Abstract
We coupled multiplex PCR and a DNA microarray to construct an assay suitable for the simultaneous detection of five important marine fish pathogens (Vibrio vulnificus, Listonella anguillarum, Photobacterium damselae subsp. damselae, Aeromonas salmonicida subsp. salmonicida, and Vibrio parahaemolyticus). The array was composed of nine short oligonucleotide probes (25-mer) complementary to seven chromosomal loci (cyt, rpoN, gyrB, toxR, ureC, dly, and vapA) and two plasmid-borne loci (fatA and A.sal). Nine primer sets were designed to amplify short fragments of these loci (100 to 177 bp) in a multiplex PCR. PCR products were subsequently labeled by nick translation and hybridized to the microarray. All strains of the five target species (n = 1 to 21) hybridized to at least one species-specific probe. Assay sensitivities ranged from 100% for seven probes to 83 and 67% for the two remaining probes. Multiplex PCR did not produce any nonspecific amplification products when tested against 23 related species of bacteria (n = 40 strains; 100% specificity). Using purified genomic DNA, we were able to detect PCR products with <20 fg of genomic DNA per reaction (equivalent to four or five cells), and the array was at least fourfold more sensitive than agarose gel electrophoresis for detecting PCR products. In addition, our method allowed the tentative identification of virulent strains of L. anguillarum serotype O1 based on the presence of the fatA gene (67% sensitivity and 100% specificity). This assay is a sensitive and specific tool for the simultaneous detection of multiple pathogenic bacteria that cause disease in fish and humans.
Vibriosis and furunculosis are two fish diseases responsible for considerable economic hardship to mariculture operations worldwide (3). Vibriosis, mainly caused by Listonella anguillarum, Vibrio vulnificus, and Photobacterium damselae subsp. damselae, is a systemic bacterial infection affecting more than 48 fish species in widely distributed regions (3, 35). Other halophilic Vibrio spp., such as Vibrio parahaemolyticus, and V. vulnificus have been identified as causing vibriosis in humans (22, 29) and have been isolated from many species of fish, shellfish, and crustaceans. Aeromonas salmonicida is the causal agent of furunculosis, a disease of major significance in the culturing of salmonid fish and other valuable marine fish species (3).
Conventional microbiological methods needed to identify these organisms are often limited by the length of time required to complete the assays. In recent years, enzyme-linked immunosorbent assays and molecular methods based on DNA probes or PCR have overcome problems associated with culture-based techniques, enabling the detection of microorganisms directly in clinical samples without the need for previous culturing. Molecular diagnosis protocols have been the most effective methods for the diagnosis of bacterial agents in maricultures because they permit more specific and sensitive detection than do serological assays. Many PCR methods have been developed for the identification of bacterial pathogens in aquacultures (30). Although many of these protocols are based on the amplification of 16S and 23S rRNA genes (2, 19, 24, 25, 31), which are found in all eubacteria, there is a high degree of genetic similarity for these genes across taxa; therefore, the specificity of the detection method can be compromised (21, 37). Alternatively, bacterium-specific genes (e.g., virulence loci) can be used as targets for PCR amplification to permit more specific detection (16) as well as subspecies and strain differentiation (9, 28, 32). Conventional PCR is used to amplify a single gene target, whereas multiplex PCR involves amplifying multiple gene products in a single reaction; the latter method has been used successfully to detect fish pathogens (4, 14, 32). Agarose gel electrophoresis is typically used to assess results from multiplex PCRs, but DNA microarrays offer a more discriminating means to examine reaction products for specific sequences.
DNA microarrays are important molecular tools that have been applied to studies of gene expression (38), phylogenetic classification (12), ecological studies (15), and the detection and genotyping of bacterial (9, 17) and viral (11) pathogens. DNA microarrays consist of ordered sets of DNA fixed to solid surfaces; generally on glass but sometimes on nylon substrates. Each spot in a microarray is composed of many identical probes that are complementary to a gene of interest. Microarrays can be used to detect cDNA (38), genomic DNA (5), and plasmid DNA (7) in the context of gene expression analysis and comparative genomics. They can also be used as end-point detectors to examine complex mixtures of PCR products (8). For the latter application, PCR products are hybridized to complementary probes and are usually detected by fluorescence imaging systems. The objectives of this work included the design and evaluation of a multiplex PCR coupled with a low-density microarray for the detection of selected marine pathogens.
MATERIALS AND METHODS
A total of 75 strains of bacteria from seven genera, mainly isolated from marine fish in the United States, Europe, and Japan, were included in this study (Table 1). The bacterial strains were obtained from the American Type Culture Collection (ATCC; Manassas, Va.); the National Collection of Industrial Marine Bacteria (NCIMB; Aberdeen, Scotland); the Japan Collection of Microorganisms (JCM; Tokyo, Japan); the Czechoslovak Collection of Microorganism (CCM); and the collection of the Department of Microbiology and Parasitology, University of Santiago de Compostela, Santiago de Compostela, Spain. The bacteria were grown on tryptic soy agar (Oxoid) supplemented with 1% (vol/vol) NaCl for 24 to 48 h at 25°C. Tenacibaculumin maritimum and Flavobacterium psychophilum strains were cultured at the appropriate temperatures in Flexibacter maritimus medium (34) and on modified Anacker-Ordal agar (40). Genomic DNA was extracted with two commercial systems, InstaGene matrix (Bio-Rad, Hercules, Calif.) and Dynabeads DNA DIRECT (Dynal, Oslo, Norway), and quantified by spectrophotometry.
