Abstract
The unrestricted population of CD4+Foxp3+ regulatory T (Treg) cells, which have been known to control the expression of autoimmune diseases and protective immunity to inflammatory reactions, has led to greater appreciation of functional plasticity. Detecting and/or isolating Ag-specific CD4+Foxp3+ Tregs at the single cell level are required to study their function and plasticity. In this study, we established and compared both MHC class II tetramer and intracellular CD154 staining, in order to detect CD4+Foxp3+ Treg specific for foreign Ag in acute and chronic infections with lymphocytic choriomeningitis virus (LCMV). Our results revealed that MHC class II tetramer staining showed a lower detection rate of LCMV GP66-77-specific CD4+ T cells because most of MHC class II tetramers were unbound and unstable when combined staining was performed with intracellular cytokines. In contrast, intracellular CD154 staining was revealed to be easier and simple for detecting LCMV GP66-77-specific CD4+ T cells, compared to MHC class II tetramer staining. Subsequently, we employed intracellular CD154 staining to detect LCMV GP66-77-specific CD4+Foxp3+ Tregs using Foxp3GFP knock-in mouse, and found that LCMV GP66-77-specific CD4+Foxp3+ Tregs and polyclonal CD4+Foxp3+ Tregs showed differential expansion in mice infected with LCMV Arms or Cl13 at acute (8 and 13 days pi) and chronic phases (35 days pi). Therefore, our results provide insight into the valuable use of intracellular CD154 staining to detect and characterize foreign Ag-specific CD4+Foxp3+ Treg in various models.
Keywords: CD4+Foxp3+ regulatory T cells, MHC class II tetramer, Intracellular CD154 staining, Exhausted T cell
INTRODUCTION
Regulatory T cells (Tregs) are important to control the expression of autoimmune diseases and protective immunity to inflammatory reactions in many tumors and infectious diseases (1). Therefore, initially described as critical for the control of autoimmunity, Treg were found upon adoptive transfer to prevent experimental autoimmune diseases (2,3). More recently, evidences has been accumulated that Treg cells suppress allogeneic immune responses and prevent transplant rejection (4). However, despite decades of research into the phenotypes and functions of different subsets of Tregs, only recently have they been sufficiently well characterized to begin considering how they might be targeted therapeutically. Thus, it has been reported that manipulating the numbers and/or function of Tregs in mice can decrease pathology in a wide range of context, such as autoimmunity, transplantation, and cancer (5-8).
Several different types of Tregs exist in human, including specialized subsets of CD4+, CD8+, double negative CD3+CD4- CD8-, γδ T cells, and natural killer T (NKT) cells. Although these different types of Tregs work together in a network to maintain immune homeostasis, the majority of the current studies focus on defining the normal function of CD4+ Tregs, because these cells mediate dominant, long-lasting, and transferable tolerance in experimental models (9). Research into how Treg can be manipulated therapeutically is most advanced for main types of CD4+ Tregs: forkhead box protein 3 (Foxp3)+ Tregs and interleukin-10 (IL-10)-producing type 1 Tregs (Tr1). CD4+ Tregs that express Foxp3 transcription factor were originally identified by their constitutive expression of the IL-2 receptor-α chain (CD25) (10), and for many years these cells have been referred to as CD4+CD25+ Tregs. CD25, however, is not a specific marker for these cells, because it is also expressed by all activated CD4+ T cells. Therefore, it has been believed that the Foxp3 transcription factor is considered as a crucial marker and a lineage commitment factor for CD4+CD25+ Tregs (11). In mice, expression of Foxp3 seems to be restricted to T cells with regulatory activity. CD4+CD25+ Treg cells expressing Foxp3 basically full into two groups: those that are pre-existent and those induced in response to the disease situations (9,10). The former CD4+Foxp3+ Treg cells arise both in the thymus [natural Treg (nTreg) cells], whereas the latter CD4+Foxp3+ Treg cells are believed to be differentiated at the periphery [inducible Treg (iTreg) cells] as a consequence of exposure to Ags (9,10). Numerous studies have proposed and demonstrated that nTreg cells are generated in the thymus through MHC class II-dependent T cell receptor (TCR) interactions resulting in high avidity selection (12,13), although additional selection mechanisms may take place. In recent years it has become evident that Foxp3+ Treg cells can also be generated outside the thymus under a variety of conditions (9,10). However, little is known about the response of Treg during exposure to foreign Ag. In organ-specific autoimmune disease, the tissue inflammation and immunopathology are driven by autoreactive effector T cells that infiltrate the target tissue. It is believed that Treg can also expand in an Ag-specific manner and traffic to the target tissue (14). Thus, understanding the functions and features of expanded CD4+Foxp3+ iTreg cells during exposure to foreign Ags may be crucial for manipulating immunopathologic diseases caused by acute and chronic infections. However, before characterization of CD4+Foxp3+ iTreg specific for foreign Ags, appropriate detection tools for foreign Ag-specific CD4+Foxp3+ iTreg cells must be developed.
Analysis of Ag-specific T cells by flow cytometry using fluorescent oligomers of major histocompatibility complex (MHC)-peptide complexes, a technique known as 'MHC tetramer staining', was introduced in 1996 by Altman et al. (15). The procedure, little changed since the original report, allows for the identification of T cells of interest on the basis of the binding specificity of their cell surface receptors for particular MHC-peptide complexes (16-18). Studies using MHC class I tetramers have demonstrated that current methods for enumerating Ag-reactive CD8+ T cells vastly underestimate the size of the Ag-specific, virally induced T cell repertoire (17,18). However, broad application of this powerful technology to CD4+ T cells beyond a few well-defined test systems has been slow because of the difficulty in producing class II MHC-based reagents, a low frequency of CD4+ T cells of interest in many biological contexts, and complications caused by low T-cell receptor (TCR)-MHC avidity.
