Abstract
Macrolide (including erythromycin and azithromycin) and lincosamide (including clindamycin) antibiotics are recommended for treatment of penicillin-allergic patients with Streptococcus pyogenes pharyngitis. Resistance to erythromycin in S. pyogenes can be as high as 48% in specific populations in the United States. Macrolide and lincosamide resistance in S. pyogenes is mediated by several different genes. Expression of the erm(A) or erm(B) genes causes resistance to erythromycin and inducible or constitutive resistance to clindamycin, respectively, whereas expression of the mef(A) gene leads to resistance to erythromycin but not clindamycin. We studied the resistance of S. pyogenes to erythromycin and clindamycin at an urban tertiary-care hospital. Of 196 sequential isolates from throat cultures, 15 (7.7%) were resistant to erythromycin. Three of these were also constitutively resistant to clindamycin and had the erm(B) gene. Five of the erythromycin-resistant isolates were resistant to clindamycin upon induction with erythromycin and had the erm(A) gene. The remaining seven erythromycin-resistant isolates were susceptible to clindamycin even upon induction with erythromycin and had the mef(A) gene. Pulsed-field gel electrophoresis analysis and emm typing demonstrated that the erythromycin-resistant S. pyogenes comprised multiple strains. These results demonstrate that multiple mechanisms of resistance to macrolide and lincosamide antibiotics are present in S. pyogenes strains in the United States.
Streptococcus pyogenes (group A streptococcus) is the most common cause of pharyngitis and also causes more serious infections such as necrotizing fasciitis and toxic shock syndrome (4, 16). S. pyogenes is uniformly susceptible to penicillin, and this is the recommended treatment for S. pyogenes pharyngitis in patients who are able to take penicillin (4). In patients who are allergic to penicillin, macrolide (including erythromycin and azithromycin) and lincosamide (including clindamycin) antibiotics have an important role in treatment of S. pyogenes infections. The Infectious Diseases Society of America guidelines recommend erythromycin for treatment of S. pyogenes pharyngitis for patients who are allergic to penicillin, and azithromycin is also approved by the U.S. Food and Drug Administration for this use (4). Azithromycin may be given once per day, making this an attractive option for treatment of S. pyogenes pharyngitis. Clindamycin is recommended for treatment of multiple recurrent episodes of S. pyogenes pharyngitis and can be used to treat some erythromycin-resistant S. pyogenes infections (4).
Resistance to macrolide and lincosamide antibiotics among S. pyogenes is a growing concern in the United States because of high rates of resistance elsewhere in the world and because of reports of resistance in the United States (3, 15). Further, studies showing that the use of macrolides is increasing in the United States, taken together with studies demonstrating that increased macrolide use is associated with higher rates of resistance in S. pyogenes, raise concern that macrolide resistance will increase in the United States (11, 21).
Two common mechanisms of resistance to macrolide antibiotics by S. pyogenes have been described. First, erythromycin ribosome methylation (erm) genes encode enzymes which dimethylate an adenine in the 23S rRNA of the 50S ribosomal subunit, blocking binding of macrolide, lincosamide, and streptogramin B antibiotics to the ribosome (the MLSB phenotype) (14, 27). The MLSB phenotype may be constitutive (cMLSB), which is usually due to expression of the erm(B) gene (14). Alternatively, the MLSB phenotype may be inducible by erythromycin (iMLSB), which is usually due to the presence of the erm(A) gene [formally called erm(TR)] (20, 21). The erm(A) gene encodes an mRNA that is translated only in the presence of an inducer such as erythromycin (14, 28). Clindamycin does not induce the translation of erm(A) mRNA; however, induction of erm(A) translation by erythromycin leads to clindamycin resistance (14). The second mechanism by which S. pyogenes strains commonly become resistant to macrolides is through acquisition of the mef(A) gene (7, 23). mef(A) encodes an efflux pump protein which pumps 14- and 15-membered ring macrolides (including erythromycin and azithromycin) out of the organism, leading to resistance to these drugs. Expression of mef(A) does not lead to resistance to clindamycin, even in the presence of erythromycin. This is referred to as the M phenotype (14).
The goals of the present study were to determine the frequency of resistance to erythromycin and clindamycin in the patients served by Children's Hospital Boston Microbiology Laboratory, to determine the mechanism of resistance among resistant isolates, and to determine the relatedness of the resistant isolates.
