Abstract
In this study, we compared the immune cell populations in rheumatoid arthritis (RA) synovial fluid, which shows lymphoid tissue-like structure, with those in tonsils, which are normal secondary lymphoid tissues. Firstly, we found that CD4-CD11b+ macrophages were the major population in RA synovial fluid and that B cells were the major population in tonsils. In addition, synovial fluid from patients with osteoarthritis, which is a degenerative joint disease, contained CD4+CD11b+ monocytes as the major immune cell population. Secondly, we categorized three groups based on the proportion of macrophages found in RA synovial fluid: (1) the macrophage-high group, which contained more than 80% macrophages; (2) the macrophage-intermediate group, which contained between 40% and 80% macrophages; and (3) the macrophage-low group, which contained less than 40% macrophages. In the macrophage-low group, more lymphoid tissue inducer (LTi)-like cells were detected, and the expression of OX40L and TRANCE in these cells was higher than that in the other groups. In addition, in this group, the suppressive function of regulatory T cells was downregulated. Finally, CXCL13 expression was higher in RA synovial fluid than in tonsils, but CCL21 expression was comparable in synovial fluid from all groups and in tonsils. These data demonstrate that increased lymphocyte infiltration in RA synovial fluid is correlated with an increase in LTi-like cells and the elevation of the chemokine expression.
Keywords: Lymphoid tissue inducer, Rheumatoid arthritis, Tonsil
INTRODUCTION
Rheumatoid arthritis (RA), which destroys cartilage, bones, and tissues in joints, is the most common systemic inflammatory disease. Approximately 1% of the worldwide population suffers from this autoimmune disease. One of the most obvious characteristics of this disease is an increase of activated macrophages in synovial tissue (1-3); however, some RA patients exhibit lymphocyte infiltration to the damaged joints and lymphoid tissue-like structures in synovial tissue (4,5). Many studies have demonstrated that the development and process of the ectopic lymphoid tissue-like structures are similar to those of normal lymphoid tissues, such as lymph nodes and spleen (6-8). Accordingly, the factors involved in the formation of normal lymphoid tissues, such as CXCL13, CCL19, CCL21, CCR7, CXCR5, lymphotoxin-α/β, and interleukin (IL)-7, are found at RA synovial membranes (9-16). In the formation of normal lymphoid tissues, lymphoid tissue inducer (LTi) cells whose name reflects the primary role of the cell in lymphoid tissue organization induce the lymphoid development by expressing CCR7, CXCR5, lymphotoxin-α/β, and IL-7 receptor (17-22). The engagement of lymphotoxin-α/β receptors on stromal cells with LTi cells upregulate CXCL13, CCL19 and CCL21 and these chemokines recruit the receptor-expressing lymphocytes to form lymphoid tissues. The LTi cells do not express lineage markers, but do express CD117, CD127 (IL-7 receptor), RORC (retinoid-related orphan receptor gamma), OX40L, CD30L and TRANCE (18-22). By the interaction with OX40 and CD30-expressing CD4 T cells, LTi cells help memory CD4 T cell development (18). Recently, LTi cells were identified in human tissues such as tonsils and lymph nodes (23,24). However, the phenotype of the human cells is not exactly same to that of the murine cells, for example, human LTi cells do not express CD30L (25,26). In addition, many subpopulations which show one or a couple of different molecular expression exist, so they are called LTi-like cells. Although the involvement of LTi cells in normal and ectopic lymphoid tissue development has been well established and reported in mice (17-22), the identification of LTi cells in human RA synovial tissues has not yet been reported.
Therefore, we examined whether LTi cells are present in human RA synovial fluid. Because human LTi cells are well described in tonsils (23,24), which are normal lymphoid tissues, we compared the immune cells infiltrate in RA synovial tissue with cells from tonsils as well as to immune cells infiltrated to synovial fluid in patients with osteoarthritis (OA), which is degenerative joint disease and the most common form of arthritis (27,28). Because the proportions of infiltrated lymphocytes and activated macrophages are different between different patients, we categorized three patient groups based on the proportion of macrophages in the synovial fluid: a macrophage-high group, which contained more than 80% macrophages; a macrophage-intermediate group, which contained between 40% and 80% macrophages, and a macrophage-low group, which contained less than 40% macrophages. According to this grouping, we analyzed the proportion and activation of LTi-like cells, CD4 T cells, and regulatory T cells (Tregs) and compared these numbers to those obtained in tonsils. Finally, we examined the expression of CCL21 and CXCL13, which are chemokines that recruit CCR7-expressing T cells and LTi cells and CXCR5-expressing B cells and LTi cells, respectively (29,30).
