Ligand-induced degradation of endogenous flagellin receptor desensitizes cells to its stimulus, likely to remove ligand-bound receptors from the site of perception, and subsequent de novo receptor synthesis resensitizes cells for a new round of stimulus perception.
Abstract
FLAGELLIN-SENSING2 (FLS2) is the plant cell surface receptor that perceives bacterial flagellin or flg22 peptide, initiates flg22-signaling responses, and contributes to bacterial growth restriction. Flg22 elicitation also leads to ligand-induced endocytosis and degradation of FLS2 within 1 h. Why plant cells remove this receptor precisely at the time during which its function is required remains mainly unknown. Here, we assessed in planta flg22-signaling competency in the context of ligand-induced degradation of endogenous FLS2 and chemical interference known to impede flg22-dependent internalization of FLS2 into endocytic vesicles. Within 1 h after an initial flg22 treatment, Arabidopsis (Arabidopsis thaliana) leaf tissue was unable to reelicit flg22 signaling in a ligand-, time-, and dose-dependent manner. These results indicate that flg22-induced degradation of endogenous FLS2 may serve to desensitize cells to the same stimulus (homologous desensitization), likely to prevent continuous signal output upon repetitive flg22 stimulation. In addition to impeding ligand-induced FLS2 degradation, pretreatment with the vesicular trafficking inhibitors Wortmannin or Tyrphostin A23 impaired flg22-elicited reactive oxygen species production that was partially independent of BRASSINOSTEROID INSENSITIVE1-ASSOCIATED KINASE1. Interestingly, these inhibitors did not affect flg22-induced mitogen-activated protein kinase phosphorylation, indicating the ability to utilize vesicular trafficking inhibitors to target different flg22-signaling responses. For Tyrphostin A23, reduced flg22-induced reactive oxygen species could be separated from the defect in FLS2 degradation. At later times (>2 h) after the initial flg22 elicitation, recovery of FLS2 protein levels positively correlated with resensitization to flg22, indicating that flg22-induced new synthesis of FLS2 may prepare cells for a new round of monitoring the environment for flg22.
Eukaryotic host cell surface receptors are key components of innate immunity in that they act as the first line of defense against invading microbial pathogens (Boller and Felix, 2009; Kumar et al., 2011). These receptors detect extracellular microbe-associated molecular patterns, also referred to as pathogen-associated molecular patterns (PAMPs), as “nonself” to alert host cells of these microbes. PAMP perception activates host immune responses that ultimately contribute to cessation of microbial infection. In plants, one of the best-studied pattern-triggered immunity systems is perception of bacterial flagellin or its active peptide-derivative flg22 by FLAGELLIN-SENSING2 (FLS2; Boller and Felix, 2009; Nicaise et al., 2009; Monaghan and Zipfel, 2012; Robatzek and Wirthmueller, 2013). FLS2 is the plasma membrane (PM)-localized receptor-like kinase (RLK) that detects apoplastic flg22 through its extracellular Leucine-rich repeat domain (Chinchilla et al., 2006; Dunning et al., 2007; Sun et al., 2012; Robatzek and Wirthmueller, 2013). Flg22 perception initiates downstream immune responses including production of reactive oxygen species (ROS), phosphorylation and activation of mitogen-activated protein kinases (MAPKs), and transcriptional changes (Nicaise et al., 2009; Tena et al., 2011; Monaghan and Zipfel, 2012), and increasing evidence suggests that these signaling events constitute a signaling network rather than a single linear pathway (Lu et al., 2009; Korasick et al., 2010; Tena et al., 2011). The regulatory RLK BRI1-ASSOCIATED KINASE1 (BAK1) forms a flg22-induced complex with FLS2 within seconds and functions very early in flg22 response pathways because BAK1 is required for all known downstream flg22-signaling responses (Chinchilla et al., 2007, 2009; Heese et al., 2007; Schulze et al., 2010; Ntoukakis et al., 2011). The importance of FLS2-dependent responses in plant immunity is underlined by the fact that in the absence of functional FLS2, cells exhibit enhanced susceptibility to bacterial infection (Zipfel et al., 2004; Li et al., 2005; Hann and Rathjen, 2007; Zeng and He, 2010).
FLS2 is one of only a few plant RLKs shown to undergo ligand-induced endocytosis and subsequent degradation (Robatzek et al., 2006; Göhre et al., 2008; Lu et al., 2011; Beck et al., 2012; Choi et al., 2013). As determined by live-cell imaging, treatment with active flg22 (derived from Pseudomonas syringae) results in internalization and subsequent degradation of ectopically expressed FLS2 tagged with GFP (FLS2-GFP) within 60 min postelicitation (Robatzek et al., 2006). By contrast, elicitation with inactive flg22 (from Agrobacterium tumefaciens) or elf26, a bacterial PAMP recognized by the plant elongation factor-Tu receptor (EFR) and known to elicit similar signaling responses as flg22 (Zipfel et al., 2006), had no effect (Robatzek et al., 2006). Very recent quantitative live-cell imaging studies further show that when ectopically expressed in Arabidopsis (Arabidopsis thaliana) and Nicotiana benthamiana, FLS2-GFP traffics through early, late, and multivesicular endosomes in response to flg22 (Beck et al., 2012; Choi et al., 2013). Vesicular trafficking inhibitors such as Wortmannin (Wm) or Tyrphostin A23 (TyrA23) impede flg22-induced internalization of FLS2-GFP into endocytic vesicles (Robatzek et al., 2006; Beck et al., 2012). More specifically, Wm is a known phosphatidylinositol3 (PI3)- and PI4-kinase inhibitor that interferes with the formation of endocytic vesicles from the PM and maturation of late endosomes and multivesicular bodies in plant cells (Emans et al., 2002; Tse et al., 2004; Wang et al., 2009; Munnik and Nielsen, 2011; Ito et al., 2012). Consistent with these functions, Wm impairs flg22-induced endocytosis of FLS2-GFP and results in reduced accumulation of FLS2-GFP in endosomes (Robatzek et al., 2006; Beck et al., 2012). Flg22-elicited internalization of FLS2-GFP is also partially reduced after treatment with TyrA23 (Beck et al., 2012). TyrA23 interferes with the interaction between receptors containing the endocytic signal YXXɸ (where Y is the amino acid Tyr, X is any amino acid, and ɸ is a bulky hydrophobic amino acid) and the µ2 subunit of the adaptor protein complex2 required for clathrin-coated vesicle formation and subsequent endocytosis (Banbury et al., 2003). In plants, TyrA23 treatment leads to reduced internalization of endocytic vesicles from the PM (Ortiz-Zapater et al., 2006; Dhonukshe et al., 2007; Konopka and Bednarek, 2008; Leborgne-Castel et al., 2008; Irani et al., 2012). In addition to its function as an endocytic internalization inhibitor, TyrA23 acts as an inhibitor of Tyr kinases in animals (Banbury et al., 2003), a role that has not been well characterized in plants (Leborgne-Castel et al., 2008). Thus, chemical interference studies demonstrate that vesicular trafficking inhibitors impair ligand-induced endocytosis of FLS2-GFP; but so far, it remains unknown whether these inhibitors also affect flg22 signaling.
The observations that FLS2 undergoes ligand-induced endocytosis and degradation appears to be an apparent paradox, whereby a functional FLS2 is required for a full immune response, yet perception of the ligand removes FLS2 from the cell surface at the time during which PAMP perception is required. One plausible explanation is that termination of PAMP signaling must be tightly controlled because constitutive activation of immune signaling diverts valuable resources from growth and development to defense mechanisms. For the FLS2/flg22 system, signal attenuation may be achieved by diverse mechanisms that may include regulating activity and/or abundance of FLS2 itself (Trujillo et al., 2008; Saijo, 2010; Lee et al., 2011; Lu et al., 2011; Sun et al., 2012). In animals, ligand-induced endocytosis of receptors can be a means to desensitize cells to further stimuli by decreasing receptor levels from the cell surface (the site of stimulus perception), to attenuate signal(s) originating from the cell surface, and/or to regulate the turnover of ligand-bound receptor(s) from the PM (Robatzek, 2007; Sorkin and von Zastrow, 2009; Scita and Di Fiore, 2010; Kumar et al., 2011). For some receptors, ligand-induced endocytosis can also serve as a means to ensure contact between receptors and endosomal signaling components to initiate signaling from endosomes (Geldner and Robatzek, 2008; McGettrick and O’Neill, 2010; Kagan, 2012). Similar mechanisms have been proposed but not yet formally investigated for the flg22/FLS2 system.
As a first step to examine potential role(s) of ligand-induced down-regulation of FLS2 in flg22 signaling in planta, we established reelicitation assays to correlate FLS2-signaling competency with endogenous receptor abundance in Arabidopsis leaves, a tissue commonly used for flg22-signaling and bacterial pathogen infection assays. Our results indicate that flg22-induced degradation of endogenous FLS2 may serve as a means to desensitize cells to stimuli. We also provide evidence that the vesicular trafficking inhibitors Wm and TyrA23, previously shown to impair flg22-induced internalization of FLS2 resulting in accumulation of FLS2 at the PM (Robatzek et al., 2006; Beck et al., 2012), negatively affect flg22-induced ROS production but not MAPK phosphorylation. In addition, subsequent flg22-elicited FLS2 protein synthesis that could be blocked by the protein synthesis inhibitor cycloheximide (CHX) prepared cells for a new round of flg22 perception, thus contributing to the resensitization of plant cells to flg22.