MATERIALS AND METHODS
Isolates.
During April through July of 2002, 196 sequential S. pyogenes isolates from throat cultures submitted to the microbiology laboratory of Children's Hospital Boston were collected. Only one specimen per patient was included in the present study. S. pyogenes were identified from typical beta-hemolytic colonies on 5% sheep blood agar (Becton Dickinson, Franklin Lakes, N.J.) with the PYR test (LifeSign, Somerset, N.J.) and serotyped for group A antigen (Remel, Lenexa, Kans.). The last names of the patients with erythromycin-resistant S. pyogenes isolates were compared to determine whether these patients were in the same immediate family.
Antimicrobial susceptibility testing.
All isolates were tested by disk diffusion on Mueller-Hinton agar with 5% sheep blood, by using a 15-μg erythromycin disk and a 2-μg clindamycin disk according to National Committee for Clinical Laboratory Standards guidelines. Isolates that were resistant or intermediate to either drug by disk diffusion were tested by using epsilometer strips to determine the MIC of erythromycin and clindamycin (AB Biodisk, Solne, Sweden). Epsilometer strips were used, according to the manufacturer's instructions, on Mueller-Hinton agar with 5% sheep blood and incubated for 24 h (Becton Dickinson).
The D test detects erythromycin-inducible clindamycin resistance (23). This test was done on all isolates that were resistant to erythromycin but susceptible to clindamycin by MIC. Isolates were plated on Mueller-Hinton agar with 5% sheep blood (Remel) and erythromycin and clindamycin disks were placed ca. 16 mm apart. Increased bacterial growth around the clindamycin disk adjacent to the erythromycin disk, forming a zone of inhibition in the shape of a D, was interpreted as a positive test.
The cMLSB phenotype was defined as resistance to both erythromycin and clindamycin, the iMLSB phenotype was defined as resistance to erythromycin, susceptibility to clindamycin, and a positive D test, and the M phenotype was defined as resistance to erythromycin, susceptibility to clindamycin, and a negative D test.
Identification of antimicrobial resistance genes.
Detection of the erm(A), erm(B), mef(A) and streptolysin O genes was done by using PCR with previously published primers (25, 26). Escherichia coli strains transfected with genes for erm(B) and mef(A), for use as positive controls, were obtained from Marilyn C. Roberts. S. pyogenes with the erm(A) gene, for use as a positive control, was obtained from Bernard Beall. Template DNA was prepared by digestion of a loopful of organisms with 0.05 mg of proteinase K (Sigma-Aldrich, St. Louis, Mo.)/ml in 0.1 M Tris buffer (Fisher Scientific, Pittsburgh, Pa.) at pH 8.5 with 0.05% Tween 20 (Sigma-Aldrich) at 60°C overnight, followed by inactivation of proteinase K at 100°C for 15 min (6). PCR was performed by using the PCR Core System 1 kit (Promega, Madison, Wis.). The MgCl2 concentration for all reactions was 2 mM, and the annealing temperatures were 50°C for erm(A) and 55°C for mef(A), erm(B), and streptolysin O. PCRs consisted of 2 min at 95°C and then 35 cycles of 1 min each at 95°C, and then the annealing temperature, and then 72°C, followed in turn by a final extension for 5 min at 72°C. PCR products were resolved on 0.9% agarose gels, along with a 100-bp molecular weight ladder standard (Invitrogen, Carlsbad, Calif.).
Pulsed-field gel electrophoresis (PFGE).
