Abstract
The pro-inflammatory cytokines tumor necrosis factor-α (TNFα) and interleukin (IL)-1β are crucial mediators involved in chronic inflammatory diseases. Inflammatory signal pathways regulate inflammatory cytokine expression-mediated by p38 mitogen activated protein kinase (p38MAPK). Therefore, considerable attention has been given to p38MAPK as a target molecule for the development of a novel anti-inflammatory therapeutics. BIRB 796, one of p38MAPK inhibitor, is a candidate of therapeutic drug for chronic inflammatory diseases. In this study, we investigated the effect of BIRB 796 on inflammatory cytokine productions by lipopolysaccharide (LPS) in different immune cell types. BIRB 796 reduced LPS-mediated IL-8 production in THP-1 cells but not in Raw 264.7 cells. Further analysis of signal molecules by western blot revealed that BIRB 796 sufficiently suppressed LPS-mediated phosphorylation of p38MAPK in both cell types whereas it failed to block inhibitor of kappa B (I-κB) degradation in Raw 264.7 cells. Taken together, these results suggest that the anti-inflammatory function of BIRB 796 depends on cell types.
Keywords: p38MAPK, BIRB 796, Inflammatory cytokine, Inflammatory signal pathways, LPS
INTRODUCTION
The pro-inflammatory cytokines including tumor necrosis factor-α (TNFα) and interleukin-1β (IL-1β) mediate the inflammatory responses-associated with the immunological recognition of antigens. Abnormal levels of pro-inflammatory cytokines are related to several diseases of autoimmunity, such as toxic shock syndrome, rheumatoid arthritis, diabetes, and inflammatory bowel disease (1). A key for potential drug intervention in these diseases is the reduction of pro-inflammatory cytokines. For example, clinical efficacy has been demonstrated with a monoclonal antibody directed against TNFα and IL-1α/β for the treatment of rheumatoid arthritis (2-4).
The p38 Ser/Thr mitogen activated protein kinase (MAPK) is a serine/threonine kinase originally identified as an enzyme in monocytes that is phosphorylated and activated by lipopolysaccharide (LPS) (5). The p38MAPK pathway transmits extracellular signals resulted in phosphorylation of transcription factors leading to alterations in inflammatory gene expression (6). The p38MAPK is practically associated with enhanced cell survival and activated by cytokine, growth factors, and a various cell stresses (7). One of representative compound SK&F 86002 (6-(4-Fluorophenyl)-5-(4-pyridyl)-2,3-dihydroimidazo[2,1-b]-thiazole) has no significant effect on DNA/RNA or protein synthesis whereas its specific inhibitory activity on IL-1 production in target cells (8). The SK&F 86002, an inhibitor of p38MAPK, was intensively searched by the pharmaceutical companies to identify potent and efficient p38MAPK inhibitors. These investigations expanded not only in chemical structures but also how the compounds interact with the target molecule. Subsequently, several compounds have progressed to clinical trials however have dropped out for various reasons such as efficacy and toxicity.
A new class of p38MAPK inhibitor BIRB 796 is one of the most potent compound of this series and it binds to p38MAPK with high affinity (9). BIRB796 inhibits p38MAPK by a novel mechanism: it indirectly competes with the binding of ATP. Previous study demonstrated that BIRB 796 suppresses endotoxin (LPS)-mediated TNFα production and prevent mouse model of collagen-induced arthritis (9). BIRB 796 also displayed anti-inflammatory effects on a trial of human endotoxemia and it has been in phase IIb/III clinical trials for treatment of rheumatoid arthritis (9-12).
The activation of p38MAPK occurs through inflammatory stimuli via receptors of Toll-like and IL-1 receptor family including TNF receptor family. However, the pathways are ubiquitous and also activated by growth factors, G-protein coupled receptors, immune receptors, and other stimuli (13,14). Thus it is uncertain that p38MAPK would be appropriate target to inhibit inflammatory cytokines. Though numerous studies have shown anti-inflammatory activities of BIRB 796, its role in inflammatory cytokine production in different cell types is still unclear. Therefore, we investigated the direct effect of BIRB 796 on mouse Raw 264.7 macrophages and human THP-1 monocytes to elucidate a possible distinctive effect of BRIB 796 signal pathways involved in inflammatory cytokine productions.