TABLE 1.
Test isolates used in this studya
| Species | Strain(s) |
|---|---|
| Aeromonas caviae | 1.25 |
| Aeromonas hydrophila | 80-A1, B-32, B-35 |
| Aeromonas salmonicida subsp. salmonicida | MT-004, RSP 7.1, SEG-9.1 |
| Aeromonas sobria | P.33 |
| Flavobacterium psychrophilum | NCIMB 1947 |
| Listonella anguillarum | 775, 11008, 43-F, 96-F, ATCC 14181, ATCC 43305 to ATCC 43314, ET-208, NCIMB 571, R-82, RG-111, RV-22, TM-14 |
| Listonella pelagia | ATCC 25916, NCIMB 1900, NF-182, RPM-87.1, RPM-138.1, ST-11 |
| Photobacterium damselae subsp. damselae | ATCC 33539, CDC2227-81, JCM 8968, RG-91, RM-51, RM-71 |
| Photobacterium damselae subsp. piscicida | ATCC 17911, DI-21, EPOY-8803-II |
| Photobacterium leiognathi | ATCC 25521 |
| Photobacterium phosphoreum | ATCC 11040 |
| Streptococcus parauberis | RA 99.1 |
| Tenacibaculum maritimum | LPV 1.7, NCIMB 2154, NCIMB 2158 |
| Vibrio aestuarianus | ATCC 35048 |
| Vibrio alginolyticus | CCM 2578 |
| Vibrio campbellii | ATCC 25920 |
| Vibrio cholerae | ATCC 14935, V-69 |
| Vibrio fischeri | ATCC 7744 |
| Vibrio harveyi | ATCC 14126 |
| Vibrio metschnikovi | ATCC 7708 |
| Vibrio natriegens | ATCC 14048 |
| Vibrio nereis | ATCC 25917 |
| Vibrio ordalii | NCIMB 2167 |
| Vibrio parahaemolyticus | ATCC 25969 |
| Vibrio proteolyticus | ATCC 15338 |
| Vibrio splendidus | ATCC 25914, ATCC 33125, RM-77, PC 399.1 |
| Vibrio tubiashii | ATCC 19106, EX-1 |
| Vibrio vulnificus | ATCC 27562, ATCC 33149, NCIMB 2136, NCIMB 2137 |
The test isolates used in this study originated from multiple countries (Denmark, Japan, Portugal, Scotland, Spain, Thailand, the United Kingdom, and the United States).
Probes and primers.
Nine PCR primer sets and nine internal probe sequences were designed by using the Primer3 program (36). PCR products ranged from 100 to 177 bp in length. Seven specific loci from chromosomal DNA (cyt, rpoN, gyrB, toxR, ureC, dly, and vapA) and two loci from plasmid DNA (fatA and A.sal) were selected for the probe and primer targets (Table 2). All oligonucleotides were purchased from Invitrogen (Carlsbad, Calif.) and were desalted without further modification.
TABLE 2.
Genes targeted for multiplex PCR and microarray hybridization
| Pathogen | Locusa | GenBank accession no. (reference) | Nameb | Sequence | Annealing temp (°C) | Product (bp) |
|---|---|---|---|---|---|---|
| Aeromonas salmonicida | vapA | M64655 (13) | F-A.sal-1 | ATTAGCCCGAACGACAACAC | 60 | 177 |
| R-A.sal-2 | GTCGTTGAATTGGCCTTCAC | 60 | ||||
| P-A.sal-vapA | AACTAAGCAGCCGGTACTGGACTTC | 65 | ||||
| A.plas. | X64214 (18) | F-A.sal-3 | TCCGTTGGATATGGCTCTTC | 60 | 101 | |
| R-A.sal-4 | TTATCGAGGCAGCCAACAAT | 60 | ||||
| P-A.sal-plas | TCGACACAAAATTCAAATTTAACCCC | 65 | ||||
| Listonella anguillarum | rpoN | U86585 (33) | F-V.ang-1 | CCAGCAAGAGATCCAAGAGG | 60 | 125 |
| R-V.ang-2 | ACACCTCAGCACTGGCTTCT | 60 | ||||
| P-V.ang-rpoN | CGCTGATGTTCATAGCATCAATGAG | 65 | ||||
| fatA | Z12000 (39) | F-V.ang-3 | GTCCGCAAGATGGAATGAAT | 60 | 137 | |
| R-V.ang-4 | ACTGCTGCCACTTCCTTTGT | 60 | ||||
| P-V.ang-fatA | AGTTCAGCAAACCTTCCCACAATTT | 65 | ||||
| Photobacterium damselae subsp. damselae | ureC | U40071 | F-P.dam-1 | CACCAGGGGTCTGGAATATG | 60 | 127 |
| R-P.dam-2 | GCTCCAGCTTCAATTTGCTC | 60 | ||||
| P-P.dam-ureC | CTGGAAGCCGTTGATGACTTACCTA | 65 | ||||
| dly | L16584 (26) | F-P.dam-3 | GCAATTGTTGGTGAACGATG | 60 | 137 | |
