prevention of rerupture and adhesion after tendon surgery.
Studies are limited to the examination of nanofiber scaffolds made of random nanofibers on articular cartilage regeneration, although these scaffolds show the potential for regeneration in vivo.
No literature has reported on the use of electrospun nanofibers for meniscal tissue regeneration in vivo. A novel electrospinning method has been developed to produce scaffolds composed of circumferentially aligned nanofibers using a rotated plate as a collector, which can mimic the circumferentially aligned collagen fibers in menisci' extracellular matrix.
Anisotropic nanofiber laminates seeded with mesenchymal stem cells are developed using a layer-by-layer stacking strategy, which could to some degree mimic the fibrous architecture of annulus fibrosus tissue.
Nanofiber scaffolds with gradations in fiber organization and mineral content are fabricated for mimicking both the fiber orientation and composition at the tendon-to-bone insertion site.
Footnotes
Financial & competing interests disclosure: The authors are supported in part by the National Center for Research Resources (grant number UL1RR033173), funded by the Office of the Director, NIH (grant number 1R15 AR063901-01), supported by the NIH roadmap for Medical Research and receive start-up funds from Marshall Institute for Interdisciplinary Research and Center for Diagnostic Nanosystems at Marshall University. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States. 2nd. Vol. 129. American Academy of Orthopaedic Surgeons; IL, USA: 2011. [Google Scholar]
- Deng M, James R, Laurencin CT, Kumbar SG. Nanostructured polymeric scaffolds for orthopaedic regenerative engineering. IEEE Trans Nanobiosci. 2012;11(1):3–14. doi: 10.1109/TNB.2011.2179554. [DOI] [PubMed] [Google Scholar]
- Dale H, Fenstad AM, Hallan G, et al. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop. 2012;83(5):449–458. doi: 10.3109/17453674.2012.733918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talmo CT, Aghazadeh M, Bono JV. Perioperative complications following total joint replacement. Clin Geriatr Med. 2012;28(3):471–487. doi: 10.1016/j.cger.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Badylak SF, Nerem RM. Progress in tissue engineering and regenerative medicine. Proc Natl Acad Sci USA. 2010;107(8):3285–3286. doi: 10.1073/pnas.1000256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 7.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci USA. 2006;103(8):2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khademhosseini A, Vacanti JP, Langer R. Progress in tissue engineering. Sci Am. 2009;300(5):64–71. doi: 10.1038/scientificamerican0509-64. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal S, Wendorff JH, Greiner A. Progress in the field of electrospinning for tissue engineering applications. Adv Mater. 2009;21(32–33):3343–3351. doi: 10.1002/adma.200803092. [DOI] [PubMed] [Google Scholar]
- 10.Hashi CK, Zhu Y, Yang GY, et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci USA. 2007;104(29):11915–11920. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnell E, Klinkhammer K, Balzer S, et al. Guidance of glial migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28(19):3012–3025. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Xin X, Hussain M, Mao JJ. Continuring differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials. 2007;28(2):316–325. doi: 10.1016/j.biomaterials.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Cao X, Shen M, Guo R, Banyai I, Shi X. Fabrication and morphology control of electrospun poly(γ-glutamic acid) nanofibers for biomedical applications. Colloids Surf B Biointerfaces. 2012;89:254–264. doi: 10.1016/j.colsurfb.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Maria AW, Dietmar WH. The return of a forgotten polymer-polycaprolactone in the 21st century. Prog Polym Sci. 2010;35:1217–1256. [Google Scholar]
