Abstract
Cellular pathways are numerous and are highly integrated in function in the control of cellular systems. They collectively regulate cell division, proliferation, survival and apoptosis of cells and mutagenesis of key genes that control these pathways can initiate neoplastic transformations. Understanding these pathways is crucial to future therapeutic and preventive strategies of the disease. Ovarian cancers are of three major types; epithelial, germ-cell and stromal. However, ovarian cancers of epithelial origin, arising from the mesothelium, are the predominant form. Of the subtypes of ovarian cancer, the high-grade serous tumors are fatal, with low survival rate due to late detection and poor response to treatments. Close examination of preserved ovarian tissues and in vitro studies have provided insights into the mechanistic changes occurring in cells mediated by a few key genes. This review will focus on pathways and key genes of the pathways that are mutated or have aberrant functions in the pathology of ovarian cancer. Non-genetic mechanisms that are gaining prominence in the pathology of ovarian cancer, miRNAs and epigenetics, will also be discussed in the review.
Keywords: Ovarian cancer, Pathways, Epigenetics, miRNA, Cell cycle, Apoptosis, Proliferation
Introduction
Ovarian neoplasms are challenging to detect and their genesis is often considered as de novo or sporadic (Scully, 1995; Liu and Ganesan, 2002; Zikan et al., 2007; Weberpals et al., 2008; Weberpals et al., 2011; Sarojini et al., 2012; Wysham et al., 2012). When detected the cancer is often advanced and is at stage III/IV category (Kurman and Shih Ie, 2010). The absence of or poor detection methods of precancerous lesions of the tissue account for the late detection of ovarian cancer (Fleischer et al., 2012; Sarojini et al., 2012). Thus, ovarian cancer is considered to be a very lethal disease of gynecological origin.
In contrast to previous reports, ovarian cancer is not considered a single disease of epithelial origin, but instead covers a group of tumors that are morphologically and genetically distinct (Kurman and Shih Ie, 2010; Kurman and Shih Ie, 2011). The hypothesis however is still highly debated. Some of the reasons include late detection, inability to detect precancerous lesions and therefore insufficient evidence of neoplastic origination. This obscurity in the pathogenesis of the disease requires the understanding of the molecular aspects that influence the cellular pathways of the ovarian tissue. Based on morphology, genetics, and site of origination, ovarian cancers of epithelial-cell origin have been categorized into two groups. The type I group are those that are strictly confined to the ovary and are low- grade serous, endometrial, mucinous, and clear-cell type (Figure 1) ( Kurman and Shih Ie, 2010; Kurman and Shih Ie, 2011; Le Page, 2010). These tumors are genetically more stable, have few to rare p53 mutations, are easily diagnosed and have a good prognosis. However, only 25% of the ovarian cancers detected are of this type. The Type 2 group contains tumors that are aggressive and comprise high-grade serous carcinomas, undifferentiated carcinomas and carcinosarcomas (Figure 1) (Le Page, 2010). These tumors constitute 75% of the ovarian cancers with a 90% death rate and the site of origination stems from tissues other than the ovary. These tumors exhibit genetic instabilities with a higher percentage of p53 mutations (Kurman and Shih Ie, 2011).
Figure 1. Proposed theories of origination of epithelial-cell ovarian cancers (EOCs).
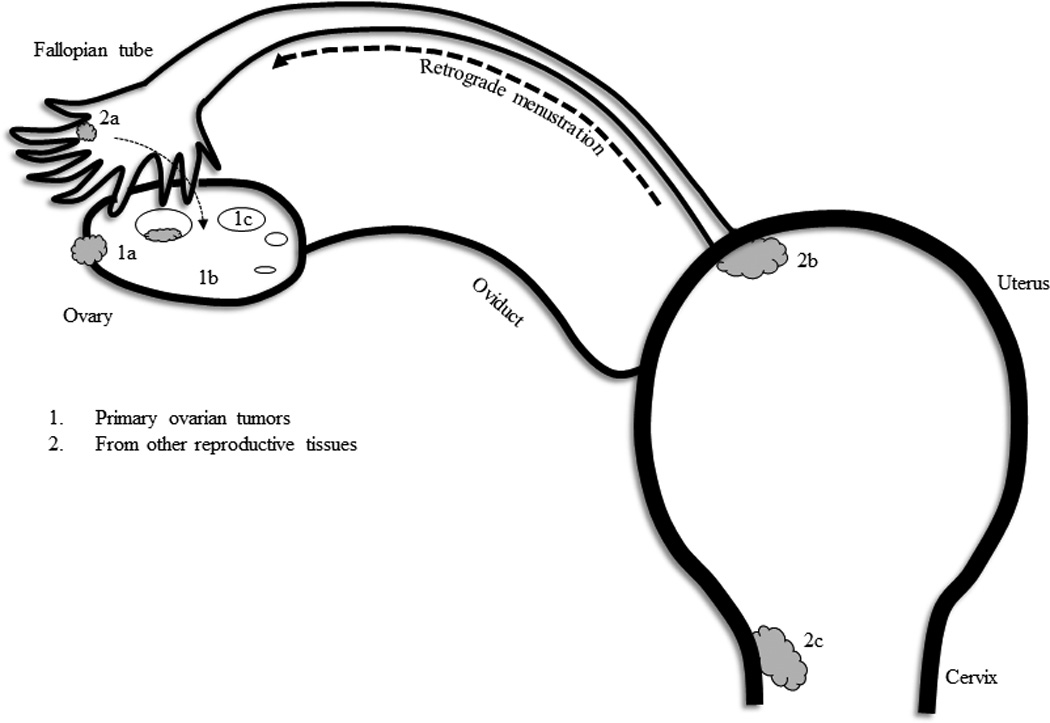
The primary ovarian cancers reside in the ovary tissue itself. However, cells from the endometrium, fallopian tube, stroma can give rise to the histological types of tumors of epithelial-cell origin. As shown in the diagrams, 1a, 1b and 1c denote the commonly seen ovarian tumors - epithelia, stroma and germ-cell tumors, respectively. However, EOCs are the predominant form and closer examination reveals that the origin could stem from other reproductive tissues such as the endometrium lining the uterus, cervical tissue or from the epithelia of the fallopian tube. Retrograde menstruation problems can be a pathway for the transfer of endometrial cells to that of the ovary. Ovulation and encystment of cells can be a mode of travel for cells from the fallopian tube. Therefore, the serous type EOCs arise from the fallopian tube (2a); the endometroid EOCs arise from the cervical or uterine tissue (2a and 2b); the mucinous and clear-cell tumors could arise from cells of 1b, 1c, (internal environment of the ovary) and 2a (cervical tissue). Hence, ovarian epithelial tumors are of the multi-histological type. Symbols used: grey filled cloud-like structure, tumors; small open oval circles, follicles in the ovary.
Since the last 30 years or so, many preclinical and clinical trials have been conducted to determine the therapeutic efficacies of drugs against ovarian tumors. However, the success rates against ovarian cancers have been few. A majority of tumors that are removed through cytoreductive surgery resurface and become aggressive and have been proposed to be mediated in part through the treatment itself (Steffensen et al., 2011a; Modugno and Edwards, 2012; Stathopoulos et al., 2012). Elucidating the underlying factors that govern the genesis, progression and metastasis of this disease would provide valuable information for the development of diagnostic, prognostic and therapeutic approaches of the disease. Further understanding the genes and pathways that become dysregulated either due to loss of heterozygosity, loss of function, amplification or mutation would facilitate progress in managing ovarian cancer. This review will focus on the pathways deregulated and instrumental in the pathogenesis of ovarian tumors and key genes that are involved in these pathways. Genetic and epigenetic factors that contribute to these changes will also be analyzed.
Genes involved in the biogenesis of ovarian cancers
p53
Mutations in cell-cycle regulatory genes, primarily tumor suppressor genes, favor an uninhibited growth of cells and serve as precursors of immortality. Combined mutations in these genes and oncogenes encourage a neoplastic phenotype. How these tight regulations become deregulated through mutations and how these mutations affect important regulatory pathways is important to a diagnostic and prognostic approach for all cancers. Unlike cancers of other origins, a majority of ovarian cancers are of epithelial-cell origin, commonly designated as epithelial ovarian carcinomas (EOCs). Nonetheless this single layer epithelia morphs to give rise to histologically different types (Kurman and Shih Ie, 2011). The transition is thought to occur through the cell cycle and genes that control metabolic and molecular pathways within the ovarian cell or via changes of the epithelia of surrounding reproductive tissue that find their way into the ovary through the inflammation and repair process of ovulation (Figures 1 and 2). Of the many regulators, tumor suppressor proteins play a pivotal role in the cell cycle process and these proteins are highly deregulated in ovarian cancers (Table 1).
Figure 2. Cell cycle pathways involved in ovarian carcinogenesis.
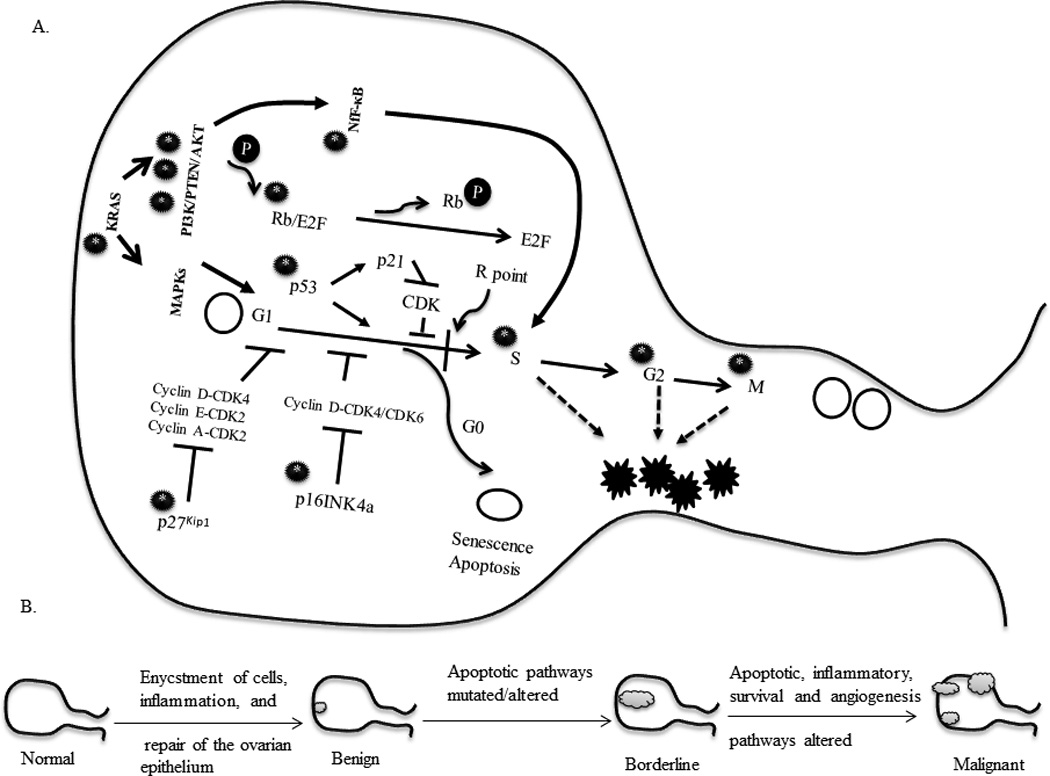
The cell cycle traverses through the G1-S-G2-M phases tightly controlled by check points between each phase through a set of regulatory proteins, primarily cyclins and kinases. The levels and expression of these regulators ensure the fate of the preceding and proceeding phases of the cycle. Cellular destinations, division, differentiation, senescence and apoptosis are controlled by a certain restriction in the late G1 phase termed the restriction (R) point. It is when cells successfully cross this threshold point, that cellular destinations are met. Various genes involved in tightly regulating the cell cycle in ovarian cancer. A. Deregulation of the key genes (black circle with a star) initiates aberrant proliferation triggering the development of neoplasms. Single or multiple pathways may be involved in the process. In early stage development there are chances of single gene mutations or epigenetic mechanisms that transform the cell into an immortal phenotype. However, when multiple pathways are hit or crucial genes such as NF-κB are mutated, far more aggressive tumors can develop. Symbols used: open circle, normal cell; open oval, cell towards senescence or apoptosis; black jagged star, cancerous cell; black circle with white star, mutated gene/pathway; black circle with ‘P’, phosphorylated. B. Gradual stages of early to late ovarian tumor development and pathway abnormalities have been highlighted in miniature figures below the main one. Symbol used: grey cloud-like structure, tumor.
Table 1.
Alterations in genes and pathways involved in the cell cycle in ovarian carcinomas.
| Gene | Cases | Percent | Observations | Ovarian Tissue analyzed |
Technique use | Reference |
|---|---|---|---|---|---|---|
| p53 | 36/46 | 78.3% | Altered expression |
Epithelial | Immunohistochemistry | (Hashiguchi et al., 2001) |
| p53 | 79/134 | 59% | High positive staining |
Serous | Immunohistochemistry and disease outcome results |
(Bali et al., 2004) |
| p53 | 54/107 | 50.4% | High expression Overexpression |
Epithelial | Immunohistochemistry | (Marks et al., 1991) |
| Nuclear p53 protein |
15/52 8/52 |
29% 15% |
Point mutations Overexpression |
Early-stage Ovarian cancer |
Immunostaining and DNA sequencing |
(Kohler et al., 1993) |
| p53 | 177/284 | 62.3% | Overexpression | Epithelial (Stage I, II, III and IV) |
Immunohistochemistry | (Hartmann et al., 1994) |
| p53 | 107/221 193/316 104/136 |
48.4% 26 % 35% |
Overexpression Overexpression Mutated |
Primary Epithelial (Stages I-IV) |
Immunohistochemistry | (Eltabbakh et al., 1997) |
| p53 and cyclin D1 |
11/18 5/18 |
61% 27.7% |
P53 mutation Overexpression of cyclin D1 |
Primary Epithelial Metastatic tumor |
Computer aided image analysis system |
(Anttila et al., 1999a) |
|
p53 and Cyclin D1 |
7/18 | 39% | Absence of p53 mutations but increased expression of Cyclin D1 |
Ovarian tumor |
cDNA sequence analysis |
(Shigemasa et al., 1999) |
| 42/90 | 47% | |||||
| p53 | 81/125 3/125 |
74% 3% |
P53 expression Positive staining |
Ovarian tumor |
cDNA sequence analysis and PCR |
(Shigemasa et al., 1999) |
| 25/125 55/81 |
23% 67.9% | Single mutation Two mutations |
||||
| p53 | 7/22 | 32% | Lacking a mutation exons 2 to 11 |
Ovarian tumor |
cDNA sequence analysis and PCR |
(Shigemasa et al., 1999) |
| 8/25 | 32% | Overexpression observed with missense mutation |
Epithelial | Immunohistochemistry | (Sagarra et al., 2002) | |
| 44/82 | 54% | Overexpression observed with truncations |
Frozen Ovarian tumor |
cDNA analysis in automated sequencer |
(Havrilesky et al., 2003) | |
| p53 | 34/66 | 51.5% | Overexpression in those lacking a mutation in exons 2 to 11 |
Frozen Ovarian tumor |
cDNA analysis in automated sequencer |
(Havrilesky et al., 2003) |
| p53 protein | 33/131 | 25% | Stained positive Promoter methylated |
Frozen Ovarian tumor |
cDNA analysis in automated sequencer |
(Havrilesky et al., 2003) |
| Abnormal p53 pathway |
nd | 80.4% | nd | Epithelial | nd | (Hashiguchi et al., 2001) |
| Abnormal Rb pathway |
nd | 60.9% | nd | Epithelial | nd | (Hashiguchi et al., 2001) |
| pRb | 5/46 106/134 |
10.9% 79% |
Protein expression |
Epithelial Serous |
Immunohistochemistry Immunohistochemistry and disease outcome results |
(Hashiguchi et al., 2001; Bali et al., 2004) |
| p16INK4a | 16/46 | 34.7% | Altered expression; no deletions |
Epithelial | Immunohistochemistry | (Hashiguchi et al., 2001; Bali et al., 2004) |
| 74/134 | 55% | Loss of function | Serous | Immunohistochemistry and disease outcome results |
||
|
p16INK4a mutation + methylation |
7/46 | 15.2% | Methylated promoter |
Epithelial | Immunohistochemistry | (Hashiguchi et al., 2001) |
| Cyclin D1/CDK4 |
14/46 | 30.4% | Overexpression | Epithelial | Immunohistochemistry | (Hashiguchi et al., 2001; Bali et al., 2004) |
| Cyclin D1 Cyclin D1 |
25/134 21/27 |
19% 78% |
Overexpression Higher than normal ovarian samples |
Serous Ovarian tumors |
Immunohistochemistry mRNA expression-PCR analysis |
(Shigemasa et al., 1999) |
| Cyclin D1 protein |
10/18 | 56% | Positive immunostaining |
Ovarian tumors |
Immunostaining | (Shigemasa et al., 1999) |
| p14ARF mutation |
10/46 | 21.7% | Altered expression |
Epithelial | Immunohistochemistry | (Hashiguchi et al., 2001) |
| p14ARF mutation + methylation |
4/46 | 8.7% | Methylated promoter |
Epithelial | ||
Nd = not determined
Tumor suppressor genes, proto-oncogenes and cell-cycle proteins cohesively regulate cell growth and division. The primary cyclins and kinase inhibitors that have a major role in ovarian cancer are cyclin D1, p16INK4a, p27 and p21 (Table 1, Figure 2), (Vikhanskaya et al., 1996; Kusume et al., 1999; Shigemasa et al., 1999; Bali et al., 2004; Barbieri et al., 2004; Hashimoto et al., 2011). The regulation and function of cyclin D1, p16INK4a, p27 and p21 have been shown to be controlled in part by the tumor suppressor gene p53, which is predominantly mutated in high grade ovarian cancers (Shigemasa et al., 1999; Bali et al., 2004). The changes in p53 as seen in ovarian cancers could be both at the gene and protein level. Point mutations, missense mutations and truncations have been observed, and overexpression of the p53 protein has been detected in many of the immunohistochemical studies that have been performed (Table 1) (Marks et al., 1991; Liu et al., 1994; Havrilesky et al., 2003). The accumulation of non-functional p53 has been shown to affect the expression and interaction of pro-apoptotic genes such as BCL2-associated X protein (BAX) and B-cell lymphoma 2 (BCL-2) (Tai et al., 1998; Schuyer et al., 2001; Ziolkowska-Seta et al., 2009). The aberrations in p53 result in the accumulation of the altered protein within the cell that has a negative effect on BAX, a transcriptional target of p53 (Ozer et al., 2012). It seems likely that p53 alterations in high grade tumors reduce BAX expression allowing the progression of solid tumors. However, immunohistological studies have not provided a definitive conclusion of a positive correlation with p53 expression and tumor prognosis and survival (Reles et al., 2001; Rose et al., 2003; Leffers et al., 2008). Contradictions in observations are possible due to the type of ovarian tissue analyzed, the method used and statistical programs employed to determine the correlation (Table 1).