MATERIALS AND METHODS
Isolation of lymphocytes from synovial fluid of RA and OA patients
RA and OA synovial fluid was obtained from Korea University Guro Hospital and all experiments were carried out with the approval of Korea University Guro Hospital Institutional Review Board (IRB). For the diagnosis of RA, clinical symptoms and radiographic evidence of bony erosions are used. According to the classification of RA by the American Rheumatism Association 1987 (31), patients are diagnosed as RA when four or more of the following seven factors are present: morning stiffness, swelling or fluid around more than three joints simultaneously, at least one swollen area, symmetric arthritis, rheumatoid nodules, increased rheumatoid factor, and X-ray changes in the hands and wrist. Whereas RA is systemic, OA is limited only to the joints, and radiologic evidences of OA include asymmetrical narrowing of the joint space and bony overgrowth. In the obtained synovial fluid, PBS was added and the diluted synovial fluid was centrifuged at 1,500 rpm for 5 minutes. After depletion of red blood cells with Gey's solution, impurities were filtered out from the cell suspensions using 70 um cell strainer (BD biosciences, San Jose, CA). The separated cell suspensions were used for flow cytometric analysis or cultured.
Isolation of lymphocytes from tonsils
Human tonsils were obtained from Gangnam Severance Hospital of Yonsei University Medical Center. Patients who have chronic tonsillitis, snoring, or mouth breathing problem underwent tonsillectomy. The experiments have been approved under the supervision of Gangnam Severance Hospital IRB. To separate cells from human tonsils, the extracted tonsils were cut into small fragments and crushed between gauze. To deplete red blood cells, Gey's solution was treated and the cell suspensions were used for flow cytometric analysis or cultured.
Flow cytometric analysis
mAbs for TCRαβ (IP26), CD3 (UCHT1), CD4 (RPA-T4), CD25 (BC96), CD19 (HIB19), CD56 (MEM188), GITR (eBioAITR), OX40 (ACT35), TRANCE (MIH24), CD127 (eBioRDR5), RORC (AFKJS-9), and Foxp3 (PCH101) were purchased from eBio-science (San Diego, CA). mAbs for CD11b (ICRF44), OX40L (IK-1), CD11c (B-ly6) were purchased from BD biosciences. Foxp3 and RORC were detected by intracellular staining according to manufacturer's instructions.
Enzyme-linked immunosorbent assay (ELISA) of CCL21 and CXCL13
Synovial fluid of RA patient was centrifuged and the supernatant was collected and stored at -20℃. Samples are thawed and the concentration of CCL21 was measured using ELISA (R&D systems, Minneapolis, MN) according to manufacturer's instructions. Briefly, a 96-well microplate was coated with 2 µg/ml mouse anti-human CCL21 capture Ab and 100 ul of test sample or standard was added and incubate. After washing, biotinylated goat anti-human CCL21 detection Ab, and then streptavidin conjugated to horseradish-peroxidase solution was added. Finally, substrate solution was added and optical density was measured by microplate reader (Bio Rad, Tokyo, Japan) at 450 nm.
To assay the concentration of CXCL13, a microplate coated with mouse mAb against human CXCL13 was used (R&D systems). Prepared samples and standards were added into each well. After incubation with mAb against human CXCL13 conjugated to horseradish peroxidise, substrate was added, and optical density was measured by microplate reader at 450 nm. A standard curve was created by reducing the data using computer software generating a four parameter logistic curve-fit and used to calculate the CCL21 and CXCL13 concentration of each sample.