- 15▪.Xie J, Li X, Xia Y. Putting electrospun nanofibers to work for biomedical research. Macromol Rapid Commun. 2008;19(22):1775–1792. doi: 10.1002/marc.200800381. Comprehensive review of electrospun nanofibers for applications in tissue engineering and drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arras MML, Grasl C, Bergmeister H, Schima H. Electrospinning of aligned fibers with adjustable orientation using auxiliary electrodes. Sci Technol Adv Mater. 2012;13:035008. doi: 10.1088/1468-6996/13/3/035008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grasel C, Arras MML, Stoiber M, Bergmeister H, Schima H. Electrodynamic control of the nanofiber alignment during electrospinning. Appl Phys Lett. 2013;102:053111. [Google Scholar]
- 18.Pham QP, Sharma U, Mikos AG. Electrospun poly(ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7:2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 19.Blakeney BA, Tambralli A, Anderson JM, et al. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials. 2011;32:1583–1590. doi: 10.1016/j.biomaterials.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker BM, Gee AO, Metter RB, et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008;29:2348–2358. doi: 10.1016/j.biomaterials.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker BM, Shah RP, Silverstein AM, et al. Sacrificial nanofibrous composites provide instruction without impediment and enable functional tissue formation. Proc Natl Acad Sci USA. 2012;109:14176–14181. doi: 10.1073/pnas.1206962109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 23.Khatiwala CB, Peyton SR, Putnam AJ. Intrinsic mechanical properties of the extracellular matrix affect the behavior of pre-osteoblastic MC3T3-E1 cells. Am J Phys Cell Physiol. 2006;290(6):C1640–C1650. doi: 10.1152/ajpcell.00455.2005. [DOI] [PubMed] [Google Scholar]
- 24.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 25.Tan EP, Lim CT. Effects of annealing on the structural and mechanical properties of electrospun polymeric nanofibers. Nanotechnology. 2006;17(10):2649–2654. doi: 10.1088/0957-4484/17/10/034. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Oh SH, Liu J, Soker S, Atala A, Yoo JJ. The use of thermal treatments to enhance the mechanical properties of electrospun poly (epsilon-caprolactone) scaffolds. Biomaterials. 2008;29(10):1422–1430. doi: 10.1016/j.biomaterials.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Abdolkarim S, Ravandi H, Sadrjahani M. Mechanical and structural characterizations of simultaneously aligned and heat treated PAN nanofibers. J Appl Polym Sci. 2012;124(5):3529–3537. [Google Scholar]
- 28.Xie J, Michael PL, Zhong S, Ma B, MacEwan MR, Lim CT. Mussel inspired protein-mediated surface modification to electrospun fibers and their potential biomedical applications. J Biomed Mater Res A. 2012;100(4):929–938. doi: 10.1002/jbm.a.34030. [DOI] [PubMed] [Google Scholar]
- 29.Liu W, Yeh YC, Lipner J, et al. Enhancing the stiffness of electrospun nanofiber scaffolds with a controlled surface coating and mineralization. Langmuir. 2011;27(15):9088–9093. doi: 10.1021/la2018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie J, Zhong S, Ma B, Shuler FD, Lim CT. Controlled biomineralization of electrospun poly(ε-caprolactone) fibers to enhance their mechanical properties. Acta Biomater. 2013;9(3):5698–5707. doi: 10.1016/j.actbio.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 31.Sui X, Wiesel E, Wagner HD. Enhanced mechanical properties of electrospun nano-fibers through NaCl mediation. J Nanosci Nanotechnol. 2011;11(9):7931–7936. doi: 10.1166/jnn.2011.4760. [DOI] [PubMed] [Google Scholar]