| R-P.dam-4 | CGTCGCATGAAATGATCTTG | 60 | ||||
| P-P.dam-dly | GTCAATATGGCCCAGATTGTTTT | 65 | ||||
| Vibrio parahaemolyticus | gyrB | AF007287 (42) | F-V.par-1 | GCTAAGCAGGGTCGTAATCG | 60 | 145 |
| R-V.par-2 | GACCGATACCACAGCCAAGT | 60 | ||||
| P-V.par-gyrB | CGCAAGAAGTTGCAACGCTTATTAC | 65 | ||||
| toxR | L11929 (27) | F-V.par-3 | CTTGGATTCCACGCGTTATT | 60 | 147 | |
| R-V.par-4 | TGATTTGCGGGTGATTTACA | 60 | ||||
| P-V.par-toxR | ATCTCAGTTCCGTCAGATTGGTGAG | 65 | ||||
| Vibrio vulnificus | cyt | M34670 (44) | F-V.vul-1 | TTCATTCGAGCGTGAATTTG | 60 | 100 |
| R-V.vul-2 | ATCAAATACCCAGCCACTGC | 60 | ||||
| P-V.vul-cyt | CCAAGAGCTTGGATGCTATTTCACC | 66 |
Genetic locus targeted by the described PCR primers and probes: cyt, cytolysine; gyrB, gyrase B; ureC, urease C; dly, phospholipase D; A.plas., A. salmonicida plasmid.
F, sequence of the forward primer; R, sequence of the reverse primer; P, oligonucleotide probe.
Microarray construction.
Slides were prepared by following the methods of Call et al. (6). Briefly, 12-well Teflon-masked slides (Erie Scientific, Portsmouth, N.H.) were sonicated for 2 min in a prewarmed solution of 2.5% Conrad 70 detergent (Fisher Scientific, Fair Lawn, N.J.) and rinsed three times in distilled H2O. After being dried with compressed air, the slides were immersed for 1 h in an acid bath (3 N HCl), rinsed three times in deionized H2O, and dried again. The slides then were derivatized by immersion in 2% epoxysilane (3-glycidoxypropyltrimethoxysilane; Sigma-Aldrich, Milwaukee, Wis.) in methanol for 15 min, rinsed twice in methanol, and dried. Oligonucleotide probes were diluted in print buffer (0.1 M Na2HPO4, 0.2 M NaCl, 0.01% sodium dodecyl sulfate) to a final concentration of 60 μM and spotted onto the slides in quadruplicate by using a MicroGrid II spotter (BioRobotics, Inc., Woburn, Mass.). Printed slides were baked for 60 min at 130°C in a vacuum oven and stored at room temperature.
Multiplex PCR.
Multiplex PCR mixtures (50-μl volume) each contained 50 to 100 ng of purified genomic DNA, 200 μM each deoxynucleoside triphosphate, 400 nM each primer, 2.5 mM MgCl2, 1× reaction buffer, and 2 U of Taq polymerase (Fisher Scientific, Pittsburgh, Pa.). Thermal cycling was performed with a Mastercycler (Eppendorf, Hamburg, Germany) and included an initial incubation at 95°C for 3 min followed by 30 amplification cycles. Cycling included denaturation for 30 s at 95°C followed by annealing for 1 min at 52, 54, 56, 58, 60, or 62°C. Extension was done for 45 s at 72°C, and cycling was concluded with a final elongation for 5 min at 72°C. All multiplex products were checked by electrophoresis on 1% agarose gels and stained with ethidium bromide (0.5 μg ml−1). Negative test strains that did not show a PCR band upon checking of gels were considered negative for all nine loci and were not labeled or hybridized to the array. PCR mixtures were ethanol precipitated, resuspended in 40 μl of sterile water, and labeled by nick translation with a BioNick labeling system (Invitrogen). The labeled products were ethanol precipitated, and the pellets were resuspended in 75 μl of hybridization buffer (4× SSC [60 mM NaCl, 0.6 mM Na citrate] [pH 7.0], 5× Denhardt's solution [0.1% polyvinylpyrrolidone, 0.1% bovine serum albumin, 0.1% Ficoll]).
Hybridization and detection.