MATERIALS AND METHODS
Protein kinase inhibitors
The compound 1-5-tert-butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2-morpholin-4-yl-ethoxy)naphthalen-1-yl]urea (BIRB 796, Millipore Corporation, Billerica, MA) and mono[4-[[1-[[3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl]thio]-1-methylethyl]thio]-2,6-bis (1,1-dimethylethyl)phenyl] ester (AGI 1067, Sigma-Aldrich, St. Louis, MO) were purchased. The compounds were diluted in dimethyl sulphoxide (DMSO; 1 mM of stock solution) and kept at 20℃ until use for cell assays.
Cell culture and LPS-induced cytokine assays
Raw 264.7 and THP-1 cells were cultured in RPIM 1640 medium at 37℃, supplemented with 10% fetal calf serum, 50 units of penicillin/ml, 50µg/ml streptomycin (Invitrogen, Carlsbad, CA) in a humidified atmosphere containing 5% CO2 at 37℃. Raw 264.7 and THP-1 cells were pretreated with the compounds prior to stimulation of LPS at a final concentration of 10 ng/ml for Raw 264.7 or 1µg/ml for THP-1 cells then the cells were further incubated for 16 hrs at 37℃ in a humidified incubator containing 5% CO2. After stimulation, levels of inflammatory cytokines in the culture supernatant were assessed by using specific sandwich enzyme-linked immunosorbent assay (ELISA) kit (R&D systems, Minneapolis, MN). Color reaction was developed with 3, 3', 5, 5'-Tetramethyl-benzidine (TMB) Liquid substrate (Sigma-Aldrich), and stopped with 0.1 molar of H2SO4. The absorbance at 450 nm was measured using an ELISA plate reader (Molecular Devices, Orleans, CA).
RT-PCR
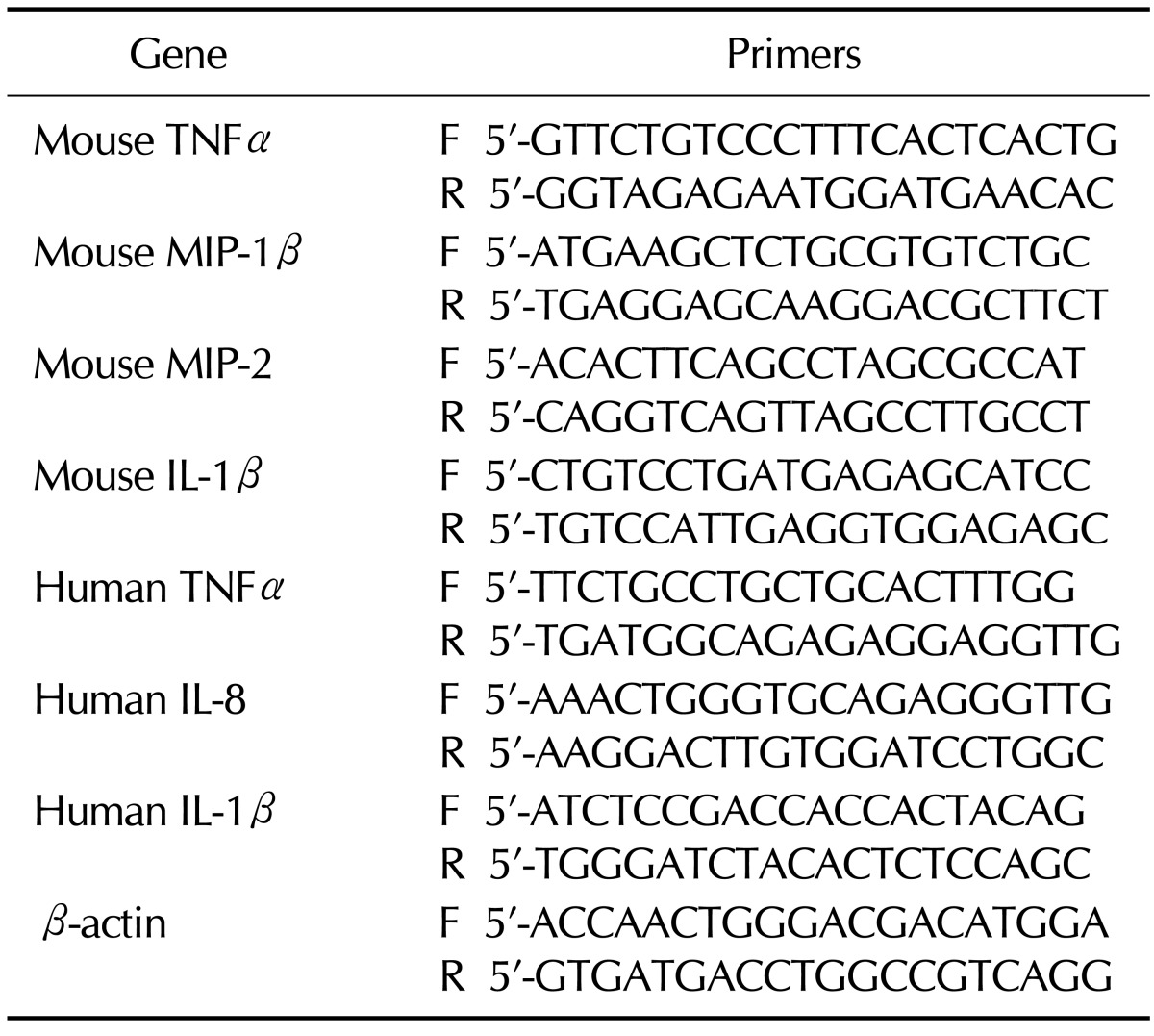