- 32.Chew SY, Hufnagel TC, Lim CT, Leong KW. Mechanical properties of single electrospun drug-encapsulated nanofibers. Nanotechnology. 2006;17(15):3880–3891. doi: 10.1088/0957-4484/17/15/045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng F, Shaw MT, Olson JR, Wei M. Hydroxyapatite needles-shaped particles/poly(l-lactic acid) electrospun scaffolds with perfect particle-along-nanofiber orientation and significantly enhanced mechanical properties. J Phys Chem C. 2011;115(32):15743–15751. [Google Scholar]
- 34.Dou Y, Wu C, Chang J. Preparation, mechanical property and cytocompatibility of poly(l-lactic acid) calcium silicate nanocomposites with controllable distribution of calcium silicate nanowires. Acta Biomater. 2012;8(11):4139–4150. doi: 10.1016/j.actbio.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Luo Y, Wang S, Shen M, et al. Carbon nanotube-incorporated multilayered cellulose acetate nanofibers for tissue engineering applications. Carbohydr Polym. 2013;91(1):419–427. doi: 10.1016/j.carbpol.2012.08.069. [DOI] [PubMed] [Google Scholar]
- 36.Dhandayuthapani B, Varghese SH, Aswathy RG, Yoshida Y, Maekawa T, Sakthikumar D. Evaluation of antithrombogenicity and hydrophilicity on Zein-SWCNT electrospun fibrous nanocomposite scaffolds. Int J Biometer. 2012;2012:345029. doi: 10.1155/2012/345029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan EP, Lim CT. Physical properties of a single polymeric nanofiber. Appl Phys Lett. 2004;84(9):1603–1605. [Google Scholar]
- 38.Shin MK, Kim SI, Kim SJ, Kim SK, Lee H, Spinks GM. Size-dependent elastic modulus of single electroactive polymer nanofibers. Appl Phys Lett. 2006;89(23):231929–231933. [Google Scholar]
- 39.Wong SC, Baji A, Leng S. Effect of fiber diameter on tensile properties of electrospun poly (ε-caprolactone) Polymer. 2008;49(21):4713–4722. [Google Scholar]
- 40.Pai CL, Boyce MC, Rutledge GC. Mechanical properties of individual electrospun PA 6(3)T fibers and their variation with fiber diameter. Polymer. 2011;52(10):2295–2301. [Google Scholar]
- 41.Naraghi M, Arshad SN, Chasiotis I. Molecular orientation and mechanical property size effects in electrospun polyacrylonitrile nanofibers. Polymer. 2011;52(7):1612–1618. [Google Scholar]
- 42.Papkov D, Zou Y, Andalib MN, Goponenko A, Cheng SZ, Dzenis YA. Simultaneously strong and tough ultrafine continuous nanofibers. ACS Nano. 2013;7(4):3324–3331. doi: 10.1021/nn400028p. [DOI] [PubMed] [Google Scholar]
- 43.Huang L, Bui NN, Manickam SS, McCutcheon JR. Controlling electrospun nanofiber morphology and mechanical properties using humidity. J Polym Sci Part B Polym Phys. 2011;49(24):1734–1744. [Google Scholar]
- 44.Inai R, Kotaki M, Ramakrishna S. Structure and properties of electrospun PLLA single nanofibers. Nanotechnology. 2005;16(2):208–213. doi: 10.1088/0957-4484/16/2/005. [DOI] [PubMed] [Google Scholar]
- 45.Xie J, Li X, Lipner J, et al. ‘Aligned-to-random’ nanofiber scaffolds for mimicking the structure of the tendon-to-bone insertion site. Nanoscale. 2010;2(6):923–926. doi: 10.1039/c0nr00192a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moffat KL, Kwei AS, Spalazzi JP, Doty SB, Levine WN, Lu HH. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng A. 2009;15(1):115–126. doi: 10.1089/ten.tea.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker BM, Nerurkar NL, Burdick JA, Elliott DM, Mauck RL. Fabrication and modeling of dynamic multipolymer nanofibrous scaffolds. J Biomech Eng. 2009;131(10):101012. doi: 10.1115/1.3192140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahoo S, Ang LT, Goh JC, Toh SL. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J Biomed Mater Res A. 2010;93(4):1539–1550. doi: 10.1002/jbm.a.32645. [DOI] [PubMed] [Google Scholar]