While recently activated CD4+ T cells express CD154 (CD40L) which provides costimulatory signals to activate B cells and Ag presenting cells (APCs), resting CD4+ T cells do not express CD154. Therefore, de novo synthesized CD154 can be used to identify Ag-specific CD4+ T cells in response to Ag stimulation (19-21). The intracellular CD154 assay is also compatible with intracellular cytokine staining, by which this assay may provide additional information about Ag-elicited CD4 function by quantifying cells that are capable of stimulating APCs through CD40. Most importantly, the intracellular CD154 assay provides a simple means to isolate viable Ag-specific CD4+ T cells. Furthermore, while few number of peptide-MHC class II multimers are available, and knowledge of the subject's MHC haplotype is required, intracellular CD154 assay does not necessarily identify functionally responsive cells. Recent studies found that CD4+CD25+Foxp3+ Treg cells have feature of Th17 cells at the various conditions. Thus, the unrestricted population of CD4+CD25+Foxp3+ Treg cells has extended concept and has led to a greater appreciation of functional plasticity. Therefore, in this study we attempted to detect foreign Ag-specific CD4+Foxp3+ iTregs using LCMV infection model. To this end, we employed two tools for detecting foreign Ag-specific CD4+Foxp3+ iTreg cells, including MHC class II tetramer staining and intracellular CD154 staining methods, and compared the abilities of two tools to characterize foreign Ag-specific CD4+Foxp3+ iTreg cells.
MATERIALS AND METHODS
Animals and ethic statement
C57BL/6 (H-2b) mice (6 to 8-week old) were purchased from Samtako Co. (O-san, Korea). Foxp3GFP knock-in mice (H-2b), which co-express EGFP and the regulatory T cell-specific transcription factor Foxp3 under the control of the endogenous promoter, were obtained from The Jackson Laboratory (Sacramento, CA). Both of mouse stains were bred and maintained in accredited facilities at the Chonbuk National University. All experimental procedures were pre-approved by the Institutional Animal Care and Use Committees (IACUC), Chonbuk National University (Permission code 2013-0040), and adhered to the guidelines set by Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). The animal facility of the Chonbuk National University is fully accredited by the Korea Association for Laboratory Animal Sciences (KALAS).
Viruses and animal infection
Lymphocytic choriomeningitis virus (LCMV) Armstrong (Arms) and Clone 13 (Cl13) strains were kindly provided by Dr. Sang Jun Ha (Yeonse University, Korea). LCMV were propagated in BHK-21 cells for 48 h using DEM supplemented with 2.5% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 U/ml). BHK-21 cultures were infected with LCMV at a multiplicity of infection of 0.01, and then incubated in a humidified CO2 incubator for 1 h at 37℃. After absorption, the 10 ml of maintenance medium containing 2.5% fetal bovine serum was added. Approximately 48 h after infection, culture media of infected BHK-21 cells were harvested and used for virus titration by plaque formation on Vero cells. The virus stocks were stored in aliquots at -80℃ until use. C57BL/6 and Foxp3GFP knock-in mice were infected intravenously (i.v.) with 2×105 PFU of LCMV Arms or 2×106 PFU of LCMV Cl13.
Reagents and antibodies
Several mAbs were obtained from eBioscience or BD Biosciences (San Diego, CA) for FACS analysis and other experiments; fluorescein isothiocyanate (FITC) conjugated-antimouse-CD4 (RM4-5), phycoerythrin (PE) conjugated-antimouse-CD154 (MR1), CD4 (RM4-5), peridinin chlorophyll protein complex (PerCP) conjugated-anti-mouse-CD4 (RM4-5), F4/80 (BM8), IFN-γ (XMG1.2), and allophycocyanin (APC) conjugated-anti-mouse-TNF-α (MP6-XT22). I-Ab MHC class II tetramer complexes that were refolded with synthetic LCMV GP66-77, GP126-140 and GP6-20 epitope peptides and subsequently conjugated with allophycocyalnin (APC) were kindly provided by NIH Tetramer Core Facility (Emory University, Atlanta, GA). LCMV GP66-77 (DIYKGVYQFKSV, DIY), GP126-140 (TSAFNKKTFDHTLMS, TSA), and GP6-20 (TMFEALPHIIDEVIN, TMF) epitope peptides (22,23) were chemically synthesized at Peptron Inc. (Daejeon, Korea).
MHC class II tetramer staining for detecting LCMV-specific CD4+ T cells
To detect LCMV Ag-specific CD4+ T and CD4+Foxp3+ Treg cells by using MHC class II tetramer staining, three-color fluorescent staining was performed. Briefly, splenocytes (2×106 cells) prepared from mice infected with LCMV were incubated with each APC-labeled MHC class II tetramers (I-Ab/DIY, I-Ab/TSA, and I-Ab/TMF) in RPMI1640 medium containing 2% FBS for 3 h at 37℃, followed by fixation with 0.2% formaldehyde fixation buffer for 10 min at 4℃. After staining with appropriate amount of PE-CD4 and PerCP-F4/80 antibodies for 20 min at 4℃, cells were washed twice with phosphate buffered saline (PBS). Flow cytometric acquisition of multiple parameters was performed on a Becton Dickinson FACSCalibur (Becton Dickinson, SanJose, CA, USA). Appropriate MHC class II tetramer controls (I-Ab/CLIP) were used in all experiments. Data were analyzed using FlowJo version 7.6.5 software (Tree Star, San Carlos, CA).
Intracellular CD154 and cytokines staining for detecting viable LCMV-specific CD4+ T cells
To detect viable CD4+ T cells specific for epitope peptide derived from LCMV, intracellular CD154 staining were used together with intracellular cytokine staining, as previously described (19-21). Briefly, cells (2×106 cells/well) were resuspended in RPMI1640 media with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100µg/ml streptomycin (all from Gibco) in 96-well U-bottomed plate. After stimulation with LCMV-derived peptide epitopes (1µg/ml) in the presence of PE-CD154 antibodies for 12 h at 37℃, the intracellular accumulation of cytokines was facilitated by the addition of monensin (2µM). Monensin was added to the Ag-stimulated cells 6 h before harvest. The cells were then washed twice with PBS, and surface stained for FITC-CD4 antibodies for 20 min at 4℃, after which they were washed twice with 1 ml of PBS containing monensin. After fixation, the cells were washed twice permeabilization buffer (eBioscience, San Diego, CA) and then stained intracellularly with PerCP-IFN-γ and APC-TNF-α antibodies in permeabilization buffer for 30 min at room temperature. Finally, the cells were washed twice with 1 ml of PBS and applied to flow cytomeric analysis.