RESULTS
Endogenous FLS2 Undergoes Ligand-Induced Degradation That Is Ligand, Time, and Dose Dependent
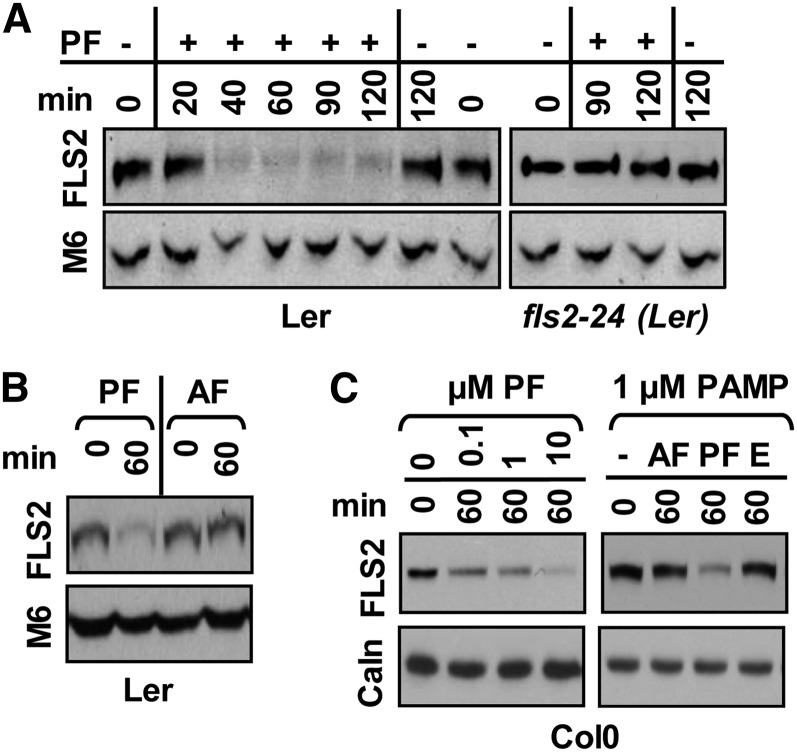
A previous live-cell imaging study using emerging young leaves (Robatzek et al., 2006) showed that in response to 10 µm flg22, ectopically expressed FLS2-GFP is internalized from the PM into small vesicles at 30 min, followed by loss of FLS2-GFP fluorescence at 60 min, consistent with ligand-induced endocytosis and subsequent degradation of FLS2-GFP. In recent quantitative live-cell imaging reports, FLS2-GFP signal could still be detected in endosomes 120 to 200 min after elicitation with 10 to 100 µm flg22 (Beck et al., 2012; Choi et al., 2013). However, little is known about the fate of endogenous, nontagged FLS2 after flg22 elicitation. To this end, we investigated whether endogenous, nontagged FLS2 underwent flg22-induced degradation in more detail. Using Arabidopsis ecotypes expressing functional endogenous FLS2 (Bauer et al., 2001), we assessed FLS2 accumulation using an αFLS2-peptide antibody that detects FLS2 in the wild type but not fls2∆ plants (Heese et al., 2007; Korasick et al., 2010). When ecotype Landsberg erecta (Ler) seedlings were treated with concentrations of flg22 (10 µm) previously used in live-cell imaging studies (Robatzek et al., 2006; Beck et al., 2012), FLS2 levels decreased within 40 min of flg22 elicitation (Fig. 1A). These results are consistent with flg22-induced degradation of endogenous FLS2. By contrast, fls2-24 seedlings expressing FLS2 mutant protein impaired in flg22 binding and signaling (Bauer et al., 2001) did not show altered FLS2 levels (Fig. 1A), confirming the specificity of the degradation response. Similarly, FLS2 underwent ligand-induced degradation in a dose-dependent manner in 4- to 5-week-old Arabidopsis ecotype Columbia (Col-0) leaves (Fig. 1C), a developmental tissue stage commonly used for flg22-signaling and pathogen infection assays. Importantly, FLS2 degradation occurred at a concentration as low as 0.1 µm, commonly used for flg22-signaling response assays (Chinchilla et al., 2007; Heese et al., 2007; Korasick et al., 2010). Confirming the specificity for the ligand receptor pair, FLS2 degradation was specific to active flg22 but not to water, inactive flg22, or the unrelated bacterial PAMP elf26 (Fig. 1, A–C). Protein levels of Mitogen-Associated Protein Kinase6 (MPK6) or the endoplasmic reticulum membrane-bound calnexin were not significantly altered by any treatment (Fig. 1, A–C). These results are consistent with endogenous, nontagged FLS2 undergoing flg22-induced degradation in a ligand-, dose-, and time-dependent manner in two different Arabidopsis ecotypes.
Figure 1.
Flg22-induced degradation of endogenous FLS2 is ligand, dose, and time dependent. A and B, Time dependency and specificity in Ler seedlings. Ler wild-type and fls2-24 mutant seedlings were elicited in the presence (+) or absence (–) of 10 μm flg22 (A) or with 1 μm of indicated PAMPs (B) for indicated times. C, Dose and ligand dependency in Col-0 leaves. Col-0 wild-type leaf strips were elicited with (+) or without (–) indicated PAMP with indicated concentration for 0 or 60 min. For immunoblot analyses, total protein extracts were probed with αFLS2 (FLS2), αCalnexin (Caln), or αMPK6 (M6) antibodies. Probing with the latter two antibodies served as loading controls. Each experiment was done at least three times with similar results. AF, inactive flg22 (from A. tumefaciens); E, elf26.
Ligand-Induced Degradation of FLS2 Desensitizes Cells to Flg22 in a PAMP- and Time-Dependent Manner
To address whether flg22-dependent degradation of FLS2 may serve as a means to desensitize host cells to flg22, we assayed MAPK phosphorylation and ROS production because they are implicated in two parallel flg22-signaling pathways and both responses are rapid and transient, in that flg22-induced activities peak 10 to 15 min postelicitation and return to nearly basal levels within 40 to 60 min (Fig. 2, A–D). For reelicitation assays, leaf tissue was first treated at 0 min with the indicated PAMP, incubated for 45 to 50 min, and then washed to remove excess ligand. To evaluate PAMP-signaling competency after the first elicitation, the same samples were reelicited with specified PAMPs at 60 min. Responses were monitored at indicated times postelicitation.
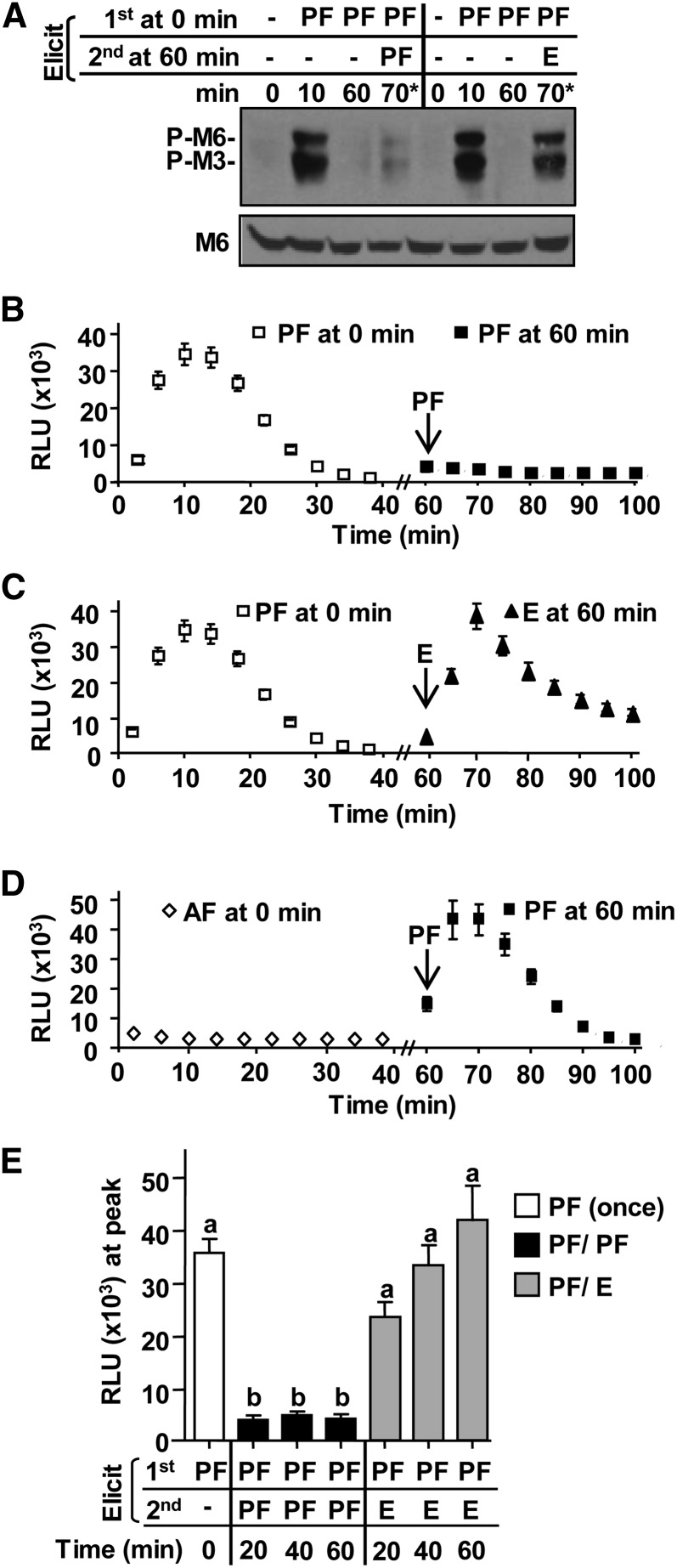
Figure 2.
