Abstract
Der f 2 is the group 2 major allergen of a house dust mite (Dermatophagoides farinae) and its function has been recently suggested. To determine the optimal condition of sensitization to recombinant Der f 2 (rDer f 2) in murine model of asthma, we compared the effectiveness with different adjuvants in BALB/c and C57BL/6 mice. Mice from both strains sensitized with rDer f 2 by intraperitoneal injection or subcutaneous injection on days 1 and 14. The dosage was 20 µg. Freund's adjuvants with pertussis toxin (FP) or alum alone were used as adjuvants. On days 28, 29, and 30, mice were challenged intranasally with 0.1% rDer f 2. We evaluated airway hyperresponsivenss, eosinophil proportion in lung lavage, airway inflammation, and serum allergen specific antibody responses. Naive mice were used as controls. Airway hyperresponsiveness was increased in C57BL/6 with FP, and BALB/c with alum (PC200: 13.5±6.3, 13.2±6.7 vs. >50 mg/ml, p<0.05). The eosinophil proportion was increased in all groups; C57BL/6 with FP, BALB/c with FP, C57BL/6 with alum, BALB/c with alum (24.8±3.6, 20.3±10.3, 11.0±6.9, 5.7±2.8, vs. 0.0±0.0%, p<0.05). The serum allergen specific IgE levels were increased in C57BL/6 with FP or alum (OD: 0.8±1.4, 1.1±0.8, vs. 0.0±0.0). C57BL/6 mice were better responders to rDer f 2 and as for adjuvants, Freund's adjuvant with pertussis toxin was better.
Keywords: Allergy, Asthma, Rodent, House dust mite, Der f 2
INTRODUCTION
Allergic diseases such as asthma, allergic rhinitis, and atopic dermatitis are increasing worldwide including in Korea. The prevalence has been doubled or tripled for recent decades (1,2). House dust mite is the most common inhalant allergen that causes asthma, allergic rhinitis, and even atopic dermatitis (3). There are two species of house dust mites, Dermatophagoides farina and Dermatophagoides pteronyssinus, in Korea. D. farinae is the predominant species (65.3%) followed by D. pteronyssinus (20.6%) (4). House dust mites have various proteins that could IgE-mediated immune responses and molecules with adjuvant-like characteristics or their affinity to adjuvant (4). Among the major allergens, group 1 and group 2 allergens constitute 40~60% of the total allergenicity in house dust mites (5).
Group 1 major allergens, Der f 1 and Der p 1, have proteolytic properties as cysteine protease; 1) they can cause disruption of the epithelium, allowing access of allergens to antigen-presenting cells; 2) Der p 1 can cleave immunomodulators such as CD23 (low-affinity IgE receptor) and CD25 (α-subunit of IL-2 receptor); 3) they can elicit inflammatory reactions by activating protease-activated receptor-2; 4) they can release inflammatory cytokines independent of PAR-2 activation; 5) Possibly Der p 1 has been shown to activated and recruit basophils to the draining lymph nodes and stimulate production of Th2-inducing cytokines such as IL-4 and thymic stromal lymphopoietin (TSLP) (4).
Group 2 major allergens, Der f 2 and Der p 2, have lipid-binding properties and show almost 90% sequence homology which made them highly cross-reactive (4). However, the function of group 2 major allergens, especially Der f 2 has not been well elucidated. Only a few studies used recombinant Der f 2 (rDer f 2) to evaluate its function (4,6-8). Reports on murine asthma model using rDer f 2 are few (9,10). There was no report on the influence of adjuvants and genetic background on the asthma model using rDer f 2 in mice. This study showed which strain and adjuvants were the best combinations in the development of murine asthma model using rDer f 2.
MATERIALS AND METHODS
Animals
Female C57BL/6 and BALB/c mice aged 8~10 weeks were used in this study. The mice were purchased from Dae Han Biolink (Choongbuk, Korea) and kept in specific pathogen free conditions in the preclinical center of the Clinical Research Institute of Seoul National University Hospital (Seoul, Korea). None of the mice were exposed to rDer f 2 before the experiment. The study was approved by the appropriate committees on animal experimentation at our institution and performed according to the guide for the care and use of laboratory animals.