RESULTS
Detection of foreign Ag-specific CD4+Foxp3+ iTregs by MHC class II tetramer staining
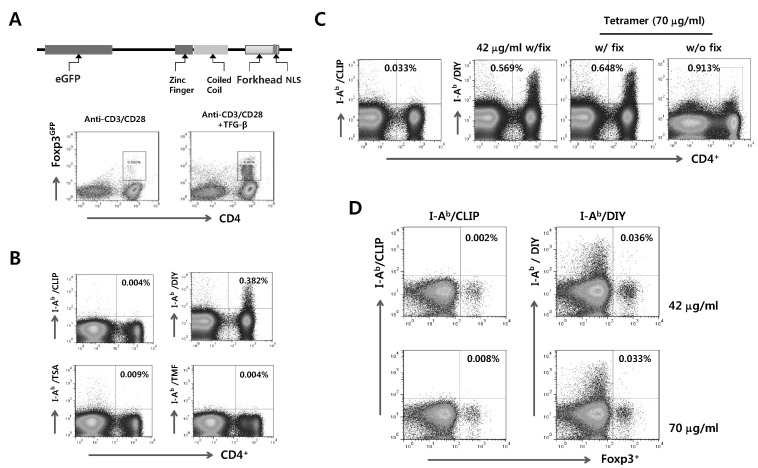
Foxp3GFP knock-in mice, which are designed to co-express EGFP and the Treg-specific transcription factor Foxp3 under the control of the endogenous promoter (Fig. 1A), have been used to characterize the differentiation of CD4+Foxp3+ Treg under various conditions. Using Foxp3GFP knock-in mice, we addressed de novo expression of transcription factor Foxp3 in sorted CD4+Foxp3- Th cells by stimulation of anti-CD3 and CD28 antibodies in the presence of TGF-β, known as a main inducer of CD4+Foxp3+ Treg cells. CD4+Foxp3- Th cells were observed to express Foxp3 molecules with 72 h-stimulation of anti-CD3 and CD28 antibodies in the presence of TGF-β (Fig. 1A), which indicated that Foxp3GFP knock-in mice could be useful for detecting and characterizing CD4+Foxp3+ nTreg and iTreg cells. Using this Foxp3GFP knock-in mouse system, we tried to detect foreign Ag-specific CD4+Foxp3+ Treg cells by using MHC class II tetramer staining method in LCMV infection model. C57BL/6 mice were infected i.v. with LCMV Arms, and the frequency of CD4+ T cells specific for three epitope, GP66-77 (DIY), GP126-140 (TSA), and GP6-20 (TMF), were detected by MHC class II tetramer (I-Ab/DIY, I-Ab/TSA, and I-Ab/TMF) (Fig. 2B). Data revealed that I-Ab/TSA and I-Ab/TMF-specific CD4+ T cells were detected at very low frequencies, whereas I-Ab/DIY-specific CD4+ T cells showed a higher frequency compared to other epitope-specific CD4+ T cells. This indicates that LCMV GP66-77 (DIY) is immune-dominant epitope of CD4+ T cell responses in LCMV Arms infection. Next, we tried to detect LCMV-specific CD4+ T cells under various conditions using I-Ab/DIY to find optimal condition of MHC class II tetramer staining. Staining with MHC class II teramer I-Ab/DIY (42 and 70 ug/ml) followed by fixation and surface CD4+ staining showed a comparable detection frequency of LCMV GP66-77-specific CD4+ T cells, but surface CD4 staining without fixation after MHC class II tetramer (I-Ab/DIY) staining showed a significant loss of LCMV GP66-77-specific CD4+ T cells (Fig. 1C). Thus, surface staining of CD4 and other molecules after MHC class II tetramer staining followed by fixation showed better results to detect LCMV GP66-77-specific CD4+ T cells.
Figure 1.
Detection of LCMV Ag-specific CD4+Foxp3+ iTreg by MHC class II tetramer staining. (A) Diagram of Foxp3GFP and iTreg detection. CD4+Foxp3- T cells sorted from Foxp3GFP knock-in mice were stimulated with anti-CD3/CD28 in the presence or absence of TGF-β for 72 h. The conversion of CD4+Foxp3- Th cells to CD4+Foxp3+ iTregs was evaluated by the expression of GFP fused by Foxp3 molecule. (B) Detection of LCMV-specific CD4+Foxp3+ iTreg specific for three epitopes. Foxp3GFP knock-in mice that had been previously infected with LCMV Armstrong (Arms) were sacrificed to prepare the splenocytes 7 days pi, and the levels of CD4+ T cells specific for different epitopes of LCMV Ag were detected by each MHC class II tetramer staining (I-Ab/DIY, I-Ab/TSA, and I-Ab/TMF). MHC class II tetramer (I-Ab/CLIP) was used for negative control. (C) Opimization of condition to detect LCMV GP66-77-specific CD4+Foxp3+ iTreg. Staining conditions for MHC class II tetramer included I-Ab/CLIP, I-Ab/DIY (42 µg/ml), and I-Ab/DIY (70 µg/ml). After staining with MHC class II tetramer, cells were fixed, stained with anti-CD40 and F4/80 antibody. Some cells were stained by anti-CD4 and F4/80 antibody without fixation. The values in dot-plot denote the average percentage of detected CD4+I-Ab/DIY+ T cells after gating on F4/80-negative cells. (D) Detection of LCMV GP66-77-specific CD4+Foxp3+ iTregs by MHC class II tetramer staining. The splenocytes from LCMV-infected Foxp3GFP knock-in mice were prepared 7 days pi and used for MHC class II tetramer (I-Ab/DIY, 42 and 70 µg/ml) staining to detect CD4+Foxp3+ Treg cells specific for LCMV GP66-77 epitope. The values in dot-plot denote the average percentage of Foxp3+I-Ab/DIY+ gated on CD4+ T cells derived from three independent experiments.