We used a combination of a Tyramide signal amplification (TSA) biotin system (Perkin-Elmer, Boston, Mass.) and fluorescence to detect hybridized targets (7). Slide wells were incubated with 35 μl of TNB buffer (0.1 M Tris-HCl, 0.15 M NaCl, 0.5% blocking reagent [TSA biotin system]) for 30 min at room temperature. A 1:10 dilution of the labeled PCR product was prepared in hybridization buffer, heat denatured (2 min at 95°C), and rapidly chilled to 4°C. After aspiration of the TNB buffer from the wells, 35 μl of each target was added to each of two wells on the printed slides. The slides were placed in a humidified chamber and incubated overnight by being submerged in a water bath at 50 or 55°C. After incubation, the hybridization solution was removed by aspiration, and the slides were washed in TNT buffer (0.1 M Tris-HCl [pH 7.5], 0.15 M NaCl, 0.05% Tween 20) three times for 1 min each time with agitation. The wells were incubated for 30 min with streptavidin conjugated to horseradish peroxidase (1:100 in TNB buffer; TSA biotin system). After another washing step, the wells were incubated for 30 min with 10% equine serum albumin (Sigma-Aldrich) in 2× SSC for 30 min. The slides were washed again and incubated with biotinylated Tyramide (1:50 in amplification buffer; TSA biotin system) for 10 min. After another washing step, the wells were incubated with streptavidin (2 μg ml−1) conjugated to Alexa Fluor 546 (Molecular Probes, Eugene, Oreg.) in 1× SSC-5× Denhardt's solution for 60 min. After a final wash in TNT buffer, the slides were spun dry and then imaged with an arrayWoRxe scanner (Applied Precision, Issaquah, Wash.).
Microarray image analysis.
Image analysis software (softWoRx Tracker; Applied Precision) was used to quantify hybridization signals. The contour function was used to accommodate variations in spot shape and size. To objectively determine whether a spot was positive, we used a variant of a k-means algorithm. Replicate spots were averaged for each hybridization experiment, and the averages were sorted from low to high. The lowest and highest values were used as “seeds” for low and high clusters, respectively. The next lowest value then was compared with the two seeds to determine to which cluster it belonged (i.e., most proximal), and the values for this cluster subsequently were pooled to calculate a new average. This process was continued until all spots were assigned to the low cluster or the high cluster, followed by calculation of final cluster averages and standard deviations. When final cluster averages differed by >3 standard deviations, we considered members of the high cluster to represent positive hybridization. In practice, we also imposed a minimum intensity requirement such that the low cluster average could not exceed 25,000 (out of a maximum of 65,535) and the high cluster average could not drop below 10,000. If either condition was not met, then the sample was reprocessed.
Assay specificity and sensitivity.
Purified DNA from 75 strains (28 species or subspecies) was used as a DNA template for multiplex PCR followed by hybridization to the microarray. In total, 21 L. anguillarum (serotypes O1 to O10), 4 V. vulnificus, 1 V. parahaemolyticus, 6 P. damselae subsp. damselae, and 3 A. salmonicida strains were included as positive test strains, and 40 strains of taxonomically or ecologically related bacteria were included as negative test strains. Statistical software from NCSS, Kaysville, Utah (2004 edition), was used to calculate sensitivity and specificity parameters as well as associated 95% confidence intervals (CIs; determined by the Wilson method [1]).
16S ribosomal DNA (rDNA) PCR.
When multiplex PCR failed to amplify any products, we used universal PCR to verify that a template was present and that the reaction was not inhibited by extraction impurities. Using primers UnivRvs_517 (ATTACCGCGGCTGCTGG) and UnivFwd_008 (AGAGTTTGATCMTGGCTCAG), we amplified a ca. 530-bp fragment in 50-μl reaction volumes containing reaction buffer (Fisher Scientific), 2 mM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, a 400 nM concentration of each primer, 1 U of Taq polymerase, and 100 ng of DNA template. The cycling conditions included an initial incubation for 2 min at 95°C followed by 28 cycles that included denaturation for 30 s at 95°C, annealing for 1 min at 62°C, and extension for 1 min at 72°C. Samples were incubated for 10 min at 72°C for a final extension. An aliquot (20 μl) was checked on gels to confirm amplification. In several instances, we sequenced the resulting product to verify identity (Amplicon Express, Pullman, Wash.).
Detector sensitivity.
To assess overall detection sensitivity under ideal conditions, template DNA (P. damselae subsp. damselae) was diluted 10-fold from 2 × 10−8 g to 2 × 10−16 g and subjected to multiplex PCR. Subsequent PCR products from these dilutions were hybridized to the array. To assess detector sensitivity relative to that of conventional agarose gel electrophoresis, ureC and dly PCR products (from P. damselae subsp. damselae) were nick translated, diluted twofold, and hybridized to the array. A parallel dilution series was prepared without nick translation for detection by agarose gel electrophoresis and ethidium bromide staining.
RESULTS
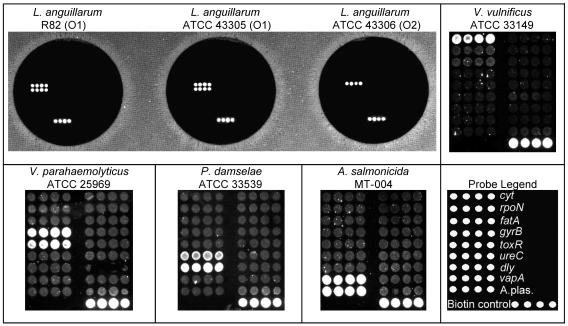
We tested PCR annealing at 54, 56, 58, 60, and 62°C with a gradient thermal cycler. The highest annealing temperature that was compatible with all primer sets in the multiplex reaction was 60°C. Microarray hybridization was tested at 50 and 55°C. At 55°C, all multiplex PCR products from the target bacteria L. anguillarum, V. parahaemolyticus, V. vulnificus, P. damselae subsp. damselae, and A. salmonicida subsp. salmonicida produced specific and clear hybridization signals on the array (Fig. 1).