An overnight culture (700 μl) of S. pyogenes was harvested, washed with buffer (10 mM Tris [pH 7.2], 20 mM NaCl, 50 mM EDTA), and resuspended in 100 μl of fresh buffer. Then, 2 μl of RNase A medium (10 mM Tris base, 0.1 mM EDTA, RNase A at 1.25 mg/ml; Sigma) was added to the suspension, which was then placed at 50°C. A total of 100 μl of 2% CleanCut agarose (Bio-Rad, Richmond, Calif.) was added, the tube was vortexed lightly, and the mixture was cast in plug molds (Bio-Rad). The plugs were placed in 250 μl of lysis solution containing lysozyme buffer (10 mM Tris [pH 7.2], 50 mM NaCl, 0.2% sodium deoxycholate, 0.5% sodium lauryl sarcosine [Bio-Rad], 2 mg of lysozyme/ml, and 400 U of mutanolysin [Sigma]/ml), followed by incubation at 37°C for 4 h. The plugs were washed briefly with wash buffer (20 mM Tris [pH 8.0], 50 mM EDTA [Bio-Rad]) and incubated overnight at 50°C in 250 μl of proteinase K reaction buffer (100 mM EDTA [pH 8.0], 0.2% sodium deoxycholate, 1% sodium lauryl sarcosine) with 10 μl of proteinase K (Bio-Rad). These plugs were then washed four times with wash buffer; the second and third washes were treated with 10 μl of phenylmethylsulfonyl fluoride stock (100 mM phenylmethanesulfonyl fluoride in 100% isopropanol) and stored at 4°C for later enzymatic treatment. Plugs were cut to size and digested in 100 μl of restriction buffer (10 mM Tris-HCl, 50 mM KCl, 7 mM MgCl2, 1 mM dithiothreitol; pH 7.75) with 40 U SmaI (Promega) overnight at 24°C (2). The plugs were then washed in 1× wash buffer, equilibrated in 0.5× TBE buffer (45 mM Tris, 45 mM borate, 1.0 mM EDTA [pH 8.3]), and loaded into a 1.2% pulsed-field certified agarose (Bio-Rad) gel with the samples flanked by bacteriophage λ DNA concatemers CI857Sam7 (Roche Molecular Biochemicals, Indianapolis, Ind.). All gels were electrophoresed in 0.5× TBE buffer at 5.1 V/cm for 33 h at 14°C with a pulse duration of 0.5 to 50 s ramped linearly in a CHEF-DR II system (Bio-Rad). PFGE results were analyzed by using Diversity Database software (Bio-Rad) by using Ward's method on a dice coefficient similarity matrix.
Determination of emm type.
DNA was extracted as for determination of antibiotic resistance by PCR. The emm gene for the M protein was amplified and sequenced as described below with the following modifications (http://www.cdc.gov/ncidod/biotech/strep/protocols.htm) (1). PCR was performed with 1.5 mM MgCl2, an annealing temperature of 45°C, and an extension time of 2 min in each cycle. The PCR product was purified by using the QIAquick SpinGel extraction kit (Qiagen) and sequenced by using the Mega BACE 1000 DNA analysis system. The sequences encoding the 50 N-terminal amino acids of the processed M protein were analyzed by a BLAST search (http://www.cdc.gov/ncidod/biotech/strep/strepblast.htm). Sequences with >95% identity to known emm types or subtypes were considered to match that type.
The emm nucleotide sequences were submitted to GenBank (accession numbers AY497025 to AY497040).
RESULTS
Of 196 S. pyogenes isolates, 15 (7.7%) were resistant to erythromycin (Table 1). Of these 15, 3 were resistant to clindamycin and 12 were susceptible to clindamycin. All clindamycin-resistant isolates were also resistant to erythromycin (cMLSB phenotype). Of the 12 erythromycin-resistant, clindamycin-sensitive isolates, 5 were positive for the D test, indicating that erythromycin induced clindamycin resistance (iMLSB phenotype), and 7 were negative for the D test (M phenotype). One isolate was intermediate for erythromycin (MIC of 0.75 μg/ml) and sensitive to clindamycin (MIC of 0.25 μg/ml). The clindamycin MIC of this erythromycin-intermediate isolate did not differ significantly from that of the other clindamycin-susceptible isolates (range, 0.125 to 0.25 μg/ml). A total of 16 (8.2%) isolates were not susceptible to erythromycin. No erythromycin-resistant organisms were from patients in the same immediate family.
TABLE 1.
Phenotype of erythromycin-resistant S. pyogenes isolates
| Phenotypea | No. (%) of isolates | Resistance gene | Mean MIC (range)b
|
|
|---|---|---|---|---|
| Erythromycin | Clindamycin | |||
| cMLSB | 3 (1.5) | erm(B) | >256 (NA) | >256 (NA) |
| iMLSB | 5 (2.6) | erm(A) | 163 (16->256) | 0.23 (0.19-0.25) |
| M | 7 (3.6) | mef(A) | 28.6 (16-48) | 0.23 (0.19-0.25) |
cMLSB isolates are resistant to erythromycin (MIC of ≥1 μg/ml) and clindamycin (MIC of ≥1 μg/ml). iMLSB isolates are resistant to erythromycin and sensitive to clindamycin and have a positive D test. M isolates are resistant to erythromycin and sensitive to clindamycin and have a negative D test. This table does not include an isolate with intermediate susceptibility to erythromycin.