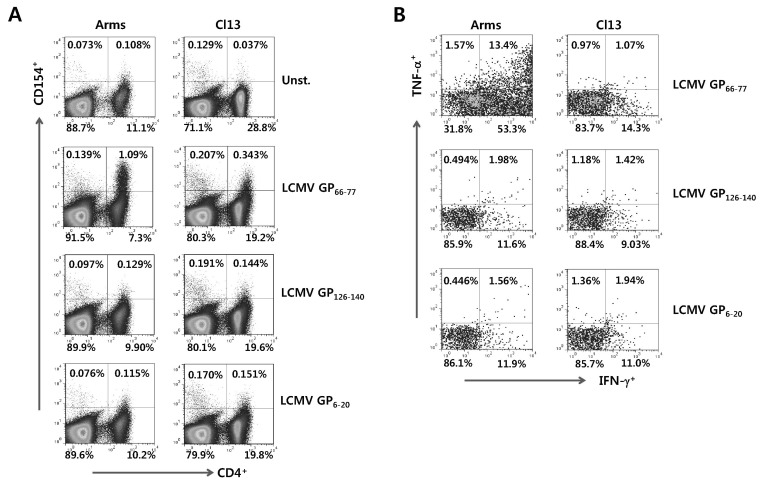
Figure 2.
Detection of LCMV-specific CD4+ T cells by intracellular CD154 staining. (A) Detection of viable LCMV-specific CD4+ T cells by intracellular CD154 staining. The splenocytes from mice infected with LCMV Arms or Cl13 were prepared 7 days pi and simulated with each specific epitope peptide (LCMV GP66-77, GP126-140, and GP6-20) in presence of PE-conjugated anti-CD154 antibody for 12 h. Splenocytes that was not stimulated with peptide in the presence of PE-conjugated anti-CD154 antibody were used for negative control. The values in dot-plot represent the percentage of CD4+ T cells specific for each LCMV epitope peptide. (B) The profile of IFN-γ and TNF-α expression in LCMV Ag-specific CD4+ T cells detected by intracellular CD154 staining. Following 12 h-stimulation of each epitope peptide in the presence of PE-conjugated CD154 antibody, the cells were stained with anti-CD4 antibody and the expression of IFN-γ and TNF-α in CD154+CD4+ T cells was determined by intracellular cytokine staining. The values in dot-plot represent the average percentage of IFN-γ and TNF-α in CD154+CD4+ T cells.
MHC class II tetramer I-Ab/DIY staining, which was established in the preliminary experiment, was employed to detect LCMV GP66-77-specific CD4+Foxp3+ iTreg in LCMV-infected Foxp3GFP knock-in mice. Two different amounts of MHC class II tetramer (I-Ab/DIY, 42 and 70 ug/ml) were used to detect LCMV GP66-77-specific CD4+Foxp3+ iTregs (Fig. 1D). Staining with I-Ab/DIY MHC class II tetramer (42 and 70 ug/ml) showed similar frequencies of LCMV GP66-77-specific CD4+Foxp3+ iTreg cells (0.036% and 0.033%, respectively). Also, I-Ab/CLIP MHC class II tetramer (42 and 70 ug/ml) used as a negative control showed detection rates of 0.002% and 0.008%, respectively. These results indicate that LCMV GP66-77-specific CD4+Foxp3+ iTreg cells could be detected by combined staining with MHC class II tetramer I-Ab/DIY and CD4 antibody, although staining with MHC class II tetramer (I-Ab/DIY) showed a low detection rate of LCMV GP66-77-specific CD4+Foxp3+ Tregs.
Detection of foreign Ag-specific CD4+CD154+ Foxp3+ iTreg
However, staining with MHC class II tetramer to detect CD4+Foxp3+ iTregs may be not useful for functional analysis because most of MHC class II tetramers were unbound when performed intracellular cytokine staining (Data not shown). For this reason, we introduced an intracellular CD154 staining method to detect CD4+Foxp3+ iTregs specific for LCMV GP66-77 epitope. This method is thought to be the most relevant to our experimental purpose, allowing for functional analysis due to the detection of viable Ag-specific CD4+ T cells by intracellular CD154 staining. Therefore, we accessed optimal staining strategies of intracellular CD154 staining method to detect CD4+ T as well as CD4+Foxp3+ Treg cells specific for LCMV GP66-77 epitope. We first detected LCMV-specific CD4+ T cells by intracellular CD154 staining in C57BL/6 mice infected with LCMV Arms or Cl13. After 7 days pi, we measured the frequency of LCMV GP66-77, GP126-140, and GP6-20-specific CD4+ T cells by 12 h-stimulation with corresponding epitope peptide in the presence of PE-conjugated CD154 antibody (Fig. 2A). Data indicate that LCMV GP126-140 and GP6-20-specific CD4+ T cells were detected at a very low frequency, whereas LCMV GP66-77-specific CD4+ T cells were detected with higher frequency, as revealed that LCMV GP66-77 epitope was immune-dominant epitope in MHC class II tetramer staining. Since the loss of CD4+ T cell function was induced during chronic LCMV infection (24-26), we also compared GP66-77-specific CD4+ T cells detected in LCMV Arms infection to those in LCMV Cl13 infection. The higher frequency of LCMV GP66-77-specific CD4+ T cells in LCMV Arm infection at the acute phase (7 days pi) was observed. To confirm the functionality of LCMV-specific CD4+ T cells detected by intracellular CD154 staining, we examined the profiling of IFN-γ and/or TNF-α expression in LCMV-specific CD4+ T cells by performing intracellular cytokine staining with intracellular CD154 staining (Fig. 2B). When compared the expression of IFN-γ and TNF-α cytokines in LCMV Arms and Cl13, higher frequency of IFN-γ- producing CD4+ T cells specific for LCMV GP66-77 epitope was detected in LCMV Arms-infected mice, compared to LCMV Cl13-infected mice. These results imply the exhaustion of LCMV-specific CD4+ T cells in LCMV Cl13-infected mice, and suggest that cellular CD154 staining could be useful to evaluate the functionality of LCMV-specific CD4+ T cells by combined staining with intracellular cytokine staining.