FIG. 1.
Positive control hybridizations. (Upper left panel) Specificity for L. anguillarum with the multiplex PCR and microarray hybridization. Genotypes for R-82 (O1), ATCC 43305 (O1), and ATCC 43306 (O2) match the respective genotypes for rpoN and fatA. (Upper right panel and lower left panel) Hybridization with probes complementary to V. vulnificus (cyt), V. parahaemolyticus (gyrB and toxR), P. damselae (ureC and dly), and A. salmonicida (vapA and A.plas.) in four different PCRs. (Lower right panel) Positions of oligonucleotide probes and the biotin control on the microarray.
We tested 75 strains of bacteria representing 28 species (Table 1). All test strains of the five target species were correctly detected by at least one species-specific marker. Because two L. anguillarum strains were negative for the fatA gene and one P. damselae subsp. damselae strain was negative for the dly gene, the calculated sensitivities for these probes were reduced (Table 3). The large CIs for all of the sensitivity calculations reflected the limited number of positive test strains that we could obtain for this study. Multiplex PCR for the 23 nontarget species produced no amplification products; thus, the specificity of the assay for the panel of strains tested in this study was 100%.
TABLE 3.
Results of multiplex PCR and microarray hybridization
| Genetic marker | No. of samplesa
|
Sensitivityb (95% CI) | Specificityc (95% CI) | |||
|---|---|---|---|---|---|---|
| Concordant
|
Discordant
|
|||||
| Positive | Negative | Positive | Negative | |||
| vapA | 3 | 72 | 0 | 0 | 1.0 (0.44-1.0) | 1.0 (0.95-1.0) |
| A.sal | 3 | 72 | 0 | 0 | 1.0 (0.44-1.0) | 1.0 (0.95-1.0) |
| rpoN | 21 | 54 | 0 | 0 | 1.0 (0.85-1.0) | 1.0 (0.93-1.0) |
| fatA | 4 | 69 | 0 | 2 | 0.67 (0.3-0.9) | 1.0 (0.95-1.0) |
| ureC | 6 | 59 | 0 | 0 | 1.0 (0.61-1.0) | 1.0 (0.95-1.0) |
| dly | 5 | 69 | 0 | 1 | 0.83 (0.44-0.97) | 1.0 (0.95-1.0) |
| gyrB | 1 | 74 | 0 | 0 | 1.0 (0.21-1.0) | 1.0 (0.95-1.0) |
| toxR | 1 | 74 | 0 | 0 | 1.0 (0.21-1.0) | 1.0 (0.95-1.0) |
| cyt | 4 | 71 | 0 | 0 | 1.0 (0.51-1.0) | 1.0 (0.95-1.0) |
Concordant refers to the number of samples that hybridized correctly to their respective probes (positive) or the number of samples that were correctly scored as negative by multiplex PCR (negative). Discordant refers to the number of samples that showed positive, nonspecific hybridization (positive) or false-negative results (negative).
Number of concordant positive samples divided by number of concordant positive samples plus number of discordant negative samples.
Number of concordant negative samples divided by number of concordant negative samples plus number of discordant positive samples.
To verify that the failure to produce products was not an artifact of PCR inhibition, all multiplex PCR-negative strains were also tested by universal 16S rDNA PCR, and an appropriately sized product was produced in all cases. The minimum DNA template required for the positive detection of multiplex products (P. damselae subsp. damselae in this case) was 20 fg of genomic DNA, which is equivalent to four or five cells. Triplicate serial dilutions of the ureC and dly PCR products demonstrated that the ureC product was detectable below 1:32, whereas the dly product was detectable only to 1:16 (based on the detection cluster algorithm). These two combined products were not visible below a 1:4 dilution when agarose gel electrophoresis was used for detection.
DISCUSSION
This is the first microarray technique described for the detection of marine fish pathogens. The availability of rapid, sensitive, and specific diagnostic methods for the detection of bacterial pathogens causing diseases is very important in aquaculture. Nevertheless, existing methods are restricted by the number of pathogens that can be detected simultaneously and by overall assay sensitivity or specificity. Like many PCR assays, the assay described here was suitable for detecting ∼5 cell equivalents under optimal conditions. Unlike conventional multiplex PCR assays, microarray detectors do not require clear length differences between PCR products; thus, the PCR can be designed around short, equally sized fragments that are amplified with similar efficiencies. In addition, because detection is based on hybridization to specific sequences rather than product length, time-consuming sequencing or blot-and-probe techniques are not necessary to confirm product identity (9, 10, 43). Products of various lengths also present a challenge for developing optimal PCR conditions (primer annealing temperatures and similar MgCl2 concentrations). While the dilution experiments presented here suggest that unequal PCR amplification efficiencies or unequal hybridization efficiencies exist for the ureC and dly targets, the current assay is sufficient for simultaneous screening for all nine pathogenic markers.