The mean MIC was calculated by using a value of 256 for isolates with an MIC of >256 μg/ml. NA, range is not applicable because all values were the same (>256 μg/ml).
The MLSB phenotype was associated with higher MIC for erythromycin than was the M phenotype. The range of erythromycin MIC for cMLSB and iMLSB isolates was 16 to more than 256 μg/ml (mean, 198 μg/ml), whereas the range for M isolates was 16 to 48 μg/ml (mean, 28.6 μg/ml).
PCR analysis was done to determine which of the genes that cause macrolide and lincosamide resistance were present in each isolate (Fig. 1). A negative control isolate, which was sensitive to both erythromycin and clindamycin, did not have the mef(A), erm(A), or erm(B) genes. The cMLSB, iMLSB and M phenotype isolates each had the erm(B), erm(A), and mef(A) genes, respectively. Each isolate had only one resistance gene. The single erythromycin-intermediate isolate did not have the erm(A), erm(B), or mef(A) genes detectable by PCR. PCR for the streptolysin O gene was positive for this isolate, demonstrating that DNA detectable by PCR was present in the sample (data not shown).
FIG. 1.
PCR testing of erythromycin-resistant S. pyogenes for macrolide and lincosamide resistance genes. DNA was amplified with primers specific for erm(B) (A), erm(A) (B), and mef(A) (C). A 100-bp ladder standard, a positive control (E. coli transfected with the indicated gene), a negative control (erythromycin-sensitive S. pyogenes), and a blank (no DNA) are included in each gel. The clinical isolates of S. pyogenes are grouped by the pattern of resistance to erythromycin and clindamycin, as indicated at top.
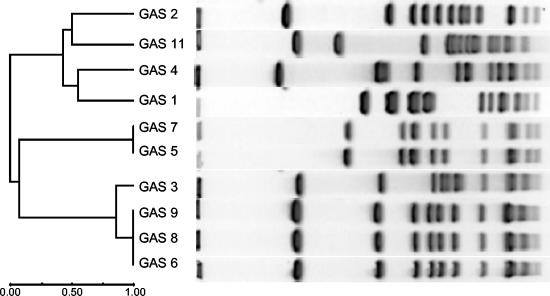
Both emm typing and PFGE demonstrated that there are multiple strains of erythromycin-resistant S. pyogenes among patients served by this hospital (Fig. 2 and Table 2). For clarity, each isolate was assigned a number; these numbers do not correspond to the order in which the isolates were collected. A control strain of S. pyogenes from the American Type Culture Collection (isolate 1, ATCC 12384) was not significantly related to the clinical isolates by PFGE. The three cMLSB isolates (isolates 2 through 4) were distinct from one another, as determined by PFGE, and had different emm types. Interestingly, the five iMLSB isolates comprised two strains by PFGE and emm typing. Two isolates (isolates 5 and 7) had emm-89 and had identical PFGE patterns, and three isolates (isolates 6, 8, and 9) had emm-12 and also had identical PFGE patterns. Each of these iMLSB isolates came from a different patient and were collected over a span of 53 days (of the total 113-day period of specimen collection). cMLSB isolate 3 differed from isolates 6, 8, and 9 by only two bands by PFGE and also had emm-12, suggesting that isolate 3 is closely related to the latter group (24). Despite trying several different digestion methods, we were able to obtain PFGE results from only one of the M phenotype isolates (isolate 11). These seven M phenotype isolates were of four different emm types; four were emm-4, two were emm-12, one was emm-6.4, and one was emm-75. In all, seven different emm types were detected among the erythromycin-resistant S. pyogenes strains.
FIG. 2.
Phylogenetic tree and PFGE results of S. pyogenes isolates that could be typed by PFGE. Isolate 1 is an unrelated control strain (ATCC 12384). Isolates 2 through 11 are erythromycin-resistant isolates: isolates 2 through 4 are cMLSB, isolates 5 through 9 are iMLSB, and isolates 10 through 16 are M isolates. Isolates 10 and 12 through 16 could not be typed by PFGE and are not shown.