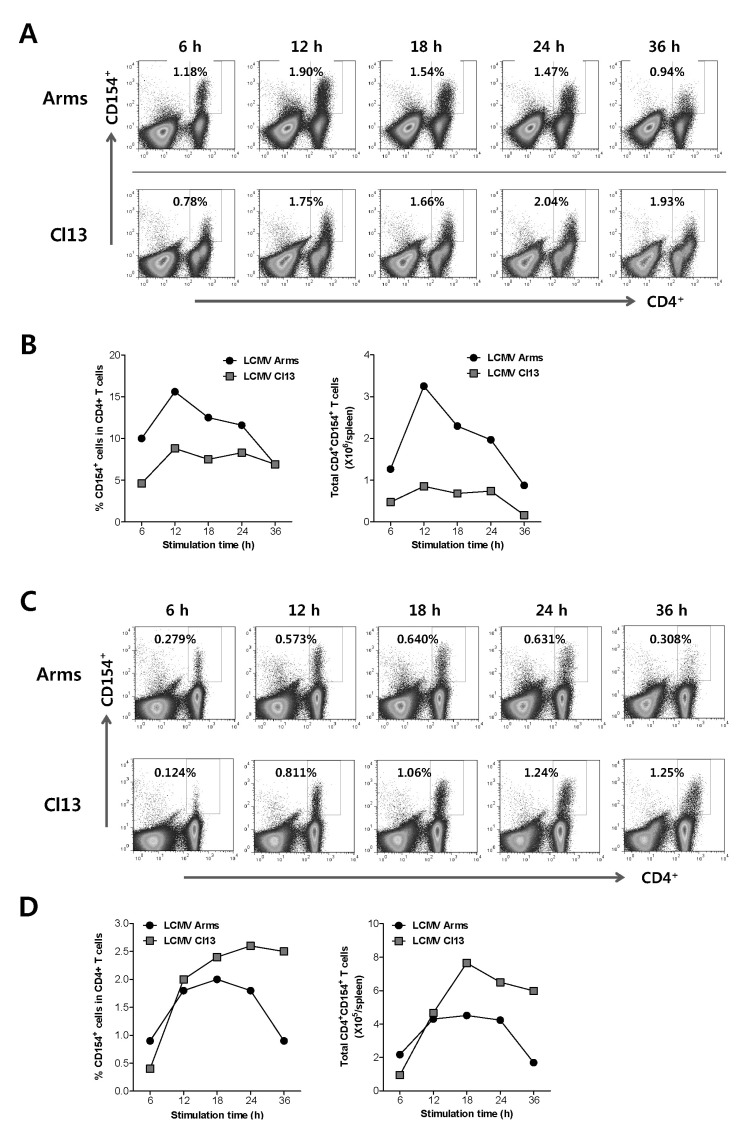
We then checked the changes in the frequency of LCMV GP66-77-specific CD4+ T cells at acute (7 days pi) and chronic phases (44 days pi) of LCMV-infected mice, depending on stimulation periods. LCMV GP66-77-specific CD4+ T cells in LCMV-infected mice 7 days pi reached a peak after 12 h-stimulation with LCMV GP66-77 epitope peptide, as shown by the frequency and absolute number of LCMV GP66-77-specific CD4+ T cells (Fig. 3A and B). However, a longer stimulation induced an increased frequency and absolute number of LCMV GP66-77-specific CD4+ T cells detected by intracellular CD154 staining when LCMV GP66-77-specific CD4+ T cells were analyzed 44 days pi (Fig. 3C and D). Thus, these results indicate that 12 h-stimulation of splenocytes prepared from LCMV-infected mice with epitope peptide can provide better results to detect LCMV-specific CD4+ T cells with intracellular CD154 staining method.
Figure 3.
Detection of LCMV GP66-77-specific CD4+CD154+Foxp3+ iTreg. (A) Detection rate of LCMV GP66-77-specific CD4+ T cells in acute LCMV infection phase, depending on stimulation period. C57BL/6 mice were infected with LCMV Armstrong (Arms) or clone 13 (Cl13), and the spelenocytes were prepared 7 days pi and used for stimulation with LCMV GP66-77 epitope peptide in the presence of PE-conjugated anti-CD154 antibody for various periods. The values in dot-plot denote the percentage of CD4+CD154+ T cells specific for LCMV GP66-77 epitope peptide. (B) The frequency and absolute number of CD4+CD154+ T cells specific for LCMV GP66-77 epitope peptide in acute LCMV infection phase. The graphs represent the average percentage and absolute number of LCMV GP66-77-specific CD4+CD154+ T cells at the indicated stimulation time point. (C) Detection rate of LCMV GP66-77-specific CD4+ T cells in chronic LCMV infection phase, depending on stimulation period. C57BL/6 mice were infected with LCMV Armstrong (Arms) or clone 13 (Cl13), and the splenocytes were prepared 44 days pi and used for stimulation with LCMV GP66-77 epitope peptide in the presence of PE-conjugated anti-CD154 antibody for various periods. The values in dot-plot denote the percentage of CD4+CD154+ T cells specific for LCMV GP66-77 epitope peptide. (D) The frequency and absolute number of CD4+CD154+ T cells specific for LCMV GP66-77 epitope peptide in chronic LCMV infection phase. The graphs represent the average percentage and absolute number of LCMV GP66-77-specific CD4+CD154+ T cells at the indicated stimulation time point.