RESULTS
Comparison of the diverse cell populations in RA and OA synovial fluid and tonsils
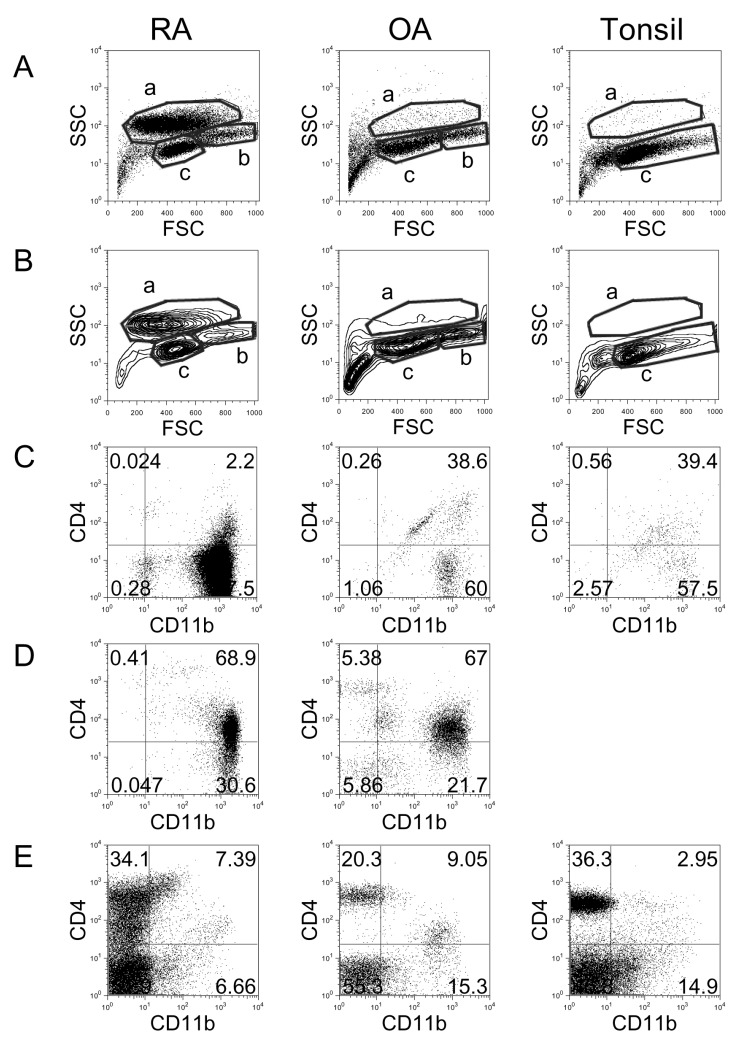
To compare the distribution of immune cells in RA synovial fluid, OA synovial fluid, and tonsils, cells were isolated and analyzed by flow cytometry (Fig. 1). Dot plot (Fig. 1A) and contour plot (Fig. 1B) analyses revealed three areas, which represented distinct cell types. The cells in 'a' area, which exhibited relatively high side-scattered light (SSC), were identified as activated macrophages (32). RA synovial fluid showed clearly increased numbers of macrophages compared with OA synovial fluid and tonsils. Staining the cells from the 'a' area with anti-CD11b and anti-CD4 revealed that most of these cells were CD4-CD11b+: 97.5% in RA, 60.0% in OA, and 57.5% in tonsils (Fig. 1C). The cells in the 'b' area, which exhibited intermediate levels of SSC and were larger in size, were possibly monocytes (32). In tonsils, the 'b' area was absent, and this absence was clear in contour plot analysis (Fig. 1B). Co-staining of the cells in the 'b' area with anti-CD11b and anti-CD4 revealed that most of these cells were CD4+CD11b+: 68.9% in RA and 67.0% in OA (Fig. 1D). The cells in the 'c' area were determined to be lymphocytes, and most of these cells were CD11b-: 86.0% in RA, 75.7% in OA, and 82.2% in tonsils (Fig. 1E).
Figure 1.
Flow cytometric analysis of cell populations in RA, OA, and tonsil showing by dot plots (A) and contour plots (B). X-axis shows forward-scattered light (FSC) and Y-axis shows side-scattered light (SSC). The cell populations were costained with anti-CD11b and anti-CD4 and analyzed in 'a' area of A and B (C), 'b' area of A and B (D), and 'c' area of A and B (E). The results are representative of each RA (n=17), OA (n=3), and tonsil (n=14) group.
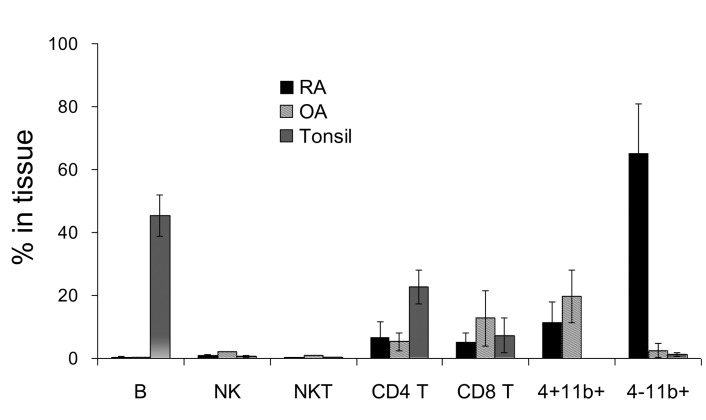
In order to compare the proportion of each cell population in RA synovial fluid, OA synovial fluid, and tonsils, the percentage of B cells, natural killer (NK) cells, NKT cells, CD4 T cells, CD8 T cells, CD4+CD11b+ monocytes, and CD4-CD11b+ macrophages were calculated (Fig. 2). Notably, cells that were found mainly in RA synovial fluid were macrophages (average 64.9%) and CD4+CD11b+ monocytes (~11.2%). In OA synovial fluid, the largest population of cells was CD4+CD11b+ monocytes (~19.6%), while the second most common population was CD8 T cells (~12.7%), which were increased compared with RA synovial fluid (~5.1%). In tonsils, B cells (~45.4%) and CD4 T cells (22.7%) were the major populations.