Our prototype assay was highly specific, with no false-positive detections for a battery of test strains (23 nontarget species or subspecies). The sensitivity was 100% for seven of the nine markers. The fatA marker hybridized only to four L. anguillarum strains, although these were all serovar O1. Two additional serovar O1 strains were negative for fatA. The fatA gene is harbored on a virulence plasmid (pJM1) that encodes an iron-sequestering system, and an estimated 90% of serotype O1 strains harbor this plasmid. Thus, we would not expect all serovar O1 strains to hybridize to both L. anguillarum probes. No other serovars hybridized to the fatA marker.
One test strain of P. damselae subsp. damselae (JCM 8968) did not hybridize to the dly probe, although the ureC probe was positive for this strain. This particular strain was originally classified as Photobacterium histaminum (20); thus, the failure to hybridize is consistent with some degree of genetic divergence. Although all three A. salmonicida strains were positive for both plasmid-borne markers (vapA and A.sal), not all strains are expected to harbor these genes (41); thus, the sensitivity reported here (100%) does not accurately reflect what would likely be encountered in a diagnostic or surveillance application.
The specificity and sensitivity estimates reported here apply to the microarray detector only. Both of these variables can be affected by numerous events “upstream” of the actual microarray hybridization. For example, during the course of this study, we encountered five instances when a strain of bacteria did not hybridize as expected to one or more probes. In all of these instances, partial sequencing of the 16S rRNA gene demonstrated that the test strains were not correctly identified, and the microarray hybridization results were consistent with the species identified by 16S rRNA gene sequencing (these strains were not included in the present analysis). Either the initial strain identification was incorrect or subsequent sample processing led to an error. In another instance, two test strains were found to be negative when first hybridized to the array but were found to be positive when checked a second time (i.e., a 2.7% error rate during the hybridization step). These errors are examples of process-level errors that can be minimized by using stringent controls and standard operating procedures in a diagnostic laboratory setting.
The high degree of specificity reported here suggests that this assay format is not prone to generating false-positives; as with any assay, if any unusual positive results are detected, then additional confirmation is advisable. A larger problem is that of false-negatives. False-negatives can arise due to naturally occurring sequence polymorphisms in PCR primer or probe hybridization sequenced. This is not a significant issue if all polymorphisms are known and can be included on the microarray or if relatively conserved genes are selected. If an array is dependent on many sequence polymorphisms within the same probe region (e.g., selected regions of the 16S rRNA gene), then naturally occurring mutations in these regions could lead to false-negatives when these variable sequences are tested with the microarray.
During the execution of any PCR assay, false-negatives can also result when coprecipitates from the template extraction interfere with the PCR (23). In the format described here, we used post hoc PCR amplification of the 16S rRNA gene to verify that PCR failure was not due to template impurities. It is clear that if prokaryotic bacterial DNA were used in the reaction, we could include a 10th primer set targeting the 16S rRNA gene as a positive control for the PCR. Nevertheless, the choice of an internal control depends on the matrix that is sampled. If tissue samples are assayed, then samples without prokaryotic DNA will still appear negative for a prokaryotic 16S rDNA marker; a eukaryotic positive control could be incorporated for this application. A partial solution would be to spike the reaction with control DNA, but this strategy can reduce sensitivity if the spiked template is preferentially amplified during the PCR (unpublished data). For the survey of environmental samples, it is appropriate to add control DNA to separate dilutions of the original extract so that PCR inhibition can be quantified (23). Consequently, the assay described here should accommodate multiple matrices (purified DNA, tissue samples, or environmental samples) with modest assay or procedural modifications.
This is the first microarray technique described for the detection of bacteria pathogenic for marine fish. The sensitivity and specificity of the described method and the simultaneous detection of five bacterial species make it suitable for preliminary diagnoses or confirmation of vibriosis and furunculosis as well as for the detection of potential human pathogens in sea farming products.
Acknowledgments
This work was supported by the Agricultural Animal Health Program (College of Veterinary Medicine, Washington State University) and the USFWS (through the WSU and UI Center for Reproductive Biology, Washington State University). S.F.G. thanks the University of Santiago de Compostela for a research fellowship in support of this work.
REFERENCES
- 1.Agresti, A., and B. Coull. 1998. Approximate is better than ′exact' for interval estimation of binomial proportions. Am. Stat. 52:119-126. [Google Scholar]
- 2.Arias, C. R., E. Garay, and R. Aznar. 1995. Nested PCR method for rapid and sensitive detection of Vibrio vulnificus in fish, sediments, and water. Appl. Environ. Microbiol. 61:3476-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austin, B., and D. Austin. 1999. Bacterial fish pathogens: disease of farmed and wild fish, 3rd ed. Springer-Praxis, London, England.