Ligand-induced desensitization to flg22 is PAMP and time dependent. A, Specificity of reelicitation of MAPK phosphorylation. Col-0 leaf strips were first elicited with 0.1 µm flg22 (PF) for 0, 10, or 60 min. Samples denoted 70* were reelicited at 60 min for 10 min with 0.1 µm of indicated PAMP; minus (–) denotes no elicitation at given time point. Total protein extracts were probed with αMPK6 (M6) or αP-p44/42 MAPK to assess MPK6 protein levels or phosphorylation of MPK6 (P-M6), MPK3 (P-M3), and an unknown MPK (P-M?). B–D, Specificity of ROS reelicitation. For first elicitation, Col-0 leaf discs were elicited with 0.1 µm of indicated PAMP at 0 min (white symbols), washed, and reelicited with indicated PAMP at 60 min (arrow; black symbols; n = 20 per treatment). All three ROS experiments in B to D were set-up in the same 96-well plate at the same time to allow direct comparison. E, Signaling competency between 20 and 60 min. All Col-0 leaf disc halves (n = 96) were elicited at 0 min with 0.1 μm active flg22 (white bar). After 15 min, all tissue was washed. Subsets of tissue (n = 16 per treatment) were then reelicited a second time at 20, 40, or 60 min with indicated PAMP. White bar represents ROS peak production 10 to 12 min after the first elicitation at 0 min (PF once). Black (PF/PF) or gray bars (PF/E) represent ROS peak production 10 to 12 min after reelicitation of tissue subsets for a second time at indicated times with active flg22 or elf26, respectively. To allow for direct comparison, all treatments in E were set-up in the same 96-well plate at the same time. Values are mean ± se, and means with different or the same letters are significantly different or not significantly different, respectively (two-tailed Student’s t test, P ≤ 0.0001). Each experiment was repeated more than three times with similar results. AF, inactive flg22; E, elf26; RLU, relative light unit.
To assess cells’ ability to reelicit MAPK activation, samples were collected just prior to elicitation (0 and 60 min) as well as 10 min postelicitation (10 and 70 min, respectively) with indicated PAMPs and analyzed for phosphorylated MAPKs using immunoblot analyses (Heese et al., 2007; Korasick et al., 2010). Cells initially treated with flg22 were flg22-signaling incompetent at 60 min (Fig. 2A; Active flg22 [PF]/PF, 70*; phosphorylated MPK6 [P-M6], P-M3) but remained signaling competent to elf26 (PF/E, 70*). Importantly, the inability to reelicit flg22-dependent MAPK phosphorylation was not due to reduced MAPK protein levels at any times (Fig. 2A; MPK6). Similarly for ROS production, after the initial fast and transient flg22-induced ROS response, reelicitation of the same tissue with flg22 at 60 min did not result in significant ROS production (Fig. 2B). Consistent with the MAPK phosphorylation results, these cells were flg22-signaling incompetent at 60 min postelicitation. Thus, loss of flg22-induced signaling at 60 min correlated with flg22-induced degradation of FLS2 at that time (Fig. 1C). By contrast, reelicitation with the unrelated PAMP peptide elf26 led to ROS production, indicating that cells treated initially with flg22 were capable of mounting PAMP-dependent ROS responses at 60 min per se (Fig. 2C). Cells initially elicited with inactive flg22 (Fig. 2D) or elf26 (Supplemental Fig. S1A) remained signaling competent for flg22, consistent with FLS2 not being degraded in response to these peptides (Fig. 1C). Flg22-signaling incompetency was observed as early as 20 min after the initial flg22 treatment, but reelicitation with elf26 resulted in significant ROS production at these times (Fig. 2E). This argues against the possibility that this flg22-signaling incompetency between 20 and 60 min may be solely due to an extended refractory period of cellular components required for PAMP-induced ROS production.
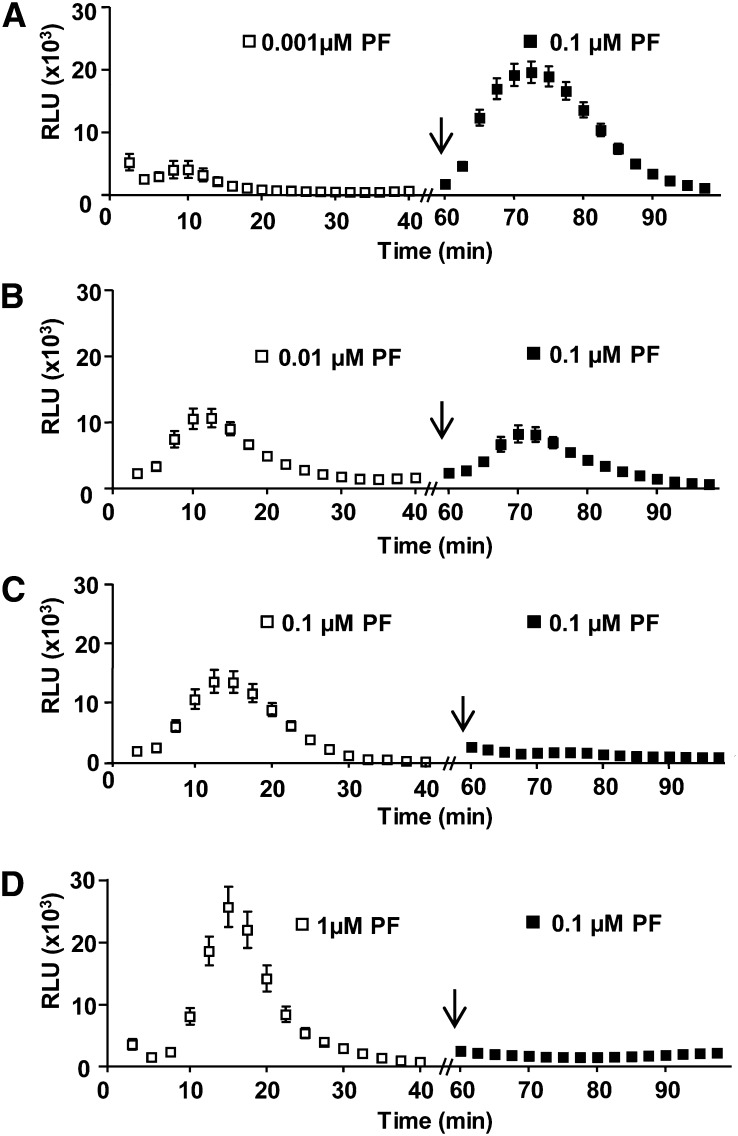
Loss of flg22-signaling competency at 60 min was dose dependent, in that the amplitude of the reelicitation ROS response was dependent on the initial flg22 concentration to which cells were exposed to at 0 min. Cells treated first with 0.001 or 0.01 µm flg22 remained signaling competent to 0.1 µm flg22 at 60 min (Fig. 3, A and B). By contrast, cells initially exposed to 0.1 or 1 µm flg22 were flg22-signaling incompetent at 60 min (Fig. 3, C and D, respectively). The dose dependency of obtaining a reelicitation response showed an inverse correlation with the dose-dependent degradation of FLS2 in response to flg22 (Fig. 1C). For subsequent experiments, concentrations of 0.1 or 1 µm flg22 were used for all elicitations.
Figure 3.
Desensitization of flg22-induced ROS production was dose dependent. A to D, For ROS production, Col-0 leaf disc halves were elicited for their first elicitation (white square) with the indicated concentration of flg22 at 0 min, washed, and reelicited at 60 min (arrow) with 0.1 μm flg22 (black square; n = 24 per treatment). To allow direct comparisons, all shown ROS experiments (A–D) were performed in the same 96-well plate at the same time. Values are mean ± se. This experiment was repeated more than three times with similar results. RLU, relative light units.
Taken together, reelicitation data using two independent PAMP-signaling assays indicate that the inability to reelicit cells with flg22 was specific to the receptor-ligand pair FLS2-flg22. These results also support the hypothesis that ligand-induced degradation of FLS2 contributes to desensitizing host cells to the stimulus.
Vesicular Trafficking Inhibitors Impede Ligand-Induced Degradation of FLS2 and Flg22-Induced ROS Production But Not MAPK Phosphorylation
A recent study provides evidence that treatment with the vesicular trafficking inhibitors Wm and TyrA23 interferes with flg22-induced endocytosis and reduce flg22-induced accumulation of FLS2 in endosomes (Beck et al., 2012). So far, however, it has not been investigated whether these treatments affect flg22 signaling. To this end, we pretreated Col-0 leaf strips with Wm and TyrA23 at previously published inhibitor concentrations and preincubation times of 1 h prior to flg22 elicitation (Beck et al., 2012). Leaf strips were then elicited with 1 µm flg22 as described in our previous experiments to monitor flg22-induced ROS production and MAPK phosphorylation.
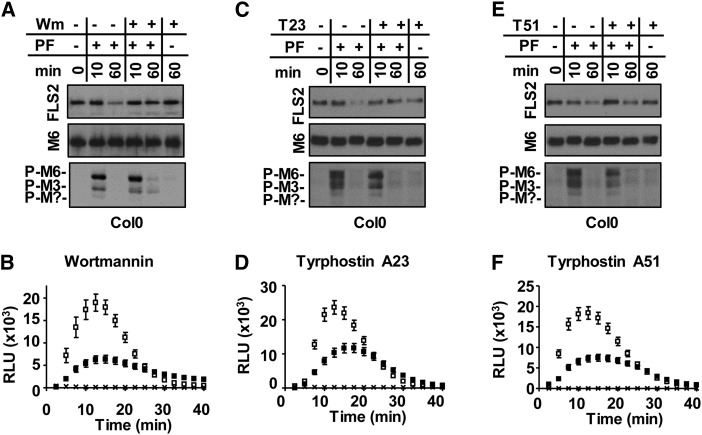
First, we showed that consistent with previous live-cell imaging studies (Robatzek et al., 2006; Beck et al., 2012), pretreatment with Wm resulted in impaired flg22-induced degradation of endogenous FLS2 as determined by immunoblot analyses (Fig. 4A). Flg22-elicited MAPK phosphorylation did not show any apparent difference between Wm- and mock-treated samples (Fig. 4A; compare –Wm/+PF and +Wm/+PF; P-M3 and P-M6 at 10 min), indicating that tissue preincubated with Wm remained flg22-signaling competent for flg22-induced MPK3 and MPK6 phosphorylation to a similar extent as mock-treated tissue. By contrast, we consistently observed a significantly decreased amplitude in flg22-elicited ROS production after Wm treatment compared with mock treatment (Fig. 4B; compare +Wm, black symbols and –Wm, white symbols; P < 0.0001); however, no change in the timing of initiation and attenuation of ROS was observed. In control experiments, no ROS production was observed over the entire time course in mock-treated samples that were pretreated with Wm and then treated with water or dimethyl sulfoxide (DMSO) instead of flg22 at 0 min (Fig. 4B; x, +Wm/–flg22).