Figure 2.
Comparison of percentage of cell populations including B cells, NK cells, NKT cells, CD4 T cells, CD8 T cells, CD4+CD11b+ monocytes, and CD4-CD11b+ macrophages in RA (n=17), OA (n=3), and tonsil (n=14). Error bar shows the standard deviation.
RA patients divided based on the proportion of macrophages
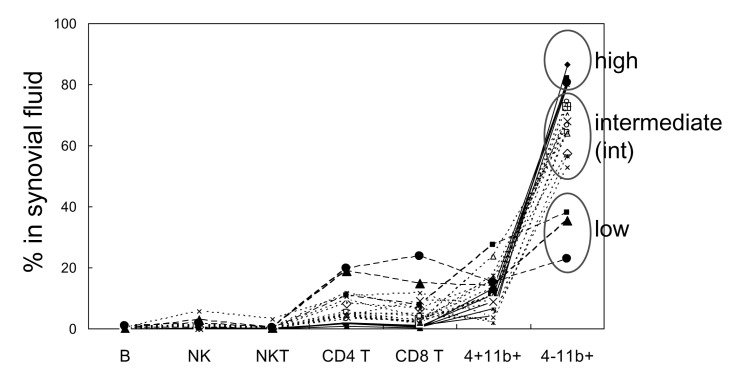
Because each RA patient exhibits a different proportion of infiltrated lymphocytes and activated macrophages, the RA patients were categorized into three groups based on the proportion of macrophages found in synovial fluid (Fig. 3). The first group was the macrophage-high group, which contained more than 80% macrophages. In this group, the proportion of lymphocytes was very low (average 2.8%). The second group showed intermediate levels (between 40% and 80%) of macrophages, and the average proportion of lymphocytes was 9.6%. Ten of the 17 examined patients were assigned to this group. The third group was the macrophage-low group, which contained less than 40% macrophages and exhibited increased infiltration of lymphocytes up to 46.1% with an average lymphocyte content of 34.1%.
Figure 3.
Comparison of percentage of cell populations in RA patients. Synovial fluids which contain over 80% of CD4-CD11b+ macrophages were grouped as macrophage high group (high, n=4), and synovial fluids which contain between 40% and 80% of macrophages were grouped as macrophage intermediate group (int, n=10). Synovial fluids which contain less than 40% of macrophages were grouped as macrophage low group (low, n=3).
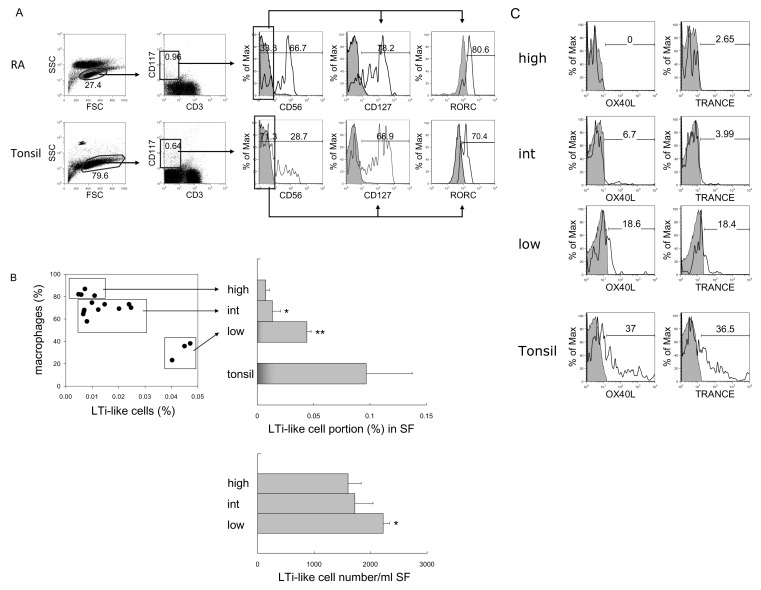
CD117+CD3-CD56-CD127+RORC+ LTi-like cells in RA synovial fluid
As we previously identified CD117+CD3-CD56-CD127+ RORC+OX40L+ LTi cells in human tonsils (23), we determined whether LTi cells are present in RA synovial fluid. When cells were gated as CD117+CD3-CD56- cells, most of them expressed CD127 (78.2%) and RORC (80.6%) (Fig. 4A), and these characteristics are very similar to tonsillar LTi cells. More LTi-like cells were found in the macrophage-low group compared with the macrophage-high and -intermediate groups (Fig. 4B). Not only the proportion but also the absolute cell number of LTi-like cells was increased in the macrophage-low group (p<0.05 compared to macrophage-high group).