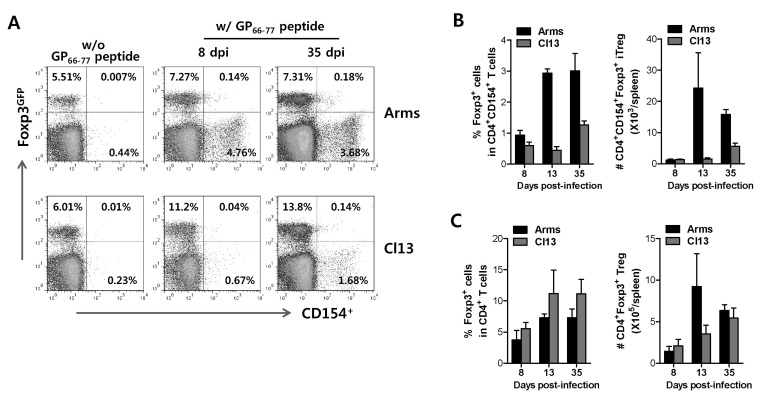
Differential expansion of foreign Ag-specific CD4+ CD154+Foxp3+ iTreg and CD4+Foxp3+ Treg in acute and chronic LCMV infection
Using intracellular CD154 staining, we determined the frequency of LCMV GP66-77-specific CD4+CD154+Foxp3+ iTreg cells at acute (8 and 13 days pi) and chronic phase (35 days pi) in Foxp3GFP knock-in mice that were previously infected with LCMV Arms or Cl13. C57BL/6 mice infected with LCMV Arms showed a lower frequency of polyclonal CD4+Foxp3+ Treg cells at the acute phase, compared to mice infected with LCMV Cl13 (Fig. 4A). In contrast, CD4+CD154+Foxp3+ iTreg cells were observed at a higher frequency in LCMV Arms-infected mice at acute phase (8 and 13 days pi) as well as chronic phase (35 days pi) (Fig. 4B), and the frequency and absolute number of such LCMV GP66-77-specific CD4+CD154+Foxp3+ iTreg cells were lower in mice infected chronically with LCMV Cl13 than those of mice infected with LCMV Arms. However, the frequency of polyclonal CD4+Foxp3+ Treg cells was interestingly detected at higher levels in mice infected with LCMV Cl13, compared to those of mice infected with LCMV Arms (Fig. 4C). It is also likely that the frequency and absolute number of LCMV GP66-77-specific CD4+CD154+Foxp3+ iTreg and polyclonal CD4+Foxp3+ Treg cells in mice infected with LCMV Cl13 was gradually increased in course of time. Therefore, these results suggest that foreign Ag-specific CD4+CD154+Foxp3+ iTreg and polyclonal CD4+Foxp3+ Treg cells could elicit differential expansion pattern in mice infected with LCMV Arms or Cl13 in acute and chronic phase.
Figure 4.
Differential expansion of LCMV GP66-77-specific CD4+Foxp3+ iTreg and CD4+Foxp3+ Treg in acute and chronic infection. (A) Frequency of LCMV GP66-77-specific CD4+CD154+Foxp3+ iTreg in acute and chronic LCMV infection. Foxp3GFP knock-in mice were infected with LCMV Armstrong (Arms) or clone 13 (Cl13) and the splenocytes from LCMV-infected Foxp3GFP knock-in mice were prepared 8 and 35 days pi and used for 12 h-stimulation with LCMV GP66-77 epitope peptide in the presence of PE-conjugated anti-CD154 antibody. The values in dot-plot denote the average of CD154+Foxp3- Th, CD154-Foxp3+ Treg, CD154+Foxp3+ iTreg cells gated on CD4+ T cells (B and C) The frequency and absolute number of LCMV GP66-77-specific CD4+CD154+Foxp3+ iTreg and nonspecific CD4+Foxp3+ Treg cells. The splenocytes of infected Foxp3GFP knock-in mice were prepared 8, 13 and 35 days pi, stimulated with LCMV GP66-77 in the presence of the presence of PE-conjugated anti-CD154 antibody. The bars in graphs represent the average±SD of LCMV GP66-77-specific CD4+CD154+Foxp3+ iTregs (B) and nonspecific CD4+Foxp3+ Treg (C) detected by intracellular CD154 staining in acute and chronic phase.
DISCUSSION
In this study, we established and compared both MHC class II tetramer and intracellular CD154 staining to detect CD4+Foxp3+ iTregs specific for LCMV GP66-77 epitope in acute and chronic infections of LCMV. It was revealed that MHC class II tetramer (I-Ab/DIY) staining showed a lower detection rate than intracellular CD154 staining, and was not suitable to characterize the functionality of LCMV GP66-77-specific CD4+ T cells because most of MHC class II tetramers were revealed to be unbound and unstable when intracellular cytokine staining was performed. In contrast, intracellular CD154 staining has been shown to become easier and simpler for detecting LCMV GP66-77-specific CD4+ T cells, compared to MHC class II tetramer staining. Subsequently, we employed intracellular CD154 staining to detect LCMV GP66-77-specfic CD4+Foxp3+ iTreg using Foxp3GFP knock-in mouse, and found that LCMV GP66-77-specific CD4+Foxp3+ iTreg and polyclonal CD4+Foxp3+ Treg showed differential expansion in mice infected with LCMV Arms or Cl13. Our results provide an insight into the valuable use of intracellular CD154 staining to detect and characterize foreign Ag-specific CD4+Foxp3+ iTreg in various models including those of infection as well as autoimmune diseases.
Classically, MHC class I tetramer staining technology enabled us to characterize epitope peptide-specific CD8+ T cells at a single cell level in a variety of studies (18). Similarly, several groups have developed MHC class II tetramer staining to detect Ag-specific CD4+ T cells (17). However, the generation and use of MHC class II tetramer staining seemed more problematic those that of MHC class I tetramer staining. Moreover, to detect Ag-specific CD4+ T cells by MHC class II tetramer, the sequence of epitope peptide and character of MHC II haplotype should be revealed. Similarly, MHC class II teramer (I-Ab/DIY) staining seemed difficult to detect LCMV GP66-77-specific CD4+ T cells by sensitivity and binding stability of tetramers under staining conditions. Moreover, since intracellular cytokine staining in responses to epitope peptide GP66-77 showed a higher frequency of functional effector CD4+ T cells, it is likely that most MHC class II (I-Ab/DIY) tetramer did not bind to cognate TCR of CD4+ T cells. This may cause the underestimate of Ag-specific CD4+ T cells in vivo.