Molecular cloning, functional expression and purification of recombinant Der f 2
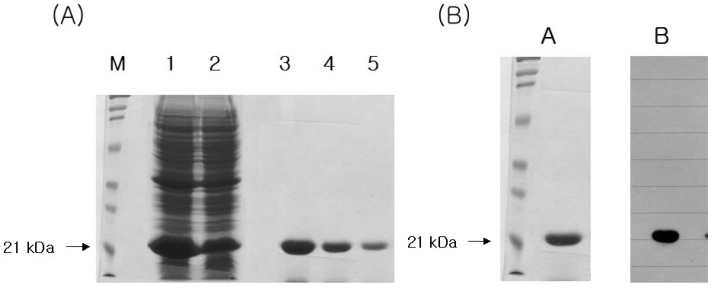
cDNA cloning, over-expression and purification of Der f 2 was performed as described previously (11,12). In brief, amplification of Der f 2 gene with the exception of the signal peptide was carried out in 50 ul volume by using Ex.Taq polymerase. Thirty cycles of PCR were performed on the cDNA of Dermatophagoides farinae using the upstream primer 5'-CAAGTCGATGTTAAAGATTG-3' and the downstream primer 5'-TTAATCACGGATTTTACCATGG-3' with denaturing for 45 s at 94℃, annealing for 45 s at 55℃, and extension for 1 min at 94℃. The PCR product was inserted into pET-15b vector for over-expression, and then transformed into E.coli JM109 (DE3). Single positive colony was grown overnight at 37℃ in 2X-YT media (16 g of Bacto-tryptone, 10 g of yeast extract, 5 g of NaCl) supplemented with 100 mg/ml ampicillin. The next day, 990 ml of 2X-YT media supplemented with 100 mg/ml ampicillin was inoculated with 10 ml of the overnight culture and grown at 30℃ with shaking at 260 rpm until O.D600=1.0. We induced gene expression by adding isopropylthio-b-D-galactoside to a final concentration of 0.1 mM, and the cultures were grown at 28℃ overnight with gentle agitation. The cells were pelleted at 1,500 g and washed twice in phosphate-buffered saline. The cell pellet was then resuspended in 50 ml of a 10 mM potassium phosphate buffer (pH 7.0), 1 mM 2-mercaptoethanol, 1 mM PMSF, and 1% (v/v) Triton X-100, and then it was sonicated while being chilled in an ice bath. We removed cell debris by centrifugation at 15,000 g for 30 min, and the supernatant were purified by using the His-bind purification kits (Novagen, Darmstadt, Germany) as explained in the manufacturer's manual. The purified recombinant proteins were confirmed by SDS-PAGE and immunoblot analysis (Fig. 1).
Figure 1.
Molecular cloning, functional expression and purification of recombinant Der f 2. (A) 12% SDS-PAGE analysis of the expressed Der f 2 allergen. M; molecular size marker, Lane 1, 2; homogenates from JM 109 transformed of pET-15b+Der f 2 cDNA in induced condions, Lane 3-5; purified recombinant Der f 2 allergens. (B) 12% SDS-PAGE and Western blot analysis of the expressed Der f 2 allergen. A; coomassie blue staining of SDS-PAGE gel. B; immunoblot with pooled patients sera sensitized against Dermatophagoides farinae.
Protocols for sensitization and rDer f-specific intranasal challenge
The protocols of sensitization and intranasal challenge were designed according to those previously recommended with some modifications (13,14). Twenty µg of rDer f 2 was injected for sensitization and boosting using Freund's adjuvants with pertussis toxin (Freund/Pertussis) or alum as adjuvants.
For alum group, 4 mg of alum hydroxide (alum, Sigma, St. Louis, USA) was dissolved in 200 µl of phosphate buffered saline (PBS). Mice were injected intraperitoneally with rDer f 2 and alum on days 1 and 14.