TABLE 2.
emm typing of erythromycin-resistant S. pyogenes isolates
| Isolate no.a | Resistance gene | emm type | % Identityb | GenBank accession no. |
|---|---|---|---|---|
| 2 | erm(B) | 18 | 99 | AY497033 |
| 3 | erm(B) | 12 | 100 | AY497034 |
| 4 | erm(B) | 73 | 100 | AY497035 |
| 5 | erm(A) | 89 | 100 | AY497036 |
| 6 | erm(A) | 12 | 100 | AY497037 |
| 7 | erm(A) | 89 | 100 | AY497038 |
| 8 | erm(A) | 12 | 100 | AY497039 |
| 9 | erm(A) | 12 | 100 | AY497025 |
| 10 | mef(A) | 4 | 100 | AY497040 |
| 11 | mef(A) | 6.4 | 99 | AY497026 |
| 12 | mef(A) | 4 | 100 | AY497027 |
| 13 | mef(A) | 4 | 100 | AY497028 |
| 14 | mef(A) | 75 | 100 | AY497029 |
| 15 | mef(A) | 12 | 100 | AY497030 |
| 16 | mef(A) | 4 | 100 | AY497031 |
| 17 | mef(A) | 12 | 100 | AY497032 |
Isolate numbers are the same as in Fig. 2. Isolate 1, an unrelated control strain used for PFGE, was not tested for emm type.
That is, the percent nucleic acid sequence identity between the isolate and the established emm gene sequence, as described in Materials and Methods.
DISCUSSION
The rate of erythromycin nonsusceptibility (8.2%) is similar to that found in other studies in North America. A recently published report indicates that 7.4% of isolates collected in 2000 and 2001 are resistant to erythromycin, but the supporting data are not yet published (22). The data from earlier years show that rates of clindamycin resistance in the United States and Canada range from 2.1 to 9% (8, 10, 13, 29, 30). One study suggests that S. pyogenes strains isolated from patients with invasive disease have higher rates of erythromycin resistance than do isolates from respiratory sources (30), and it is important to note that some studies combine data from respiratory and invasive isolates (8, 9). The impetus for the present study was an article that showed an alarmingly high rate of resistance (48%) to erythromycin within an elementary school in Pittsburgh (15). This was due to a single strain of S. pyogenes that had the mef(A) gene. A random 100 pharyngeal isolates of S. pyogenes submitted to the clinical microbiology laboratory of the Children's Hospital of Pittsburgh also had a high rate (38%) of erythromycin resistance, suggesting that the resistant organism is widespread in the community (15). In contrast, we found that the rate of erythromycin resistance in S. pyogenes in Boston remains relatively low, a finding consistent with a recent national study (3). Together, these data suggest that local monitoring of the rate of erythromycin resistance is needed for physicians to make reasonable decisions about empirical therapy for S. pyogenes with erythromycin.
We found a low rate of constitutive resistance to clindamycin in S. pyogenes, as has also been noted in other studies in North America (3). Two large studies that included isolates from several states in the United States collected in 1989 to 1992 and in 1994 to 1997 did not detect any clindamycin-resistant isolates, although 3.5 and 2.6% of the isolates, respectively, were not susceptible to erythromycin (8, 13). Of 3,205 S. pyogenes isolates collected in Ontario, Canada, in 1997, 20 (0.06%) were constitutively or erythromycin inducibly resistant to clindamycin (10). The erm(B) gene, usually associated with constitutive clindamycin resistance, was present in 2 of 496 (0.4%) isolates collected in Canada in 1998 (29). The strain of erythromycin-resistant S. pyogenes described in Pittsburgh is sensitive to clindamycin since it has the mef(A) gene (15).
Our data demonstrate that there are multiple strains of macrolide-resistant S. pyogenes in the population served by this hospital. A total of seven erythromycin-resistant strains were identified by emm typing and PFGE. Interestingly, the five isolates with the erm(A) gene (iMLSB) were found to include only two strains as determined by both PFGE and emm typing testing. These data suggest that strains of erythromycin-resistant S. pyogenes may be transmitted between people within the Boston area.
An isolate of S. pyogenes with intermediate susceptibility to erythromycin that did not carry the erm(A), erm(B), or mef(A) genes was found. The mechanism by which this isolate is nonsusceptible to erythromycin is therefore unclear. This isolate did not have increased resistance to clindamycin, so altered entry or secretion of erythromycin is a more likely explanation than alterations to the ribosome, although the latter cannot be ruled out by our data.