For RT-PCR, Raw 264.7 and THP-1 cells (1×106 cells) in 6 well-plates were pretreated with the compounds (10µM) for 1 h at 37℃ and then the cells were stimulated with Escherichia coli O55:B5 LPS (Sigma-Aldrich) at a final concentration as described above. Total RNA was isolated with Tri-Reagent (Sigma-Aldrich) from the cells after for 4 hrs stimulation. MMLV-RT (Beams Bio, Korea) was used for converting 2µg of total RNA to first strand cDNA, and then PCR reaction was performed at 94℃ for 45 s, 70℃ for 2 min, and 59℃ for 1 min for 30 cycles. The different pair of primers for several cytokines and chemokines for human and mouse was designed on the basis of published gene sequences as reported in Table I and beta-actin. PCR products were electrophoresed and exposed to UV imager for photograph.
Table I.
Specific primers for cytokines RT-PCR
Western blot
For detecting the phosphorylation of p38MAPK in THP-1 and Raw 264.7 cells, the cells were stimulated with LPS and the compounds as described above for 30 minutes. Cells were lysed with kinase lysis buffer (Cell Signaling Technology, Beverly, MA) then subjected to 10% SDS-PAGE. The samples were transferred to nitrocellulose membranes. The membranes were blocked in 3% BSA/TBST (Santa Cruz Biotechnology, Santa Cruz, CA) then probed first with rabbit anti-phospho-p38MAPK (Cell Signaling Technology). The membranes were reprobed with rabbit anti-I-κB (Santa Cruz Biotechnology). For normalization of each protein, the membranes were probed finally with goat anti-actin (Santa Cruz Biotechnology).