For Freund/Pertussis group, 100 µl of complete Freund's adjuvant (Sigma, St. Louis, USA) was injected subcutaneously at the time of sensitization on day 1. On days 2 and 4, 300 ng of Pertussis toxin (Sigma, St. Louis, USA) was injected intraperitoneally. On day 14, 100 µl of Incomplete Freund's adjuvant (Sigma, St. Louis, USA) was injected subcutaneously with rDer f 2.
On day 28, intranasal challenge was performed with 50 µl of 0.1% rDer f 2 and for consecutive 3 days after light anesthesia with ether.
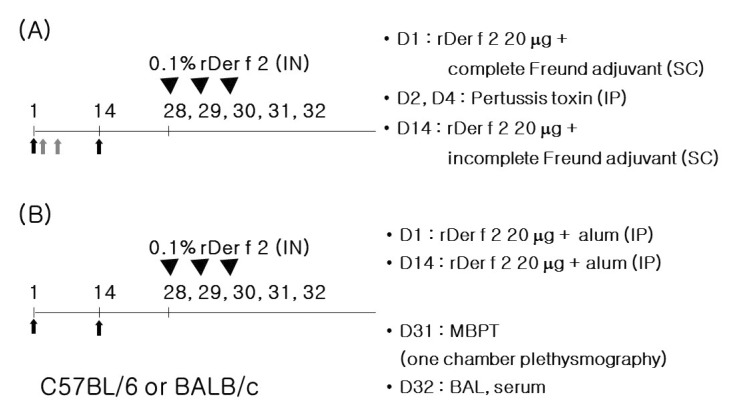
We evaluated airway hyperresponsivenss (day 31), eosinophil proportion in lung lavage, airway inflammation, and serum allergen specific antibody responses (day 32). Naive mice were used as controls. The mice were divided into 6 groups according to the protocols (6 mice for each experimental group, 4 mice for control groups, Fig. 2).
Figure 2.
Experimental design. Twenty µg of recombinant Der f 2 was injected for sensitization and boosting using Freund adjuvants with pertussis toxin (A) or alum (B) as adjuvants. On day 28, intranasal (IN) challenge was performed with 50 µl of 0.1% recombinant Der f 2 and for consecutive 3 days.
Evaluation of asthma phenotypes
Airway hyperresponsiveness
Twenty-four hours after the final intranasal challenge with rDer f 2, airway hyperresponsiveness was assessed by methacholine-induced airflow obstruction using single chamber whole body plethysmography (Allmedicus, Anyang, Korea) as previously described (15). Increases in enhanced pause (Penh) were measured as an index of airway resistance {Penh=[(Te/RT.1)×(PEF/PIF)], where Penh=enhanced pause, Te=expiratory time (s), RT=relaxation time (s), PEF=peak expiratory flow (ml/s), PIF=peak inspiratory flow (ml/s)}. Increasing doses of methacholine (ranging from 2.5 to 50 mg/ml; Sigma, St. Louis, USA) were administered by nebulization for 3 minutes, and the values of Penh were calculated over the subsequent 3 minutes. During the experiment, the activity of the mice and the barometric plethysmograph flow tracings were monitored. For the quantification of the dose-response to methacholine, the linear regression of Penh on log was calculated for individual mice. The log dose corresponding to an increase in Penh of 200%, respectively, was determined, and the average log doses of the different groups were compared. The results are presented as PC200, which is the concentration of methacholine required to increase the baseline Penh by 200%.
Inflammatory cells in bronchoalveolar lavage
Forty-eight hours after the final intranasal challenge with rDer f 2, mice tracheae were cannulated and the lungs were lavaged five times with 0.4 ml aliquots of pyrogen-free saline. After Diff-quikR staining (Dade Behring AG, Dudingen, Switzerland) of lung lavage cells in a cytospin preparation, two investigators blindly counted more than 300 inflammatory cells under a light microscope and classified them as macrophages, lymphocytes, neutrophils, and eosinophils (16,17).