Figure 4.
Identification and comparison of LTi/LTi-like cells in RA and tonsil. (A) Flow cytometric analysis of LTi-like cells. According to FSC and SSC, lymphocytes were selected and CD3-negative and CD117-positive population were gated. The expression of CD127 and RORC in CD117+CD3-CD56- cells was analyzed. Shaded histograms indicate isotype staining. (B) Correlation between the portion of macrophages and LTi-like cells in synovial fluid (SF). Percentage and absolute cell numbers of LTi-like cells in macrophage-high, intermediate (int), and low groups of RA synovial fluid were compared with tonsils. The statistical evaluation of the data was performed with Student's t-test and one-way analysis of variance. *p<0.05, and **p<0.005 compared to macrophage-high group. (C) Expression of OX40L and TRANCE in LTi-like cells in macrophage-high, intermediate, and low groups of RA synovial fluid and tonsil. Shaded histograms indicate isotype staining.
In contrast to tonsillar LTi cells, which express high levels of OX40L and TRANCE, LTi-like cells in RA synovial tissue expressed minimal levels of OX40L and TRANCE. In fact, low expression of these factors was found in the macrophage-low group, and no expression was detected in the macrophage-high group (Fig. 4C). Thus, we categorized these cells as LTi-like cells.
Comparison of activated CD4 T cells and Tregs
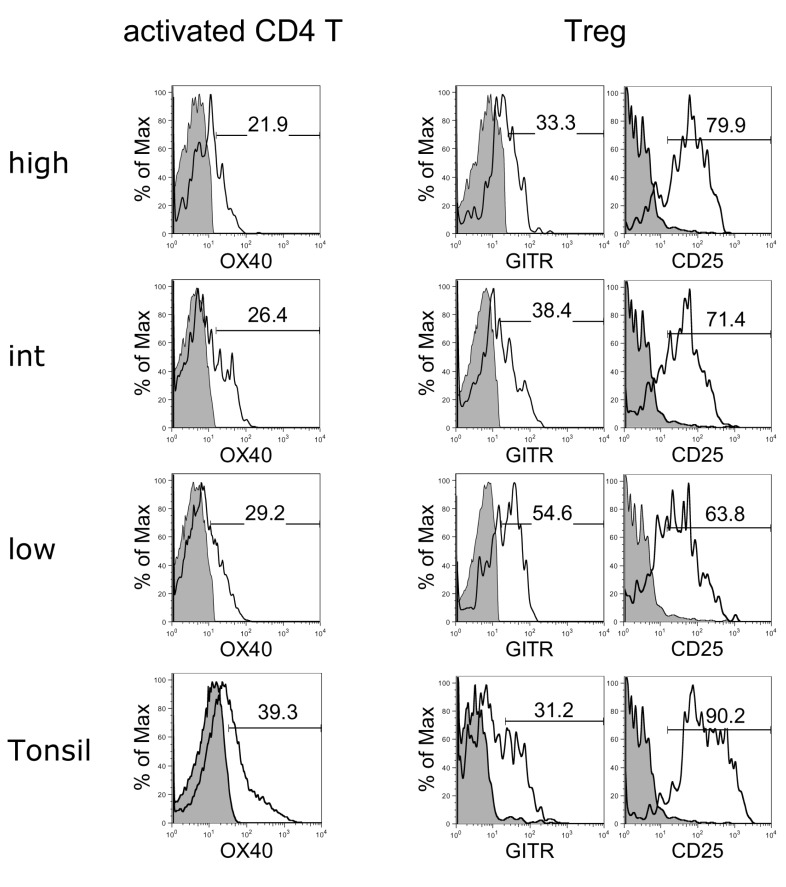
To analyze the activation state of CD4 T cells and CD3+Foxp3+ Tregs, the activation markers of these cells were evaluated and compared between the three macrophage groups of RA synovial fluid and tonsils (Fig. 5). All groups of CD4 T cells expressed similar levels of OX40 as tonsillar CD4 T cells. In comparison, Tregs in the macrophage-low group expressed higher levels of GITR and lower levels of CD25 compared to the other groups, suggesting that the suppressive function of Tregs in this group was downregulated (33).