RESULTS
BIRB 796 inhibited LPS-induced cytokine secretion in THP-1 cells but not in Raw 264.7 cells
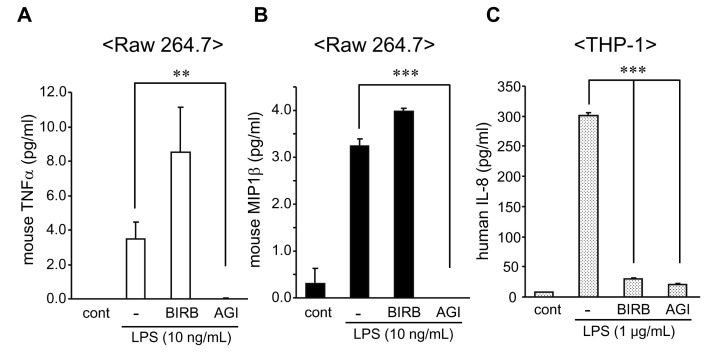
We investigated the effect of BIRB 796 and AGI 1067 on the production of inflammatory cytokine induced by LPS in different cell lines using quantitative cytokine assay. We first examined the levels of LPS-induced cytokines in Raw 264.7 and THP-1 cells pre-treated with BIRB 796 and AGI 1067. AGI 1067 is a metabolically stable derivative of the antioxidant drug. It is a member of a novel class of orally active, antioxidant, anti-inflammatory compounds termed vascular protectants and exhibits anti-atherosclerotic properties in multiple animal models and in humans (15). The level of cytokines in culture supernatants were assessed by sandwich ELISA kits. As shown in Fig. 1, AGI 1067 sufficiently blocked the secretion of mouse TNFα and MIP-1β as well as human IL-8 in the cultured medium. The inhibitory effect of BIRB 796 was observed only in THP-1 cells (Fig. 1C). Unlike the suppression of IL-8 production in THP-1 cells (Fig. 1C), there were no inhibition of MIP-1β and TNFα in BIRB 796-pretreated Raw 264.7 cells compared to that of LPS-treated controls (Fig. 1A and B). BIRB 796 slightly enhanced cytokine productions in mouse Raw 264.7 cells.
Figure 1.
Inhibitory effect of BIRB 796 and AGI 1067 on cytokine production in Raw 264.7 and THP-1 cells. Raw 264.7 and THP-1 cells were pre-incubated with BIRB 796 and AGI 1067 (10µM) for 1 h prior to stimulation of LPS (10 ng/ml) for Raw 264.7 and LPS (1µg/ml) for THP-1 cells. Levels of mouse TNFα (A), mouse IL-1β (B) and human IL-8 (C) were determined by ELISA kits from R&D Systems. Mean±SEM of cytokine levels were measured (**p<0.01; ***p <0.001). Representative data from 1 of 3 independent experiments performed in duplicate are shown.
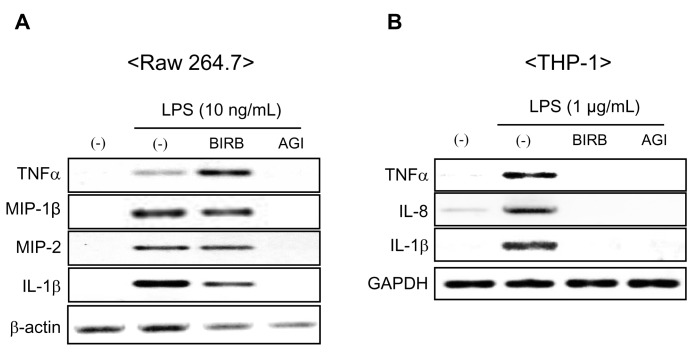
BIRB 796 inhibited LPS-induced cytokine transcripts in THP-1 cells but not in Raw 264.7 cells
To analyze the effects of two compounds on cytokine gene expression, we examined cytokine mRNA expression in THP-1 and Raw 264.7 cells by RT-PCR. The transcripts of LPS-mediated mouse TNFα, IL-1β, MIP-1β and MIP-2 was not suppressed by BIRB 796, although LPS significantly increased the levels of cytokines mRNA (Fig. 2A). Unlike other transcripts, mouse TNFα mRNA level was enhanced by BIRB 796. The LPS treatment specifically increased mRNA level of human TNFα, IL-8 and IL-1β in THP-1 cells (Fig. 2B). The transcript of LPS-mediated human TNFα, IL-8 and IL-1β were sufficiently reduced by both BIRB 796 and AGI 1067 in THP-1 cells.
Figure 2.
Inhibitory effect of BIRB 796 and AGI 1067 on cytokine transcripts in Raw 264.7 and THP-1 cells. Raw 264.7 and THP-1 cells were pre-incubated with BIRB 796 and AGI 1067 (10µM) for 1 h and then stimulated with LPS (10 ng/ml) for Raw 264.7 and LPS (1µg/ml) for THP-1 cells for 4 hrs. The transcript of cytokines in Raw 264.7 (A) and THP-1 (B) were detected by RT-PCR. Representative data from 1 of 3 independent experiments are shown.