- 49.Ji W, Sun Y, Yang F, et al. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm Res. 2011;28(6):1259–1272. doi: 10.1007/s11095-010-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahoo S, Ang LT, Cho-Hong Goh J, Toh SL. Bioactive nanofibers for fibroblastic differentiation of mesenchymal precursor cells for ligament/tendon tissue engineering applications. Differentiation. 2010;79(2):102–110. doi: 10.1016/j.diff.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Fu YC, Nie H, Ho ML, Wang CK, Wang CH. Optimized bone regeneration based on sustained release from three-dimensional fibrous PLGA/HAp composite scaffolds loaded with BMP-2. Biotechnol Bioeng. 2008;99(4):996–1006. doi: 10.1002/bit.21648. [DOI] [PubMed] [Google Scholar]
- 52.Nie H, Ho ML, Wang CK, Wang CH, Fu YC. BMP2 plasmid loaded PLGA/HAp composite scaffolds for treatment of bone defects in nude mice. Biomaterials. 2009;30(5):892–901. doi: 10.1016/j.biomaterials.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 53.Zeng J, Xu X, Chen X, et al. Biodegradable electrospun fibers for drug delivery. J Control Release. 2003;92(3):227–231. doi: 10.1016/s0168-3659(03)00372-9. [DOI] [PubMed] [Google Scholar]
- 54.Kim K, Luu YK, Chang C, et al. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release. 2004;98(1):47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Wang S, Zhao Y, Shen M, Shi X. Electrospun hybride nanofibers doped with nanoparticles or nanotubes for biomedical applications. Ther Deliv. 2012;3(10):1155–1169. doi: 10.4155/tde.12.103. [DOI] [PubMed] [Google Scholar]
- 56.Wang S, Zheng F, Huang Y, et al. Encapsulation of amoxicillin within Iaponite-doped poly(lactic-co-glycolic acid) nanofibers: preparation, characterization, and antibacterial activity. ACS Appl Mater Interfaces. 2012;4(11):6393–6401. doi: 10.1021/am302130b. [DOI] [PubMed] [Google Scholar]
- 57.Zheng F, Wang S, Wen S, Shen M, Zhu M, Shi X. Characterization and antibacterial activity of amoxicillin-loaded electrospun nano-hydroxyapatite/poly(lactic-co-glycolic acid) composite nanofibers. Biomaterials. 2013;34(4):1402–1412. doi: 10.1016/j.biomaterials.2012.10.071. [DOI] [PubMed] [Google Scholar]
- 58.Hong KH. Preparation and properties of electrospun poly(vinyl alcohol)/silver fiber web as wound dressings. Polym Eng Sci. 2007;47(1):43–49. [Google Scholar]
- 59.Shi Q, Vitchuli N, Nowak J, et al. Durable antibacterial Ag/polyacrylonitrile (Ag/PAN) hybrid nanofibers prepared by atmospheric plasma treatment and electrospinning. Eur Polym J. 2011;47:1402–1409. [Google Scholar]
- 60.Theisen C, Fuchs-Winkelmann S, Knappstein K, et al. Influence of nanofibers on growth and gene expression of human tendon derived fibroblast. Biomed Eng. 2010;9:9. doi: 10.1186/1475-925X-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erisken C, Zhang X, Moffat KL, Levine WN, Lu HH. Scaffold fiber diameter regulates human tendon fibroblast growth and differentiation. Tissue Eng Part A. 2013;19:519–528. doi: 10.1089/ten.tea.2012.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaiswal D, Brown JL. Nanofiber diameter-dependent MAPK activity in osteoblasts. J Biomed Mater Res A. 2012;100(11):2921–2918. doi: 10.1002/jbm.a.34234. [DOI] [PubMed] [Google Scholar]
- 63.Li WJ, Jiang YL, Tuan RS. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006;12(7):1775–1785. doi: 10.1089/ten.2006.12.1775. [DOI] [PubMed] [Google Scholar]
- 64.Riboldi SA, Sampaolesi M, Neuenschwander P, Cossu G, Mantero S. Electrospun degradable polyesterurethane membranes: potential scaffolds for skeletal muscle tissue engineering. Biomaterials. 2005;26(22):4606–4615. doi: 10.1016/j.biomaterials.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 65.Huang NF, Patel S, Thakar RG, et al. Myotube assembly on nanofibrous and micropatterned polymers. Nano Lett. 2006;6(3):537–542. doi: 10.1021/nl060060o. [DOI] [PubMed] [Google Scholar]
- 66.Huber A, Pickett A, Shakesheff KM. Reconstruction of spatially orientated myotubes in vitro using electrospun, parallel microfiber arrays. Eur Cells Mater. 2007;14:56–63. doi: 10.22203/ecm.v014a06. [DOI] [PubMed] [Google Scholar]