The BCL family of apoptotic proteins along with p53 can serve as tools for histotyping (Skirnisdottir et al., 2002). The expression of p53-BCL-2 and BCL-2-BAX have been shown to have a strong association with tumor grade and histopathological subtyping, factors that could be vital for identifying the specific epithelial ovarian carcinoma for adjuvant or combined therapies (Skirnisdottir et al., 2002). What has been observed is that ovarian tumors are initially very responsive to treatment but later become chemoresistant. Possibly the treatment itself may cause a few cells within the tumor mass to harbor mutations in p53 that, in addition to epigenetic silencing of promoter regions of apoptotic favorable genes such as p16 or Rb, may account for the relapse and progression of the tumor. Ovarian cancers are categorized as low or high-grade tumors of various histological subtypes (Le Page, 2010). These range from well-differentiated, to moderately differentiated to non-differentiated tumors, respectively, and morphologically are identified as benign, borderline or malignant ovarian tumors, (Figure 2) (Le Page, 2010). Studies have shown that in low-grade ovarian tumors, p53 mutations are absent, but transitioning into the aggressive type, a significant increase in mutations of the gene are observed, with a majority of them being of the serous epithelial type (Marks et al., 1991; Kohler et al., 1993). The factors that contribute to this observed increase in p53 mutations are still very unclear. p53 is a quintessential player in the cell cycle and its role in maintaining healthy cell populations is very apparent. Since ovarian cancers are of multi-histological phenotype the alterations of p53 in tumors of this type may be varied. An altered state of p53 is observed both at the gene and at the protein level in type 2 ovarian cancers (Yemelyanova et al., 2011; Jones et al., 2012; Kuhn et al., 2012) . Most mutations observed in p53 gene are point mutations that cause the accumulation of the non-functional protein in the cell due to increased stability. In some instances, the binding domains of p53 could be altered such that p53 cannot bind to the DNA binding elements of its target genes hindering subsequent pathways that encourage apoptosis. In other scenarios, the functional role of the non-functional p53 is based on p16INK4a, a cyclin kinase inhibitor within the cell (Leong et al., 2009).
p21 and p27
Cyclin dependent kinase (CDK) inhibitors are major co-regulatory proteins in the cell cycle along with the p16, p53 and retinoblastoma (Rb) pathways (Sherr, 1996; Bartkova et al., 2003; Jayasurya et al., 2004). p21 is a direct transcriptional target of p53 (Gallagher et al., 2012). In the presence of wild-type (WT) p53, p21 induction ensues followed by the inhibition of cyclin E/CDK2 preventing the G1-S transition, encouraging the apoptotic phenotype of cells (Hindley and Philpott, 2012). CDK inhibitors p21 and p27 control various phases of the cell cycle based on the cyclin with which they associate. p21 association with cyclin D-CDK4/6 inhibits G1-S transition; with cyclin E-CDK2 the late G1-S transition; with cyclin A-CDK2 preventing the S-G2 transition; and with cyclinA-cdc2 the G2-M transition (Cariou et al., 2000; Sandhu et al., 2000). p27 exhibits the same level of control with cyclins D, E and A from the G1 to the G2 phase (Cariou et al., 2000). Although the roles of p21 and p27 have been discussed in many cancers, they appear to have secondary roles in the etiology of ovarian cancer rather than in the genesis (Milde-Langosch et al., 2003; Plisiecka-Halasa et al., 2003; Oudit et al., 2004; Lee et al., 2007). The role of p27 in ovarian cancer is somewhat contradictory. On examination of the protein level and localization of p27 in 150 advanced epithelium ovarian cancers (EOCs), lower nuclear p27 expression was associated with an improved prognosis (Psyrri et al., 2005). However, another study found that negative p27 expression was associated with poor prognosis (Shigemasa et al., 2001). Possibly, different grading scales, techniques and samples assessed might account for these inconsistencies. Loss of function or accumulation of these proteins in ovarian cancers has not been reported. In some cases, the absence of or low expression of p21 with p53 expression appears to place patients at a higher risk for disease recurrence (Anttila et al., 1999b). Studies have also shown that high p21 expression correlates with early stage ovarian tumors and that only high p27 expression was necessary for disease free survival (Schmider-Ross et al., 2006). Overall, there appears to be no correlation between p21 and p27 expression and in terms of ovarian cancers, as yet these proteins are not especially useful tools for the prognosis of the disease (Baekelandt et al., 1999).
Pathways implicated in the biogenesis of ovarian cancer
p16INK4a and Rb pathway
In normal cells, p16INK4a regulates cell proliferation by promoting genes of the apoptotic pathways (Leong et al., 2009). However, in ovarian cancer cells, it has been observed that p16INK4a is mutated or its promoter region is hypermethylated switching off its expression. Transfection studies using ovarian cancer cell lines have shown that in the absence of WT p53, p16 INK4a is upregulated to rescue the apoptotic pathway (Modesitt et al., 2001; Ramirez et al., 2001). In cells that contain double defects of both p53 and p16 INK4a, cell proliferation is rampant and the tumors are more aggressive (Modesitt et al., 2001; Ramirez et al., 2001). Far advanced tumors have been shown to have low expression of p16INK4a but higher expressions of retinoblastoma (Rb) (Todd et al., 2000). p16INK4a is considered to be a direct target of Rb expression (Todd et al., 2000). However, some studies have contradicted this finding. It appears that p16INK4a and Rb mutually regulate the expression of one another. While trying to understand the mechanistic pathways involved in ovarian cancers, the Rb-p16INK4a pathway in conjunction with p53 has been implicated in the genesis of a majority of cancers and has a pivotal role in the ovarian epithelial specific type (Konecny et al., 2011). The p16INK4a inhibitor of kinase prevents the association of the cyclin D1-CDK4/6 complex necessary for the transition of cells from the G1-S phase, in conjunction with hyperphosphorylated Rb. Studies performed to determine the expression patterns of these keys genes have shown that loss of p16 INK4a function through promoter methylation is more frequent than mutations and deletions, in comparison to Rb, where hemizygous deletions are more pronounced with few mutations (Todd et al., 2000; Hashiguchi et al., 2001). The level of cyclin D1 necessary for cyclin-CDK complex formation is overexpressed and has been found to be associated with aggressive phenotype and poor prognosis in about 19% of ovarian cancers. (Todd et al., 2000; Konecny et al., 2011). As discussed, although alterations/loss of function appear to be the highlighted changes associated in the Rb-p16 INK4a pathway, most ovarian cancers appear to co-express Rb and p16 proteins, with overexpression of Rb found in advanced ovarian tumors (Todd et al., 2000). Post-translational modifications that affect protein function are also an important factor to consider in the analysis of tumor biogenesis. The Rb protein release from the Rb-E2F complex occurs by the hyperphosphorylation of its serine/threonine residues. A further examination of the phosphorylation levels of Rb protein therefore needs to be examined. Post-translational modifications have been found to affect protein function based on the residues phosphorylated as seen in p53 (Smeenk et al., 2011). In addition, epigenetic alterations as seen in the p16INK4a gene, may also regulate Rb and p53 function through CCCTC binding factor (CTCF) (De La Rosa-Velazquez et al., 2007; Soto-Reyes and Recillas-Targa, 2010).
KRAS/MAPK/ERK pathway
Kirsten rat sarcoma oncogene (KRAS) activation triggers a sequence of events through the RAF/MEK and mitogen activated protein kinases (MAPK) pathways, and in conjunction with mammalian target of rapamycin (mTOR), a target of the protein AK strain thymoma (AKT) pathway, control cell proliferation (Table 2) (Janku et al., 2012). Point mutations in KRAS provide an advantage for the survival and progression of tumors (Mane et al., 1990; Edkins et al., 2006; Harris and McCormick, 2010; Li et al., 2011; Oliveira-Cunha et al., 2012). KRAS mutations have been implicated in the genesis of low-grade ovarian tumors, inducing an overactive proliferative phenotype (Vereczkey et al., 2011; Stewart et al., 2012). In addition to breast cancer associated protein 1 (BRCA1) and 2 (BRCA2) that have familial roles in sporadic germline-based breast and ovarian cancers, KRAS is now considered the third player (Kundu et al., 2012; Pilarski et al., 2012). KRAS expression levels differ based on the histopathological type of ovarian tissue and the expression levels may help determine the various type of ovarian epithelial cancers (Auner et al., 2009; Steffensen et al., 2011b; Nowak-Markwitz and Spaczynski, 2012). These signatures have a specific pattern in tandem with the expression of RAF/MAPK components, phosphatase and tensin homolog (PTEN) levels and AKT (Table 2) and may help differentiate normal from borderline to early-stage cancers. Determining the signatorial patterns is relatively difficult due to heterogeneity of the tumors.
Table 2.
Alterations in PI3K/PTEN/AKT pathway in ovarian cancers
| Gene | Type of alteration |
Frequency found |
Cellular Phenotype affected |
Pathway affected |
Reference |
|---|---|---|---|---|---|
| PTEN | Mutation | Less than 1% 20% |
Proliferation | RAS/PI3K | (Landen C.N. Jr. et al., 2008; Urick et al., 2011) |
| PTEN | Deletion | 7% | Proliferation | RAS/PI3K, Homologous repair (HR) altered |
(Urick et al., 2011) |
| PI3KCa | Amplified | 18% | Proliferation | RAS/PI3K | (Urick et al., 2011) |
| AKT | Amplified | 30% | Proliferation | PI3kinase/AKT | (Landen C.N. Jr. et al., 2008) |
| AKT1 | Amplified | 3% | Proliferation | RAS/PI3K | (Urick et al., 2011) |
| AKT2 | Amplified | 6% | Proliferation | RAS/PI3K | (Urick et al., 2011) |
| KRAS | Amplified | 11% 50% |
Proliferation/ survival |
RAS alterations |
(Landen C.N. Jr. et al., 2008; Urick et al., 2011) |
| Rb | Mutation | 2% | Cell cycle progression |
Rb | (Urick et al., 2011) |
| Rb | Deletion | 8% | Cell cycle progression |
Rb | (Urick et al., 2011) |
| BRCA1 | Mutated/ hypermethylated |
23% | Apoptosis | HR-pathway | (Urick et al., 2011) |
| BRCA2 | Mutated | 11% | Apoptosis | HR-pathway | (Urick et al., 2011) |
A mutant form of KRAS, called KRAS-variant, carries a mutation in its sequence that has the binding site of a micro RNA (miRNA) termed lethal-7 (Let-7), limiting the association of Let-7 with KRAS (Ratner et al., 2010). Consequently the levels of KRAS rises followed by the induction of the nuclear transcription factor (NF-κB) that promotes the induction of anti-apoptotic genes. It is quite probable that in late stage ovarian cancers, the earlier mutations in KRAS can stimulate and induce the over-activation of NF-κB in cancer stem cells (CSCs) that survive chemotherapy. However, immunohistochemical-based correlation assessments between the KRAS expression and alterations in NF-κB components and PTEN have not yet been able to confirm a direct association (Laudanski et al., 2011). KRAS-variants have also been shown to target NF-κB expression independent of the phosphatidylinositide-3 kinase PI3K-AKT pathway (Keane and Ratner, 2010; Ratner et al., 2010; Kinross et al., 2011; Mizumoto et al., 2011; Pharoah et al., 2011; Weidhaas and Slack, 2011; Pilarski et al., 2012; Ratner et al., 2012). The disparities in KRAS expression and association patterns with downstream targets may stem from the type of tissues analyzed. In a study conducted on 489 high grade ovarian adenocarcinomas, Spellman et al. (2011) showed that in 45% cases that contained altered PI3K / RAS signaling, less than 1% was due to mutations and 11% were amplifications. Also, the downstream target, serine/threonine-protein kinase B-Raf (BRAF) was mutated (0.5%). In addition to PI3K/RAS signaling, KRAS induces mitogen activated protein kinase/extracellular signal-regulated kinases (MAPK/ERK) through BRAF. Stimulation of KRAS via GTPase activity activates BRAF. The stimulation can be mediated by cytokines, growth factors or proto-oncogenes. The downstream target of BRAF, MEK stimulates ERK when activated. ERK targets transcription factors that are involved in cell proliferation such as Myc (Smolle et al., 2013). In ovarian carcinomas, activation of the ERK pathway stems from hormonal activation of G-protein coupled receptors (Smolle et al., 2013). MAPKs, part of the MAPK/ERK pathway, are involved in the transduction of signals through hormones. MAPKs are of two types, tyrosine protein kinase and G-protein coupled receptor. In ovarian cancers, hormones such as follicle stimulating hormone (FSH) and luteinizing hormone (LH) may participate in signal transduction via MAPKs, and are thought to be responsible for ovarian cancer cell growth in carcinomas that express the receptors (Hilliard et al., 2013).
PI3K/PTEN/AKT pathway
Phosphatidylinositide-3 kinase (PI3K) and phosphatase and tensin homolog (PTEN) proteins influence multiple pathways within the cell that have many effects on the cellular phenotype through protein AK strain thymoma (AKT), also known as protein kinase B (PKB). These genes are crucially required to transcend the input from external stimuli (growth factors) and convey them to AKT. Phosphorylation of key amino acid residues in these proteins triggers the downstream activation processes. PTEN is a tumor suppressor gene and either mutations in the gene or loss of function of the protein is observed in many cancers, including ovarian cancers (Table 2) (Saga et al., 2002; Meng et al., 2006; Yan et al., 2006; Paige and Brown, 2008). Loss of function in this gene is mediated through epigenetic silencing and its role in ovarian cancer needs to be further assessed (Kurose et al., 2001) . AKT is an oncogene, and its function and activation depends on the function of PTEN (Meng et al., 2006; Blanco-Aparicio et al., 2007). However, when PTEN becomes inactivated, or loss of function is observed, AKT is overexpressed which serves as an impetus for tumor formation by changes in sub-pathways such as NF-κB, mammalian target of rapamycin (mTOR), and p53, that in turn regulates proliferation, cell division, and cell apoptosis (Tables 1 and 2) (Blanco-Aparicio et al., 2007; Hussain et al., 2012). PTEN and AKT are genes of a pathway controlled by PI3K phosphorylation of its internal amino acid residues, the signals of which depend on either growth receptors or tyrosine kinase receptors (Xu et al., 2004; Lee et al., 2005; Meng et al., 2006).