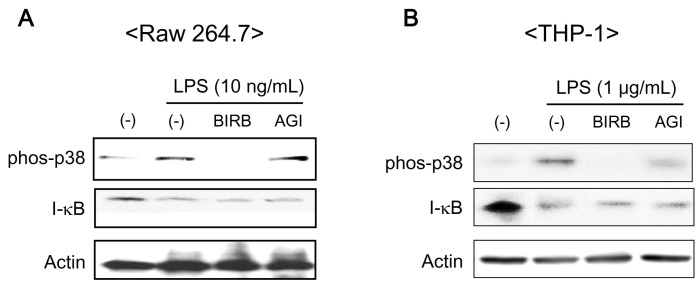
BIRB 796 and AGI 1067 inhibited phosphorylation of p38MAPK but failed to inhibit I-κB degradation in Raw 264.7 and THP-1 cells
The previous results showed that BIRB 796 and AGI 1067 inhibit LPS-induced cytokines release depending on cell type. To identify mechanism of the difference, we examined the phosphorylation of p38MAPK and degradation of I-κB using western blot analysis. The phosphorylation of p38MAPK is prerequisite for induction of p38MAPK catalytic activity. BIRB 796 and AGI 1067 are known well as inhibitors of p38MAPK previously (16,17). We also obtained similar results in Raw 264.7 (Fig. 3A) and THP-1 cells (Fig. 3B). LPS phosphorylated p38MAPK in THP-1 cell was more significant than that of Raw 264.7 cells. BIRB 796 inhibited LPS-induced phosphorylation of p38MAPK completely in both cell types whereas AGI 1067 slightly suppressed LPS-induced phosphorylation of p38MAPK in THP-1 cells but not in Raw 264.7 cells (Fig. 3A and B, lane 4 in upper panel).
Figure 3.
Effect of BIRB 796 and AGI 1067 on the activation of p38MAPK and degradation of I-κB in Raw 264.7 and THP-1 cells. (A) Raw 264.7 and (B) THP-1 cells were pre-incubated with BIRB 796 and AGI 1067 (10µM) for 1 h and then stimulated with LPS (10 ng/ml) for Raw 264.7 and LPS (1µg/ml) for THP-1 cells for 30 min. The cells were lyzed and total cell lysates of 20µg per lane were electrophoresed in 10% SDS-PAGE. The phosphorylation of p38MAPK and degradation of I-κB were detected using western blot with specific antibodies. Representative data from 1 of 3 independent experiments are shown.
It is well known that LPS induces cytokine expression via NF-κB activation in monocytes and macrophage cells. The activation of NF-κB involves the inducible phosphorylation and subsequent degradation of I-κB. We confirmed LPS-mediated degradation of I-κB by western blot (Fig. 3A and B, in middle panel). As depicted in Fig. 3, BIRB 796 and AGI 1067 had no effects on I-κB degradation in Raw 264.7 and THP-1 cells.
DISCUSSION
The successful therapies of anti-cytokine agents, such as Enbrel (TNF receptor-p75 Fc fusion protein), Infliximab (chimeric anti-human TNFα monoclonal antibody), Adalimumab (human anti-human TNFα monoclonal antibody), and Anakinra (human IL-1 receptor antagonist) has ushered in a new era in the treatment of chronic inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, and psoriasis (18). The success of these novel treatments has demonstrated the clinical benefits that can be gained from therapeutic intervention in inflammatory cytokines activities, underscoring the crucial role of inflammatory cytokines, IL-1β and TNFα, in autoimmune diseases (18). However, all of the anti-cytokine drugs available to date are proteins, and suffer to a varying degree from the general disadvantages associated with protein drugs: stability, cellular penetration, oral absorption and mandatory subcutaneous or intravenous administration, and high costs of manufacturing. Therefore, small molecular, orally active anti-cytokine agents, which target specific signaling and/or biosynthetic pathways of inflammatory cytokines, could provide an alternative drugs to treat inflammatory autoimmune diseases.