Figure 5.
Flow cytometric analyses of OX40 expression on activated CD4 T cells and GITR and CD25 expression on Tregs in macrophage-high, intermediate (int), and low groups of RA synovial fluid and tonsil. Shaded histograms indicate isotype staining. The results are representative of each macrophage high (n=4), intermediate (n=10), and low (n=3) group.
Expression of CXCL13 and CCL21 in RA synovial fluid
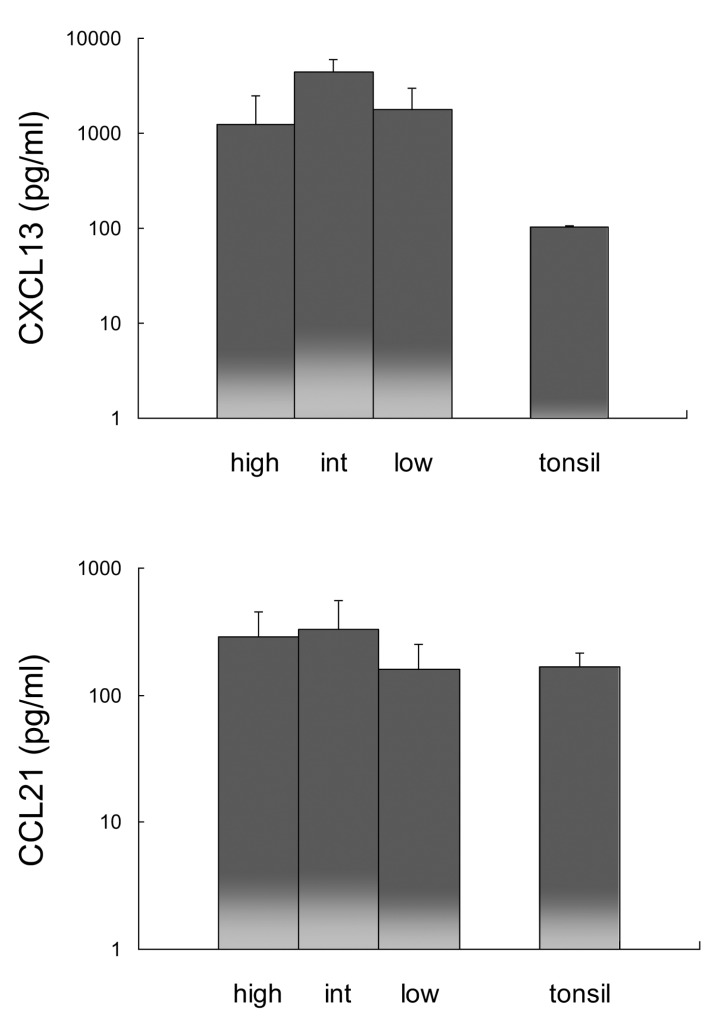
Since CXCL13 and CCL21 recruit CXCR5- and CCR7-expressing cells, CXCL13 and CCL21 expression was analyzed in RA synovial fluid and compared with expression in tonsils (Fig. 6). The expression of CXCL13 in RA synovial fluid was more than 12 times greater than that in tonsils, while the expression of CCL21 was comparable in all RA synovial fluid groups and tonsils.
Figure 6.
Comparison of CXCL13 and CCL21 expression in macrophage-high, intermediate (int), and low groups of RA synovial fluid and tonsil. Error bar shows the standard deviation.