Serum anti-rDer f 2 specific IgE
Forty-eight hours after the final intranasal challenge with rDer f 2, blood samples were obtained from the mice via the inferior vena cava. Serum anti-rDer f 2 specific IgE was measured by ELISA as previously described with some modifications (18). Briefly, microtiter plates (Nunc, Roskilde, Denmark) were coated overnight with 10 µg/well of rDer f 2 in a 50 mM carbonate buffer (pH 9.6) at 4℃. Nonspecific binding was blocked with 2% bovine serum albumin for 1 hour at 20℃. After incubation of test sera for 2 hours, the plates were incubated with horse radish peroxidase-labeled goat anti-mouse IgE (Pharmingen, San Diego, USA) for 1 hour at 20℃. The reaction was developed with a tetramethylbenzidine (Sigma, St. Louis, USA) substrate and then stopped by adding 2 N H2SO4. Subsequently, the optical density was measured at 490 nm. The antibody titers of the samples were related to pooled standards that were generated in the laboratory and expressed as arbitrary units (AU) according to each O.D. value.
Statistical analysis
Statistical analysis was performed using the Kruskal-Wallis and the Mann-Whitney tests. Statistical significance was accepted at p<0.05. Analysis was performed using SPSS 9.0. Values for all measurements are expressed as the means±the standard error of the mean.
RESULTS AND DISCUSSION
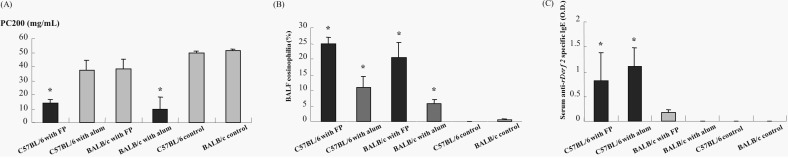
The airway responsiveness was increased in the groups of C57BL/6 with Freund/Pertussis and BALB/c with alum and the control groups (p<0.05). No difference of PC200 was found between them (p>0.05). The PC200 of C57BL/6 with alum, BALB/c with Freund/Pertussis were not significantly different from those of the control groups (p>0.05) (Fig. 3A).
Figure 3.
Evaluation of asthma phenotypes. (A) Airway hyperresponsiveness measured using whole body plethysmograph, (B) airway eosinophilia from bronchioalveolar lavage fluid, (C) Serum anti-recombinant Der f 2 (rDer f2) specific IgE measured by ELISA. Each bar indicates the mean (±SEM) value. *p<0.05, compared with the control groups. BALF, bronchoalveolar lavage fluid.
The percentages of BALF eosinophil were higher in all experimentally sensitized groups compared with those of the control groups (p<0.05). The BALF eosinophil levels in the groups of C57BL/6 or BALB/c with Freund/Pertussis were higher than in the groups of C57BL/6 or BALB/c with alum (Fig. 3B).
The serum anti-rDer f 2 specific IgE levels were higher in C57BL/6 with Freund/Pertussis or alum than in the control groups (p<0.05). There was no significant difference in the serum anti-rDer f 2 levels between the C57BL/6 with Freund/Pertussis or alum (p>0.05) (Fig. 3C).
We evaluated the influence of the adjuvants and genetic background on the murine model of asthma using rDer f 2, especially evaluating the important features of asthma - airway hyperresponsivenss, eosinophilic airway inflammation and serum allergen-specific IgE levels, which has not been reported so far. This study showed that C57BL/6 mice with Freund adjuvant and pertussis toxin were the best model among the experimental groups. In terms of airway hypersensitivity, C57BL/6 with Freund/Pertussis and BALB/c with alum were good combinations. Freund and pertussis toxin was more effective adjuvants for inducing airway eosinophilia in both strains although alum also induced airway eosinophilia. C57BL/6 mice were ideal for inducing serum anti-rDer f 2-specific IgE using Freund/Pertussis or alum.