A number of molecular targets have been identified for the development of such small molecular anti-cytokine agents. Previous studies have been focused on inhibitors of inflammatory cytokines via targeting either p38MAPK, TNFα converting enzyme (TACE), or IL-1β converting enzyme. The p38MAPK occupies a central role in the signaling network responsible for the induction of inflammatory cytokines such as IL-1β and TNFα, and also regulates the biosynthesis of inflammatory cytokines at both the transcriptional and translational level (19). Drug selectivity is important for the inhibition of protein kinase as a means of therapeutic intervention. Protein kinases have high homology across the entire group, especially in the catalytic core (20). However the selectivity of BIRB 796 for inhibition of p38MAPK is specific. Another protein kinases such as extracellular signal-regulated kinase, protein kinase A and I kappa-B kinase are not affected by BIRB 796 (9).
The isoforms of p38MAPK comprise p38MAPKα, p38MAPKβ, p38MAPKγ, and p38MAPKδ. Each p38MAPK isoform may possess different biological function and distinctive physiological substrate; but they phosphorylate substrates containing the minimal consensus sequence Ser/Thr-Pro. Although BIRB 796 inhibits all isoforms of p38MAPK other signaling molecules, it has more specifically effect on p38MAPKα and p38MAPKβ rather than SAPK3/p38MAPKγ and SAPK4/p38MAPKδ isoform including other members of the MAPK family (16).
In present study, the degree of cytokine inhibition was distinct albeit BIRB 796 inhibits phosphorylation of p38MAPK in both Raw 264.7 and THP-1 cells. Although Raw 264.7 and THP-1 cells are all immune cells originated from monocytes they probably are not identical in cellular properties due to a distinctive differentiation stage. Several studies have demonstrated that differentiation stage-specific activation of p38MAPK isoforms exist in immune cells (21,22). The difference of cytokine release is inhibited by BIRB 796 between Raw 264.7 and THP-1 cells could be because of cell-type specific isoforms of p38MAPK. The difference of BIRB 796 and AGI 1067 in cytokine suppression is probably due to that AGI 1067 is initially developed as anti-oxidant agent and its anti-inflammatory function is characterized as a redox signal modulation (23).
The present study demonstrates that the inhibitory efficacy of p38MAPK by BIRB 796 depends on cell types though additional studies are necessary for characterization of the precise role of BIRB 796 in cytokine productions. This result suggests the importance of using various cell types to determine anti-inflammatory effects, which depends on target molecules in each cell type.
ACKNOWLEDGEMENTS
This work was supported by the Konkuk University research support program.
Abbreviations
- TNFα
tumor necrosis factor-α
- IL
interleukin
- p38MAPK
p38 mitogen activated protein kinase
- LPS
lipopolysaccharide
- ELISA
enzyme-linked immunosorbent assay
- MIP-1β
macrophage inflammatory protein-1β
- I-κB
inhibitor of kappa B
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
Footnotes
The authors have no financial conflict of interest.
References
- 1.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 2.Jarvis B, Faulds D. Etanercept: a review of its use in rheumatoid arthritis. Drugs. 1999;57:945–966. doi: 10.2165/00003495-199957060-00014. [DOI] [PubMed] [Google Scholar]
- 3.Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, Jackson CG, Lange M, Burge DJ. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA. Interleukin-18 and the treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 2004;30:417–434. ix. doi: 10.1016/j.rdc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Schafer PH, Wang L, Wadsworth SA, Davis JE, Siekierka JJ. T cell activation signals up-regulate p38 mitogen-activated protein kinase activity and induce TNF-alpha production in a manner distinct from LPS activation of monocytes. J Immunol. 1999;162:659–668. [PubMed] [Google Scholar]