DISCUSSION
RA, which is one of the most common autoimmune diseases, is characterized by chronic systemic inflammation with synovial inflammation and joint destruction. Because RA is a chronic inflammation disease, many types of immune cells in filtrate the damaged synovial tissue and in some cases, form lymphoid tissue-like structures (4,5). Although lymphoid infiltration into the tissue is not related to clinical symptoms (34), we hypothesized that RA patients could be categorized based on the proportion of immune cells and their activation conditions in synovial fluid, and it could be related to the LTi cell numbers. In this study, we identified clear differences in the distribution of macrophages and lymphocytes in RA synovial fluid. In particular, LTi/LTi-like cells, which are critical for induction of normal lymphoid tissue organogenesis (17-22), were increased in synovial fluid that contained more infiltrated lymphocytes. Compared with tonsillar LTi cells, synovial LTi/LTi-like cells in the macrophage-low, lymphocyte-high group expressed minimal levels of OX40L, which is an important ligand for CD4 T cell memory generation (18), while the cells in the macrophage-high group did not express this ligand. In addition, the suppressive function of Tregs in the macrophage-low, lymphocyte-high group was downregulated, suggesting that inflammatory responses are more active in this group. Although CXCL13 and CCL21 expression was not significantly different between the RA groups, CXCL13 expression was upregulated compared with that in tonsils, and CCL21 expression was comparable in synovial fluid from all groups and in tonsils, suggesting that these chemokine expression attracted CXCR5 and CCR7 expressing LTi/LTi-like cells (22).
In conclusion, LTi-like cells in RA synovial fluid that contains more infiltrated lymphocytes exhibited a more similar phenotype to tonsillar LTi cells compared to those in other RA tissues. It suggests that increased lymphocyte infiltration is correlated with an increase in LTi-like cells, and it is involved in the formation of normal lymphoid tissue-like structure.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2011-0024205).
Abbreviations
- RA
rheumatoid arthritis
- IL
interleukin
- LTi
lymphoid tissue inducer
- RORC
retinoid-related orphan receptor gamma, OA, osteoarthritis
- OA
osteoarthritis
- Tregs
regulatory T cells
- IRB
Institutional Review Board
- SSC
side-scattered light
- NK
natural killer
Footnotes
The authors have no financial conflict of interest.
References
- 1.Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994;53:39–44. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutolo M, Sulli A, Barone A, Seriolo B, Accardo S. Macrophages, synovial tissue and rheumatoid arthritis. Clin Exp Rheumatol. 1993;11:331–339. [PubMed] [Google Scholar]
- 4.Young CL, Adamson TC, 3rd, Vaughan JH, Fox RI. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1984;27:32–39. doi: 10.1002/art.1780270106. [DOI] [PubMed] [Google Scholar]
- 5.Schroder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1996;93:221–225. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O'Fallon WM, Goronzy JJ, Weyand CM. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167:1072–1080. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- 7.Cupedo T, Jansen W, Kraal G, Mebius RE. Induction of secondary and tertiary lymphoid structures in the skin. Immunity. 2004;21:655–667. doi: 10.1016/j.immuni.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Timmer TC, Baltus B, Vondenhoff M, Huizinga TW, Tak PP, Verweij CL, Mebius RE, van der Pouw Kraan TC. Inflammation and ectopic lymphoid structures in rheumatoid arthritis synovial tissues dissected by genomics technology: identification of the interleukin-7 signaling pathway in tissues with lymphoid neogenesis. Arthritis Rheum. 2007;56:2492–2502. doi: 10.1002/art.22748. [DOI] [PubMed] [Google Scholar]
- 9.Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgwick JD, Cyster JG. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wengner AM, Hopken UE, Petrow PK, Hartmann S, Schurigt U, Brauer R, Lipp M. CXCR5- and CCR7-dependent lymphoid neogenesis in a murine model of chronic antigen-induced arthritis. Arthritis Rheum. 2007;56:3271–3283. doi: 10.1002/art.22939. [DOI] [PubMed] [Google Scholar]
- 11.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 12.Shi K, Hayashida K, Kaneko M, Hashimoto J, Tomita T, Lipsky PE, Yoshikawa H, Ochi T. Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol. 2001;166:650–655. doi: 10.4049/jimmunol.166.1.650. [DOI] [PubMed] [Google Scholar]