In recent years, de novo synthesized CD154 was shown to be a useful marker to identify Ag-specific CD4+ T cells in a stimulation assay (19-21). Indeed, CD154 staining was previously limited by the highly transient nature of CD154 expression (27,28), which is rapidly internalized and/or degraded after surface expression (28) or is sometimes secreted (29). To overcome this limitation, simple incorporation of fluorescently conjugated antibody to CD154 and monensin in the culture during Ag stimulation showed stable staining of CD154 molecule (21). Using this intracellular CD154 staining, we could stably detect CD4+ T cells specific for several LCMV epitope peptides. The advantage of intracellular CD154 staining is that the defined sequence of epitope peptide is not absolutely required for detecting Ag-specific CD4+ T cells. Also, this assay is compatible with intracellular cytokines staining (19-21), although it cannot be used with brefeldin A, which completely blocks surface expression of CD154. Using combined staining of intracellular CD154 with intracellular cytokine (IFN-γ and TNF-α), we found that most of LCMV GP66-77-specific CD4+ T cells were dysfunctional in mice which were chronically infected with LCMV Cl13. This result is in accordance with the generation of exhausted CD8+ T cells specific for LCMV in chronic LCMV infection (24-26). Subsequently, this intracellular CD154 staining to detect Ag-specific CD4+ T cells was subjected to detect CD4+Foxp3+ iTregs specific for LCMV GP66-77 epitope peptide in Foxp3GFP knock-in mice infected with LCMV. Interestingly, we found the different expression of Foxp3 transcription molecule in polyclonal CD4+ and LCMV GP66-77-specific CD4+CD154+ T cells at acute (8 and 13 days) and chronic (35 day) phase after infecting mice with LCMV Arms or Cl13. This result suggests that differential expansion of polyclonal and LCMV GP66-77-specific CD4+Foxp3+ iTreg could affect viral persistence during process of acute and chronic LCMV infection.
In CD4+Foxp3+ Treg study, an unresolved issue that remains is whether foreign Ag-specific CD4+Foxp3+ Treg cells are present in peripheral tissues, because such cells cannot be directly detected. A few study groups identified foreign Ag-specific CD4+ T cells in peripheral lymphoid tissues, but failed to show their presence at a single cell level (30). It is speculated that these foreign Ag-specific Tregs may originate from activated CD4+ effector T cells and are iTregs (30-32). Otherwise, foreign Ag-specific Tregs could be derived from conversion of naïve Foxp3+ nTregs into memory nTregs, in a similar fashion to the development of Ag-reactive memory CD4+ effector T cells that develop from Foxp3- naïve CD4+ T cells (33). According to a previous report, the unchanged highly demethylated TSDR Foxp3 within CD154+ Tregs is in favor of the development of foreign Ag-specific Tregs from nTregs in CMV infection, rather than the development from CD4+ effector T cells (34). However, it is uncertain whether LCMV GP66-77-specific CD4+CD154+Foxp3+ Tregs were generated from nTreg or from CD4+ effector T cells in response to Ag stimulation, because the demethylation status of Foxp3 was not assessed. One intriguing finding in this study is that the differential ratio of LCMV GP66-77-specific CD4+ Th to CD154+Foxp3+ Tregs was found in mice infected with between LCMV Arms and Cl13. i.e., approximately 0.029 and 0.059 ratio of LCMV GP66-77-specific CD4+CD154+Foxp3+ Treg to CD4+CD154+ Th at acute phase (8 days pi of LCMV Arms vs Cl13) and approximately 0.048 and 0.083 ratio of LCMV GP66-77-specific CD4+CD154+ Foxp3+ Treg to CD4+CD154+ Th at chronic phase (35 days pi of LCMV Arms vs Cl13). The proportion of LCMV GP66-77-specific CD4+CD154+Foxp3+ Treg in Ag-specific CD154+CD4+ population appeared to be gradually increased, as the duration of infection passed. The formation of Ag-specific Treg to Th in a fixed ratio between acute and chronic phases indicates that despite acute and chronic infection, foreign Ag-specific CD4+CD154+Foxp3+ Tregs may be conversely increased at the late phase of infection, in order to break the exuberant effector response. Hence, LCMV GP66-77-specific CD4+CD154+Foxp3+ Treg compartment was determined to be filled with Ag-specific CD4+CD154+ Th cells as a mirror image of the memory CD4+ effector population. Also, our results support the results from TCR repertoire analysis of effector CD4+ T and Treg cells, which did not show any evidence for affinity for autoreactive Ag (35), and the results from Vβ T cell analysis which showed that human CD4+CD25++ Tregs equally share complex and comparable repertoires with CD4+CD25- Th counterparts (36). Moreover, our results are strengthened by the result that CMV seropositivity resulted in a similar shift in Vβ repertoire in effector CD4+ Th and Treg cells (36). Direct identification of foreign Ag-specific CD4+Foxp3+ Treg at a single cell level using fine analytic tools will facilitate further Treg research and provide more complete understanding of the significance and interaction of Tregs in human disease and immune responses. Furthermore, isolation and propagation of viable foreign Ag-specific CD4+Foxp3+ Tregs are of potential benefit in future immunotherapy with CD4+Foxp3+ Treg cells.
ACKNOWLEDGEMENTS
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MISP) (2012R1A2A1A03670284 and 2013R1A4A1069486).
Abbreviations
- APC
allophycocyanin
- FITC
fluorescein isothiocyanate
- Foxp3
forkhead box protein 3
- IFN-γ
interferon-gamma
- LCMV
lymphocytic choriomeningitis virus
- MHC
major histocompatibility complex
- PE
phycoerythrin
- PerCP
peridinin chlorophyll protein complex
- TCR
T cell receptor
- TNF-α
tumor necrosis factor-alpha
- Treg
regulatory T cell
Footnotes
The authors have no financial conflict of interest.