- 6.Debnath J, Chamorro M, Czar MJ, Schaeffer EM, Lenardo MJ, Varmus HE, Schwartzberg PL. rlk/TXK encodes two forms of a novel cysteine string tyrosine kinase activated by Src family kinases. Mol Cell Biol. 1999;19:1498–1507. doi: 10.1128/mcb.19.2.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendelson KG, Contois LR, Tevosian SG, Davis RJ, Paulson KE. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc Natl Acad Sci U S A. 1996;93:12908–12913. doi: 10.1073/pnas.93.23.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JC, Griswold DE, Votta B, Hanna N. Inhibition of monocyte IL-1 production by the anti-inflammatory compound, SK&F 86002. Int J Immunopharmacol. 1988;10:835–843. doi: 10.1016/0192-0561(88)90007-0. [DOI] [PubMed] [Google Scholar]
- 9.Pargellis C, Tong L, Churchill L, Cirillo PF, Gilmore T, Graham AG, Grob PM, Hickey ER, Moss N, Pav S, Regan J. Inhibition of p38 MAP kinase by utilizing a novel allosteric binding site. Nat Struct Biol. 2002;9:268–272. doi: 10.1038/nsb770. [DOI] [PubMed] [Google Scholar]
- 10.Branger J, van den Blink B, Weijer S, Madwed J, Bos CL, Gupta A, Yong CL, Polmar SH, Olszyna DP, Hack CE, van Deventer SJ, Peppelenbosch MP, van der Poll T. Anti-inflammatory effects of a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. J Immunol. 2002;168:4070–4077. doi: 10.4049/jimmunol.168.8.4070. [DOI] [PubMed] [Google Scholar]
- 11.Branger J, van den Blink B, Weijer S, Gupta A, van Deventer SJ, Hack CE, Peppelenbosch MP, van der Poll T. Inhibition of coagulation, fibrinolysis, and endothelial cell activation by a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. Blood. 2003;101:4446–4448. doi: 10.1182/blood-2002-11-3338. [DOI] [PubMed] [Google Scholar]
- 12.van den Blink B, Branger J, Weijer S, Gupta A, van Deventer SJ, Peppelenbosch MP, van der Poll T. P38 mitogen activated protein kinase is involved in the down-regulation of granulocyte CXC chemokine receptors 1 and 2 during human endotoxemia. J Clin Immunol. 2004;24:37–41. doi: 10.1023/B:JOCI.0000018061.58504.75. [DOI] [PubMed] [Google Scholar]
- 13.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 15.Sundell CL, Somers PK, Meng CQ, Hoong LK, Suen KL, Hill RR, Landers LK, Chapman A, Butteiger D, Jones M, Edwards D, Daugherty A, Wasserman MA, Alexander RW, Medford RM, Saxena U. AGI-1067: a multifunctional phenolic antioxidant, lipid modulator, anti-inflammatory and antiatherosclerotic agent. J Pharmacol Exp Ther. 2003;305:1116–1123. doi: 10.1124/jpet.102.048132. [DOI] [PubMed] [Google Scholar]
- 16.Kuma Y, Sabio G, Bain J, Shpiro N, Marquez R, Cuenda A. BIRB796 inhibits all p38MAPK isoforms in vitro and in vivo. J Biol Chem. 2005;280:19472–19479. doi: 10.1074/jbc.M414221200. [DOI] [PubMed] [Google Scholar]
- 17.Luyendyk JP, Piper JD, Tencati M, Reddy KV, Holscher T, Zhang R, Luchoomun J, Chen X, Min W, Kunsch C, Mackman N. A novel class of antioxidants inhibit LPS induction of tissue factor by selective inhibition of the activation of ASK1 and MAP kinases. Arterioscler Thromb Vasc Biol. 2007;27:1857–1863. doi: 10.1161/ATVBAHA.107.143552. [DOI] [PubMed] [Google Scholar]
- 18.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 19.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, Strickler JE, McLaughlin MM, Siemens IR, Fisher SM, Livi GP, White JR, Adams JL, Young PR. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 20.Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 21.Hale KK, Trollinger D, Rihanek M, Manthey CL. Differential expression and activation of p38 mitogen-activated protein kinase alpha, beta, gamma, and delta in inflammatory cell lineages. J Immunol. 1999;162:4246–4252. [PubMed] [Google Scholar]
- 22.Uddin S, Ah-Kang J, Ulaszek J, Mahmud D, Wickrema A. Differentiation stage-specific activation of p38 mitogen-activated protein kinase isoforms in primary human erythroid cells. Proc Natl Acad Sci U S A. 2004;101:147–152. doi: 10.1073/pnas.0307075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem. 2002;277:22131–22139. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]