- 67.Aviss KJ, Gough JE, Downes S. Aligned electrospun polymer fibres for skeletal regeneration. Eur Cells Mater. 2010;19:193–204. doi: 10.22203/ecm.v019a19. [DOI] [PubMed] [Google Scholar]
- 68.Ricotti L, Polini A, Genchi GG, et al. Proliferation and skeletal myotube formation capability of C2C12 and H9c2 cells on isotropic and anisotropic electrospun nanofibrous PHB scaffolds. Biomed Mater. 2012;7(3):035010. doi: 10.1088/1748-6041/7/3/035010. [DOI] [PubMed] [Google Scholar]
- 69.Jun I, Jeong S, Shin H. The stimulation of myoblast differentiation by electrically conductive sub-micron fibers. Biomaterials. 2009;30(11):2038–2047. doi: 10.1016/j.biomaterials.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 70.Ku SH, Lee SH, Park CB. Synergic effects of nanofiber alignment and electroactivity on myoblast differentiation. Biomaterials. 2012;33(26):6098–6104. doi: 10.1016/j.biomaterials.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 71.Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJ. The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008;29(19):2899–2906. doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 72▪▪.Yin Z, Chen X, Chen JL, et al. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31(8):2163–2175. doi: 10.1016/j.biomaterials.2009.11.083. Reports the influences of fiber alignment on tendon stem cell differentiation. [DOI] [PubMed] [Google Scholar]
- 73.Ravichandran R, Venugopal JR, Sundarrajian S, Mukherjee S, Ramakrishna S. Precipitation of nanohydroxyapatite on PLLA/PBLG/collagen nanofibrous structures for the differentiation of adipose derived stem cells to osteogenic lineage. Biomaterials. 2012;33(3):846–855. doi: 10.1016/j.biomaterials.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 74▪.Cobun JM, Gibson M, Monagle S, Patterson Z, Elisseeff JH. Bioinspired nanofibers support chondrogenesis for articular cartilage repair. Proc Natl Acad Sci USA. 2012;109(25):10012–10017. doi: 10.1073/pnas.1121605109. Demonstrates that the implantation of nanofiber scaffolds into damaged cartilage in the knees of rats results in greater production of a more durable type of collagen compared with surgically repaired cartilage tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.James R, Kumbar SG, Laurencin CT, Balian G, Chhabra AB. Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomed Mater. 2011;6(2):025011. doi: 10.1088/1748-6041/6/2/025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sommerfeldt DW, Rubin CT. Biology of bone and how it orchestrates the form and function of the skeleton. Eur Spine J. 2001;10(2):S86–S95. doi: 10.1007/s005860100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77▪.Zhong SP, Zhang YZ, Lim CT. Fabrication of large pores in electrospun nanofibrous scaffolds for cellular infiltration: a review. Tissue Eng B. 2012;18(2):77–87. doi: 10.1089/ten.TEB.2011.0390. Highlights methods of producing nanofiber scaffolds with high porosity. [DOI] [PubMed] [Google Scholar]
- 78.Baker BM, Shah RP, Silverstein AM, Esterhai JL, Burdick JA, Mauck RL. Sacrificial nanofibrous composites provide instruction without impediment and enable functional tissue formation. Proc Natl Acad Sci USA. 2012;109(35):14176–14181. doi: 10.1073/pnas.1206962109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frohhbergh ME, Katsman A, Botta GP, et al. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials. 2012;33(36):9167–9178. doi: 10.1016/j.biomaterials.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nam J, Johnson J, Lannutti JJ, Agarwal S. Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers. Acta Biomater. 2011;7(4):1516–1524. doi: 10.1016/j.actbio.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang D, Tong A, Zhou L, Fang F, Guo G. Osteogenic differentiation of human placenta-derived mesenchymal stem cells (PMSCs) on electrospun nanofiber meshes. Cytotechnology. 