Figure 4.
Effects of chemical inhibitors on ligand-induced degradation of FLS2 and flg22 signaling. A and B, Pretreatment with 30 µm Wm. C and D, Pretreatment with 100 µm TyrA23. E and F, Pretreatment with 100 µm TyrA51. For immunoblot analyses (A, C, and E), Col-0 leaf strips were treated with (+) or without (–) chemical inhibitors and elicited with (+) or without (–) 1 µm flg22 for indicated times in minutes. Total protein extracts were probed with αFLS2, αP-p44/42 MAPK to assess FLS2 protein degradation, or flg22-induced phosphorylation of MPK6 (P-M6), MPK3 (P-M3), and an unknown MPK (P-M?). Individual MAPKs were identified by apparent mass. Immunoblots probed with αMPK6 (M6) confirmed MPK6 accumulation and served as loading control. For flg22-induced ROS production (B, D, and F), Col-0 leaf disc halves were treated in the presence (black squares) or absence (white squares) of chemical inhibitors and elicited with 1 µm flg22 at 0 min (n = 24 per treatment). Mock treated samples (x) were pretreated with either inhibitor and treated with DMSO instead of flg22 at 0 min (n = 24 per treatment). To allow for correct comparisons, ROS experiments shown in the same section were performed in the same 96-well plate at the same time. Values are mean ± se. Each experiment was done at least three times with similar results. T23, TyrA23; T51, TyrA51; RLU, relative light units.
Pretreatment with TyrA23 showed similar flg22-elicited response defects as those observed after Wm preincubation. Tissue preincubated with TyrA23 displayed impaired ligand-induced degradation of FLS2 yet unaltered MAPK phosphorylation in response to flg22 (Fig. 4C). TyrA23 pretreatment also led to a significantly reduced amplitude of flg22-induced ROS production (Fig. 4D; compare +T23, black symbols and –T23, white symbols; P < 0.0001; see also Supplemental Fig. S2, A and B). To investigate whether the TyrA23-reduced ROS response could be attributed to TyrA23 being an inhibitor of endocytic internalization or of Tyr kinase activity, we performed the control experiment by pretreating Col-0 leaf strips with Tyrphostin A51 (TyrA51). TyrA51 is a tyrphostin analog known to block Tyr kinase activity and receptor signaling, but in contrast to TyrA23, TyrA51 does not inhibit endocytic internalization of receptors (Banbury et al., 2003; Ortiz-Zapater et al., 2006; Dhonukshe et al., 2007; Konopka and Bednarek, 2008; Leborgne-Castel et al., 2008; Irani et al., 2012). Similar to TyrA23, TyrA51 pretreatment consistently resulted in decreased flg22-induced ROS production compared with mock-treated Col-0 samples (Fig. 4F; see also Supplemental Fig. S2, A and B). Importantly, we observed no reproducible statistically significant differences in flg22-elicited ROS production between TyrA23- and TyrA51-treated samples (Supplemental Fig. S2, A and B; P > 0.5). Similar to TyrA23, TyrA51 did not show any apparent impairment of flg22-elicited MAPK phosphorylation (Fig. 4E). But in contrast to TyrA23, TyrA51 did not impair ligand-induced degradation of FLS2 (Fig. 4E; Supplemental Fig. S2B). Thus, these results indicate that in TyrA23-treated samples, the reduced flg22-elicited ROS production was likely due to the function of TyrA23 as a Tyr kinase inhibitor rather than to its role in blocking ligand-induced endocytosis of FLS2. Taking results from these inhibitor studies together, Col-0 leaf strips exposed to the vesicular trafficking inhibitors Wm and TyrA23 remained signaling competent for MPK3 and MPK6 phosphorylation but were impaired in flg22-induced ROS production.
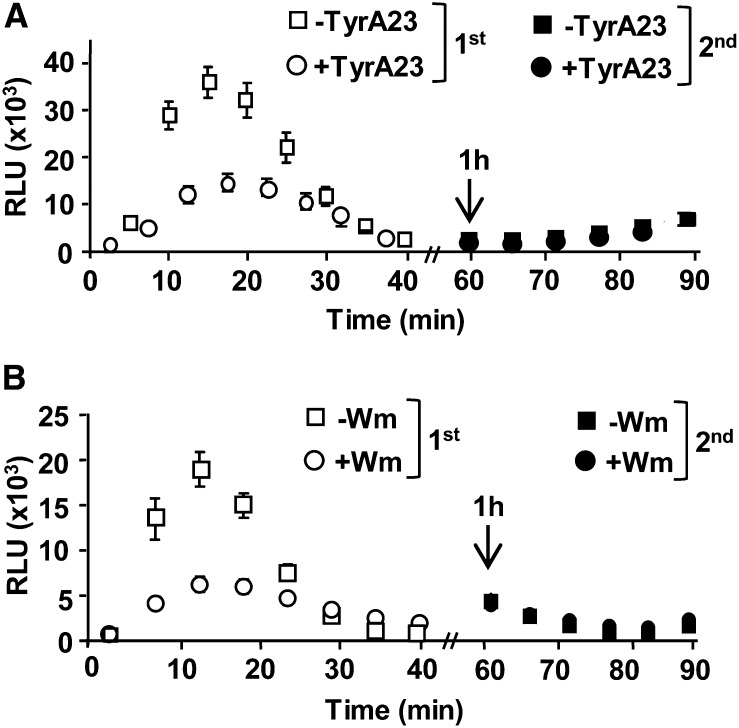
To further determine whether pretreatment with Wm and TyrA23 affected the cell’s ability to reelicit flg22-induced ROS production, we combined inhibitor pretreatment with reelicitation assays. As discussed above, pretreatment with these inhibitors interferes with ligand-induced endocytosis of FLS2, resulting in FLS2 localization at the PM (Robatzek et al., 2006; Beck et al., 2012). For the reelicitation assays, leaf strips were preincubated with inhibitors for 1 h as specified above, washed, and then elicited with 1 µm flg22 to induce ROS production (designated as the first elicitation at 0 min). After 45 to 50 min after the first elicitation, samples were washed, reelicited with 1 µm flg22 at 60 min, and monitored for ROS production (designated as the second elicitation at 60 min). Consistent with our data shown in Figure 4, B and D, TyrA23 or Wm pretreatment resulted in significantly reduced ROS production after the first elicitation with flg22 compared with tissue that was mock pretreated (Fig. 5, A and B, respectively; P < 0.0001). For pretreatment using either inhibitor, reelicitation at 60 min with a second round of flg22 did not induce any ROS production after 10 to 20 min (corresponding to time points at 70–80 min). In addition, no statistically significant difference was observed during the entire time course of the second reelicitation between Col-0 samples that were pretreated with or without these vesicular trafficking inhibitors. ROS production after the second elicitation (at 70- to 80-min time points) was consistently significantly reduced compared with ROS production after the first elicitation (10- to 20-min time points; P < 0.0001). From these reelicitation assays, we conclude that upon pretreatment with Wm and TyrA23, FLS2 was unable to initiate a second elicitation within 1 h after an initial elicitation. Thus, cells appeared to be flg22-signaling incompetent even under conditions at which this receptor remained at the PM due to interference with flg22-induced endocytosis of FLS2 from the PM into endocytic vesicles.
Figure 5.
Cells remain flg22-signaling incompetent after pretreatment with vesicular trafficking inhibitors Wm and TyrA23 following an initial flg22 elicitation. A, Flg22-induced ROS reelicitation after pretreatment with 100 µm TyrA23. B, Flg22-induced ROS reelicitation after pretreatment with 30 µm Wm. For ROS production in A and B, Col-0 leaf disc halves were pretreated for 1 h with (circles) or without (square) chemical inhibitors, washed, elicited with 1 μm flg22 at 0 min (first elicitation, white symbols), and then reelicited at 60 min (arrow; second elicitation, black symbols) with 1 μm flg22. To allow for correct comparisons, ROS experiments shown in the same section (A or B) were performed in the same 96-well plate at the same time (n = 24 per treatment). Experiment was repeated at least three times with similar results. RLU, relative light units.