House dust mite is the most common inhalant allergen in the world as well as in Korea (3). Allergens from mite groups 1, 3, 6, and 9 were identified as cysteine protease, trypsin, chymotrypsin, and collagenolytic serine protease, respectively (4). Group 2, 13, and 14 allergens are associated with lipid-binding activity (4). Der f 2 as well as Der P 2 was localized in the midgut and fecal pellets of the mite (19,20). The exact function of Der f 2 has not been known yet. The group 2 major mite allergens, Der f 2 and Der p 2, are 14 kD protein and show structural homology with MD-2, the lipopolysaccharide (LPS)-binding component of the Toll-like receptor (TLR) 4 signaling complex (4). It has been reported that Der f 2 binds to LPS in a molar ratio of 1 : 1 and that LPS binds Der f 2 between the two large beta-sheets, similar to its binding to MD-2, the LPS-binding component of the innate immunity receptor TLR4 (21). There is interesting reports that Der f 2 showed stronger IgE reactivity than Der f 1 in Korean mite-allergy patients (22,23). rDer f 2 produced in E. coli is recognized by 90~100% of serum IgE from Korean D. farinae-sensitized subjects (4). Recently it has been reported that Der f 2 induced interleukin-13 expression by activating the PI3K/Akt pathway and by phospholipase D1 through activating transcription factor-2 activation in human bronchial epithelial cells (24,25).
There has been several murine models using Der f 2 but only few researchers evaluated only limited aspects of allergic reactions in mice using rDer f 2 (7,9,10). However, this study shows unique data on the influence of the adjuvants and genetic background on the murine model of asthma using recombinant Der f 2 evaluating the important features of asthma.
C57BL/6 mice is a black with H2b allele which has been reported to be a good responder to house dust mite extract allergen but a poor responder to ovalbumin (26,27). BALB/c mice, a white with H2d allele, have been reported to be a good responder to ovalbumin but a poor responder to house dust mite extract allergen (28). As shown in this study, C57BL/6 mice showed generally good response to rDer f 2 with Freund/Pertussis but not with alum. In terms of airway hyperresponsiveness, BALB/c with alum showed a better result than C57BL/6 with alum. It is interesting that BALB/c with alum showed increased airway hyperresponsiveness with less eosinophilic airway inflammation than C57BL/6 with alum. It is well known that airway hyperresponsiveness and airway inflammation are independent pathways: There are several hypotheses on that (17). It was also interesting that BALB/c with alum showed airway hyperresponsiveness in the almost absence of rDer f 2-specific IgE. As presented in Fig. 3C, there was a small increase in rDer f 2-specific IgE, which could mean that BALB/c with alum was enough to induce local rDer f2-specific IgE production after intranasal challenge but was not enough to induce systemic rDer f2-specific IgE production to be detected from serum.
In conclusion, the influence of the adjuvants and genetic background were important factors on the murine model of asthma using rDer f 2. The optimal condition for the asthma model using rDer f 2 in mouse was using C57BL/c mice treated with Freund adjuvant with pertussis toxin.
ACKNOWLEDGEMENTS
This study was supported by a research grant from the Ministry of Health and Welfare (HMP-00-CH-06-0006), and by a grant of Seoul National University Bundang Hospital (02-2006-024), Korea.
Abbreviations
- rDer f 2
recombinant Der f 2
- FP
Freund's adjuvants with pertussis toxin
- CD
cluster of differentiation
- TSLP
thymic stromal lymphopoietin
Footnotes
The authors have no financial conflict of interest.