2012;64(6):701–710. doi: 10.1007/s10616-012-9450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schofer MD, Veltum A, Theisen C, et al. Functionalisation of PLLA nanofiber scaffolds using a possible cooperative effect between collagen type I and BMP-2: impact on growth and osteogenic differentiation of human mesenchymal stem cells. J Mater Sci Mater Med. 2011;22(7):1753–1762. doi: 10.1007/s10856-011-4341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piskin E, Isoglu IA, Bolgen N, et al. In vivo performance of simvastatin-loaded electrospun spiral-wound polycaprolactone scaffolds in reconstruction of cranial bone defects in the rat model. J Biomed Mater Res A. 2009;90(4):1137–1151. doi: 10.1002/jbm.a.32157. [DOI] [PubMed] [Google Scholar]
- 84.Schofer MD, Roessler PP, Schaefer J, et al. Electrospun PLLA nanofiber scaffolds and their use in combination with BMP-2 for reconstruction of bone defects. PLoS One. 2011;6(9):e25462. doi: 10.1371/journal.pone.0025462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schofer MD, Tunnermann L, Kaiser H, et al. Functionalisation of PLLA nanofiber scaffolds using a possible cooperative effect between collagen type I and BMP-2: impact on colonization and bone formation in vivo. J Mater Sci Mater Med. 2012;23(9):2227–2233. doi: 10.1007/s10856-012-4697-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rainer A, Spadaccio C, Sedati P, et al. Electrospun hydroxyapatite-functionalized PLLA scaffold: potential applications in sternal bone healing. Ann Biomed Eng. 2011;39(7):1882–1890. doi: 10.1007/s10439-011-0289-2. [DOI] [PubMed] [Google Scholar]
- 87.Kolambkar YM, Dupont KM, Boerckel JD, et al. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32(1):65–74. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu YC, Nie H, Ho ML, Wang CK, Wang CH. Optimized bone regeneration based on sustained release from three-dimensional fibrous PLGA/Hap composite scaffolds loaded with BMP-2. Biotechnol Bioeng. 2008;99(4):996–1006. doi: 10.1002/bit.21648. [DOI] [PubMed] [Google Scholar]
- 89.James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33(1):102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 90.Manning CN, Schwartz AG, Liu W, et al. Controlled delivery of mesenchymal stem cells and growth factors using a tendon-specific nanofiber scaffold. Acta Biomater. 2013;9(6):6905–6914. doi: 10.1016/j.actbio.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.James R, Hogan M, Keller TC, Balian G, Laurencin CT, Chhabra A. Combined treatment of a tendon gap with a biomimetic electrospun scaffold, stromal cells and GDF5. Presented at: 2013 ORS Annual Meeting; San Antonio, TX, USA. 25–29 January 2013. [Google Scholar]
- 92▪.Ni T, Senthil-Kumar P, Dubbin K, et al. A photoactivated nanofiber graft material for augmented Achilles tendon repair. Laser Surg Med. 2012;44(8):645–652. doi: 10.1002/lsm.22066. Reports the fabrication of a photoactivated nanofiber graft material for the prevention of rerupture and adhesion after tendon surgery. [DOI] [PubMed] [Google Scholar]
- 93.Liu S, Zhao J, Ruan H, et al. Biomimetic sheath membrane via electrospinning for antiadhesion of repaired tendon. Biomacromolecules. 2012;13(11):3611–3619. doi: 10.1021/bm301022p. [DOI] [PubMed] [Google Scholar]
- 94.Liu S, Qin M, Hu C, et al. Tendon healing and anti-adhesion properties of electrospun fibrous membranes containing bFGF loaded nanoparticles. Biomaterials. 2013;34(19):4690–4701. doi: 10.1016/j.biomaterials.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 95.Surrao DC, Hayami JW, Waldman SD, Amsden BG. Self-crimping, biodegradable, electrospun polymer microfibers. Biomacromolecules. 2010;11(12):3624–3629. doi: 10.1021/bm101078c. [DOI] [PubMed] [Google Scholar]