TyrA23- and Wm-Dependent Inhibition of Flg22-Induced ROS Production Is in Part BAK1 Independent
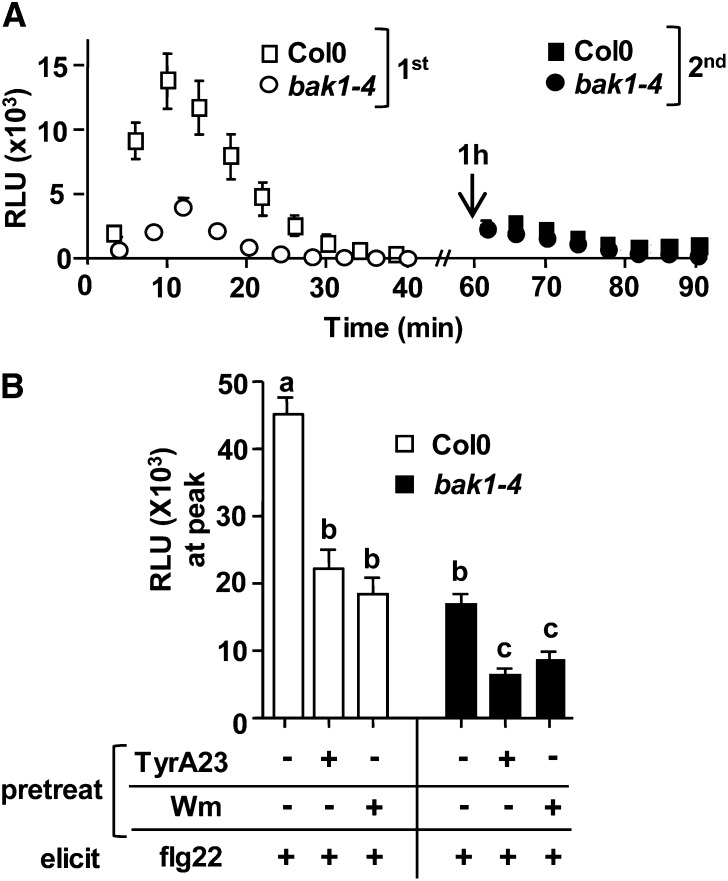
Previous studies reported that the regulatory RLK BAK1 is required for ligand-induced endocytosis of FLS2 because in bak1 mutant plants, FLS2 remains at the PM and is not degraded after flg22 elicitation (Chinchilla et al., 2007; Lu et al., 2011). We confirmed that under our experimental conditions using 4- to 5-week-old bak1-4 leaves, FLS2 was not degraded after 1 h of flg22 elicitation (Supplemental Fig. S2D). Next, we examined whether after an initial flg22 treatment, bak1-4 mutant cells remained ROS-signaling competent using the experimental reelicitation set-up described above. Consistent with previous results (Chinchilla et al., 2007; Heese et al., 2007; Shan et al., 2008), bak1-4 mutants showed significantly reduced but detectable ROS production when elicited at 0 min with flg22 (Fig. 6A; P < 0.0001). After the initial elicitation, bak1-4 mutant tissue was unable to mount a ROS response when samples were reelicited at 60 min with a second round of flg22 (Fig. 6A; 70- to 80-min time point), and no significant difference in ROS production was observed between reelicited bak1-4 and wild-type (Col-0) tissues during the duration of the ROS measurements after the reelicitation (Fig. 6A). From these reelicitation assays, we conclude that similar to Wm and TyrA23 pretreatment, FLS2 was unable to initiate ROS signaling in bak1-4 mutant plants when exposed to a second flg22 elicitation within 1 h after an initial treatment with flg22.
Figure 6.
TyrA23- and Wm-dependent inhibition of flg22-induced ROS is partially independent of BAK1. A, Flg22-induced ROS reelicitation in bak1-4 mutant plants. Col-0 or bak1-4 leaf disc halves were elicited with 1 μm flg22 at 0 min (first elicitation, white symbols) and then reelicited at 60 min (arrow; second elicitation, black symbols) with 1 μm flg22. B, Flg22-induced ROS production after pretreatment with (+) or without (–) 100 µm TyrA23 or 30 µm Wm in Col-0 or bak1-4. For ROS production, Col-0 or bak1-4 leaf disc halves were pretreated for 1 h with (+) or without (–) chemical inhibitors, washed, and then elicited once with 1 μm flg22 at 0 min. ROS peaks (10–12 min postelicitation) are shown in bar graph representation. To allow direct comparisons, ROS experiments shown in the same section were performed in the same 96-well plate at the same time (n = 24 per treatment). Values are mean ± se, and means with different or the same letters are significantly different or not significantly different, respectively (two-tailed Student’s t test, P ≤ 0.001). Experiments were repeated at least three times with similar results. RLU, relative light units.
When compared directly within the same experimental plate set-up, we noticed that during the first flg22 elicitation (elicitation at 0 min), the amplitudes of the initial ROS production were consistently not statistically significantly different between wild-type Col-0 plants pretreated with TyrA23 and nonpretreated bak1-4 mutant plants (Fig. 6B; black bar, –TyrA23/+flg22 and white bar, +TyrA23/+flg22; P > 0.15). Notably, BAK1 undergoes Tyr phosphorylation required for flg22 signaling (Oh et al., 2010, 2011), and BAK1, but not FLS2, contains a YXXɸ motif in its cytoplasmic tail (Geldner and Robatzek, 2008; Robatzek and Wirthmueller, 2013), thus raising the possibility that BAK1 may be the target of the observed TyrA23-dependent inhibitory effects on flg22-ROS production in Col-0. To this end, we directly compared ROS production in Col-0 and bak1-4 leaf strips that were either pretreated with TyrA23 (+TyrA23) or mock pretreated (–TyrA23) prior to their elicitation with 1 µm flg22. Importantly, bak1-4 leaf tissue that was exposed to TyrA23 consistently showed an additional highly significant reduction in flg22-induced ROS production compared with mock-pretreated bak1-4 plants (Fig. 6B; black bar, +TyrA23/+flg22 and +TyrA23/–flg22; P < 0.0001). Furthermore, when either plant line was preincubated with TyrA23, bak1-4 consistently showed an additional significant reduction in flg22-induced ROS production compared with Col-0 (Fig. 6B; white and black bar, +TyrA23/+flg22; P < 0.0001). Similar results were obtained when comparing Col-0 and bak1-4 tissue in the absence and presence of Wm (Fig. 6B; compare white and black bars, –Wm and +Wm/+flg22), indicating that this was not a TyrA23-specific response defect. Based on these chemical interference results in Col-0 and bak1-4 plants, we conclude that BAK1 is unlikely the sole target of TyrA23- and Wm-dependent inhibition of flg22-induced ROS production.
The Protein Synthesis Inhibitor CHX Blocks Ligand-Induced Degradation of FLS2 and Attenuation of Flg22-Signaling Responses
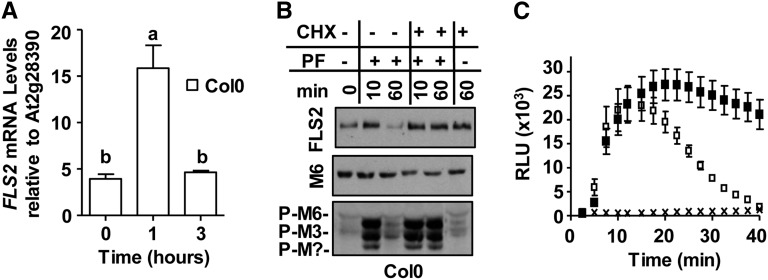
We noticed that flg22 elicitation resulted in a significant decrease but not always a complete loss of FLS2 protein accumulation at 1 h postelicitation (Fig. 1). Because previous studies have shown that flg22 induces FLS2 mRNA accumulation (Zipfel et al., 2004; Denoux et al., 2008), we confirmed first that under the experimental conditions used for our reelicitation assays, flg22 elicitation led to a statistically significant increase in FLS2 mRNA levels within 1 h in Col-0, as determined by quantitative real-time (qRT)-PCR (Fig. 7A; 0 and 1 h, P < 0.0001). This increase was transient in that there was no statistically significant difference in FLS2 mRNA levels at 3 h postelicitation compared with 0 h (Fig. 7A; 0 and 3 h, P > 0.2). Next, we used immunoblot analyses to examine whether FLS2 protein detected at 1 h after flg22 elicitation represented newly synthesized FLS2 by treating Col-0 leaf strips with the protein translation inhibitor CHX. Interestingly, in the presence of CHX, FLS2 protein did not undergo apparent ligand-induced degradation at 1 h in response to flg22 compared with mock-treated samples (Fig. 7B; FLS2, –CHX/+flg22 and +CHX/+flg22). Similar results were obtained for Ler seedlings (Supplemental Fig. S3A; FLS2). CHX treatment also inhibited attenuation of flg22-signaling responses in that MPK3 and MPK6 remained phosphorylated at 60 or 90 min post flg22 elicitation in the presence of CHX (+CHX/+PF) but not in the absence (–CHX/+PF) in Col-0 leaf tissue or Ler seedlings, respectively (Fig. 7B; Supplemental Fig. S3A; P-M6 and P-M3). At least for MPK6, this lack in phosphorylation attenuation could not be attributed to changed MAPK protein levels because MPK6 protein levels did not increase at 60 min in the presence of CHX compared with the absence of CHX at 60 min postelicitation (Fig. 7B; Supplemental Fig. S3A; M6). Similarly, the duration of flg22-induced ROS production was greatly prolonged in the presence but not in the absence of CHX (Fig. 7C). Treatment with CHX alone did not significantly induce MAPK phosphorylation or ROS production (Fig. 7, B and C, respectively). In contrast to duration, the amplitude of flg22-induced ROS production was not significantly affected. Elevated ROS levels after treatment with flg22 plus CHX were not observed in respiratory burst oxidase-D (rbohD) null mutant plants (Supplemental Fig. S3B), which lack functional RbohD, the PM-localized NADPH oxidase responsible for PAMP-dependent extracellular ROS production (Nühse et al., 2007; Zhang et al., 2007; Mersmann et al., 2010; Torres, 2010). Thus, prolonged ROS production in CHX-treated tissue was fully dependent on RbohD. These results are consistent with the idea that de novo synthesis of yet unknown protein(s) contribute to ligand-induced degradation of FLS2 and attenuation of flg22-induced ROS and MAPK phosphorylation.
Figure 7.