Widespread use of macrolide antibiotics appears to have selected for high levels (>10%) of macrolide and lincosamide resistance in several countries in Europe and Asia (5, 12, 17-19). Recent Infectious Diseases Society of America guidelines for the treatment of S. pyogenes pharyngitis recommend the use of penicillin for patients who are not allergic to penicillin (4). Erythromycin is recommended for penicillin-allergic patients, and clindamycin is among the treatments recommended for patients with multiple recurrent episodes of S. pyogenes pharyngitis (4). Since our data, and those of others, demonstrate that macrolide- and lincosamide-resistant S. pyogenes is present in the United States, it is important that these antibiotics be used prudently to prevent the selection of antibiotic-resistant S. pyogenes.
REFERENCES
- 1.Beall, B., R. Facklam, and T. Thompson. 1996. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J. Clin. Microbiol. 34:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bert, F., C. Branger, and N. Lambert-Zechovsky. 1997. Pulsed-field gel electrophoresis is more discriminating than multilocus enzyme electrophoresis and random amplified polymorphic DNA analysis for typing pyogenic streptococci. Curr. Microbiol. 34:226-229. [DOI] [PubMed] [Google Scholar]
- 3.Biedenbach, D. J., J. M. Stephen, and R. N. Jones. 2003. Antimicrobial susceptibility profile among β-hemolytic Streptococcus spp. collected in the SENTRY Antimicrobial Surveillance Program-North America, 2001. Diagn. Microbiol. Infect. Dis. 46:291-294. [DOI] [PubMed] [Google Scholar]
- 4.Bisno, A. L., M. A. Gerber, J. M. Gwaltney, Jr., E. L. Kaplan, and R. H. Schwartz. 2002. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin. Infect. Dis. 35:113-125. [DOI] [PubMed] [Google Scholar]
- 5.Canton, R., E. Loza, M. I. Morosini, and F. Baquero. 2002. Antimicrobial resistance amongst isolates of Streptococcus pyogenes and Staphylococcus aureus in the PROTEKT antimicrobial surveillance programme during 1999-2000. J. Antimicrob. Chemother. 50(Suppl. S1):9-24. [DOI] [PubMed] [Google Scholar]
- 6.Chung, W. O., C. Werckenthin, S. Schwarz, and M. C. Roberts. 1999. Host range of the ermF rRNA methylase gene in bacteria of human and animal origin. J. Antimicrob. Chemother. 43:5-14. [DOI] [PubMed] [Google Scholar]
- 7.Clancy, J., J. Petitpas, F. Dib-Hajj, W. Yuan, M. Cronan, A. V. Kamath, J. Bergeron, and J. A. Retsema. 1996. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol. Microbiol. 22:867-879. [DOI] [PubMed] [Google Scholar]
- 8.Coonan, K. M., and E. L. Kaplan. 1994. In vitro susceptibility of recent North American group A streptococcal isolates to eleven oral antibiotics. Pediatr. Infect. Dis. J. 13:630-635. [DOI] [PubMed] [Google Scholar]
- 9.de Azavedo, J. C., M. McGavin, C. Duncan, D. E. Low, and A. McGeer. 2001. Prevalence and mechanisms of macrolide resistance in invasive and noninvasive group B streptococcus isolates from Ontario, Canada. Antimicrob. Agents Chemother. 45:3504-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Azavedo, J. C., R. H. Yeung, D. J. Bast, C. L. Duncan, S. B. Borgia, and D. E. Low. 1999. Prevalence and mechanisms of macrolide resistance in clinical isolates of group A streptococci from Ontario, Canada. Antimicrob. Agents Chemother. 43:2144-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Rey, C., L. Aguilar, F. Baquero, J. Casal, and J. E. Martin. 2002. Pharmacoepidemiological analysis of provincial differences between consumption of macrolides and rates of erythromycin resistance among Streptococcus pyogenes isolates in Spain. J. Clin. Microbiol. 40:2959-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsueh, P. R., L. J. Teng, L. N. Lee, P. C. Yang, S. W. Ho, H. C. Lue, and K. T. Luh. 2002. Increased prevalence of erythromycin resistance in streptococci: substantial upsurge in erythromycin-resistant M phenotype in Streptococcus pyogenes (1979-1998) but not in Streptococcus pneumoniae (1985-1999) in Taiwan. Microb. Drug Resist. 8:27-33. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan, E. L., D. R. Johnson, M. C. Del Rosario, and D. L. Horn. 1999. Susceptibility of group A beta-hemolytic streptococci to thirteen antibiotics: examination of 301 strains isolated in the United States between 1994 and 1997. Pediatr. Infect. Dis. J. 18:1069-1072. [DOI] [PubMed] [Google Scholar]