References
- 1.Cho SH, Kim YK, Chang YS, Kim SS, Min KU, Kim YY. Asthma insights and reality in Korea. Korean J Med. 2006;70:69–77. [Google Scholar]
- 2.Kim YY. Past, present, and future of allergy in Korea. Allergy Asthma Immunol Res. 2010;2:155–164. doi: 10.4168/aair.2010.2.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim TB, Kim KM, Kim SH, Kang HR, Chang YS, Kim CW, Bahn JW, Kim YK, Kang HT, Cho SH, Park HS, Lee JM, Choi IS, Min KU, Hong CS, Kim NS, Kim YY. Sensitization rates for inhalant allergens in Korea; a multi-center study. J Asthma Allergy Clin Immunol. 2003;23:483–493. [Google Scholar]
- 4.Jeong KY, Park JW, Hong CS. House dust mite allergy in Korea: The most important inhalant allergen in current and future. Allergy Asthma Immunol Res. 2012;4:313–325. doi: 10.4168/aair.2012.4.6.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong KY, Choi SY, Lee JH, Lee IY, Yong TS, Lee JS, Hong CS, Park JW. Standardization of House Dust Mite Extracts in Korea. Allergy Asthma Immunol Res. 2012;4:346–350. doi: 10.4168/aair.2012.4.6.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu CC, Liao EC, Lee MF, Tsai JJ. Augmentation of regulatory T cells in allergic individuals by recombinant Der f 2 peptide with fungal immunomodulatory peptide fve. Ann Allergy Asthma Immunol. 2009;102:216–222. doi: 10.1016/S1081-1206(10)60084-1. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi Y, Takai T, Ota M, Kato T, Takeda K, Mitsuishi K, Ikeda S, Okumura K, Ogawa H. Application of immunoreaction enhancer solutions to an enzyme-linked immunosorbent assay for antigen-specific IgE in mice immunized with recombinant major mite allergens or ovalbumin. Int Arch Allergy Immunol. 2006;141:322–330. doi: 10.1159/000095458. [DOI] [PubMed] [Google Scholar]
- 8.Jin HS, Yong TS, Park JW, Hong CS, Oh SH. Immune reactivity of recombinant group 2 allergens of house dust mite, Dermatophagoides pteronyssinus, and Dermatophagoides farinae. J Investig Allergol Clin Immunol. 2003;13:36–42. [PubMed] [Google Scholar]
- 9.Yasue M, Yokota T, Kajiwara Y, Suko M, Okudaira H. Inhibition of airway inflammation in rDer f 2-sensitized mice by oral administration of recombinant der f 2. Cell Immunol. 1997;181:30–37. doi: 10.1006/cimm.1997.1184. [DOI] [PubMed] [Google Scholar]
- 10.Yasue M, Yokota T, Fukada M, Takai T, Suko M, Okudaira H, Okumura Y. Hyposensitization to allergic reaction in rDer f 2-sensitized mice by the intranasal administration of a mutant of rDer f 2, C8/119S. Clin Exp Immunol. 1998;113:1–9. doi: 10.1046/j.1365-2249.1998.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon SG, Bahn JH, Jang JS, Jang SH, Lee BR, Lee KS, Park J, Kang TC, Won MH, Kim HB, Kwon OS, Cho SW, Choi SY. Molecular cloning and functional expression of bovine brain GABA transaminase. Mol Cells. 2001;12:91–96. [PubMed] [Google Scholar]
- 12.Jeon SG, Bahn JH, Jang JS, Park J, Kwon OS, Cho SW, Choi SY. Human brain GABA transaminase tissue distribution and molecular expression. Eur J Biochem. 2000;267:5601–5607. doi: 10.1046/j.1432-1327.2000.01626.x. [DOI] [PubMed] [Google Scholar]
- 13.Yu CK, Yang BC, Lee SC, Wang JY, Hsiue TR, Lei HY. Dermatophagoides-farinae-induced pulmonary eosinophilic inflammation in mice. Int Arch Allergy Immunol. 1997;112:73–82. doi: 10.1159/000237434. [DOI] [PubMed] [Google Scholar]