- 96.Liu Y, Zhang X, Xia Y, Yang H. Magnetic-filed-assisted electrospinning of aligned straight and wavy polymeric nanofibers. Adv Mater. 2010;22(22):2454–2457. doi: 10.1002/adma.200903870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kock L, van Donkelaar CC, Ito K. Tissue engineering of functional articular cartilage: the current status. Cell Tissue Res. 2012;347(3):613–627. doi: 10.1007/s00441-011-1243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Toyokawa N, Fujioka H, Kokubu T, et al. Electrospun synthetic polymer scaffold for cartilage repair without cultured cells in an animal model. Arthroscopy. 2010;26(3):375–383. doi: 10.1016/j.arthro.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 99.Shafee A, Soleimani M, Chamheidari GA, et al. Electrospun nanofiber-based regeneration of cartilage enhanced by mesenchymal stem cells. Biomed Mater Res A. 2011;99(3):467–478. doi: 10.1002/jbm.a.33206. [DOI] [PubMed] [Google Scholar]
- 100.Xu T, Binder KW, Albanna MZ, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2013;5(1):015001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 101.Klein TJ, Malda J, Sah RL, Hutmacher DW. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng Part B Rev. 2009;15(2):143–157. doi: 10.1089/ten.teb.2008.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Minns RJ, Steven FS. The collagen fibril organization in human articular cartilage. J Anat. 1977;123(2):437–457. [PMC free article] [PubMed] [Google Scholar]
- 103.Petersen W, Tillmann B. Collagenous fibril texture of the human knee joint menisci. Anat Embryol (Berl) 1998;197(4):317–324. doi: 10.1007/s004290050141. [DOI] [PubMed] [Google Scholar]
- 104.Noyes FR, Barber-Westin SD. Repair of complex and avascular meniscal tears and meniscal transplantation. J Bone Joint Surg Am. 2010;92:1012–1029. [PubMed] [Google Scholar]
- 105.Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28(11):1967–1977. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nerurkar NL, Sen S, Baker BM, Elliott DM, Mauck RL. Dynamic culture enhances stem cell infiltration and modulates extracellular matrix production on aligned electrospun nanofibrous scaffolds. Acta Biomater. 2011;7(2):485–491. doi: 10.1016/j.actbio.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Driscoll TP, Nerurkar NL, Jacobs NT, Elliott DM, Mauck RL. Fiber angle and aspect ratio influence the shear mechanics of oriented electrospun nanofibrous scaffolds. J Mech Behav Biomed Mater. 2011;4(8):1627–1636. doi: 10.1016/j.jmbbm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fisher MB, Henning EA, Söeqaard N, Esterhai JL, Mauck RL. Organized nanofibrous scaffolds that mimic the macroscopic and microscopic architecture of the knee meniscus. Acta Biomater. 2013;9(1):4496–4504. doi: 10.1016/j.actbio.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Whatley RR, Wen X. Intervertebral disc (IVD): structure, degeneration, repair and regeneration. Mat Sci Eng C. 2012;32(2):61–77. [Google Scholar]
- 110.Benjamin M, Ralphs JR. Biology of fibrocartilage cells. Int Rev Cytol. 2004;233:1–45. doi: 10.1016/S0074-7696(04)33001-9. [DOI] [PubMed] [Google Scholar]
- 111.Bowles RD, Gebhard HH, Härtl R, Bonassar LJ. Tissue-engineered intervertebral disc produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proc Natl Acad Sci USA. 2011;108(32):13106–13111. doi: 10.1073/pnas.1107094108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112▪▪.Nerurkar NL, Baker BM, Sen S, Wible EE, Elliott DM, Mauck RL. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat Mater. 2009;8(12):986–992. doi: 10.1038/nmat2558. Demonstrates the recapitulation of the annulus fibrosus using a nanofibrous biologic laminate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nesti LJ, Li JW, Shanti RM, et al. Intervertebral disc tissue engineering using a novel hyaluronic acid-nanofibrous scaffold (HANFS) amalgam. Tissue Eng A. 2008;14(9):1527–1537. doi: 10.1089/ten.tea.2008.