PI3K is a heterodimeric protein comprised of a regulatory subunit, p85, and a catalytic subunit, p110 (Berenjeno and Vanhaesebroeck, 2009; He et al., 2010; Folgiero et al., 2012; Hofmann and Jucker, 2012; Li et al., 2012; Takayama et al., 2012). The p110 catalytic subunit exits as isoforms α, β, γ and δ (Li et al., 2012). This enzyme phosphorylates the hydroxyl group of inositol of phosphatidylinositol triggering endocellular pathways from external stimuli. For the activation of PI3K, autophosphorylation of tyrosine kinase receptors (RTKs) is essential (Smith et al., 2002). The p85 subunit of PI3K tethers to the phosphorylated residues of RTKs. Once the docking of its regulatory subunit occurs, p110 begins its phosphorylation of phosphatidylinositol-4,5 biphosphate (PIP)2 to phosphatidylinositol-3,4,5- triphosphate (PIP)3. (PIP)3 is essential for the activation of AKT (Carnero, 2010). However, AKT activation requires phosphorylation activities of (PIP)3 and mTOR (Carnero, 2010). PTEN, an intermediate factor of the PI3K/PTEN/AKT pathway, plays an important role in ensuring that the activity of AKT is held in check by dephosphorylating (PIP)3 (Stocker et al., 2002). While assessing the roles of PI3K/PTEN/AKT in ovarian carcinogenesis, amplifications of PI3K, p110 α subunit, and loss of PTEN function are found (Blanco-Aparicio et al., 2007). When events such as these occur, AKT levels cannot be controlled triggering pathways of survival, progression and invasion through sub-pathways controlled by AKT (Blanco-Aparicio et al., 2007). It has been observed that inhibition of p110 α subunit or AKT, can induce cell cycle regulatory proteins, p21 and p27, inhibit phosphorylation of Rb and reduce the levels of cyclins D1 and CDK4 that essentially serve the apoptotic pathway (Meng et al., 2006). Some of the resistance offered by ovarian tumors to treatment by certain drugs stem from the amplification of PIK3Ca, a gene that encodes PI3K p110 α subunit, and AKT expression with reduced PTEN levels (Janku et al., 2011; Yamamoto et al., 2011; Abe et al., 2013). In such instances, cell cycle regulatory functions are impaired, and pro-apoptotic proteins such as Bax translocation is inhibited (Lee et al., 2005). PI3K mutations in ovarian tumors are rare, but an increase in gene copy number has been observed. The amplifications are more pronounced in high grade ovarian tumors than low grade tumors along with AKT phosphorylation contributing to survival, and progression of the disease (Huang et al., 2011; Abe et al., 2013). There does not appear to be a collective association of the three proteins together in ovarian carcinogenesis and are thought to act independently (Carden et al., 2012). However, the alterations in the expression of these proteins have been shown to have a relationship between the resistance offered against therapies and the recurrence of the disease (Dent et al., 2009).
Mammalian target of rapamycin (mTOR) promotes cell growth and proliferation in association with the PI3K/AKT pathway (Dobbin and Landen, 2013). The mammalian target of rapamycin has the ability to phosphorylate AKT at the serine residue 473 through its mammalian target of rapamycin complex 2 (mTORC2) complex. The mammalian target of rapamycin complex 1 (mTORC1), raptor, and mTORC2, rictor, differ in composition by a few elements that make up the respective complexes, in addition to difference in resistant to rapamycin (Mabuchi et al., 2011). mTORC1 promotes cell growth and cell mass by the activation of molecules involved in protein synthesis (No et al., 2011). mTORC2 promotes cell survival and proliferation through AKT activation. Under normal physiological conditions in the ovary, the PI3K/AKT/mTOR pathway protects the primordial follicles from destruction. However, in the presence of environmental toxins, overstimulation of the pathway results in follicular proliferation, depletion of primordial follicles and an induction of a condition called premature ovarian failure (Borman et al., 2000; Sobinoff et al., 2011). Together, PI3K/AKT/mTOR contributes to cell survival, and proliferation. The PI3K/AKT/mTOR pathway is involved in type I and type II ovarian cancers. Single mutations of a member of the pathway coupled with a mutation with members of another pathway promote ovarian hyperplasia, and double mutations within the same pathway are necessary for ovarian tumorigenesis. Mutations in parallel pathways that are involved in cross-talk are found to be mutated in ovarian carcinomas. The integrated genomic analysis study of ovarian carcinomas showed that at least 45% of the cases contained mutations in the PI3K/RAS signaling pathway, where PTEN deletions (7%); mutations (<1%), PIK3CA amplifications (18%); mutations (<1%), AKT isoform amplifications AKT 1 and AKT 2 (3 and 6 % respectively), were observed in conjunction with KRAS amplification (11%) (Spellman et al., 2011). KRAS independently controls survival through BRAF but can also activate PIK3CA that induces AKT and cell cycle progression (Spellman et al., 2011). Similarly, in the PI3K/AKT/mTOR signaling, a study by Kinross et al. (2013) using a mouse model demonstrated that PTEN double deletions with PIK3CA activation was necessary for ovarian serous adenocarcinomas and granulosa cell tumors (Dobbin and Landen, 2013). Therefore, a single event does not appear to influence ovarian tumorigenesis but multiple hits in the pathways that regulate growth, proliferation, and survival are required for tumorigenesis and progression. Thus, a one cure all for ovarian tumors cannot exist. Rather, ovarian tumor type-pathway specific form of treatment may be more appropriate.
Hedgehog pathway
Hedgehog is a signaling pathway that controls development and is expressed during embryonal development (Chen et al., 2007). Adult tissues have significantly reduced expression of the protein and alterations, either by mutations of mediators of the pathway or overexpression of the ligand receptor, appear to promote ovarian tumorigenesis (Chen et al., 2013a). Sonic hedgehog (SHh), the Indian hedgehog (IHh) and the dessert hedgehog (DHh) have distinct biological roles and induce effects through 10-pass transmembrane patch (Ptch) (Chen et al., 2013a). The canonical stimulation of Hh pathway induces the 7-passtransmembrane smoothend (Smo)-triggered Gli activation of Hh targeted genes through Ptch inhibition (Chen et al., 2013a). The hedgehog signaling pathway is important to organ development at early embryonal stages and is selectively expressed during tissue maintenance and repair and in stem cells (Song et al., 2011; Coni et al., 2013). The pathway can be activated by ligand-dependent or independent mechanisms (Ehtesham et al., 2007; Christiansen et al., 2012). Overexpression of the ligand receptor or mutations of the members in the pathway can contribute to cancer progression. Activation through epidermal growth factor receptor (EGFR) constitute the non-canonical mode of Hh signaling (Mangelberger et al., 2012). The expression of Hh in adult ovarian tissue has not been observed. However, the signaling mechanism is likely to be activated within the stem cell population in the ovarian tissue that is necessary for the repair of the ovarian surface epithelium (OSE). Cancers arising from OSE have an epithelial phenotype. They are thought to arise through mutated Hh signaling and produce spheroid like structures with cancer stem cell-like properties (Ray et al., 2011). Suppressor of fused (Su(fu)), a tumor suppressor, is a repressor of the Hh pathway and loss of Su(fu) accounts for increased Hh signaling (Cheng and Yue, 2008). Reports suggest that Hh helps with clonal growth, and that the expression of members of the pathway, Gli1 and patched correlate with poor outcome. The three ligands of Hh signaling have distinct biological functions and yet, it appears that of the three ligands, SHh is the prominent ligand-overexpressed. It remains to be ascertained if the ligand-type overexpressed is ovarian-tumor-type specific or if the overexpression of any one ligand suffices to induce an ovarian tumor phenotype. Studies have shown that using SHh and IHh agonists, the number of spheroid formations increase (Ray et al., 2011). SHh expression has also been found to be expressed in ovarian dermoid and requires the induction of Gli (Sabol et al., 2012). DHh has been shown to correlate with poor prognosis (Chen et al., 2013a). Finally, the role of Hh pathway dysfunction in ovarian carcinogenesis is still not completely understood as its association with other signaling pathways in addition to its non-canonical pathway adds to the complexity.
The Wnt pathway is important to ovarian follicular development (Sarkar et al., 2010). The Wnt pathway is activated downstream of Hh pathway and Gli transcription factors, terminal activators of Hh, induce Wnt ligands. Also, glycogen synthase kinase-3B (GSK-3B) of Wnt pathway regulates molecules of Hh signaling (Sarkar et al., 2010). The Wnt/B-catenin, the canonical pathway of Wnt signaling influences oncogenic Hh signaling. Thus cross-talk activity between the two pathways is apparent. An analysis of 26 and 20 genes of the Hh and Wnt pathway, respectively, of matched fresh frozen and paraffin embedded ovarian endometrial carcinomas showed that the expression of tumor-specific genes associated with Hh and Wnt pathway were not consistent and those that showed statistical significance had varied genetic profiles (Steg et al., 2006). Overexpression of genes SMO, GLI, GLI2, GLI3, and Wnt7A, Frizzled homolog (FRZD1), low-density lipoprotein receptor-related protein 6 (LRP6), and Frizzled related protein (FRZB) that was detected in both freshly frozen and FFPE tissue, were not statistically significant. Similar observations was seen with genes DHH, IHH, SHH, PTCH, PTCH2, GLI, GLI3, and SMO specific to the Hh pathway (Steg et al., 2006). For suitable anti-pathway therapies, further studies that determine specific change from inter-individual variations are warranted. From a treatment perspective, an ovarian tumor type-pathway specific form of treatment is therefore more appropriate.
Multiple Drug resistant (MDR) pathway
ATP-binding cassette (ABC) transporters are implicated in multidrug resistance (MDR) of many tumors. Ovarian cancers by far appear to build up resistance to many treatments and ABC upregulation is thought to play a major role in this process (Januchowski et al., 2013). A comparative study analyzing benign versus recurrent ovarian tumors showed that of the 9 ABC transporters, 4 (ABCC1, ABCC2, ABCC3, and ABCB3) of them are significantly elevated in recurrent ovarian tumors (Auner et al., 2010). Analysis of these 4 transporters in recurrent versus primary ovarian lesions transporters showed significant differences but were minor and insignificant with respect to benign tumors. Changes in ABC transporters appear to stem from treatment rather than in situ tumor development. Abnormal expression of the transcription factor Gli1, a downstream target of Hh signaling, has been shown to induce MDR resistance in a subset of ovarian cancers and that the promoter regions of ABCB1 and ABCG2 genes contain Gli1 binding specific consensus sequence (Sims-Mourtada et al., 2007; Chen et al., 2013b).
Notch Pathway
Activation of Notch signaling pathway is important to cell fate determination and organogenesis in embryogenesis (Reynaud-Deonauth et al., 2002; Petersen et al., 2006). Downstream targets of Notch signaling such as hairy and enhancer of split-1 (HES1) are expressed in many ovarian cancers indicative of the role of Notch (Schreck et al., 2010; Wang et al., 2010a). Notch pathway is regulated by ligands such as Jagged1, 2 and Delta-like 1, 3, 4 (Fleming et al., 1997; Lendahl, 1998; Gray et al., 1999; Yamaguchi et al., 2002). When Notch receptors are activated by ligand binding, the transmembrane portion of the receptor undergoes postranslational modifications and a complex cleavage process (Pratt et al., 2011). The cleaved C- terminal domain then translocates to the nucleus and induces downstream targets such as HES1, a transcriptional factor (Schreck et al., 2010). Elements of the Notch pathway are expressed in EOCs (Hopfer et al., 2005). Notch pathway signaling appears to be fundamentally important to cell survival, motility and development of vasculature (Shin et al., 2008; Li et al., 2010). The chemoresistance observed to platinum-based therapy of ovarian cancers stems from the expansion of cancer stem cells that have Notch activated (Notch 3) (McAuliffe et al., 2012). A study analyzing pathway deregulations in ovarian tissue specimens showed that of the tumors analyzed, 22% had altered Notch signaling, mostly as amplifications in the ligand Jagged 1 and 2 and Notch 3 (Spellman et al., 2011). Overactivation of Notch ligands (Jagged 1 and 2) has been shown to be mediated through the stimulation of p73, a member of p53 (Sasaki et al., 2002).
Forkhead Box M1pathway (FOXM1)
FOXM1 is a transcription factor that regulates genes that control the cell cycle and thus proliferation and tumor progression (Mencalha et al., 2012; Yang et al., 2013) and its role in angiogenesis has also been observed (Li et al., 2009). Serous ovarian cancers express high levels of the protein that correlates with tumor progression (Lok et al., 2011). In the study by Spellman et al. (2011), 85% of the cases studied showed alterations in FOXM1 pathway. Of the important elements of the FOXM1 pathway, baculoviral inhibitor of apoptosis repeat-containing 5 (BIRC5/survivin) and cell cyclin B1 (CCNB1) were found to be highly up-regulated when compared to aurora kinase-B (AURKB), cell division cycle 25 homolog B (CDC25B) and polo-like kinase 1 (PLK1) (Spellman et al., 2011). Collectively, the proteins are required for regulated cell cycle progression and aberrations lead to deregulated tumor progression. FOXM1 is also involved in the induction of breast cancer type 2 susceptibility protein (BRCA2) a downstream target that regulates DNA repair (Kwok et al., 2010) (Millour et al., 2011). FOXM1 is involved in cell migration/invasion in ovarian cancers via ERK that acts upstream of FOXM1 (Lok et al., 2011) and regulates cell proliferation through a number of elements in the pathway.
Breast cancer type 1/2 susceptibility protein (BRCA1)/(BRCA2) and homologous repair and nucleotide excision repair pathway
BRCA1 and BRCA 2 are tumor suppressors that readily associate with p53 to serve apoptotic functions (Zhang et al., 1998; Abramovitch and Werner, 2003; Navaraj et al., 2009). Most of the mutations seen in ovarian tumors are somatic mutations (Zweemer et al., 1999; Amikura et al., 2006; Usha et al., 2011; Szabova et al., 2012). However, mutations in BRCA1 and BRCA2 are heritable germline mutations. Only 10% of the ovarian tumors that arise are hereditable, which involve the BRCA1/2 mutations (Zhou et al., 2013). The remaining 90% arise through somatic mutations affecting proteins such as BRCA1/2 or p53 (Zikan et al., 2008). A study conducted by Zweemer et. al. determined the relationship between p53 accumulation and the presence of BRCA1, BRCA2 or both mutations, respectively (Zweemer et al., 1999). When compared alone to the specific mutation itself, it was found that p53 accumulation was more pronounced with BRCA1 than BRCA2 mutation (Zweemer et al., 1999). However, similar observations are seen in the absence of BRCA mutations (Cramer, 2012). Thus the correlation between p53 and BRCA mutated genes need further clarification. BRCA1 expression is favored over BRCA2 expression in terms of staging the cancer. p53 accumulation is more apparent with BRCA1 mutations in late stage or stage III ovarian cancers (Johannsson et al., 1997; Kaern et al., 2005; Wysham et al., 2012).
BRCA proteins are associated with DNA repair and exist as a complex with other repair proteins (Brugarolas and Jacks, 1997; Jasin, 2002; Tutt and Ashworth, 2002). Hereditable ovarian cancer involving BRCA requires two-hits for tumor formation. A single BRCA1 affected allele may not be sufficient to promote tumor formation. However, DNA stability is affected as DNA repair is affected that can encourage tumor formation through the loss of function of the second allele or mutations in genes governing the repair pathway. The paradoxical role of BRCA in cancers is apparent (Powell and Kachnic, 2003). Hereditable and somatic mutations in BRCA are responsible for breast and ovarian cancer formation, and yet, cells that carry WT BRCA with other pathway anomalies are less sensitive to treatment or develop chemoresistance. The observations are controversial as some studies point out that the BRCA status (proficient versus absent) of the cells do not have a significant correlation to treatment outcome, whereas others have shown that the absence of BRCA1 enhances the sensitivity to treatment by agents that induce DNA damage, including ionizing radiations (Wiltshire et al., 2007; Johnson et al., 2011). Spellman et al. (2011) showed that the presence of BRCA1 mutations was associated with better survival outcomes as compared to those patients that carried WT BRCA1.