Ligand-induced degradation of FLS2 and attenuation of flg22-signaling responses is impaired by treatment with the protein synthesis inhibitor CHX. A, Flg22-induced expression levels of FLS2 mRNA. Col-0 leaf strips were elicited for 40 to 45 min with 1 μm flg22, washed, and incubated in the absence of flg22 until indicated times. Samples were processed for qRT-PCR using At2g28390 as the reference gene. For each time point, results of at least three independent experiments containing three biological and three technical repeats are shown. Values are mean ± se, and means with different or the same letters are significantly different or not significantly different, respectively (two-tailed Student’s t test, P ≤ 0.003). B, Effect of CHX on ligand-induced degradation of FLS2 and flg22-signaling responses. For immunoblot analyses, total protein extracts were probed with αFLS2, αP-p44/42 MAPK, or αMPK6 (M6) as in Figure 4. C, Effect of CHX on flg22-induced ROS production. Col-0 leaf disc halves (n = 24 per treatment) were cotreated in the presence (black squares) or absence (white squares) of 50 µm CHX and elicited with 1 µm flg22 at 0 min. Mock-treated samples (x) were treated with CHX and DMSO (instead of flg22) at 0 min (n = 24 per treatment). To allow for correct comparisons, ROS experiments shown in C were performed in the same 96-well plate at the same time. RLU, relative light units; P-M?, phosphorylated unknown MPK.
Resensitization of Cells to Flg22 Positively Correlates with de Novo Protein Synthesis of FLS2
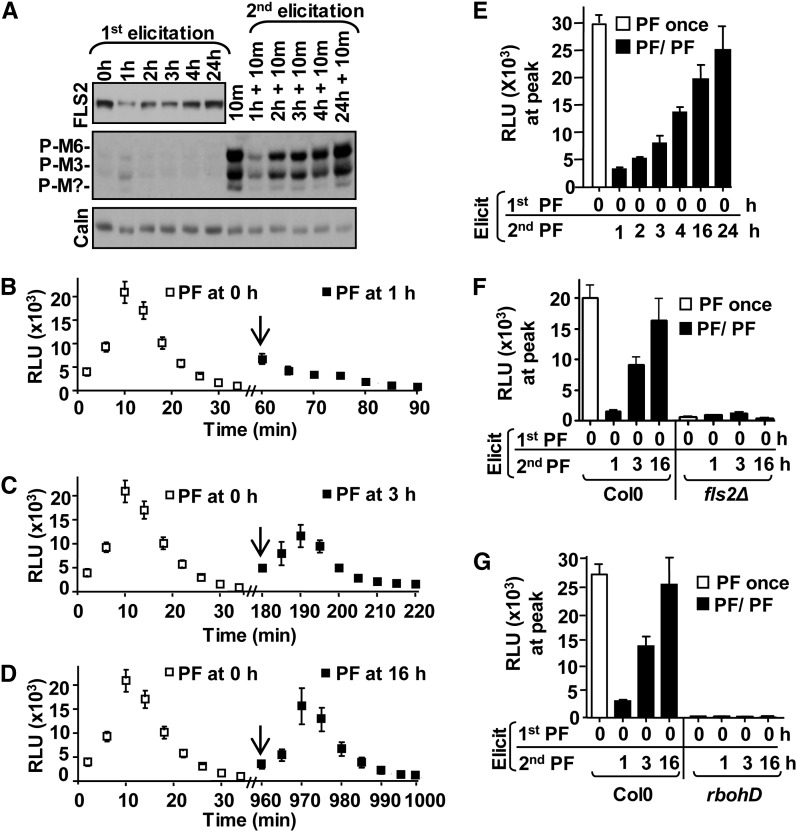
So far, it remains unknown whether the increase in FLS2 mRNA (Fig. 6A; Zipfel et al., 2004; Denoux et al., 2008) correlates with increased FLS2 protein accumulation and, if so, whether newly synthesized receptors are flg22-signaling competent and result in resensitization of the tissue. To this end, samples were pulse treated with flg22 for 45 to 50 min and incubated for up to 24 h in the absence of ligand. After the initial flg22-dependent FLS2 degradation (1 h), FLS2 protein levels began to be restored over 24 h postelicitation (Fig. 8A). No protein of similar apparent Mr was detected in fls2∆ null mutants (Supplemental Fig. S3C), confirming that the protein of interest was FLS2. To verify that FLS2 accumulation at later times was due to de novo protein synthesis in Col-0 leaf strips, we adjusted our experimental design because CHX inhibited flg22 degradation of FLS2 (Fig. 7B). Tissue was first incubated with flg22 for 1 h in the absence of CHX. At 1 h postelicitation, corresponding to the time at which FLS2 was mostly degraded, samples were incubated for an additional 2 h in the absence (–) or presence (+) of CHX. When comparing FLS2 protein accumulation at 3 h (Supplemental Fig. S3D), CHX-treated samples (+CHX/+flg22) did not accumulate additional FLS2 protein, whereas in the absence of CHX (–CHX/+flg22), FLS2 protein levels increased at 3 h postelicitation. These results are in agreement with de novo FLS2 protein synthesis at later times after an initial ligand-induced degradation of FLS2 (1 h).
Figure 8.
FLS2 protein replenishment leads to resensitization to flg22 that is FLS2 and RbohD dependent. A, After initial ligand-induced degradation of FLS2, subsequent increased new FLS2 protein levels positively correlated with flg22-induced MAPK phosphorylation. After a first flg22 elicitation at 0 min for 45 to 50 min, Col-0 leaf strips were washed and incubated in the absence of flg22 until indicated times. For reelicitation, samples were reelicited with flg22 at indicated hours for 10 min. Immunoblot analysis of total protein extracts was done as in Figure 4. B to D, Resensitization of flg22-induced ROS production. All Col-0 leaf disc halves were elicited with flg22 at 0 h for 45 min (n = 60 per treatment). After a wash step, subsets of leaf disc halves (n = 20 per treatment) were reelicited at 1 (B), 3 (C), or 16 h (D). Arrows indicate times of second elicitation with flg22. ROS experiments shown in B, C, and D were set-up in the same 96-well plate to allow for direct comparison. E, Bar graph representation showing ROS peak (10–12 min) after first elicitation and second reelicitation with active flg22 at indicated hours (n = 10 per treatment). F, ROS resensitization is FLS2 dependent. G, ROS resensitization is RbohD dependent. For F and G, wild-type (Col-0), fls2, or rbohD leaf tissue was treated as in B to D at indicated times. For E to G, white bars represent ROS peak production 10 to 12 min after the first elicitation at 0 min (PF once), and black bars represent ROS peak production 10 to 12 min after reelicitation of tissue subsets for a second time at indicated times (PF/PF). ROS experiments shown in the same panel were set-up in the same 96-well plate. All experiments were elicited with 0.1 μm flg22 and done at least three times with similar results. Values are mean ± se. RLU, relative light unit; P-M?, phosphorylated unknown MPK; Caln, calnexin.
To assess whether newly synthesized FLS2 was signaling competent, samples were first elicited at 0 h with flg22 for 45 min, washed, and kept in water until 1, 2, 3, 4, or 24 h. For MAPK activation, control samples (only first elicitation) were collected at indicated times after initial flg22 elicitation. Samples from tissue reelicited with flg22 were collected 10 min after the second elicitation. A 10-min sample (i.e. initial 10-min response to flg22) served as a positive control. Consistent with Figure 3D, MAPK phosphorylation was detected at 10 min after the first elicitation (Fig. 8A, 10 min) but only minimally upon reelicitation at 1 h (1 h plus 10 min). By contrast, flg22 was able to reelicit strong MAPK phosphorylation at later times between 2 and 24 h, and the intensity of MAPK phosphorylation correlated with increased FLS2 protein accumulation. Using a similar experimental design for ROS reelicitation, tissue that was initially treated with flg22 also displayed significant flg22-induced ROS production after reelicitation at 3 and 16 h (Fig. 8, C and D). This gradual increase in ROS production over time, reflecting recovery of flg22-signaling competency, is highlighted in the bar graph representation of ROS production (Fig. 7E). No ROS production was observed in fls2∆ (Fig. 8F) or rbohD (Fig. 8G) null mutants, indicating that ROS reelicitation responses were dependent on functional FLS2 and RbohD. The RbohD requirements also confirmed that ROS detected at later times were not due to other mechanisms of ROS production. In conclusion, results from two independent reelicitation assays correlated restoration of flg22-signaling competency with increased accumulation of newly synthesized FLS2. Our results are consistent with the hypothesis that after the initial flg22-dependent endocytic degradation of FLS2 (at 1 h), de novo synthesis of FLS2 at later times (>1 h) may be a means to prepare cells for a new round of flg22 perception, thus to resensitize cells to the stimulus.
DISCUSSION
In this study, we provided evidence that in Arabidopsis tissue commonly used for flg22-signaling and bacterial pathogen infection assays, endogenous, nontagged FLS2 protein was degraded in a ligand-, time-, and dose-dependent manner within 60 min postelicitation (Fig. 1). Thus, our results are in agreement with endogenous FLS2 undergoing ligand-induced endocytic degradation in a flg22-dependent manner shown in a previous live-cell imaging study using ectopically expressed FLS2-GFP (Robatzek et al., 2006). Furthermore, our study demonstrated that degradation of endogenous FLS2 occurred after elicitation with active flg22 but not inactive flg22 (Felix et al., 1999) or the unrelated PAMP peptide elf26 that is perceived by and signals through a different RLK, namely EFR (Zipfel et al., 2006). Our work provided ligand specificity and dose dependency, thus expanding on a previous study reporting that in 12-day-old seedlings, endogenous FLS2 is degraded 30 min postelicitation with 1 µm active flg22 (Lu et al., 2011). Another finding of our study was that ligand-induced degradation of endogenous FLS2 was observed even at 0.1 µm flg22, a concentration commonly used for flg22 response assays and 100- to 1,000-fold lower than that used for live-cell imaging studies of FLS2-GFP (Robatzek et al., 2006; Beck et al., 2012; Choi et al., 2013).