References
- 1.Valencia X, Lipsky PE. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol. 2007;3:619–626. doi: 10.1038/ncprheum0624. [DOI] [PubMed] [Google Scholar]
- 2.Huan J, Culbertson N, Spencer L, Bartholomew R, Burrows GG, Chou YK, Bourdette D, Ziegler SF, Offner H, Vandenbark AA. Decreased Foxp3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 3.Xu W, Lan Q, Chen M, Chen H, Zhu N, Zhou X, Wang J, Fan H, Yan CS, Kuang JL, Warburton D, Togbe D, Ryffel B, Zheng SG, Shi W. Adoptive transfer of induced-Treg cells effectively attenuates murine airway allergic inflammation. PLoS One. 2012;7:e40314. doi: 10.1371/journal.pone.0040314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adeegbe D, Levy RB, Malek TR. Allogeneic T regulatory cell-mediated transplantation tolerance in adoptive therapy depends on dominant peripheral suppression and central tolerance. Blood. 2010;115:1932–1940. doi: 10.1182/blood-2009-08-238584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y, Liang W, Thomson AW, Chen J, Hu X. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol. 2012 doi: 10.1002/ana.23815. in press: doi: 10.1002/ana.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y, Xu W, Xiong S. Adoptive Transfer of Regulatory T Cells Protects against Coxsackievirus B3-Induced Cardiac Fibrosis. PLoS One. 2013;8:e74955. doi: 10.1371/journal.pone.0074955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasconcelos JF, Souza BS, Lins TF, Garcia LM, Kaneto CM, Sampaio GP, de Alcantara AC, Meira CS, Macambira SG, Ribeiro-Dos-Santos R, Soares MB. Administration of granulocyte colony-stimulating factor induces immunomodulation, recruitment of T regulatory cells, reduction of myocarditis and decrease of parasite load in a mouse model of chronic Chagas disease cardiomyopathy. FASEB J. 2013 doi: 10.1096/fj.13-229351. in press: doi: 10.1096/fj.13-229351. [DOI] [PubMed] [Google Scholar]
- 8.Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Gobel K, Schuhmann MK, Langhauser F, Helluy X, Schwarz T, Bittner S, Mayer CT, Brede M, Varallyay C, Pham M, Bendszus M, Jakob P, Magnus T, Meuth SG, Iwakura Y, Zernecke A, Sparwasser T, Nieswandt B, Stoll G, Wiendl H. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121:679–691. doi: 10.1182/blood-2012-04-426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav M, Stephan S, Bluestone JA. Peripherally induced tregs - role in immune homeostasis and autoimmunity. Front Immunol. 2013;4:232. doi: 10.3389/fimmu.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 11.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci USA. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pacholczyk R, Kern J. The T-cell receptor repertoire of regulatory T cells. Immunology. 2008;125:450–458. doi: 10.1111/j.1365-2567.2008.02992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song KD, Hwang S, Yun CH. T cell receptor signaling that regulates the development of intrathymic natural regulatory T cells. Immune Netw. 2011;11:336–341. doi: 10.4110/in.2011.11.6.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 16.Krueger LA, Nugent CT, Hampl J. Identification of human antigen-specific T cells using MHC class I and class II tetramers. Curr Protoc Cytom. 2004;Chapter 6:Unit 6.18. doi: 10.1002/0471142956.cy0618s30. [DOI] [PubMed] [Google Scholar]
- 17.Cecconi V, Moro M, Del Mare S, Dellabona P, Casorati G. Use of MHC class II tetramers to investigate CD4+ T cell responses: problems and solutions. Cytometry A. 2008;73:1010–1018. doi: 10.1002/cyto.a.20603. [DOI] [PubMed] [Google Scholar]
- 18.Guillaume P, Dojcinovic D, Luescher IF. Soluble MHC-peptide complexes: tools for the monitoring of T cell responses in clinical trials and basic research. Cancer Immun. 2009;9:7–12. [PMC free article] [PubMed] [Google Scholar]
- 19.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005;11:1113–1117. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 20.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A, Thiel A. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 21.Chattopadhyay PK, Yu J, Roederer M. Live-cell assay to detect antigen-specific CD4+ T-cell responses by CD154 expression. Nat Protoc. 2006;1:1–6. doi: 10.1038/nprot.2006.1. [DOI] [PubMed] [Google Scholar]
- 22.Holst PJ, Christensen JP, Thomsen AR. Vaccination against lymphocytic choriomeningitis virus infection in MHC class II-deficient mice. J Immunol. 2011;186:3997–4007. doi: 10.4049/jimmunol.1001251. [DOI] [PubMed] [Google Scholar]
- 23.McDermott DS, Varga SM. Quantifying antigen-specific CD4 T cells during a viral infection: CD4 T cell responses are larger than we think. J Immunol. 2011;187:5568–5576. doi: 10.4049/jimmunol.1102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 25.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Zinselmeyer BH, Heydari S, Sacristan C, Nayak D, Cammer M, Herz J, Cheng X, Davis SJ, Dustin ML, McGavern DB. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med. 2013;210:757–774. doi: 10.1084/jem.20121416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- 28.Yellin MJ, Sippel K, Inghirami G, Covey LR, Lee JJ, Sinning J, Clark EA, Chess L, Lederman S. CD40 molecules induce down-modulation and endocytosis of T cell surface T cell-B cell activating molecule/CD40-L. Potential role in regulating helper effector function. J Immunol. 1994;152:598–608. [PubMed] [Google Scholar]
- 29.Graf D, Muller S, Korthauer U, van Kooten C, Weise C, Kroczek RA. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur J Immunol. 1995;25:1749–1754. doi: 10.1002/eji.1830250639. [DOI] [PubMed] [Google Scholar]
- 30.Hartigan-O'Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, Olek S, Dietmaier W, Andreesen R, Edinger M. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39:1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 33.Vukmanovic-Stejic M, Agius E, Booth N, Dunne PJ, Lacy KE, Reed JR, Sobande TO, Kissane S, Salmon M, Rustin MH, Akbar AN. The kinetics of CD4+Foxp3+ T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J Clin Invest. 2008;118:3639–3650. doi: 10.1172/JCI35834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Litjens NH, Boer K, Betjes MG. Identification of circulating human antigen-reactive CD4+ FOXP3+ natural regulatory T cells. J Immunol. 2012;188:1083–1090. doi: 10.4049/jimmunol.1101974. [DOI] [PubMed] [Google Scholar]
- 35.Pacholczyk R, Kern J, Singh N, Iwashima M, Kraj P, Ignatowicz L. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity. 2007;27:493–504. doi: 10.1016/j.immuni.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasow KA, Chen X, Knowles J, Wichlan D, Handgretinger R, Riberdy JM. Human CD4+CD25+ regulatory T cells share equally complex and comparable repertoires with CD4+CD25- counterparts. J Immunol. 2004;172:6123–6128. doi: 10.4049/jimmunol.172.10.6123. [DOI] [PubMed] [Google Scholar]