- 13.Pickens SR, Chamberlain ND, Volin MV, Pope RM, Talarico NE, Mandelin AM, 2nd, Shahrara S. Characterization of interleukin-7 and interleukin-7 receptor in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2011;63:2884–2893. doi: 10.1002/art.30493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsui T, Akahoshi T, Namai R, Hashimoto A, Kurihara Y, Rana M, Nishimura A, Endo H, Kitasato H, Kawai S, Takagishi K, Kondo H. Selective recruitment of CCR6-expressing cells by increased production of MIP-3 alpha in rheumatoid arthritis. Clin Exp Immunol. 2001;125:155–161. doi: 10.1046/j.1365-2249.2001.01542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruhl H, Mack M, Niedermeier M, Lochbaum D, Schölmerich J, Straub RH. Functional expression of the chemokine receptor CCR7 on fibroblast-like synoviocytes. Rheumatology (Oxford) 2008;47:1771–1774. doi: 10.1093/rheumatology/ken383. [DOI] [PubMed] [Google Scholar]
- 16.Calmon-Hamaty F, Combe B, Hahne M, Morel J. Lymphotoxin alpha stimulates proliferation and pro-inflammatory cytokine secretion of rheumatoid arthritis synovial fibroblasts. Cytokine. 2011;53:207–214. doi: 10.1016/j.cyto.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 18.Kim MY, Gaspal FM, Wiggett HE, McConnell FM, Gulbranson-Judge A, Raykundalia C, Walker LS, Goodall MD, Lane PJ. CD4(+)CD3(-) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–654. doi: 10.1016/s1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 19.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 20.Kim MY, McConnell FM, Gaspal FM, White A, Glanville SH, Bekiaris V, Walker LS, Caamano J, Jenkinson E, Anderson G, Lane PJ. Function of CD4+CD3- cells in relation to B- and T-zone stroma in spleen. Blood. 2007;109:1602–1610. doi: 10.1182/blood-2006-04-018465. [DOI] [PubMed] [Google Scholar]
- 21.Meier D, Bornmann C, Chappaz S, Schmutz S, Otten LA, Ceredig R, Acha-Orbea H, Finke D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007;26:643–654. doi: 10.1016/j.immuni.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Kim MY, Rossi S, Withers D, McConnell F, Toellner KM, Gaspal F, Jenkinson E, Anderson G, Lane PJ. Heterogeneity of lymphoid tissue inducer cell populations present in embryonic and adult mouse lymphoid tissues. Immunology. 2008;124:166–174. doi: 10.1111/j.1365-2567.2007.02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, Han S, Withers DR, Gaspal F, Bae J, Baik S, Shin HC, Kim KS, Bekiaris V, Anderson G, Lane P, Kim MY. CD117(+) CD3(-) CD56(-) OX40Lhigh cells express IL-22 and display an LTi phenotype in human secondary lymphoid tissues. Eur J Immunol. 2011;41:1563–1572. doi: 10.1002/eji.201040915. [DOI] [PubMed] [Google Scholar]
- 24.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 25.Kim MY, Kim KS, McConnell F, Lane P. Lymphoid tissue inducer cells: architects of CD4 immune responses in mice and men. Clin Exp Immunol. 2009;157:20–26. doi: 10.1111/j.1365-2249.2009.03932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Withers DR, Gaspal FM, Bekiaris V, McConnell FM, Kim M, Anderson G, Lane PJ. OX40 and CD30 signals in CD4+ T-cell effector and memory function: a distinct role for lymphoid tissue inducer cells in maintaining CD4+ T-cell memory but not effector function. Immunol Rev. 2011;244:134–148. doi: 10.1111/j.1600-065X.2011.01057.x. [DOI] [PubMed] [Google Scholar]
- 27.Radin EL, Paul IL, Rose RM. Role of mechanical factors in pathogenesis of primary osteoarthritis. Lancet. 1972;1:519–522. doi: 10.1016/s0140-6736(72)90179-1. [DOI] [PubMed] [Google Scholar]
- 28.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 29.Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003;197:1191–1198. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohl L, Henning G, Krautwald S, Lipp M, Hardtke S, Bernhardt G, Pabst O, Forster R. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J Exp Med. 2003;197:1199–1204. doi: 10.1084/jem.20030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 32.Wells DA, Benesch M, Loken MR, Vallejo C, Myerson D, Leisenring WM, Deeg HJ. Myeloid and monocytic dyspoiesis as determined by flow cytometric scoring in myelodysplastic syndrome correlates with the IPSS and with outcome after hematopoietic stem cell transplantation. Blood. 2003;102:394–403. doi: 10.1182/blood-2002-09-2768. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 34.Thurlings RM, Wijbrandts CA, Mebius RE, Cantaert T, Dinant HJ, van der Pouw-Kraan TC, Verweij CL, Baeten D, Tak PP. Synovial lymphoid neogenesis does not define a specific clinical rheumatoid arthritis phenotype. Arthritis Rheum. 2008;58:1582–1589. doi: 10.1002/art.23505. [DOI] [PubMed] [Google Scholar]