To date, our understanding of ligand-induced endocytosis of FLS2 is based largely on information gained from live-cell imaging analysis. While these studies have provided invaluable information on the spatial and temporal dynamics of FLS2 after flg22 elicitation in vivo (Robatzek et al., 2006; Beck et al., 2012; Choi et al., 2013), they have not addressed in detail whether ligand-induced endocytosis of FLS2 influences signaling to recurrent or different PAMPs. This work complements previous imaging studies, in that we used biochemical and physiological signaling assays to assess whether Arabidopsis tissue remained signaling competent or endured a desensitization or refractory period after an initial flg22 stimulus. Using two independent flg22-signaling responses (ROS production and MAPK phosphorylation), we demonstrated that wild-type Col-0 leaf tissue that initially responded to active flg22 was unable to reelicit a subsequent signaling response when exposed to a second round of active flg22 between 20 to 60 min after the initial flg22 elicitation. This flg22-dependent desensitization response was dose dependent and correlated with the dose dependency observed for the flg22-induced degradation of FLS2. Elicitation with 0.1 or 1 µm flg22 consistently resulted in a significant decrease but not a complete loss in FLS2 protein accumulation at 60 min. Based on the inability to reelicit flg22 signaling after an initial flg22 treatment at those concentrations, a subset of FLS2 molecules may be in an altered conformational state or sequestered to a subcellular location that prevents these receptors from perceiving flg22 and/or inducing flg22 events. In the latter scenario, FLS2 may be present in endocytic vesicles en route for protein degradation or in parts of the secretory pathway en route to the PM, the site of flg22 perception. Consistent with this hypothesis, lack of signal initiation at 20 min after the initial flg22 elicitation correlated with the time at which FLS2 is reported in intracellular vesicles (Robatzek et al., 2006). In contrast to elicitation with active flg22, cells initially elicited with elf26 or inactive flg22 remained signaling competent to a second elicitation with active flg22 (Fig. 2; Supplemental Fig. S1). Similarly, after a first exposure to flg22, cells were able to mount a subsequent elf26-induced ROS response within 20 min (Fig. 2E). These signaling results were consistent with the observation that after an initial elicitation with inactive flg22 or elf26, FLS2 protein was not degraded but accumulated to comparable levels as prior to the initial treatment (Fig. 1).
Taken together, these results indicate that flg22-induced degradation of FLS2 affected homologous but not heterologous desensitization of cells to bacterial PAMPs (Figs. 2 and 3; Supplemental Fig. S1). Homologous or heterologous desensitization refers to the inability of cells to respond to the same or a different stimulus, respectively, after cells have been exposed to an initial stimulus (Chandra et al., 2000). Thus, our signaling data were in agreement with previous studies reporting dose-dependent homologous but not heterologous desensitization to specific PAMP stimuli in tomato (Solanum lycopersicum), tobacco (Nicotiana tabacum), or soybean (Glycine max) cell-cultured cells (Legendre et al., 1993; Granado et al., 1995; Felix et al., 1998; Chandra et al., 2000; Kadota et al., 2006); however, none of these studies have addressed desensitization to flg22. Furthermore, we performed in planta experiments utilizing Arabidopsis leaf tissue, thus enabling us to start to address the genetic requirements for the desensitization (and resensitization, see below) responses for the FLS2/flg22 system. We found that under conditions in which the initial flg22-elicited ROS production and MAPK phosphorylation are reduced (bak1 loss-of-function mutant), homologous desensitization to flg22 still occurred. Our study also investigated responses for a bone fide receptor-ligand pair for which both the receptor (FLS2) and its ligand (flg22) were known at the time of the experiments, and importantly, ligand-induced endocytosis of its receptor has been reported. Such knowledge and the availability of an αFLS2 antibody allowed us to correlate flg22-signaling competency (desensitization and resensitization) with receptor abundance in the context of ligand-induced degradation of FLS2. Interestingly, a very recent study demonstrated that pretreatment with flg22 enhances ROS production to subsequent perception of AtPeps (Flury et al., 2013). AtPeps are small, plant-derived peptides recognized by RLKs, specifically, the Arabidopsis peptide receptors AtPEPR1 and AtPEPR2, and are proposed to function as endogenous amplifiers of innate immune response in plants (Boller and Felix, 2009). In agreement with our data, Flury et al. (2013) noted homologous desensitization in Arabidopsis leaf discs in response to various stimuli including flg22, but so far, a further or more detailed analysis of these homologous desensitization responses or the cellular fate of any of the involved RLKs (including AtPEPRs) has not been reported.
Homologous desensitization and the observation of a refractory period to a given stimulus may occur through a combination of molecular mechanisms that may include, but are not limited to, the removal and degradation of ligand-bound activated receptors from the cell surface, attenuation of signaling through the regulation of receptor activity, and/or receptor abundance at the site of perception. Our data are in agreement with flg22-induced degradation of FLS2 serving as a mechanism to desensitize cells to flg22 and, upon repetitive flg22 stimulation, to prevent continuous receptor-stimulated signal output. It is also tempting to speculate that ligand-induced degradation of FLS2 may contribute to flg22 signal attenuation because in the presence of the protein synthesis inhibitor CHX, we observed prolonged ROS production and MAPK phosphorylation as well as a block in FLS2 degradation after flg22 elicitation (Fig. 7). But one needs to be cautious with such interpretation because CHX is known to have pleiotropic effects in that it inhibits translation of a large number of target proteins. Furthermore, as evident from our Wm and TyrA23 results (Fig. 4), a block in flg22-induced degradation of FLS2 does not necessarily result in prolonged ROS production or MAPK phosphorylation. As an alternative explanation, CHX treatment (Fig. 7, B and C) may likely block the de novo synthesis of protein(s) that contribute to ligand-induced degradation of FLS2 as well as flg22-signaling responses and perhaps function independently. Regulation of flg22-signaling responses appears to be under the control of multiple negative regulators including phosphatases and E3 ligases (Gómez-Gómez et al., 2001; Schwessinger and Zipfel, 2008; Trujillo et al., 2008; Lu et al., 2011), and some of these may be short lived. In agreement, flg22-induced gene transcription has also been proposed to be under the control of rapidly turned-over repressors, based on the substantial overlap in transcriptional up-regulation of genes in response to CHX and flg22 (Navarro et al., 2004). De novo synthesis of short-lived phosphatases is also known to be required for attenuation of MAPK phosphorylation (Schweighofer et al., 2007), and the phosphatase inhibitor type 2A cantharidin disrupts flg22-induced FLS2 endocytosis and results in sustained ROS production after flg22 elicitation (Serrano et al., 2007). As is likely for CHX, this phosphatase inhibitor would probably also have multiple targets. Furthermore, the U-box E3 ligases Plant U-Box12 (PUB12) and PUB13, which form a BAK1-dependent complex with FLS2 and polyubiquitinate FLS2 in response to flg22, function as negative regulators of flg22-signaling responses (Lu et al., 2011). But in contrast to CHX-treated tissue (Fig. 7), mutations in PUB12 or PUB13 lead to an increase in amplitude rather than duration of flg22-induced ROS production (Lu et al., 2011), suggesting that these E3 ligases and CHX appear to affect flg22 responses differently, potentially by targeting diverse components of the flg22-signaling network.
Another potential role for ligand-induced endocytic degradation of FLS2 may be to remove ligand-bound receptor molecules from the site of stimulus perception, the PM. Consistent with flg22 binding to intact Arabidopsis tissue being nonreversible (Bauer et al., 2001), we found that Arabidopsis tissue was unable to mount a second round of flg22-induced ROS production, even under conditions in which cells have been previously shown to sequester FLS2 at the PM. These conditions included wild-type Col-0 tissue pretreated with Wm or TyrA23 and subsequent elicitation with flg22 (Fig. 5) as well as bak1 mutant plants after an initial flg22 elicitation (Fig. 6A; see also below). It should be noted that so far, it remains unknown, whether in response to CHX or in pub12/pub13 mutant plants, FLS2 accumulates at the PM or within an endosomal compartment due to a block in flg22-induced internalization of FLS2 from the PM or trafficking within endosomal compartments, respectively. In conclusion, we propose that the observed flg22-induced receptor degradation may play a significant role in turning over ligand-occupied FLS2.
It is becoming increasingly clear that flg22 perception leads to activation of a flg22-signaling network rather than a single linear signaling pathway and that MAPK phosphorylation and ROS production appear to be part of two separate signaling branches of this network (Lu et al., 2009; Korasick et al., 2010; Tena et al., 2011). It is possible that mechanisms regulating these signal pathways may be distinct and separable. In agreement with this hypothesis, we found that Col-0 leaf strips exposed to the vesicular trafficking inhibitors Wm and TyrA23 showed reduced ROS production yet unaltered MAPK phosphorylation when elicited with flg22. Importantly, flg22-induced phosphorylation of MAPK showed that the overall ability of cells to perceive flg22 was not impaired. Although we cannot formally exclude the possibility, it is unlikely that Wm and TyrA23 acted on the same component(s) involved in ROS signaling because these inhibitors are known to affect different cellular targets and have distinct mode of actions (Banbury et al., 2003; Munnik and Nielsen, 2011). Interestingly, in addition to their inhibitory function on stimulated endocytosis, Wm and TyrA23 have been previously reported to negatively affect ROS production in a RbohD-dependent manner in response to salt stimulus (Leshem et al., 2007) and to the fungal elicitor cryptogein (Leborgne-Castel et al., 2008), respectively. Similar to our findings, pretreatment with TyrA51 resulted in impaired cryptogein-induced ROS production but did not interfere significantly with endocytic vesicle formation in response to cryptogein through a yet unknown receptor (Leborgne-Castel et al., 2008).