Current therapies against ovarian cancer involve cisplatin treatment (Laios et al., 2013; Song et al., 2013). Patients that are initially responsive become resistant to the treatment. Platinum-based cisplatin therapy involves the induction of DNA damage through inter and intrastrand crosslinking between purines (Basu and Krishnamurthy, 2010) . The 1, 3 and the 1, 2-intrastrand crosslinks are excised and removed through nucleotide excision repair (NER) with the former lesions being easier to remove due to less distortion of the helix (Enoiu et al., 2012). The 1, 3-interstrand lesion are complex and require homologous recombination where double stranded breaks (DSB) are involved (Hinz, 2010). The NER system is robust in terms of lesion recognition and requires a host of various NER components. The up-regulation of these elements by DNA-induced damage could account for the gain of resistance to treatment. In terms of DSBs, Rad51 recombinase is required and acts in conjunction with BRCA2 (Davies et al., 2001). Therefore, loss of function of BRCA2 or mutations that silence the expression of the protein favors sensitivity to treatment and thus women with BRCA1/2 mutations have a better diagnosis and are more responsive to platinum-based therapy. The direct correlation between the expression of NER genes or its members and resistance to therapy does not always hold true. The data obtained from a study analyzing NER efficiency and cisplatin resistance showed that altering the HRR pathway, but not NER member expression, could enhance the sensitivity of cisplatin-resistant tumors to platinum-based agents (Wang et al., 2011). These observations have been corroborated by the Spellman et al. study that showed that of the samples tested 23% carried mutations/epigenetic altered states in BRCA1, while 11% carried mutations in BRCA2 (Spellman et al., 2011) and that 51% of the cases had altered HRR pathway that involved BRCA2 and Rad51C (Spellman et al., 2011).
Epigenetics and ovarian cancer
Histones and DNA are primary targets of epigenetic regulation (Seeber and van Diest, 2012). Genes that harbor CpG islands are susceptible to epigenetic modifications, consisting primarily of DNA methylation (Maradeo and Cairns, 2011). Both types, histone-based and DNA-based methylation marks, are largely known to inhibit gene expression through transcriptional inhibition (Seeber and van Diest, 2012). Of the amino acid residues, lysines are more prone to epigenetic modifications via methylation, acetylation, phosphorylation sumoylation and unbiquitination (Saldanha and Tollefsbol, 2012). However, the well-studied acetylation modifications primarily affect the exposure of the DNA to the transcriptome machinery. In some cases, non-histone proteins like p53 when acetylated affect its cellular functions through its DNA binding interactions and stability (Ito et al., 2002; Scoumanne and Chen, 2008; Pirola et al., 2012; Kim et al., 2013) . Alterations to the epigenome have been shown to be instrumental in the genesis of diseased phenotypes. The current status of epigenomic research shows that DNA methylation, histone modifications, miRNA collectively alter epigenetic profiles of tissues that promote tumorigenesis. Epigenetic modifications are promising targets for treatment as the mechanisms can be reversed through epigenetic enzyme targeted therapy.
The role of methylation in cancer formation can be gene or loci specific. In general, hypermethylation of tumor suppressor genes and hypomethylation of oncogenes contribute to pathway deregulations that promote tumor formation. In the genesis of ovarian cancers, overexpression or silencing of members of the pathways that control proliferation and growth promote tumor initiation and progression. In mammalian germ cell-derived ovarian tumor, promotion is associated with the deregulation of pRb pathway where p16 reduction is observed. The reduction is found to be associated with the hypermethylation of p16 (INK4A) promoter region that correlates with cell promotion (Kawauchi et al., 2004). Increased methylation of tumor suppressor genes have also been observed in ovarian tumors (Chmelarova et al., 2012; Ozdemir et al., 2012). Increase in promoter methylation of O6-methylguanine DNA methyltransferase (MGMT), paired box 5 (PAX5), Cadherin 13, H-Cadherin (Rose et al.) (CDH13), Wilms tumor 1 (WT1), Thrombospondin 1 (THBS1), and GATA5 have been observed in endometroid ovarian cancer as compared to serous ovarian cancer (Chmelarova et al., 2012). In the same study, surprisingly, commonly deregulated genes in ovarian cancer ataxia telangiectasia mutated (ATM), TP53, PTEN, Von Hippel–Lindau tumor suppressor (VLH), glutathione S-transferase pi (GSTP1), RB1a, MGMTb, and PYCARD that encodes apoptosis-associated speck-like protein containing a CARD did not show significant methylation above the cut off value of 15% (Chmelarova et al., 2012). Another study showed that tumor suppressors cyclin-dependent kinase inhibitor 2B (CDKN2B), CDH13 and RASSF1, a gene that encodes Ras association domain-containing protein 1 have significant hypermethylation and that CDKN2B promoter hypermethylation was observed in clear cell carcinomas as compared to other histological types (Ozdemir et al., 2012). Hypermethylation of BRCA1 has been shown to be frequent in spontaneous breast and ovarian cancers (Wang et al., 2010b). Demethylation of BRCA1 appears to decrease chemosensitivity of platinum-sensitive cells associated with partial increase of BRCA1. Thus BRCA1 hypermethylation favors treatment sensitivity and has been shown to function independently of PI3K-Akt pathway (Wang et al., 2010b). Methylation analysis of ovarian tumors of genes involved in the Wnt pathway demonstrated that the naked cuticle homolog 1 (NKD1) and disheveled homolog (DVL1) methylation increased risk of disease progression (Dai et al., 2011). Hypermethylation of members of SHh pathway, zinc finger protein 1 (ZIC1), results in poor progression free survival (PFS) (Huang et al., 2013). In addition to the role in PFS, silencing of SHh members, ZIC1 and zinc finger protein 4 (ZIC4) by methylation correlate with increased proliferation, migration and invasion (Huang et al., 2013). Therefore, methylation shows promise as a marker for PFS. However, studies using larger sample size may be required to support the observation. The relationship between DNA hypermethylation generally favors reduced gene expression. The examination of 1505 CpG sites between ovarian cancer cell lines and primary ovarian tumors showed that ovarian cancer cell lines seem to exhibit distinct methylation profiles as compared to the primary ovarian tumors (Houshdaran et al., 2010). Ovarian cancer cell lines tend to have higher methylation patterns as compared to primary tumors. An explanation may be that ovarian derived cancer cell lines are pure cultures that represent one type of cell population. However, primary tumors are a heterogeneous mass of cells with a mixture of other cells as well. Therefore, careful interpretation is essential as to the use of methylation as a marker for PFS and also in terms of therapeutic treatments with various compounds that target methylation. These studies make it clear that preclinical findings cannot be directly applicable to in situ tumors. This may also account for the reason why so many treatments that work successfully in vitro fail to show promise in vivo.
Methylation of lysine residues takes many different forms; mono, bi and tri valencies (Zhang et al., 2012). However, the position and number of lysines methylated determine the methylation-based gene activity status. Bi- and tri-methylation of histone H3 lysine 27 (H3K27(me3)) contributes to gene silencing and tri-methylation of histone H3 lysine 4 residue (H3K4(me3)) activates gene expression (Payne and Braun, 2006; Lilja et al., 2013). In ovarian tumors, especially in the case of a subset of cells that escape chemotherapy, these epigenetic alterations are observed (Lotem and Sachs, 2006; Balch and Nephew, 2010; Min et al., 2012). What have been analyzed in studies thus far are comparisons of gene sets at various stages of ovarian cancer to normal tissue. The bivalent marks assessed in these studies are based on comparison of ovarian cancer stem cells to patterns in human embryonic stem cells (hES) (Chapman-Rothe et al., 2012). These findings are important as chemoresistance and recurrence of more aggressive tumors could stem from the population of cancer stem cells that have epigenetic plasticity. The process of ovulation and the stress exerted upon the organ due to ovulation requires the need of continual cell replacement. Continual change and plasticity of tissues are maintained by stem cells and studies have established the presence of such in the endometrium of uterine tissue (Teixeira et al., 2008). In all likelihood a similar scenario could exist in the ovary. Recent findings of the presence of adult stem cells in the ovaries is quite exciting and may provide a key link into how tumors arise in the ovary (Djordjevic et al., 2012; Foster et al., 2012). Linking the methylation patterns to these adult stem cells, normal epigenetic marks may undergo a change mediated by environmental cues (external/internal) e.g., parity, inflammation, toward a more tumorigenic phenotype by the suppression or loss of tumor suppressor genes. Also, tumor suppressor genes that harbor these specific bi-and tri-methyl marks are more pronounced to gene silencing through epigenetics affecting pathways such as the PI3K pathway (Min et al., 2012; Seeber and van Diest, 2012) .
In normal tissues, DNA hypomethylation of tumor suppressor genes is observed, which is reversed in tumorigenic tissues, where hypermethylation of CpG-promoter rich genes and global hypomethylation is predominant (Bammidi et al., 2012; Ozdemir et al., 2012). The patterns can be readily reversed by targeting enzymes that regulate these transient modifications. DNA methyltransferases (DNMTs), histone acetyl transferases (HATs) and histone deacetylases (HDACs) serve as targets for therapies against many cancers (Cherblanc et al., 2012) However, single therapies have been found to be less effective against solid tumors as compared to combined therapy of conventional drugs with epigenetic therapies or combined epigenetic therapies.
There is a possible cross talk between DNA methylation and histone modification that dictates the dynamic states of chromatin and the genes associated with tumor biology. Hypoacetylation of H3 and H4 in association with GATA4 and 6 transcription factors have been found in a variety of ovarian cancer cells (Caslini et al., 2006). Methylation patterns such as trimethylation of H3K27 or dimethylation of H3K4 have been found to exist in carcinogenic ovarian tissue (Marsit et al., 2006; Chapman-Rothe et al., 2012). Hypermethylation of tumor suppressor genes such as PTEN and p16INK4a have been observed contributing to the loss of function of these proteins in EOCs (Yang et al., 2006; Tam et al., 2007) and the absence of these proteins contribute to deregulated pathways that have been discussed earlier in the review.
Micro RNAs (miRNAs) are tightly controlled in normal cells but become highly deregulated in cancer cells. They are single stranded non-coding RNA molecules about 22 nucleotides in length and regulate the levels of gene expression by performing silencer-like type functions, degrading the mRNA to which they bind (Kuhlmann et al., 2012). They bind either to certain sequences within the mRNA or to the 3’-untranslated region of the gene. The role of some of the miRNAs in the etiology of various cancers have been well established (Kuhlmann et al., 2012). A detailed review of miRNAs and their role in ovarian cancer has been discussed in detail in Chen et.al (Chen et al., 2011). The posttranscriptional modification of genes by miRNA and the presence of varied miRNA expression levels within solid tumors provides a map of miRNA signatures for specific cancers (Baer et al., 2013). Formulating drugs against these miRNAs may provide for a therapeutic approach. Table 3 lists the various miRNAs that play a significant role in ovarian cancers and the targets they affect in the process. For example, p27 is a cell cycle regulatory protein whose post-transcriptional level is altered by the deregulated expression of miRNA 221 and miRNA 222 in ovarian cancers. They inhibit the expression of p27 that is essential to the control of cellular apoptosis thereby promoting cell proliferation (le Sage et al., 2007).
Table 3.
Effects of miRNA deregulation in ovarian cancer
| miRNA | Gene acted on | Phenotype observed | Reference |
|---|---|---|---|
| miR-214 | p53 | Chemoresistance and metastasis | (Xu et al., 2012a) |
| miR-31 | p14 p16 p53 | Inhibits proliferation and induces apoptosis |
(Creighton et al., 2010) |
| miR-214 | PTEN | Induces proliferation and cisplastin resistance. Activates AKT pathway |
(Yang et al., 2008) |
| miR-199a*, miR-200a |
nd | Tumor progression | (Yang et al., 2008) |
| miR-182 and miR-96 |
p27 through Forkhead box O3 (FOXO3) |
Cancer transformation and progression |
(Xu et al., 2012b) |
| miR-34b/34c | p53 | Controls cell proliferation and adhesion-independent growth |
(Corney et al., 2007) |
| miR-101 | p21 | Inhibits growth, induces p21 | (Semaan et al., 2011) |
| miR- 93 | PTEN | Activates AKT; tumorigenesis and cisplatin resistance |
(Fu et al., 2012) |
| miR-146a | NF-κB | Suppression of metastases | (Kayani et al., 2011) |
| miR-199 | IKKβ | Regulates IKKβ expression | (Chen et al., 2008) |
Epigenetic marks may prove useful in the diagnosis, prognosis and prediction of the disease. In the early stages of ovarian cancer, individuals are responsive to the treatment but eventually become chemoresistant, and the presence of cancer stem cells (CSCs) in the tumor mass may be responsible for the observed chemoresistance. Altering the epigenome of these CSCs may prove to be an alternate approach to targeting advanced ovarian cancers and re-sensitizing cells to chemical treatments and regression of tumors.
Future Directions
Ovarian cancers are lethal diseases as they slip detection and are far advanced when detected. Research in ovarian cancer has just scratched the surface in terms of understanding the pathways deregulated in the disease. Clearly, there does not appear to be a strong association between deregulated patterns and the gene specific expression and subcellular correlation patterns. Thus, treatment approaches are still not very effective. This may be due to the fact that most preclinical studies of the disease have been based on the immunohistochemistry of the tissue. Utilizing more quantitative technologies such as microarray systems, western blots, real-time PCR, or whole-genome sequence analysis might provide different insights into the etiology and pathology of the disease. The information generated from these technologies can provide a wealth of information that relates to the mitotic and apoptotic deregulations of cellular pathways, histone signatures, DNA methylation patterns and miRNA expression patterns governing gene expression in ovarian cancer and its subtypes making treatments and therapies more customized.
Acknowledgments
This work was supported in part by grants from the National Cancer Institute (RO1 CA129415) and the American Institute for Cancer Research (10A020).