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vadala G, Mozetic P, Rainer A, et al. Bioactive electrospun scaffold for annulus fibrosus repair and regeneration. Eur Spine J. 2012;21:S20–S26. doi: 10.1007/s00586-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hollingsworth NT, Wagner DR. Modeling shear behavior of the annulus fibrosus. J Mech Behav Biomed Mater. 2011;4(7):1103–1114. doi: 10.1016/j.jmbbm.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 116.Bosworth LA, Turner LA, Cartmell SH. State of the art composites comprising electrospun fibers coupled with hydrogels: a review. Nanomedicine. 2012;9:322–335. doi: 10.1016/j.nano.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 117.Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons-tendon ‘entheses’. Comp Biochem Physiol A Mol Integr Physiol. 2002;133(4):931–945. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 118.Thomopoulos S, Genin GM, Galatz LM. The development and morphogenesis of the tendon-to-bone insertion – what development can teach us about healing. J Musculoskelet Neuronal Interact. 2010;10(1):35–45. [PMC free article] [PubMed] [Google Scholar]
- 119.Smith L, Xia Y, Galatz LM, Genin GM, Thomopoulos S. Tissue engineering strategies for the tendon/ligament-to-bone insertion. Connect Tissue Res. 2012;53(2):95–105. doi: 10.3109/03008207.2011.650804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Inui A, Kokubu T, Mifune Y, et al. Regeneration of rotator cuff tear using electrospun poly(d,l-lactide-co-glycolide) scaffolds in a rabbit model. Arthroscopy. 2012;28(12):1790–1799. doi: 10.1016/j.arthro.2012.05.887. [DOI] [PubMed] [Google Scholar]
- 121.Beason DP, Connizzo BK, Dourte LM, et al. Fiber-aligned polymer scaffolds for rotator cuff repair in a rat model. J Shoulder Elbow Surg. 2012;21(2):245–250. doi: 10.1016/j.jse.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 122.Moffat KL, Zhang X, Greco S, et al. In vitro and in vivo evaluation of a bi-phase nanofiber scaffold for integrative rotator cuff repair. Presented at: 57th Annual Meeting of the Ortheopaedic Research Society; Long Beach, CA, USA. 13–16 January 2011. [Google Scholar]
- 123▪▪.Li X, Xie J, Lipner J, Yuan X, Thomopoulos S, Xia Y. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Lett. 2009;9(7):2763–2768. doi: 10.1021/nl901582f. Demonstrates the fabrication of nanofiber scaffolds with gradations in mineral content and their functional consequences with regards to mechanical properties and cellular activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xie J, Ma B, Michael PL, Shuler FD. Fabrication of nanofiber scaffolds with gradations in fiber organization and their potential applications. Macromol Biosci. 2012;12(10):1336–1341. doi: 10.1002/mabi.201200115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wei G, Ma PX. Nanostructured biomaterials for regeneration. Adv Funct Mater. 2008;18(22):3566–3582. doi: 10.1002/adfm.200800662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126▪.Xie J, Ma B, Michael PL. Fabrication of novel 3D nanofiber scaffolds with anisotropic property and regular pores and their potential applications. Adv Healthcare Mater. 2012;1(5):674–678. doi: 10.1002/adhm.201200100. Reports a novel approach combining 3D weaving and electrospinning techniques for fabrication of 3D nanofiber scaffolds. [DOI] [PubMed] [Google Scholar]
- 127.Jayasinghe SN. Cell electrospinning: a novel tool for functionalizing fibers, scaffolds and membranes with living cells and other advanced materials for regenerative biology and medicine. Analyst. 2013;138(8):2215–2123. doi: 10.1039/c3an36599a. [DOI] [PubMed] [Google Scholar]
- 128.Poncelet D, de Vos P, Suter N, Jayasinghe SN. Bio-electrospraying and cell electrospinning: progress and opportunities for basic biology and clinical sciences. Adv Healthcare Mater. 2012;1(1):27–34. doi: 10.1002/adhm.201100001. [DOI] [PubMed] [Google Scholar]