- 14.Leclercq, R. 2002. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 34:482-492. [DOI] [PubMed] [Google Scholar]
- 15.Martin, J. M., M. Green, K. A. Barbadora, and E. R. Wald. 2002. Erythromycin-resistant group A streptococci in schoolchildren in Pittsburgh. N. Engl. J. Med. 346:1200-1206. [DOI] [PubMed] [Google Scholar]
- 16.Mulla, Z. D. 2002. Invasive group A streptococcal disease and intensive care unit admissions. Intensive Care Med. 28:1822-1824. [DOI] [PubMed] [Google Scholar]
- 17.Nagai, K., P. C. Appelbaum, T. A. Davies, L. M. Kelly, D. B. Hoellman, A. T. Andrasevic, L. Drukalska, W. Hryniewicz, M. R. Jacobs, J. Kolman, J. Miciuleviciene, M. Pana, L. Setchanova, M. K. Thege, H. Hupkova, J. Trupl, and P. Urbaskova. 2002. Susceptibility to telithromycin in 1,011 Streptococcus pyogenes isolates from 10 central and Eastern European countries. Antimicrob. Agents Chemother. 46:546-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Trallero, E., M. Urbieta, M. Montes, I. Ayestaran, and J. M. Marimon. 1998. Emergence of Streptococcus pyogenes strains resistant to erythromycin in Gipuzkoa, Spain. Eur. J. Clin. Microbiol. Infect. Dis. 17:25-31. [DOI] [PubMed] [Google Scholar]
- 19.Reinert, R. R., R. Lutticken, A. Bryskier, and A. Al-Lahham. 2003. Macrolide-resistant Streptococcus pneumoniae and Streptococcus pyogenes in the pediatric population in Germany during 2000-2001. Antimicrob. Agents Chemother. 47:489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppala. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seppala, H., M. Skurnik, H. Soini, M. C. Roberts, and P. Huovinen. 1998. A novel erythromycin resistance methylase gene (ermTR) in Streptococcus pyogenes. Antimicrob. Agents Chemother. 42:257-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shulman, S. T., R. Tanz, and W. Kabat. 2002. Erythromycin-resistant group A streptococci. N. Engl. J. Med. 347:614-615. [PubMed] [Google Scholar]
- 23.Sutcliffe, J., A. Tait-Kamradt, and L. Wondrack. 1996. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob. Agents Chemother. 40:1817-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyler, S. D., W. M. Johnson, J. C. Huang, F. E. Ashton, G. Wang, D. E. Low, and K. R. Rozee. 1992. Streptococcal erythrogenic toxin genes: detection by polymerase chain reaction and association with disease in strains isolated in Canada from 1940 to 1991. J. Clin. Microbiol. 30:3127-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber, P., J. Filipecki, E. Bingen, F. Fitoussi, G. Goldfarb, J. P. Chauvin, C. Reitz, and H. Portier. 2001. Genetic and phenotypic characterization of macrolide resistance in group A streptococci isolated from adults with pharyngo-tonsillitis in France. J. Antimicrob. Chemother. 48:291-294. [DOI] [PubMed] [Google Scholar]
- 27.Weisblum, B. 1995. Erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 39:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisblum, B. 1995. Insights into erythromycin action from studies of its activity as inducer of resistance. Antimicrob. Agents Chemother. 39:797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss, K., J. De Azavedo, C. Restieri, L. A. Galarneau, M. Gourdeau, P. Harvey, J. F. Paradis, K. Salim, and D. E. Low. 2001. Phenotypic and genotypic characterization of macrolide-resistant group A streptococcus strains in the province of Quebec, Canada. J. Antimicrob. Chemother. 47:345-348. [DOI] [PubMed] [Google Scholar]
- 30.York, M. K., L. Gibbs, F. Perdreau-Remington, and G. F. Brooks. 1999. Characterization of antimicrobial resistance in Streptococcus pyogenes isolates from the San Francisco Bay area of northern California. J. Clin. Microbiol. 37:1727-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]