Abbreviations
- CDK
cyclin dependent kinases
- p27kip1
cyclin-dependent kinase inhibitor1B
- p16INK4a
inhibitor of kinases 4a
- KRAS
Kirsten rat sarcoma oncogene
- NF-κB
Nuclear factor kappa B
- PI3K
phosphatidylinositide-3 kinase
- PTEN
phosphatase and tensin homolog
- E2F
transcription factor
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Abe A, Minaguchi T, Ochi H, Onuki M, Okada S, Matsumoto K, Satoh T, Oki A, Yoshikawa H. PIK3CA overexpression is a possible prognostic factor for favorable survival in ovarian clear cell carcinoma. Hum Pathol. 2013;44:199–207. doi: 10.1016/j.humpath.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Abramovitch S, Werner H. Functional and physical interactions between BRCA1 and p53 in transcriptional regulation of the IGF-IR gene. Horm Metab Res. 2003;35:758–762. doi: 10.1055/s-2004-814154. [DOI] [PubMed] [Google Scholar]
- Amikura T, Sekine M, Hirai Y, Fujimoto S, Hatae M, Kobayashi I, Fujii T, Nagata I, Ushijima K, Obata K, Suzuki M, Yoshinaga M, Umesaki N, Satoh S, Enomoto T, Motoyama S, Nishino K, Haino K, Tanaka K, Japanese Familial Ovarian Cancer Study G. Mutational analysis of TP53 and p21 in familial and sporadic ovarian cancer in Japan. Gynecol Oncol. 2006;100:365–371. doi: 10.1016/j.ygyno.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Anttila MA, Ji H, Juhola MT, Saarikoski SV, Syrjanen KJ. The prognostic significance of p53 expression quantitated by computerized image analysis in epithelial ovarian cancer. Int J Gynecol Pathol. 1999a;18:42–51. doi: 10.1097/00004347-199901000-00006. [DOI] [PubMed] [Google Scholar]
- Anttila MA, Kosma VM, Hongxiu J, Puolakka J, Juhola M, Saarikoski S, Syrjanen K. p21/WAF1 expression as related to p53, cell proliferation and prognosis in epithelial ovarian cancer. Br J Cancer. 1999b;79:1870–1878. doi: 10.1038/sj.bjc.6690298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auner V, Kriegshauser G, Tong D, Horvat R, Reinthaller A, Mustea A, Zeillinger R. KRAS mutation analysis in ovarian samples using a high sensitivity biochip assay. BMC Cancer. 2009;9:111–118. doi: 10.1186/1471-2407-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auner V, Sehouli J, Oskay-Oezcelik G, Horvat R, Speiser P, Zeillinger R. ABC transporter gene expression in benign and malignant ovarian tissue. Gynecol Oncol. 2010;117:198–201. doi: 10.1016/j.ygyno.2009.10.077. [DOI] [PubMed] [Google Scholar]
- Baekelandt M, Holm R, Trope CG, Nesland JM, Kristensen GB. Lack of independent prognostic significance of p21 and p27 expression in advanced ovarian cancer: an immunohistochemical study. Clin Cancer Res. 1999;5:2848–2853. [PubMed] [Google Scholar]
- Baer C, Claus R, Plass C. Genome-Wide Epigenetic Regulation of miRNAs in Cancer. Cancer Res. 2013;73:473–477. doi: 10.1158/0008-5472.CAN-12-3731. [DOI] [PubMed] [Google Scholar]
- Balch C, Nephew KP. The role of chromatin, microRNAs, and tumor stem cells in ovarian cancer. Cancer Biomark. 2010;8:203–221. doi: 10.3233/CBM-2011-0214. [DOI] [PubMed] [Google Scholar]
- Bali A, O'Brien PM, Edwards LS, Sutherland RL, Hacker NF, Henshall SM. Cyclin D1, p53, and p21Waf1/Cip1 expression is predictive of poor clinical outcome in serous epithelial ovarian cancer. Clin Cancer Res. 2004;10:5168–5177. doi: 10.1158/1078-0432.CCR-03-0751. [DOI] [PubMed] [Google Scholar]
- Bammidi LS, Neerukonda GN, Murthy S, Kanapuram RD. p16 gene alterations in human ovarian cancers: comparison between tissue and blood samples. Int J Gynecol Cancer. 2012;22:553–560. doi: 10.1097/IGC.0b013e31823fa90c. [DOI] [PubMed] [Google Scholar]
- Barbieri F, Lorenzi P, Ragni N, Schettini G, Bruzzo C, Pedulla F, Alama A. Overexpression of cyclin D1 is associated with poor survival in epithelial ovarian cancer. Oncology. 2004;66:310–315. doi: 10.1159/000078332. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Rajpert-De Meyts E, Skakkebaek NE, Lukas J, Bartek J. Deregulation of the G1/S-phase control in human testicular germ cell tumours. APMIS. 2003;111:252–265. doi: 10.1034/j.1600-0463.2003.1110129.x. discussion 265–256. [DOI] [PubMed] [Google Scholar]
- Basu A, Krishnamurthy S. Cellular responses to Cisplatin-induced DNA damage. J Nucleic Acids. 2010;2010 doi: 10.4061/2010/201367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenjeno IM, Vanhaesebroeck B. PI3K regulatory subunits lose control in cancer. Cancer Cell. 2009;16:449–450. doi: 10.1016/j.ccr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379–1386. doi: 10.1093/carcin/bgm052. [DOI] [PubMed] [Google Scholar]
- Borman SM, Christian PJ, Sipes IG, Hoyer PB. Ovotoxicity in female Fischer rats and B6 mice induced by low-dose exposure to three polycyclic aromatic hydrocarbons: comparison through calculation of an ovotoxic index. Toxicol Appl Pharmacol. 2000;167:191–198. doi: 10.1006/taap.2000.9006. [DOI] [PubMed] [Google Scholar]
- Brugarolas J, Jacks T. Double indemnity: p53, BRCA and cancer. p53 mutation partially rescues developmental arrest in Brca1 and Brca2 null mice, suggesting a role for familial breast cancer genes in DNA damage repair. Nat Med. 1997;3:721–722. doi: 10.1038/nm0797-721. [DOI] [PubMed] [Google Scholar]
- Carden CP, Stewart A, Thavasu P, Kipps E, Pope L, Crespo M, Miranda S, Attard G, Garrett MD, Clarke PA, Workman P, de Bono JS, Gore M, Kaye SB, Banerji U. The association of PI3 kinase signaling and chemoresistance in advanced ovarian cancer. Mol Cancer Ther. 2012;11:1609–1617. doi: 10.1158/1535-7163.MCT-11-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou S, Donovan JC, Flanagan WM, Milic A, Bhattacharya N, Slingerland JM. Down-regulation of p21WAF1/CIP1 or p27Kip1 abrogates antiestrogen-mediated cell cycle arrest in human breast cancer cells. Proc Natl Acad Sci U S A. 2000;97:9042–9046. doi: 10.1073/pnas.160016897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnero A. The PKB/AKT pathway in cancer. Curr Pharm Des. 2010;16:34–44. doi: 10.2174/138161210789941865. [DOI] [PubMed] [Google Scholar]
- Caslini C, Capo-chichi CD, Roland IH, Nicolas E, Yeung AT, Xu XX. Histone modifications silence the GATA transcription factor genes in ovarian cancer. Oncogene. 2006;25:5446–5461. doi: 10.1038/sj.onc.1209533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Page C, DGH, Provencher Diane M, Mes-Masson Anne-Marie. Predictive and Prognostic Protein Biomarkers in Epithelial Ovarian Cancer: Recommendation for Future Studies. Cancers. 2010;2:913–954. doi: 10.3390/cancers2020913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman-Rothe N, Curry E, Zeller C, Liber D, Stronach E, Gabra H, Ghaem-Maghami S, Brown R. Chromatin H3K27me3/H3K4me3 histone marks define gene sets in high-grade serous ovarian cancer that distinguish malignant, tumour-sustaining and chemo-resistant ovarian tumour cells. Oncogene. 2012;1–7 doi: 10.1038/onc.2012.477. [DOI] [PubMed] [Google Scholar]
- Chen H, Hardy TM, Tollefsbol TO. Epigenomics of ovarian cancer and its chemoprevention. Front Genet. 2011;2:1–8. doi: 10.3389/fgene.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Gao G, Luo S. Hedgehog signaling pathway and ovarian cancer. Chin J Cancer Res. 2013a;25:346–353. doi: 10.3978/j.issn.1000-9604.2013.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Alvero AB, Silasi DA, Kelly MG, Fest S, Visintin I, Leiser A, Schwartz PE, Rutherford T, Mor G. Regulation of IKKbeta by miR-199a affects NF-kappaB activity in ovarian cancer cells. Oncogene. 2008;27:4712–4723. doi: 10.1038/onc.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Horiuchi A, Kikuchi N, Osada R, Yoshida J, Shiozawa T, Konishi I. Hedgehog signal pathway is activated in ovarian carcinomas, correlating with cell proliferation: it's inhibition leads to growth suppression and apoptosis. Cancer Sci. 2007;98:68–76. doi: 10.1111/j.1349-7006.2006.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bieber MM, Teng NN. Hedgehog signaling regulates drug sensitivity by targeting ABC transporters ABCB1 and ABCG2 in epithelial ovarian cancer. Mol Carcinog. 2013b:1–8. doi: 10.1002/mc.22015. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Yue S. Role and regulation of human tumor suppressor SUFU in Hedgehog signaling. Adv Cancer Res. 2008;101:29–43. doi: 10.1016/S0065-230X(08)00402-8. [DOI] [PubMed] [Google Scholar]
- Cherblanc F, Chapman-Rothe N, Brown R, Fuchter MJ. Current limitations and future opportunities for epigenetic therapies. Future Med Chem. 2012;4:425–446. doi: 10.4155/fmc.12.7. [DOI] [PubMed] [Google Scholar]
- Chmelarova M, Krepinska E, Spacek J, Laco J, Nekvindova J, Palicka V. Methylation analysis of tumour suppressor genes in ovarian cancer using MS-MLPA. Folia Biol (Praha) 2012;58:246–250. doi: 10.14712/fb2012058060246. [DOI] [PubMed] [Google Scholar]
- Christiansen AE, Ding T, Bergmann A. Ligand-independent activation of the Hedgehog pathway displays non-cell autonomous proliferation during eye development in Drosophila. Mech Dev. 2012;129:98–108. doi: 10.1016/j.mod.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coni S, Infante P, Gulino A. Control of stem cells and cancer stem cells by Hedgehog signaling: pharmacologic clues from pathway dissection. Biochem Pharmacol. 2013;85:623–628. doi: 10.1016/j.bcp.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007;67:8433–8438. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- Cramer DW. The epidemiology of endometrial and ovarian cancer. Hematol Oncol Clin North Am. 2012;26:1–12. doi: 10.1016/j.hoc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton CJ, Fountain MD, Yu Z, Nagaraja AK, Zhu H, Khan M, Olokpa E, Zariff A, Gunaratne PH, Matzuk MM, Anderson ML. Molecular profiling uncovers a p53-associated role for microRNA-31 in inhibiting the proliferation of serous ovarian carcinomas and other cancers. Cancer Res. 2010;70:1906–1915. doi: 10.1158/0008-5472.CAN-09-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Teodoridis JM, Zeller C, Graham J, Hersey J, Flanagan JM, Stronach E, Millan DW, Siddiqui N, Paul J, Brown R. Systematic CpG islands methylation profiling of genes in the wnt pathway in epithelial ovarian cancer identifies biomarkers of progression-free survival. Clin Cancer Res. 2011;17:4052–4062. doi: 10.1158/1078-0432.CCR-10-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AA, Masson JY, McIlwraith MJ, Stasiak AZ, Stasiak A, Venkitaraman AR, West SC. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol Cell. 2001;7:273–282. doi: 10.1016/s1097-2765(01)00175-7. [DOI] [PubMed] [Google Scholar]
- De La Rosa-Velazquez IA, Rincon-Arano H, Benitez-Bribiesca L, Recillas-Targa F. Epigenetic regulation of the human retinoblastoma tumor suppressor gene promoter by CTCF. Cancer Res. 2007;67:2577–2585. doi: 10.1158/0008-5472.CAN-06-2024. [DOI] [PubMed] [Google Scholar]
- Dent P, Grant S, Fisher PB, Curiel DT. PI3K: A rational target for ovarian cancer therapy? Cancer Biol Ther. 2009;8:27–30. doi: 10.4161/cbt.8.1.7365. [DOI] [PubMed] [Google Scholar]
- Djordjevic B, Stojanovic S, Conic I, Jankovic-Velickovic L, Vukomanovic P, Zivadinovic R, Vukadinovic M. Current approach to epithelial ovarian cancer based on the concept of cancer stem cells. J BUON. 2012;17:627–636. [PubMed] [Google Scholar]
- Dobbin ZC, Landen CN. The Importance of the PI3K/AKT/MTOR Pathway in the Progression of Ovarian Cancer. Int J Mol Sci. 2013;14:8213–8227. doi: 10.3390/ijms14048213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edkins S, O'Meara S, Parker A, Stevens C, Reis M, Jones S, Greenman C, Davies H, Dalgliesh G, Forbes S, Hunter C, Smith R, Stephens P, Goldstraw P, Nicholson A, Chan TL, Velculescu VE, Yuen ST, Leung SY, Stratton MR, Futreal PA. Recurrent KRAS codon 146 mutations in human colorectal cancer. Cancer Biol Ther. 2006;5:928–932. doi: 10.4161/cbt.5.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehtesham M, Sarangi A, Valadez JG, Chanthaphaychith S, Becher MW, Abel TW, Thompson RC, Cooper MK. Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene. 2007;26:5752–5761. doi: 10.1038/sj.onc.1210359. [DOI] [PubMed] [Google Scholar]
- Eltabbakh GH, Belinson JL, Kennedy AW, Biscotti CV, Casey G, Tubbs RR, Blumenson LE. p53 overexpression is not an independent prognostic factor for patients with primary ovarian epithelial cancer. Cancer. 1997;80:892–898. [PubMed] [Google Scholar]
- Enoiu M, Jiricny J, Scharer OD. Repair of cisplatin-induced DNA interstrand crosslinks by a replication-independent pathway involving transcription-coupled repair and translesion synthesis. Nucleic Acids Res. 2012;40:8953–8964. doi: 10.1093/nar/gks670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer AC, Lyshchik A, Hirari M, Moore RD, Abramson RG, Fishman DA. Early detection of ovarian cancer with conventional and contrast-enhanced transvaginal sonography: recent advances and potential improvements. J Oncol. 2012;2012:1–11. doi: 10.1155/2012/302858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RJ, Purcell K, Artavanis-Tsakonas S. The NOTCH receptor and its ligands. Trends Cell Biol. 1997;7:437–441. doi: 10.1016/S0962-8924(97)01161-6. [DOI] [PubMed] [Google Scholar]
- Folgiero V, Di Carlo SE, Bon G, Spugnini EP, Di Benedetto A, Germoni S, Pia Gentileschi M, Accardo A, Milella M, Morelli G, Bossi G, Mottolese M, Falcioni R. Inhibition of p85, the non-catalytic subunit of phosphatidylinositol 3-kinase, exerts potent antitumor activity in human breast cancer cells. Cell Death Dis. 2012;3:1–9. doi: 10.1038/cddis.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R, Buckanovich RJ, Rueda BR. Ovarian cancer stem cells: Working towards the root of stemness. Cancer Lett. 2012;338:147–157. doi: 10.1016/j.canlet.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Fu X, Tian J, Zhang L, Chen Y, Hao Q. Involvement of microRNA-93, a new regulator of PTEN/Akt signaling pathway, in regulation of chemotherapeutic drug cisplatin chemosensitivity in ovarian cancer cells. FEBS Lett. 2012;586:1279–1286. doi: 10.1016/j.febslet.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Gallagher MF, Heffron CC, Laios A, O'Toole SA, Ffrench B, Smyth PC, Flavin RJ, Elbaruni SA, Spillane CD, Martin CM, Sheils OM, O'Leary JJ. Suppression of cancer stemness p21-regulating mRNA and microRNA signatures in recurrent ovarian cancer patient samples. J Ovarian Res. 2012;5:1–11. doi: 10.1186/1757-2215-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GE, Mann RS, Mitsiadis E, Henrique D, Carcangiu ML, Banks A, Leiman J, Ward D, Ish-Horowitz D, Artavanis-Tsakonas S. Human ligands of the Notch receptor. Am J Pathol. 1999;154:785–794. doi: 10.1016/S0002-9440(10)65325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, McCormick F. The molecular pathology of cancer. Nat Rev Clin Oncol. 2010;7:251–265. doi: 10.1038/nrclinonc.2010.41. [DOI] [PubMed] [Google Scholar]