- 4.Brasher, C. W., A. DePaola, D. D. Jones, and A. K. Bej. 1998. Detection of microbial pathogens in shellfish with multiplex PCR. Curr. Microbiol. 37:101-107. [DOI] [PubMed] [Google Scholar]
- 5.Call, D., M. Borucki, and T. Besser. 2003. Mixed-genome microarrays reveal multiple serotype and lineage-specific differences among strains of Listeria monocytogenes. J. Clin. Microbiol. 41:632-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Call, D., D. Chandler, and F. Brockman. 2001. Fabrication of DNA microarrays using unmodified oligonucleotide probes. BioTechniques 30:368-379. [DOI] [PubMed] [Google Scholar]
- 7.Call, D. R., M. Bakko, M. Krug, and M. Roberts. 2003. Identifying antimicrobial resistance genes using DNA microarrays. Antimicrob. Agents Chemother. 47:3290-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Call, D. R., M. Borucki, and F. Loge. 2003. Detection of bacterial pathogens in environmental samples using DNA microarrays. J. Microbiol. Methods 53:235-243. [DOI] [PubMed] [Google Scholar]
- 9.Call, D. R., F. J. Brockman, and D. P. Chandler. 2001. Detecting and genotyping Escherichia coli O157:H7 using multiplexed PCR and nucleic acid microarrays. Int. J. Food Microbiol. 67:71-80. [DOI] [PubMed] [Google Scholar]
- 10.Chizhikov, V., A. Rasooly, K. Chumakov, and D. Levy. 2001. Microarray analysis of microbial virulence factors. Appl. Environ. Microbiol. 67:3258-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chizhikov, V., M. Wagner, A. Ivshina, Y. Hoshino, A. Z. Kapikian, and K. Chumakov. 2002. Detection and genotyping of human group A rotaviruses by oligonucleotide microarray hybridization. J. Clin. Microbiol. 40:2398-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho, J. C., and J. M. Tiedje. 2001. Bacterial species determination from DNA-DNA hybridization by using genome fragments and DNA microarrays. Appl. Environ. Microbiol. 67:3677-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu, S., S. Cavaignac, J. Feutrier, B. M. Phipps, M. Kostrzynska, W. W. Kay, and T. J. Trust. 1991. Structure of the tetragonal surface virulence array protein and gene of Aeromonas salmonicida. J. Biol. Chem. 266:15258-15265. [PubMed] [Google Scholar]
- 14.Del Cerro, A., I. Marquez, and J. A. Guijarro. 2002. Simultaneous detection of Aeromonas salmonicida, Flavobacterium psychrophilum, and Yersinia ruckeri, three major fish pathogens, by multiplex PCR. Appl. Environ. Microbiol. 68:5177-5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson, G. 2002. Microarrays in ecology and evolution: a preview. Mol. Ecol. 11:17-24. [DOI] [PubMed] [Google Scholar]
- 16.González, S., C. R. Osorio, and Y. Santos. 2003. Development of a PCR-based method for the detection of Listonella anguillarum in fish tissues and blood samples. Dis. Aquat. Org. 55:109-115. [DOI] [PubMed] [Google Scholar]
- 17.Guschin, D. Y., B. K. Mobarry, D. Proudnikov, D. A. Stahl, B. E. Rittmann, and A. D. Mirzabekov. 1997. Oligonucleotide microchips as genosensors for determinative and environmental studies in microbiology. Appl. Environ. Microbiol. 63:2397-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiney, M., M. T. Dawson, D. M. Heery, P. R. Smith, F. Gannon, and R. Powell. 1992. DNA probe for Aeromonas salmonicida. Appl. Environ. Microbiol. 58:1039-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoie, S., M. Heum, and O. Thorensen. 1997. Evaluation of a polymerase chain reaction-based assay for the detection of Aeromonas salmonicida subsp. salmonicida in Atlantic salmon, Salmo salar. Dis. Aquat. Org. 30:27-35. [Google Scholar]
- 20.Kimura, B., S. Hokimoto, H. Takahashi, and T. Fujii. 2000. Photobacterium histaminum Okuzumi et al. 1994 is a later subjective synonym of Photobacterium damselae subsp. damselae (Love et al. 1981) Smith et al. 1991. Int. J. Syst. Evol. Microbiol. 50:1339-1342. [DOI] [PubMed] [Google Scholar]
- 21.Kita-Tsukamoto, K., H. Oyaizu, K. Nanba, and U. Simidu. 1993. Phylogenetic relationships of marine bacteria, mainly members of the family Vibrionaceae, determined on the basis of 16S rRNA sequences. Int. J. Syst. Bacteriol. 43:8-19. [DOI] [PubMed] [Google Scholar]
- 22.Klontz, K. C., S. Lieb, M. Schreiber, H. T. Janowski, L. M. Baldy, and R. A. Gunn. 1988. Syndromes of Vibrio vulnificus infections. Clinical and epidemiologic features in Florida cases, 1981-1987. Ann. Intern. Med. 109:318-323. [DOI] [PubMed] [Google Scholar]
- 23.Loge, F. J., D. E. Thompson, and D. R. Call. 2002. PCR detection of specific pathogens in water: a risk-based analysis. Environ. Sci. Technol. 36:2754-2759. [DOI] [PubMed] [Google Scholar]