- 14.Chang YS, Kim YK, Bahn JW, Kim SH, Park HW, Kim TB, Cho SH, Min KU, Kim YY. Comparison of asthma phenotypes using different sensitizing protocols in mice. Korean J Intern Med. 2005;20:152–158. doi: 10.3904/kjim.2005.20.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang YS, Kim YK, Kim SH, Park HW, Min KU, Kim YY, Cho SH. Murine subcutaneous immunotherapy models with beneficial immunological and physiological effects. Asia Pac Allergy. 2013;3:50–58. doi: 10.5415/apallergy.2013.3.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park Y, Chang YS, Lee SW, Cho SY, Kim YK, Min KU, Kim YY, Cho SH, Sung YC. The enhanced effect of a hexameric deoxyriboguanosine run conjugation to CpG oligodeoxynucleotides on the protection against allergic asthma. J Allergy Clin Immunol. 2001;108:570–576. doi: 10.1067/mai.2001.118517. [DOI] [PubMed] [Google Scholar]
- 17.Chang YS, Kim YK, Kim TB, Kang HR, Kim SS, Bahn JW, Min KU, Kim YY, Cho SH. Airway inflammation and allergen specific IgE production may persist longer than airway hyperresponsiveness in mice. J Korean Med Sci. 2004;19:69–73. doi: 10.3346/jkms.2004.19.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang YS, Kim YK, Bahn JW, Kim SH, Park HW, Kim TB, Cho SH, Min KU, Kim YY. Comparison of asthma phenotypes using different sensitizing protocols in mice. Korean J Intern Med. 2005;20:152–158. doi: 10.3904/kjim.2005.20.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park GM, Lee SM, Lee IY, Ree HI, Kim KS, Hong CS, Yong TS. Localization of a major allergen, Der p 2, in the gut and faecal pellets of Dermatophagoides pteronyssinus. Clin Exp Allergy. 2000;30:1293–1297. doi: 10.1046/j.1365-2222.2000.00883.x. [DOI] [PubMed] [Google Scholar]
- 20.Jeong KY, Lee IY, Ree HI, Hong CS, Yong TS. Localization of Der f 2 in the gut and fecal pellets of Dermatophagoides farinae. Allergy. 2002;57:729–731. doi: 10.1034/j.1398-9995.2002.23623.x. [DOI] [PubMed] [Google Scholar]
- 21.Ichikawa S, Takai T, Yashiki T, Takahashi S, Okumura K, Ogawa H, Kohda D, Hatanaka H. Lipopolysaccharide binding of the mite allergen Der f 2. Genes Cells. 2009;14:1055–1065. doi: 10.1111/j.1365-2443.2009.01334.x. [DOI] [PubMed] [Google Scholar]
- 22.Hong CS, Park JW, Nahm DH. Measurement of IgE and IgG subclass antibodies to whole body antigen and two major allergens (Der fI & Der fII) of Dermatophagoides farinae in normal subjects and asthmatics. Yonsei Med J. 1994;35:453–463. doi: 10.3349/ymj.1994.35.4.453. [DOI] [PubMed] [Google Scholar]
- 23.Nahm DH, Park JW, Hong CS, Lee SY, Lee KY. Specific IgE antibodies to D. farinae whole body extract, Der f I and Der f II in child age groups. Pediatr Allergy Respir Dis. 1995;5:117–124. [Google Scholar]
- 24.Ro EJ, Cha PH, Kim HY, Cho YH, Park JW, Han JS, Choi KY. House dust mite allergen Der f 2 induces interleukin-13 expression by activating the PI3K/Akt pathway. Immunol Res. 2013;56:181–188. doi: 10.1007/s12026-013-8392-0. [DOI] [PubMed] [Google Scholar]
- 25.Park SY, Cho JH, Oh DY, Park JW, Ahn MJ, Han JS, Oh JW. House dust mite allergen Der f 2-induced phospholipase D1 activation is critical for the production of interleukin-13 through activating transcription factor-2 activation in human bronchial epithelial cells. J Biol Chem. 2009;284:20099–20110. doi: 10.1074/jbc.M109.010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien R, Ooi MA, Clarke AH, Thomas WR. Immunologic responses following respiratory sensitization to house dust mite allergens in mice. Immunol Cell Biol. 1996;74:174–179. doi: 10.1038/icb.1996.24. [DOI] [PubMed] [Google Scholar]
- 27.Lee YL, Fu CL, Ye YL, Chiang BL. Administration of IL-12 prevent mite Der p 1 allergen-IgE antibody production and airway eosinophil infiltration in an animal model of airway inflmmation. Scand J Immunol. 1999;49:229–236. doi: 10.1046/j.1365-3083.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Lamm WJ, Albert RK, Chi EY, Henderson WR, Jr, Lewis DB. Influence of the route of allergen administration and genetic background on the murine allergic pulmonary response. Am J Respir Crit Care Med. 1997;155:661–669. doi: 10.1164/ajrccm.155.2.9032210. [DOI] [PubMed] [Google Scholar]