- Hartmann LC, Podratz KC, Keeney GL, Kamel NA, Edmonson JH, Grill JP, Su JQ, Katzmann JA, Roche PC. Prognostic significance of p53 immunostaining in epithelial ovarian cancer. J Clin Oncol. 1994;12:64–69. doi: 10.1200/JCO.1994.12.1.64. [DOI] [PubMed] [Google Scholar]
- Hashiguchi Y, Tsuda H, Yamamoto K, Inoue T, Ishiko O, Ogita S. Combined analysis of p53 and RB pathways in epithelial ovarian cancer. Hum Pathol. 2001;32:988–996. doi: 10.1053/hupa.2001.27115. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Yanaihara N, Okamoto A, Nikaido T, Saito M, Takakura S, Yasuda M, Sasaki H, Ochiai K, Tanaka T. Cyclin D1 predicts the prognosis of advanced serous ovarian cancer. Exp Ther Med. 2011;2:213–219. doi: 10.3892/etm.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havrilesky L, Darcy k M, Hamdan H, Priore RL, Leon J, Bell J, Berchuck A, Gynecologic Oncology Group S. Prognostic significance of p53 mutation and p53 overexpression in advanced epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2003;21:3814–3825. doi: 10.1200/JCO.2003.11.052. [DOI] [PubMed] [Google Scholar]
- He J, de la Monte S, Wands JR. The p85beta regulatory subunit of PI3K serves as a substrate for PTEN protein phosphatase activity during insulin mediated signaling. Biochem Biophys Res Commun. 2010;397:513–519. doi: 10.1016/j.bbrc.2010.05.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard TS, Modi DA, Burdette JE. Gonadotropins activate oncogenic pathways to enhance proliferation in normal mouse ovarian surface epithelium. Int J Mol Sci. 2013;14:4762–4782. doi: 10.3390/ijms14034762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley C, Philpott A. Co-ordination of cell cycle and differentiation in the developing nervous system. Biochem J. 2012;444:375–382. doi: 10.1042/BJ20112040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz JM. Role of homologous recombination in DNA interstrand crosslink repair. Environ Mol Mutagen. 2010;51:582–603. doi: 10.1002/em.20577. [DOI] [PubMed] [Google Scholar]
- Hofmann BT, Jucker M. Activation of PI3K/Akt signaling by n-terminal SH2 domain mutants of the p85alpha regulatory subunit of PI3K is enhanced by deletion of its c-terminal SH2 domain. Cell Signal. 2012;24:1950–1954. doi: 10.1016/j.cellsig.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Hopfer O, Zwahlen D, Fey MF, Aebi S. The Notch pathway in ovarian carcinomas and adenomas. Br J Cancer. 2005;93:709–718. doi: 10.1038/sj.bjc.6602719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houshdaran S, Hawley S, Palmer C, Campan M, Olsen MN, Ventura AP, Knudsen BS, Drescher CW, Urban ND, Brown PO, Laird PW. DNA methylation profiles of ovarian epithelial carcinoma tumors and cell lines. PLoS One. 2010;5:1–16. doi: 10.1371/journal.pone.0009359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhang L, Greshock J, Colligon TA, Wang Y, Ward R, Katsaros D, Lassus H, Butzow R, Godwin AK, Testa JR, Nathanson KL, Gimotty PA, Coukos G, Weber BL, Degenhardt Y. Frequent genetic abnormalities of the PI3K/AKT pathway in primary ovarian cancer predict patient outcome. Genes Chromosomes Cancer. 2011;50:606–618. doi: 10.1002/gcc.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RL, Gu F, Kirma NB, Ruan J, Chen CL, Wang HC, Liao YP, Chang CC, Yu MH, Pilrose JM, Thompson IM, Huang HC, Huang TH, Lai HC, Nephew KP. Comprehensive methylome analysis of ovarian tumors reveals hedgehog signaling pathway regulators as prognostic DNA methylation biomarkers. Epigenetics. 2013;8:624–634. doi: 10.4161/epi.24816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain AR, Ahmed SO, Ahmed M, Khan OS, Al Abdulmohsen S, Platanias LC, Al-Kuraya KS, Uddin S. Cross-talk between NFkB and the PI3-kinase/AKT pathway can be targeted in primary effusion lymphoma (PEL) cell lines for efficient apoptosis. PLoS One. 2012;7:1–12. doi: 10.1371/journal.pone.0039945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Kawaguchi Y, Lai CH, Kovacs JJ, Higashimoto Y, Appella E, Yao TP. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 2002;21:6236–6245. doi: 10.1093/emboj/cdf616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Hong DS, Naing A, Falchook GS, Moroney JW, Piha-Paul SA, Wheler JJ, Moulder SL, Fu S, Kurzrock R. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther. 2011;10:558–565. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku F, Wheler JJ, Westin SN, Moulder SL, Naing A, Tsimberidou AM, Fu S, Falchook GS, Hong DS, Garrido-Laguna I, Luthra R, Lee JJ, Lu KH, Kurzrock R. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol. 2012;30:777–782. doi: 10.1200/JCO.2011.36.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januchowski R, Wojtowicz K, Sujka-Kordowska P, Andrzejewska M, Zabel M. Biomed Res Int. 2013:241763. doi: 10.1155/2013/241763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M. Homologous repair of DNA damage and tumorigenesis: the BRCA connection. Oncogene. 2002;21:8981–8993. doi: 10.1038/sj.onc.1206176. [DOI] [PubMed] [Google Scholar]
- Jayasurya R, Francis G, Kannan S, Lekshminarayanan K, Nalinakumari KR, Abraham T, Abraham EK, Nair MK. p53, p16 and cyclin D1: molecular determinants of radiotherapy treatment response in oral carcinoma. Int J Cancer. 2004;109:710–716. doi: 10.1002/ijc.20042. [DOI] [PubMed] [Google Scholar]
- Johannsson OT, Idvall I, Anderson C, Borg A, Barkardottir RB, Egilsson V, Olsson H. Tumour biological features of BRCA1-induced breast and ovarian cancer. Eur J Cancer. 1997;33:362–371. doi: 10.1016/s0959-8049(97)89007-7. [DOI] [PubMed] [Google Scholar]
- Johnson N, Li YC, Walton ZE, Cheng KA, Li D, Rodig SJ, Moreau LA, Unitt C, Bronson RT, Thomas HD, Newell DR, D'Andrea AD, Curtin NJ, Wong KK, Shapiro GI. Compromised CDK1 activity sensitizes BRCA-proficient cancers to PARP inhibition. Nat Med. 2011;17:875–882. doi: 10.1038/nm.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Wang TL, Kurman RJ, Nakayama K, Velculescu VE, Vogelstein B, Kinzler KW, Papadopoulos N, Shih Ie M. Low-grade serous carcinomas of the ovary contain very few point mutations. J Pathol. 2012;226:413–420. doi: 10.1002/path.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaern J, Aghmesheh M, Nesland JM, Danielsen HE, Sandstad B, Friedlander M, Trope C. Prognostic factors in ovarian carcinoma stage III patients. Can biomarkers improve the prediction of short- and long-term survivors? Int J Gynecol Cancer. 2005;15:1014–1022. doi: 10.1111/j.1525-1438.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Liu XP, Kawasaki K, Hirakawa T, Amada S, Furuya T, Oga A, Sasaki K. Significance of beta-catenin and pRB pathway components in malignant ovarian germ cell tumours: INK4A promoter CpG island methylation is associated with cell proliferation. J Pathol. 2004;204:268–276. doi: 10.1002/path.1629. [DOI] [PubMed] [Google Scholar]
- Kayani M, Kayani MA, Malik FA, Faryal R. Role of miRNAs in breast cancer. Asian Pac J Cancer Prev. 2011;12:3175–3180. [PubMed] [Google Scholar]
- Keane FK, Ratner ES. The KRAS-Variant Genetic Test As a Marker of Increased Risk of Ovarian Cancer. Rev Obstet Gynecol. 2010;3:118–121. [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Yoon EK, Chung HJ, Park SY, Hong KM, Lee CH, Lee YS, Choi K, Yang Y, Kim K, Kim IH. p53 acetylation enhances Taxol-induced apoptosis in human cancer cells. Apoptosis. 2013;18:110–120. doi: 10.1007/s10495-012-0772-8. [DOI] [PubMed] [Google Scholar]
- Kinross KM, Brown DV, Kleinschmidt M, Jackson S, Christensen J, Cullinane C, Hicks RJ, Johnstone RW, McArthur GA. In vivo activity of combined PI3K/mTOR and MEK inhibition in a Kras(G12D);Pten deletion mouse model of ovarian cancer. Mol Cancer Ther. 2011;10:1440–1449. doi: 10.1158/1535-7163.MCT-11-0240. [DOI] [PubMed] [Google Scholar]
- Kohler MF, Kerns BJ, Humphrey PA, Marks JR, Bast RC, Jr, Berchuck A. Mutation and overexpression of p53 in early-stage epithelial ovarian cancer. Obstet Gynecol. 1993;81:643–650. [PubMed] [Google Scholar]
- Konecny GE, Winterhoff B, Kolarova T, Qi J, Manivong K, Dering J, Yang G, Chalukya M, Wang HJ, Anderson L, Kalli KR, Finn RS, Ginther C, Jones S, Velculescu VE, Riehle D, Cliby WA, Randolph S, Koehler M, Hartmann LC, Slamon DJ. Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. Clin Cancer Res. 2011;17:1591–1602. doi: 10.1158/1078-0432.CCR-10-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann JD, Rasch J, Wimberger P, Kasimir-Bauer S. microRNA and the pathogenesis of ovarian cancer--a new horizon for molecular diagnostics and treatment? Clin Chem Lab Med. 2012;50:601–615. doi: 10.1515/cclm-2011-0847. [DOI] [PubMed] [Google Scholar]
- Kuhn E, Kurman RJ, Vang R, Sehdev AS, Han G, Soslow R, Wang TL, Shih Ie M. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma--evidence supporting the clonal relationship of the two lesions. J Pathol. 2012;226:421–426. doi: 10.1002/path.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu ST, Nallur S, Paranjape T, Boeke M, Weidhaas JB, Slack FJ. KRAS alleles: the LCS6 3'UTR variant and KRAS coding sequence mutations in the NCI-60 panel. Cell Cycle. 2012;11:361–366. doi: 10.4161/cc.11.2.18794. [DOI] [PubMed] [Google Scholar]
- Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42:918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose K, Zhou XP, Araki T, Cannistra SA, Maher ER, Eng C. Frequent loss of PTEN expression is linked to elevated phosphorylated Akt levels, but not associated with p27 and cyclin D1 expression, in primary epithelial ovarian carcinomas. Am J Pathol. 2001;158:2097–2106. doi: 10.1016/S0002-9440(10)64681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusume T, Tsuda H, Kawabata M, Inoue T, Umesaki N, Suzuki T, Yamamoto K. The p16-cyclin D1/CDK4-pRb pathway and clinical outcome in epithelial ovarian cancer. Clin Cancer Res. 1999;5:4152–4157. [PubMed] [Google Scholar]
- Kwok JM, Peck B, Monteiro LJ, Schwenen HD, Millour J, Coombes RC, Myatt SS, Lam EW. FOXM1 confers acquired cisplatin resistance in breast cancer cells. Mol Cancer Res. 2010;8:24–34. doi: 10.1158/1541-7786.MCR-09-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laios A, Mohamed BM, Kelly L, Flavin R, Finn S, McEvoy L, Gallagher M, Martin C, Sheils O, Ring M, Davies A, Lawson M, Gleeson N, D'Arcy T, d'Adhemar C, Norris L, Langhe R, Saadeh FA, O'Leary JJ, O'Toole SA. Pre-Treatment of Platinum Resistant Ovarian Cancer Cells with an MMP-9/MMP-2 Inhibitor Prior to Cisplatin Enhances Cytotoxicity as Determined by High Content Screening. Int J Mol Sci. 2013;14:2085–2103. doi: 10.3390/ijms14012085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landen CN, Jr, Anil MJB, Sood K. Early Events in the Pathogenesis of Epithelial Ovarian Carcinoma. J of Clin Oncol. 2007;26:995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- Laudanski P, Kowalczuk O, Klasa-Mazurkiewicz D, Milczek T, Rysak-Luberowicz D, Garbowicz M, Baranowski W, Charkiewicz R, Szamatowicz J, Chyczewski L. Selective gene expression profiling of mTOR-associated tumor suppressor and oncogenes in ovarian cancer. Folia Histochem Cytobiol. 2011;49:317–324. doi: 10.5603/fhc.2011.0044. [DOI] [PubMed] [Google Scholar]
- le Sage C, Nagel R, Egan DA, Schrier M, Mesman E, Mangiola A, Anile C, Maira G, Mercatelli N, Ciafre SA, Farace MG, Agami R. Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J. 2007;26:3699–3708. doi: 10.1038/sj.emboj.7601790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Engelman JA, Cantley LC. Biochemistry. PI3K charges ahead. Science. 2007;317:206–207. doi: 10.1126/science.1146073. [DOI] [PubMed] [Google Scholar]
- Lee S, Choi EJ, Jin C, Kim DH. Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecol Oncol. 2005;97:26–34. doi: 10.1016/j.ygyno.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Leffers N, Lambeck AJ, de Graeff P, Bijlsma AY, Daemen T, van der Zee AG, Nijman HW. Survival of ovarian cancer patients overexpressing the tumour antigen p53 is diminished in case of MHC class I down-regulation. Gynecol Oncol. 2008;110:365–373. doi: 10.1016/j.ygyno.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Lendahl U. A growing family of Notch ligands. Bioessays. 1998;20:103–107. doi: 10.1002/(SICI)1521-1878(199802)20:2<103::AID-BIES1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Leong WF, Chau JF, Li B. p53 Deficiency leads to compensatory up-regulation of p16INK4a. Mol Cancer Res. 2009;7:354–360. doi: 10.1158/1541-7786.MCR-08-0373. [DOI] [PubMed] [Google Scholar]
- Li GH, Fan YZ, Liu XW, Zhang BF, Yin DD, He F, Huang SY, Kang ZJ, Xu H, Liu Q, Wu YL, Niu XL, Zhang L, Liu L, Hao MW, Han H, Liang YM. Notch signaling maintains proliferation and survival of the HL60 human promyelocytic leukemia cell line and promotes the phosphorylation of the Rb protein. Mol Cell Biochem. 2010;340:7–14. doi: 10.1007/s11010-010-0394-9. [DOI] [PubMed] [Google Scholar]
- Li HT, Lu YY, An YX, Wang X, Zhao QC. KRAS, BRAF and PIK3CA mutations in human colorectal cancer: relationship with metastatic colorectal cancer. Oncol Rep. 2011;25:1691–1697. doi: 10.3892/or.2011.1217. [DOI] [PubMed] [Google Scholar]
- Li J, Song J, Cassidy MG, Rychahou P, Starr ME, Liu J, Li X, Epperly G, Weiss HL, Townsend CM, Jr, Gao T, Evers BM. PI3K p110alpha/Akt signaling negatively regulates secretion of the intestinal peptide neurotensin through interference of granule transport. Mol Endocrinol. 2012;26:1380–1393. doi: 10.1210/me.2012-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhang N, Jia Z, Le X, Dai B, Wei D, Huang S, Tan D, Xie K. Critical role and regulation of transcription factor FoxM1 in human gastric cancer angiogenesis and progression. Cancer Res. 2009;69:3501–3509. doi: 10.1158/0008-5472.CAN-08-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja T, Wallenborg K, Bjorkman K, Albage M, Eriksson M, Lagercrantz H, Rohdin M, Hermanson O. Novel alterations in the epigenetic signature of MeCP2-targeted promoters in lymphocytes of Rett syndrome patients. Epigenetics. 2013;8:246–251. doi: 10.4161/epi.23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FS, Kohler MF, Marks JR, Bast RC, Jr, Boyd J, Berchuck A. Mutation and overexpression of the p53 tumor suppressor gene frequently occurs in uterine and ovarian sarcomas. Obstet Gynecol. 1994;83:118–124. [PubMed] [Google Scholar]
- Liu Y, Ganesan TS. Tumour suppressor genes in sporadic epithelial ovarian cancer. Reproduction. 2002;123:341–353. doi: 10.1530/rep.0.1230341. [DOI] [PubMed] [Google Scholar]