- 24.Magnusson, H. B., O. H. Fridjonsson, O. S. Andresson, E. Benediktsdottir, S. Gudmundsdottir, and V. Andresdottir. 1994. Renibacterium salmoninarum, the causative agent of bacterial kidney disease in salmonid fish, detected by nested reverse transcription-PCR of 16S rRNA sequences. Appl. Environ. Microbiol. 60:4580-4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall, S., S. Heath, V. Henriquez, and C. Orrego. 1998. Minimally invasive detection of Piscirickettsia salmonis in cultivated salmonids via the PCR. Appl. Environ. Microbiol. 64:3066-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNamara, P. J., W. A. Cuevas, and J. G. Songer. 1995. Toxic phospholipases D of Corynebacterium pseudotuberculosis, C. ulcerans and Arcanobacterium haemolyticum: cloning and sequence homology. Gene 156:113-118. [DOI] [PubMed] [Google Scholar]
- 27.Miller, V. L., R. K. Taylor, and J. J. Mekalanos. 1987. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell 48:271-279. [DOI] [PubMed] [Google Scholar]
- 28.Mooney, J., E. Powell, C. Clabby, and R. Powell. 1995. Detection of Aeromonas salmonicida in wild Atlantic salmon using a specific DNA probe test. Dis. Aquat. Org. 21:131-135. [Google Scholar]
- 29.Nakashima, S., and K. Takimoto. 1987. The epidemiological data of food poisoning in 1986. Food Sanit. Res. 37:50-76. [Google Scholar]
- 30.Osorio, C., and A. Toranzo. 2002. DNA-based diagnostics in sea farming, p. 253-310. In M. Fingerman and R. Nagabhushanam (ed.), Seafood safety and human health. Science Publishers, Inc., Enfield, N.H.
- 31.Osorio, C. R., M. D. Collins, A. E. Toranzo, J. L. Barja, and J. L. Romalde. 1999. 16S rRNA gene sequence analysis of Photobacterium damselae and nested PCR method for rapid detection of the causative agent of fish pasteurellosis. Appl. Environ. Microbiol. 65:2942-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osorio, C. R., A. E. Toranzo, J. L. Romalde, and J. L. Barja. 2000. Multiplexed PCR assay for ureC and 16S rRNA genes clearly discriminates between both subspecies of Photobacterium damselae. Dis. Aquat. Org. 40:177-183. [DOI] [PubMed] [Google Scholar]
- 33.O'Toole, R., D. L. Milton, P. Horstedt, and H. Wolf-Watz. 1997. rpoN of the fish pathogen Vibrio (Listonella) anguillarum is essential for flagellum production and virulence by the water-borne but not intraperitoneal route of inoculation. Microbiology 143:3849-3859. [DOI] [PubMed] [Google Scholar]
- 34.Pazos, F., Y. Santos, Macías, S. Núñez, and A. E. Toranzo. 1996. Evaluation of media for the successful culture of Flexibacter maritimus. J. Fish Dis. 19:193-197. [Google Scholar]
- 35.Pedersen, K., B. Austin, D. A. Austin, and J. L. Larsen. 1999. Vibrios associated with mortality in cultured plaice Pleuronectes platessa fry. Acta Vet. Scand. 40:263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers, p. 365-386. In S. Krawetz and S. Misener (ed.), Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 37.Ruimy, R., V. Breittmayer, P. Elbaze, B. Lafay, O. Boussemart, M. Gauthier, and R. Christen. 1994. Phylogenetic analysis and assessment of the genera Vibrio, Photobacterium, Aeromonas, and Plesiomonas deduced from small-subunit rRNA sequences. Int. J. Syst. Bacteriol. 44:416-426. [DOI] [PubMed] [Google Scholar]
- 38.Schena, M. 2000. Microarray biochip technology. Eaton Publishing, Natick, Mass.
- 39.Tolmasky, M. E., L. A. Actis, and J. H. Crosa. 1993. A single amino acid change in AngR, a protein encoded by pJM1-like virulence plasmids, results in hyperproduction of anguibactin. Infect. Immun. 61:3228-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toranzo, A. E., and J. L. Barja. 1993. Fry mortality syndrome (FMS) in Spain. Isolation of the causative Flexibacter psychrophilus. Bull. Eur. Assoc. Fish Pathol. 13:30-32. [Google Scholar]
- 41.Toranzo, A. E., Y. Santos, S. Nuñez, and J. Barja. 1991. Biochemical and serological characteristics, drug resistance and plasmid profiles of Spanish isolates of Aeromonas salmonicida. Gyobyo Kenkyu 26:55-60. [Google Scholar]
- 42.Venkateswaran, K., N. Dohmoto, and S. Harayama. 1998. Cloning and nucleotide sequence of the gyrB gene of Vibrio parahaemolyticus and its application in detection of this pathogen in shrimp. Appl. Environ. Microbiol. 64:681-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volokhov, D., A. Rasooly, K. Chumakov, and V. Chizhikov. 2002. Identification of Listeria species by microarray-based assay. J. Clin. Microbiol. 4:4720-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto, K., A. C. Wright, J. B. Kaper, and J. G. Morris. 1990. The cytolysin gene of Vibrio vulnificus: sequence and relationship to the Vibrio cholerae El Tor hemolysin gene. Infect. Immun. 58:2706-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]