- Lok GT, Chan DW, Liu VW, Hui WW, Leung TH, Yao KM, Ngan HY. Aberrant activation of ERK/FOXM1 signaling cascade triggers the cell migration/invasion in ovarian cancer cells. PLoS One. 2011;6:1–10. doi: 10.1371/journal.pone.0023790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J, Sachs L. Epigenetics and the plasticity of differentiation in normal and cancer stem cells. Oncogene. 2006;25:7663–7672. doi: 10.1038/sj.onc.1209816. [DOI] [PubMed] [Google Scholar]
- Mabuchi S, Hisamatsu T, Kimura T. Targeting mTOR signaling pathway in ovarian cancer. Curr Med Chem. 2011;18:2960–2968. doi: 10.2174/092986711796150450. [DOI] [PubMed] [Google Scholar]
- Mane SM, Meltzer SJ, Gutheil JC, Kapil V, Lee EJ, Needleman SW. RAS gene activation in acute myelogenous leukemia: analysis by in vitro amplification and DNA base sequence determination. Genes Chromosomes Cancer. 1990;2:71–77. doi: 10.1002/gcc.2870020113. [DOI] [PubMed] [Google Scholar]
- Mangelberger D, Kern D, Loipetzberger A, Eberl M, Aberger F. Cooperative Hedgehog-EGFR signaling. Front Biosci (Landmark Ed) 2012;17:90–99. doi: 10.2741/3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maradeo ME, Cairns P. Translational application of epigenetic alterations: ovarian cancer as a model. FEBS Lett. 2011;585:2112–2120. doi: 10.1016/j.febslet.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks JR, Davidoff AM, Kerns BJ, Humphrey PA, Pence JC, Dodge RK, Clarke-Pearson DL, Iglehart JD, Bast RC, Jr, Berchuck A. Overexpression and mutation of p53 in epithelial ovarian cancer. Cancer Res. 1991;51:2979–2984. [PubMed] [Google Scholar]
- Marsit CJ, Houseman EA, Christensen BC, Eddy K, Bueno R, Sugarbaker DJ, Nelson HH, Karagas MR, Kelsey KT. Examination of a CpG island methylator phenotype and implications of methylation profiles in solid tumors. Cancer Res. 2006;66:10621–10629. doi: 10.1158/0008-5472.CAN-06-1687. [DOI] [PubMed] [Google Scholar]
- McAuliffe SM, Morgan SL, Wyant GA, Tran LT, Muto KW, Chen YS, Chin KT, Partridge JC, Poole BB, Cheng KH, Daggett J, Jr, Cullen K, Kantoff E, Hasselbatt K, Berkowitz J, Muto MG, Berkowitz RS, Aster JC, Matulonis UA, Dinulescu DM. Targeting Notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc Natl Acad Sci U S A. 2012;109:E2939–E2948. doi: 10.1073/pnas.1206400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencalha AL, Binato R, Ferreira GM, Du Rocher B, Abdelhay E. Forkhead box M1 (FoxM1) gene is a new STAT3 transcriptional factor target and is essential for proliferation, survival and DNA repair of K562 cell line. PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0048160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 2006;18:2262–2271. doi: 10.1016/j.cellsig.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Milde-Langosch K, Hagen M, Bamberger AM, Loning T. Expression and prognostic value of the cell-cycle regulatory proteins, Rb, p16MTS1, p21WAF1, p27KIP1, cyclin E, and cyclin D2, in ovarian cancer. Int J Gynecol Pathol. 2003;22:168–174. doi: 10.1097/00004347-200304000-00009. [DOI] [PubMed] [Google Scholar]
- Millour J, de Olano N, Horimoto Y, Monteiro LJ, Langer JK, Aligue R, Hajji N, Lam EW. ATM and p53 regulate FOXM1 expression via E2F in breast cancer epirubicin treatment and resistance. Mol Cancer Ther. 2011;10:1046–1058. doi: 10.1158/1535-7163.MCT-11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, So KA, Ouh YT, Hong JH, Lee JK. The effects of DNA methylation and epigenetic factors on the expression of CD133 in ovarian cancers. J Ovarian Res. 2012;5:1–8. doi: 10.1186/1757-2215-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto Y, Kyo S, Kiyono T, Takakura M, Nakamura M, Maida Y, Mori N, Bono Y, Sakurai H, Inoue M. Activation of NF-kappaB is a novel target of KRAS-induced endometrial carcinogenesis. Clin Cancer Res. 2011;17:1341–1350. doi: 10.1158/1078-0432.CCR-10-2291. [DOI] [PubMed] [Google Scholar]
- Modesitt SC, Ramirez P, Zu Z, Bodurka-Bevers D, Gershenson D, Wolf JK. In vitro and in vivo adenovirus-mediated p53 and p16 tumor suppressor therapy in ovarian cancer. Clin Cancer Res. 2001;7:1765–1772. [PubMed] [Google Scholar]
- Modugno F, Edwards RP. Ovarian cancer: prevention, detection, and treatment of the disease and its recurrence. Molecular mechanisms and personalized medicine meeting report. Int J Gynecol Cancer. 2012;22:S45–S57. doi: 10.1097/IGC.0b013e31826bd1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaraj A, Finnberg N, Dicker DT, Yang W, Matthew EM, El-Deiry WS. Reduced cell death, invasive and angiogenic features conferred by BRCA1-deficiency in mammary epithelial cells transformed with H-Ras. Cancer Biol Ther. 2009;8:2417–2444. doi: 10.4161/cbt.8.24.10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- No JH, Jeon YT, Park IA, Kim YB, Kim JW, Park NH, Kang SB, Han JY, Lim JM, Song YS. Activation of mTOR signaling pathway associated with adverse prognostic factors of epithelial ovarian cancer. Gynecol Oncol. 2011;121:8–12. doi: 10.1016/j.ygyno.2010.12.364. [DOI] [PubMed] [Google Scholar]
- Nowak-Markwitz E, Spaczynski M. [Ovarian cancer--modern approach to its origin and histogenesis] Ginekol Pol. 2012;83:454–457. [PubMed] [Google Scholar]
- Oliveira-Cunha M, Hadfield KD, Siriwardena AK, Newman W. EGFR and KRAS mutational analysis and their correlation to survival in pancreatic and periampullary cancer. Pancreas. 2012;41:428–434. doi: 10.1097/MPA.0b013e3182327a03. [DOI] [PubMed] [Google Scholar]
- Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Ozdemir F, Altinisik J, Karateke A, Coksuer H, Buyru N. Methylation of tumor suppressor genes in ovarian cancer. Exp Ther Med. 2012;4:1092–1096. doi: 10.3892/etm.2012.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H, Yenicesu G, Arici S, Cetin M, Tuncer E, Cetin A. Immunohistochemistry with apoptotic-antiapoptotic proteins (p53, p21, bax, bcl-2), c-kit, telomerase, and metallothionein as a diagnostic aid in benign, borderline, and malignant serous and mucinous ovarian tumors. Diagn Pathol. 2012;7:1–10. doi: 10.1186/1746-1596-7-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige AJ, Brown R. Pharmaco(epi)genomics in ovarian cancer. Pharmacogenomics. 2008;9:1825–1834. doi: 10.2217/14622416.9.12.1825. [DOI] [PubMed] [Google Scholar]
- Payne C, Braun RE. Histone lysine trimethylation exhibits a distinct perinuclear distribution in Plzf-expressing spermatogonia. Dev Biol. 2006;293:461–472. doi: 10.1016/j.ydbio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Petersen PH, Tang H, Zou K, Zhong W. The enigma of the numb-Notch relationship during mammalian embryogenesis. Dev Neurosci. 2006;28:156–168. doi: 10.1159/000090761. [DOI] [PubMed] [Google Scholar]
- Pharoah PD, Palmieri RT, Ramus SJ, Gayther SA, Andrulis IL, Anton-Culver H, al et. The role of KRAS rs61764370 in invasive epithelial ovarian cancer: implications for clinical testing. Clin Cancer Res. 2011;17:3742–3750. doi: 10.1158/1078-0432.CCR-10-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilarski R, Patel DA, Weitzel J, McVeigh T, Dorairaj JJ, Heneghan HM, Miller N, Weidhaas JB, Kerin MJ, McKenna M, Wu X, Hildebrandt M, Zelterman D, Sand S, Shulman LP. The KRAS-variant is associated with risk of developing double primary breast and ovarian cancer. PLoS One. 2012;7:1–5. doi: 10.1371/journal.pone.0037891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirola L, Zerzaihi O, Vidal H, Solari F. Protein acetylation mechanisms in the regulation of insulin and insulin-like growth factor 1 signalling. Mol Cell Endocrinol. 2012;362:1–10. doi: 10.1016/j.mce.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Plisiecka-Halasa J, Karpinska G, Szymanska T, Ziolkowska I, Madry R, Timorek A, Debniak J, Ulanska M, Jedryka M, Chudecka-Glaz A, Klimek M, Rembiszewska A, Kraszewska E, Dybowski B, Markowska J, Emerich J, Pluzanska A, Goluda M, Rzepka-Gorska I, Urbanski K, Zielinski J, Stelmachow J, Chrabowska M, Kupryjanczyk J. P21WAF1, P27KIP1, TP53 and C-MYC analysis in 204 ovarian carcinomas treated with platinum-based regimens. Ann Oncol. 2003;14:1078–1085. doi: 10.1093/annonc/mdg299. [DOI] [PubMed] [Google Scholar]
- Powell SN, Kachnic LA. Roles of BRCA1 and BRCA2 in homologous recombination, DNA replication fidelity and the cellular response to ionizing radiation. Oncogene. 2003;22:5784–5791. doi: 10.1038/sj.onc.1206678. [DOI] [PubMed] [Google Scholar]
- Pratt EB, Wentzell JS, Maxson JE, Courter L, Hazelett D, Christian JL. The cell giveth and the cell taketh away: an overview of Notch pathway activation by endocytic trafficking of ligands and receptors. Acta Histochem. 2011;113:248–255. doi: 10.1016/j.acthis.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psyrri A, Bamias A, Yu Z, Weinberger PM, Kassar M, Markakis S, Kowalski D, Efstathiou E, Camp RL, Rimm DL, Dimopoulos MA. Subcellular localization and protein levels of cyclin-dependent kinase inhibitor p27 independently predict for survival in epithelial ovarian cancer. Clin Cancer Res. 2005;11:8384–8390. doi: 10.1158/1078-0432.CCR-05-1270. [DOI] [PubMed] [Google Scholar]
- Ramirez PT, Gershenson DM, Tortolero-Luna G, Ramondetta LM, Fightmaster D, Wharton JT, Wolf JK. Expression of cell-cycle mediators in ovarian cancer cells after transfection with p16(INK4a), p21(WAF1/Cip-1), and p53. Gynecol Oncol. 2001;83:543–548. doi: 10.1006/gyno.2001.6438. [DOI] [PubMed] [Google Scholar]
- Ratner E, Lu L, Boeke M, Barnett R, Nallur S, Chin LJ, Pelletier C, Blitzblau R, Tassi R, Paranjape T, Hui P, Godwin AK, Yu H, Risch H, Rutherford T, Schwartz P, Santin A, Matloff E, Zelterman D, Slack FJ, Weidhaas JB. A KRAS-variant in ovarian cancer acts as a genetic marker of cancer risk. Cancer Res. 2010;70:6509–6515. doi: 10.1158/0008-5472.CAN-10-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner ES, Keane FK, Lindner R, Tassi RA, Paranjape T, Glasgow M, et al. A KRAS variant is a biomarker of poor outcome, platinum chemotherapy resistance and a potential target for therapy in ovarian cancer. Oncogene. 2012;31:4559–4566. doi: 10.1038/onc.2011.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Meng E, Reed E, Shevde LA, Rocconi RP. Hedgehog signaling pathway regulates the growth of ovarian cancer spheroid forming cells. Int J Oncol. 2011;39:797–804. doi: 10.3892/ijo.2011.1093. [DOI] [PubMed] [Google Scholar]
- Reles A, Wen WH, Schmider A, Gee C, Runnebaum IB, Kilian U, Jones LA, El-Naggar A, Minguillon C, Schonborn I, Reich O, Kreienberg R, Lichtenegger W, Press MF. Correlation of p53 mutations with resistance to platinum-based chemotherapy and shortened survival in ovarian cancer. Clin Cancer Res. 2001;7:2984–2997. [PubMed] [Google Scholar]
- Reynaud-Deonauth S, Zhang H, Afouda A, Taillefert S, Beatus P, Kloc M, Etkin LD, Fischer-Lougheed J, Spohr G. Notch signaling is involved in the regulation of Id3 gene transcription during Xenopus embryogenesis. Differentiation. 2002;69:198–208. doi: 10.1046/j.1432-0436.2002.690413.x. [DOI] [PubMed] [Google Scholar]
- Rose SL, Robertson AD, Goodheart MJ, Smith BJ, DeYoung BR, Buller RE. The impact of p53 protein core domain structural alteration on ovarian cancer survival. Clin Cancer Res. 2003;9:4139–4144. [PubMed] [Google Scholar]
- Sabol M, Car D, Musani V, Ozretic P, Oreskovic S, Weber I, Levanat S. The Hedgehog signaling pathway in ovarian teratoma is stimulated by Sonic Hedgehog which induces internalization of Patched. Int J Oncol. 2012;41:1411–1418. doi: 10.3892/ijo.2012.1554. [DOI] [PubMed] [Google Scholar]
- Saga Y, Mizukami H, Suzuki M, Kohno T, Urabe M, Ozawa K, Sato I. Overexpression of PTEN increases sensitivity to SN-38, an active metabolite of the topoisomerase I inhibitor irinotecan, in ovarian cancer cells. Clin Cancer Res. 2002;8:1248–1252. [PubMed] [Google Scholar]
- Sagarra RA, Andrade LA, Martinez EZ, Pinto GA, Syrjanen KJ, Derchain SF. P53 and Bcl-2 as prognostic predictors in epithelial ovarian cancer. Int J Gynecol Cancer. 2002;12:720–727. doi: 10.1046/j.1525-1438.2002.01135.x. [DOI] [PubMed] [Google Scholar]
- Saldanha SN, Tollefsbol TO. The role of nutraceuticals in chemoprevention and chemotherapy and their clinical outcomes. J Oncol. 2012:192464. doi: 10.1155/2012/192464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu C, Donovan J, Bhattacharya N, Stampfer M, Worland P, Slingerland J. Reduction of Cdc25A contributes to cyclin E1-Cdk2 inhibition at senescence in human mammary epithelial cells. Oncogene. 2000;19:5314–5323. doi: 10.1038/sj.onc.1203908. [DOI] [PubMed] [Google Scholar]
- Sarkar FH, Li Y, Wang Z, Kong D. The role of nutraceuticals in the regulation of Wnt and Hedgehog signaling in cancer. Cancer Metastasis Rev. 2010;29:383–394. doi: 10.1007/s10555-010-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarojini S, Tamir A, Lim H, Li S, Zhang S, Goy A, Pecora A, Suh KS. Early detection biomarkers for ovarian cancer. J Oncol. 2012;2012:1–15. doi: 10.1155/2012/709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Ishida S, Morimoto I, Yamashita T, Kojima T, Kihara C, Tanaka T, Imai K, Nakamura Y, Tokino T. The p53 family member genes are involved in the Notch signal pathway. J Biol Chem. 2002;277:719–724. doi: 10.1074/jbc.M108080200. [DOI] [PubMed] [Google Scholar]
- Schmider-Ross A, Pirsig O, Gottschalk E, Denkert C, Lichtenegger W, Reles A. Cyclin-dependent kinase inhibitors CIP1 (p21) and KIP1 (p27) in ovarian cancer. J Cancer Res Clin Oncol. 2006;132:163–170. doi: 10.1007/s00432-005-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck KC, Taylor P, Marchionni L, Gopalakrishnan V, Bar EE, Gaiano N, Eberhart CG. The Notch target Hes1 directly modulates Gli1 expression and Hedgehog signaling: a potential mechanism of therapeutic resistance. Clin Cancer Res. 2010;16:6060–6070. doi: 10.1158/1078-0432.CCR-10-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyer M, van der Burg ME, Henzen-Logmans SC, Fieret JH, Klijn JG, Look MP, Foekens JA, Stoter G, Berns EM. Reduced expression of BAX is associated with poor prognosis in patients with epithelial ovarian cancer: a multifactorial analysis of TP53, p21, BAX and BCL-2. Br J Cancer. 2001;85:1359–1367. doi: 10.1054/bjoc.2001.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoumanne A, Chen X. Protein methylation: a new mechanism of p53 tumor suppressor regulation. Histol Histopathol. 2008;23:1143–1149. doi: 10.14670/hh-23.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully RE. Early de novo ovarian cancer and cancer developing in benign ovarian lesions. Int J Gynaecol Obstet. 1995;49:S9–S15. doi: 10.1016/0020-7292(95)02404-z. [DOI] [PubMed] [Google Scholar]
- Seeber LM, van Diest PJ. Epigenetics in ovarian cancer. Methods Mol Biol. 2012;863:253–269. doi: 10.1007/978-1-61779-612-8_15. [DOI] [PubMed] [Google Scholar]
- Semaan A, Qazi AM, Seward S, Chamala S, Bryant CS, Kumar S, Morris R, Steffes CP, Bouwman DL, Munkarah AR, Weaver DW, Gruber SA, Batchu RB. MicroRNA-101 inhibits growth of epithelial ovarian cancer by relieving chromatin-mediated transcriptional repression of p21(waf(1)/cip(1)) Pharm Res. 2011;28:3079–3090. doi: 10.1007/s11095-011-0547-x. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Shigemasa K, Shiroyama Y, Sawasaki T, Fujii T, Nagai N, Parmley TH, O'Brien TJ, Ohama K. Underexpression of cyclin-dependent kinase inhibitor p27 is associated with poor prognosis in serous ovarian carcinomas. Int J Oncol. 2001;18:953–958. doi: 10.3892/ijo.18.5.953. [DOI] [PubMed] [Google Scholar]
- Shigemasa K, Tanimoto H, Parham GP, Parmley TH, Ohama K, O'Brien TJ. Cyclin D1 overexpression and p53 mutation status in epithelial ovarian cancer. J Soc Gynecol Investig. 1999;6:102–108. doi: 10.1016/s1071-5576(99)00002-7. [DOI] [PubMed] [Google Scholar]
- Shin DM, Shaffer DJ, Wang H, Roopenian DC, Morse HC., 3rd NOTCH is part of the transcriptional network regulating cell growth and survival in mouse plasmacytomas. Cancer Res. 2008;68:9202–9211. doi: 10.1158/0008-5472.CAN-07-6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims-Mourtada J, Izzo JG, Ajani J, Chao KS. Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene. 2007;26:5674–5679. doi: 10.1038/sj.onc.1210356. [DOI] [PubMed] [Google Scholar]
- Skirnisdottir I, Seidal T, Gerdin E, Sorbe B. The prognostic importance of p53, bcl-2, and bax in early stage epithelial ovarian carcinoma treated with adjuvant chemotherapy. Int J Gynecol Cancer. 2002;12:265–276. doi: 10.1046/j.1525-1438.2002.01121.x. [DOI] [PubMed] [Google Scholar]
- Smeenk L, van Heeringen SJ, Koeppel M, Gilbert B, Janssen-Megens E, Stunnenberg HG, Lohrum M. Role of p53 serine 46 in p53 target gene regulation. PLoS One. 2011;6:1–14. doi: 10.1371/journal.pone.0017574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V, Hobbs S, Court W, Eccles S, Workman P, Kelland LR. ErbB2 overexpression in an ovarian cancer cell line confers sensitivity to the HSP90 inhibitor geldanamycin. Anticancer Res. 2002;22:1993–1999. [PubMed] [Google Scholar]
- Smolle E, Taucher V, Pichler M, Petru E, Lax S, Haybaeck J. Targeting signaling pathways in epithelial ovarian cancer. Int J Mol Sci. 2013;14:9536–9555. doi: 10.3390/ijms14059536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobinoff AP, Mahony M, Nixon B, Roman SD, McLaughlin EA. Understanding the Villain: DMBA-induced preantral ovotoxicity involves selective follicular destruction and primordial follicle activation through PI3K/Akt and mTOR signaling. Toxicol Sci. 2011;123:563–575. doi: 10.1093/toxsci/kfr195. [DOI] [PubMed] [Google Scholar]
- Song T, Kim MK, Lee YY, Choi CH, Kim TJ, Lee JW, Kim BG, Bae DS. Phase II study of ifosfamide and cisplatin for the treatment of recurrent ovarian cancer. Cancer Chemother Pharmacol. 2013;72:653–660. doi: 10.1007/s00280-013-2241-7. [DOI] [PubMed] [Google Scholar]
- Song Z, Yue W, Wei B, Wang N, Li T, Guan L, Shi S, Zeng Q, Pei X, Chen L. Sonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PLoS One. 2011;6:1–13. doi: 10.1371/journal.pone.0017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Reyes E, Recillas-Targa F. Epigenetic regulation of the human p53 gene promoter by the CTCF transcription factor in transformed cell lines. Oncogene. 2010;29:2217–2227. doi: 10.1038/onc.2009.509. [DOI] [PubMed] [Google Scholar]
- Spellman PT, et al. Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos GP, Papadimitriou C, Aravantinos G, Rigatos SK, Malamos N, Stathopoulos JG, Kaparelou M, Koutantos J, Andreadis C. Maintenance chemotherapy or not in ovarian cancer stages IIIA, B, C, and IV after disease recurrence. J BUON. 2012;17:735–739. [PubMed] [Google Scholar]
- Steffensen KD, Alvero AB, Yang Y, Waldstrom M, Hui P, Holmberg JC, Silasi DA, Jakobsen A, Rutherford T, Mor G. Prevalence of epithelial ovarian cancer stem cells correlates with recurrence in early-stage ovarian cancer. J Oncol. 2011a;2011:620523. doi: 10.1155/2011/620523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen KD, Waldstrom M, Grove A, Lund B, Pallisgard N, Jakobsen A. Improved classification of epithelial ovarian cancer: results of 3 danish cohorts. Int J Gynecol Cancer. 2011b;21:1592–1600. doi: 10.1097/IGC.0b013e31822a0f6b. [DOI] [PubMed] [Google Scholar]
- Steg A, Wang W, Blanquicett C, Grunda JM, Eltoum IA, Wang K, Buchsbaum DJ, Vickers SM, Russo S, Diasio RB, Frost AR, LoBuglio AF, Grizzle WE, Johnson MR. Multiple gene expression analyses in paraffin-embedded tissues by TaqMan low-density array: Application to hedgehog and Wnt pathway analysis in ovarian endometrioid adenocarcinoma. J Mol Diagn. 2006;8:76–83. doi: 10.2353/jmoldx.2006.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CJ, Leung Y, Walsh MD, Walters RJ, Young JP, Buchanan DD. KRAS mutations in ovarian low-grade endometrioid adenocarcinoma: association with concurrent endometriosis. Hum Pathol. 2012;43:1177–1183. doi: 10.1016/j.humpath.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Stocker H, Andjelkovic M, Oldham S, Laffargue M, Wymann MP, Hemmings BA, Hafen E. Living with lethal PIP3 levels: viability of flies lacking PTEN restored by a PH domain mutation in Akt/PKB. Science. 2002;295:2088–2091. doi: 10.1126/science.1068094. [DOI] [PubMed] [Google Scholar]
- Szabova L, Yin C, Bupp S, Guerin TM, Schlomer JJ, Householder DB, Baran ML, Yi M, Song Y, Sun W, McDunn JE, Martin PL, Van Dyke T, Difilippantonio S. Perturbation of Rb, p53, and Brca1 or Brca2 cooperate in inducing metastatic serous epithelial ovarian cancer. Cancer Res. 2012;72:4141–4153. doi: 10.1158/0008-5472.CAN-11-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YT, Lee S, Niloff E, Weisman C, Strobel T, Cannistra SA. BAX protein expression and clinical outcome in epithelial ovarian cancer. J Clin Oncol. 1998;16:2583–2590. doi: 10.1200/JCO.1998.16.8.2583. [DOI] [PubMed] [Google Scholar]
- Takayama G, Ohtani M, Minowa A, Matsuda S, Koyasu S. Class I PI3K-mediated Akt and ERK signals play a critical role in Fc{varepsilon}RI-induced degranulation in mast cells. Int Immunol. 2012;25:215–220. doi: 10.1093/intimm/dxs105. [DOI] [PubMed] [Google Scholar]
- Tam KF, Liu VW, Liu SS, Tsang PC, Cheung AN, Yip AM, Ngan HY. Methylation profile in benign, borderline and malignant ovarian tumors. J Cancer Res Clin Oncol. 2007;133:331–341. doi: 10.1007/s00432-006-0178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira J, Rueda BR, Pru JK. StemBook. Cambridge (MA): 2008. Uterine stem cells. [PubMed] [Google Scholar]
- Todd MC, Sclafani RA, Langan TA. Ovarian cancer cells that coexpress endogenous Rb and p16 are insensitive to overexpression of functional p16 protein. Oncogene. 2000;19:258–264. doi: 10.1038/sj.onc.1203289. [DOI] [PubMed] [Google Scholar]
- Tutt A, Ashworth A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol Med. 2002;8:571–576. doi: 10.1016/s1471-4914(02)02434-6. [DOI] [PubMed] [Google Scholar]
- Urick ME, Rudd ML, Godwin AK, Sgroi D, Merino M, Bell DW. PIK3R1 (p85alpha) is somatically mutated at high frequency in primary endometrial cancer. Cancer Res. 2011;71:4061–4067. doi: 10.1158/0008-5472.CAN-11-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usha L, Sill MW, Darcy KM, Benbrook DM, Hurteau JA, Michelin DP, Mannel RS, Hanjani P, De Geest K, Godwin AK. A Gynecologic Oncology Group phase II trial of the protein kinase C-beta inhibitor, enzastaurin and evaluation of markers with potential predictive and prognostic value in persistent or recurrent epithelial ovarian and primary peritoneal malignancies. Gynecol Oncol. 2011;121:455–461. doi: 10.1016/j.ygyno.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereczkey I, Serester O, Dobos J, Gallai M, Szakacs O, Szentirmay Z, Toth E. Molecular characterization of 103 ovarian serous and mucinous tumors. Pathol Oncol Res. 2011;17:551–559. doi: 10.1007/s12253-010-9345-8. [DOI] [PubMed] [Google Scholar]
- Vikhanskaya F, Erba E, D'Incalci M, Broggini M. Changes in cyclins and cyclin-dependent kinases induced by DNA damaging agents in a human ovarian cancer cell line expressing mutated or wild-type P53. Exp Cell Res. 1996;227:380–385. doi: 10.1006/excr.1996.0288. [DOI] [PubMed] [Google Scholar]
- Wang QE, Milum K, Han C, Huang YW, Wani G, Thomale J, Wani AA. Differential contributory roles of nucleotide excision and homologous recombination repair for enhancing cisplatin sensitivity in human ovarian cancer cells. Mol Cancer. 2011;10:1–12. doi: 10.1186/1476-4598-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fu Y, Chen X, Ye J, Lu B, Ye F, Lu W, Xie X. The expressions of bHLH gene HES1 and HES5 in advanced ovarian serous adenocarcinomas and their prognostic significance: a retrospective clinical study. J Cancer Res Clin Oncol. 2010a;136:989–996. doi: 10.1007/s00432-009-0744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YQ, Zhang JR, Li SD, He YY, Yang YX, Liu XL, Wan XP. Aberrant methylation of breast and ovarian cancer susceptibility gene 1 in chemosensitive human ovarian cancer cells does not involve the phosphatidylinositol 3'-kinase-Akt pathway. Cancer Sci. 2010b;101:1618–1623. doi: 10.1111/j.1349-7006.2010.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weberpals JI, Clark-Knowles KV, Vanderhyden BC. Sporadic epithelial ovarian cancer: clinical relevance of BRCA1 inhibition in the DNA damage and repair pathway. J Clin Oncol. 2008;26:3259–3267. doi: 10.1200/JCO.2007.11.3902. [DOI] [PubMed] [Google Scholar]
- Weberpals JI, Tu D, Squire JA, Amin MS, Islam S, Pelletier LB, O'Brien AM, Hoskins PJ, Eisenhauer EA. Breast cancer 1 (BRCA1) protein expression as a prognostic marker in sporadic epithelial ovarian carcinoma: an NCIC CTG OV.16 correlative study. Ann Oncol. 2011;22:2403–2410. doi: 10.1093/annonc/mdq770. [DOI] [PubMed] [Google Scholar]
- Weidhaas JB, Slack FJ. KRAS rs61764370 in Epithelial Ovarian Cancer-Letter. Clin Cancer Res. 2011;17:1–2. doi: 10.1158/1078-0432.CCR-11-1195. [DOI] [PubMed] [Google Scholar]
- Wiltshire T, Senft J, Wang Y, Konat GW, Wenger SL, Reed E, Wang W. BRCA1 contributes to cell cycle arrest and chemoresistance in response to the anticancer agent irofulven. Mol Pharmacol. 2007;71:1051–1060. doi: 10.1124/mol.106.029504. [DOI] [PubMed] [Google Scholar]
- Wysham WZ, Mhawech-Fauceglia P, Li H, Hays L, Syriac S, Skrepnik T, Wright J, Pande N, Hoatlin M, Pejovic T. BRCAness profile of sporadic ovarian cancer predicts disease recurrence. PLoS One. 2012;7:1–7. doi: 10.1371/journal.pone.0030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CX, Xu M, Tan L, Yang H, Permuth-Wey J, Kruk PA, Wenham RM, Nicosia SV, Lancaster JM, Sellers TA, Cheng JQ. MicroRNA miR-214 regulates ovarian cancer cell stemness by targeting p53/Nanog. J Biol Chem. 2012a;287:34970–34978. doi: 10.1074/jbc.M112.374611. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xu G, Zhang W, Bertram P, Zheng XF, McLeod H. Pharmacogenomic profiling of the PI3K/PTEN-AKT-mTOR pathway in common human tumors. Int J Oncol. 2004;24:893–900. [PubMed] [Google Scholar]
- Xu X, Dong Z, Yang Y, Yuan Z, Qu X, Kong B. The upregulation of signal transducer and activator of transcription 5-dependent microRNA-182 and microRNA-96 promotes ovarian cancer cell proliferation by targeting forkhead box O3 upon leptin stimulation. Int J Biochem Cell Biol. 2012b;45:536–545. doi: 10.1016/j.biocel.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Yamaguchi E, Chiba S, Kumano K, Kunisato A, Takahashi T, Takahashi T, Hirai H. Expression of Notch ligands, Jagged1, 2 and Delta1 in antigen presenting cells in mice. Immunol Lett. 2002;81:59–64. doi: 10.1016/s0165-2478(01)00326-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Tsuda H, Takano M, Iwaya K, Tamai S, Matsubara O. PIK3CA mutation is an early event in the development of endometriosis-associated ovarian clear cell adenocarcinoma. J Pathol. 2011;225:189–194. doi: 10.1002/path.2940. [DOI] [PubMed] [Google Scholar]
- Yan X, Fraser M, Qiu Q, Tsang BK. Over-expression of PTEN sensitizes human ovarian cancer cells to cisplatin-induced apoptosis in a p53-dependent manner. Gynecol Oncol. 2006;102:348–355. doi: 10.1016/j.ygyno.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Yang C, Chen H, Yu L, Shan L, Xie L, Hu J, Chen T, Tan Y. Inhibition of FOXM1 transcription factor suppresses cell proliferation and tumor growth of breast cancer. Cancer Gene Ther. 2013;20:117–124. doi: 10.1038/cgt.2012.94. [DOI] [PubMed] [Google Scholar]
- Yang H, Kong W, He L, Zhao JJ, O'Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Liu VW, Wang Y, Tsang PC, Ngan HY. Differential DNA methylation profiles in gynecological cancers and correlation with clinico-pathological data. BMC Cancer. 2006;6:1–10. doi: 10.1186/1471-2407-6-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, Shih Ie M, Kurman RJ. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24:1248–1253. doi: 10.1038/modpathol.2011.85. [DOI] [PubMed] [Google Scholar]
- Zhang H, Somasundaram K, Peng Y, Tian H, Zhang H, Bi D, Weber BL, El-Deiry WS. BRCA1 physically associates with p53 and stimulates its transcriptional activity. Oncogene. 1998;16:1713–1721. doi: 10.1038/sj.onc.1201932. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wen H, Shi X. Lysine methylation: beyond histones. Acta Biochim Biophys Sin (Shanghai) 2012;44:14–27. doi: 10.1093/abbs/gmr100. [DOI] [PubMed] [Google Scholar]
- Zhou L, Graves M, Macdonald G, Cipollone J, Mueller CR, Roskelley CD. Microenvironmental regulation of BRCA1 gene expression by c-Jun and Fra2 in pre-malignant human ovarian surface epithelial cells. Mol Cancer Res. 2013;11:272–281. doi: 10.1158/1541-7786.MCR-12-0395. [DOI] [PubMed] [Google Scholar]
- Zikan M, Janatova M, Pavlista D, Pohlreich P. High frequency of BRCA1/2 and p53 somatic inactivation in sporadic ovarian cancer. J Genet. 2007;86:169–171. doi: 10.1007/s12041-007-0022-y. [DOI] [PubMed] [Google Scholar]
- Zikan M, Pohlreich P, Freitag P, Janousek M, Pavlista D, Fischerova D, Jancarkova N, Slama J, Pinkavova I, Cibula D. [Inactivation of BRCA1, BRCA2 and p53 genes in sporadic ovarian cancer] Ceska Gynekol. 2008;73:298–302. [PubMed] [Google Scholar]
- Ziolkowska-Seta I, Madry R, Kraszewska E, Szymanska T, Timorek A, Rembiszewska A, Kupryjanczyk J. TP53, BCL-2 and BAX analysis in 199 ovarian cancer patients treated with taxane-platinum regimens. Gynecol Oncol. 2009;112:179–184. doi: 10.1016/j.ygyno.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Zweemer RP, Shaw PA, Verheijen RM, Ryan A, Berchuck A, Ponder BA, Risch H, McLaughlin JR, Narod SA, Menko FH, Kenemans P, Jacobs IJ. Accumulation of p53 protein is frequent in ovarian cancers associated with BRCA1 and BRCA2 germline mutations. J Clin Pathol. 1999;52:372–375. doi: 10.1136/jcp.52.5.372. [DOI] [PMC free article] [PubMed] [Google Scholar]