Considering that both TyrA23 and TyrA51 are known inhibitors of Tyr kinase activity (Banbury et al., 2003) and both of these tyrphostins impaired flg22-induced ROS production, Tyr kinase activity appears to be necessary to initiate a full and robust ROS production following flg22 elicitation. By contrast, flg22-induced endocytic degradation of FLS2 was only impaired after pretreatment with TyrA23 (but not TyrA51), suggesting that Tyr kinase activity may not play a significant role in flg22-induced endocytic degradation of FLS2. Because pretreatment with TyrA23 or TyrA51 did not appear to alter MAPK phosphorylation, we propose that Tyr kinase activity and/or the endocytic degradation of FLS2 may not be necessary to initiate MAPK phosphorylation. Furthermore, our results support the hypothesis that the inhibitory effect of TyrA23 on ligand-induced degradation of FLS2 could be attributed to an inhibition of endocytic vesicle formation by disrupting interaction between the µ2 adapter protein and YXXɸ-containing receptors (Banbury et al., 2003; Ortiz-Zapater et al., 2006; Konopka and Bednarek, 2008). Such YXXɸ internalization motif is present in BAK1 but absent from FLS2. Despite the fact that BAK1 contains a YXXɸ motif (Geldner et al., 2007) and shows Tyr phosphorylation required for some flg22-signaling responses (Oh et al., 2010, 2011), our chemical interference results in Col-0 and bak1-4 plants indicated that BAK1 was unlikely the only target of TyrA23-dependent inhibition of flg22-induced ROS production. Thus, we propose that other, yet unknown YXXɸ-containing protein(s) or component(s) targeted by TyrA23 or Wm appear to participate in flg22-induced ROS production that function either downstream of or in a parallel pathway to BAK1.
At later times (>2 h) after the initial flg22 treatment, time-dependent resensitization of flg22 signaling correlated with increased accumulation of FLS2 protein after the initial flg22-induced degradation of FLS2. Consistent with the increased accumulation of FLS2 protein being due to new FLS2 protein synthesis, it could be blocked by the protein synthesis inhibitor CHX (Supplemental Fig. S3D; Beck et al., 2012). Based on the resensitization of Col-0 tissue to flg22 being FLS2 dependent (Fig. 8F; Supplemental Fig. S3C), we propose that this new synthesis of FLS2 protein served as a means to prepare cells for a new round of flg22 perception. This hypothesis is congruent, as cells are likely to encounter flagellated bacteria multiple times throughout their lifecycle. During an initial encounter, ligand-induced degradation of FLS2 may aid in turning over bound receptors and/or preventing continuous flg22 signaling, which is known to be detrimental for plants because it diverts valuable cellular resources from growth and development to signaling. Subsequent replenishment of signaling-competent FLS2 is conceivable because cells unable to reinstate degraded receptors with newly synthesized FLS2 would lose their ability to perceive flagellin and initiate immune responses during subsequent infections. In agreement, cells lacking functional FLS2 exhibit enhanced susceptibility to bacterial infection (Zipfel et al., 2004; Li et al., 2005; Hann and Rathjen, 2007; Zeng and He, 2010). Such replenishment scenario may provide insight into why pathogenic bacteria evolved mechanisms to inject effectors into the host cell that target components of the FLS2 receptor complex for degradation (Göhre et al., 2008; Shan et al., 2008; Zhang et al., 2010). Because effector delivery is assumed to occur after pattern-triggered immunity response initiation (Block and Alfano, 2011), effectors that target the FLS2 complex components may interfere with the reestablishment of flg22-signaling competency of cells rather than with the initial detection of bacteria and flg22-response initiation.
In summary, we provide in planta evidence that endogenous FLS2 underwent ligand-induced degradation in a ligand-, time-, and dose-dependent manner. Results from flg22 reelicitation assays for two independent flg22-signaling responses (MAPK phosphorylation and ROS production) support the hypothesis that ligand-induced degradation of FLS2 plays a role in desensitizing host cells to the stimulus. Degradation of ligand-bound FLS2 is likely required for receptor turnover from the cell surface. Subsequent replenishment of newly synthesized FLS2 to the site of stimulus perception resulted in resensitization, probably to prepare cells for a new round of flg22 perception and signaling. We also report that flg22-induced ROS production and FLS2 degradation responses can be separated from MAPK phosphorylation through the use of vesicular trafficking inhibitors (Wm and TyrA23), thus highlighting the value of utilizing vesicular trafficking inhibitors to gain insights into their effect on stimulus-dependent immune signaling as well as trafficking events.
MATERIALS AND METHODS
Chemicals and PAMPs
Unless otherwise specified, all chemicals were from Fisher Scientific. CHX (Sigma-Aldrich) was used at a final concentration of 50 μm for Col-0 leaves and 100 μm for Ler seedlings at indicated times. TyrA23 and TyrA51 were purchased from Santa Cruz Biotechnology and used at a final concentration of 100 µm (Beck et al., 2012). Wm (Sigma-Aldrich) was used at a final concentration of 30 µm (Beck et al., 2012). For CHX treatment, Col-0 leaf tissue was cotreated by floating tissue in solution containing both CHX and flg22. For all other inhibitor treatments, Col-0 leaf tissue was pretreated by floating leaf strips in water-containing inhibitor for 1 h prior to subsequent elicitation with indicated flg22 concentration. PAMP peptides (Korasick et al., 2010) were made by GenScript and used at indicated concentrations and times.
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) seedling and plant growth was at 22°C as described (Heese et al., 2007), except that after transplanting, plants were grown in an 8-h-light/16-h-dark photoperiod at 82 μmol m–2 s–1. fls2-24 is in Ler background (Gómez-Gómez et al., 1999); fls2Δ, bak1-4, and rbohD are in Col-0 background (Torres et al., 2002; Heese et al., 2007). Fully expanded rosette leaves were used from 4- to 5-week-old plants for all samples unless otherwise noted. After cutting leaf tissue or transfer of seedlings, all samples were floated on distilled water overnight at 22°C in continuous light to reduce wounding response prior to experimental assays.
Immunoblot Analysis and Antibodies
For sample elicitation for immunoblot analysis, three leaf discs (each 1.5 cm2, cut into five strips) of 4- to 5-week-old plants or four to five intact seedlings (7–8 d old) were elicited with indicated concentrations of specified PAMP for 45 to 50 min, washed twice with 1 mL distilled water to remove excess PAMP, and incubated in distilled water at 22°C until flash frozen in liquid nitrogen at indicated times. For chemical interference assays, samples were pretreated for 1 h with specified chemical inhibitors prior to flg22 elicitation. For reelicitation of MAPK phosphorylation, samples were initially elicited and washed as indicated above, but then incubated with the indicated PAMP for an additional 10 min prior to freezing. All samples were stored at –80°C. Sample preparation and immunoblot analyses of total proteins using antibody concentrations were done as described (Heese et al., 2007). An exception is that αP-p44/42 MAPK (1:3,000; #4370; Cell Signaling Tech) was used to detect phosphorylated MAPKs.
Apoplastic ROS Production
One-half of a cut leaf disc (1.1 cm2) of 4- to 5-week-old plants were used for individual ROS production measurements using a luminol-based assay (Heese et al., 2007). For ROS reelicitation, samples were first elicited for 40 min with indicated PAMP at specified concentration, washed twice with 150 μL of distilled water, and placed at 22°C under continuous light until indicated times of reelicitation. For chemical interference assays, samples were pretreated for 1 h with specified chemical inhibitors prior to flg22 elicitation. To allow for correct comparison, ROS experiments shown within the same figure were set-up at the same time and performed in the same 96-well plate. Although absolute values of ROS production (displayed as relative light units) varied from experiment to experiment, the actual trends were always the same.
qRT-PCR Analysis
For pulse elicitation, four leaf discs (each 1.1 cm2, cut into five strips) of 4- to 5-week-old plants were elicited with 1 µm flg22 for 40 min, washed twice, and kept in distilled water at 22°C until flash frozen in liquid nitrogen at indicated times. Total RNA was isolated and real-time PCR reactions were performed and analyzed as described (Korasick et al., 2010; Anderson et al., 2011) with FLS2-f 5′-TCTGATGAAACTTAGAGGCAAAGCG-3′ and FLS2-r 5′-CGTAACAGAGTTTGGCAAAGTCG-3′ primers using the expression of At2G28390 (SAND family protein gene) to normalize all qRT-PCR results (Anderson et al., 2011).
Statistical Analysis
Each experiment was done at least three independent times with similar results. Statistical significances based on unpaired two-sample Student’s t test were determined with Graph Pad Prism4 software.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. flg22-dependent desensitization is PAMP-dependent.
Supplemental Figure S2. Specificity of Tyrphostin A23 and Tyrphostin A51 in flg22-induced ROS production and ligand-induced degradation of FLS2.
Supplemental Figure S3. Prolonged activation of flg22-signaling responses in the presence of CHX is RbohD-dependent.
Acknowledgments
We thank David Korasick (University of Missouri) for technical assistance, Drs. Scott Peck, Walter Gassmann, and Gary Stacey and their lab members (University of Missouri) for helpful discussions and comments, Dr. Scott Peck (University of Missouri) for rbohD plant line and MPK6 antibody, and Dr. Neil E. Hoffman (U.S. Department of Agriculture, Maryland) for calnexin antibody.
Glossary
- PAMP
pathogen-associated molecular pattern
- PM
plasma membrane
- RLK
receptor-like kinase
- ROS
reactive oxygen species
- MAPK
mitogen-activated protein kinase
- Wm
Wortmannin
- TyrA23
Tyrphostin A23
- TyrA51
Tyrphostin A51
- CHX
cycloheximide
- Ler
ecotype Landsberg erecta
- Col-0
ecotype Columbia
- qRT
quantitative real-time
- DMSO
dimethyl sulfoxide
References
- Anderson JC, Bartels S, González Besteiro MA, Shahollari B, Ulm R, Peck SC. (2011) Arabidopsis MAP Kinase Phosphatase 1 (AtMKP1) negatively regulates MPK6-mediated PAMP responses and resistance against bacteria. Plant J 67: 258–268 [DOI] [PubMed] [Google Scholar]
- Banbury DN, Oakley JD, Sessions RB, Banting G. (2003) Tyrphostin A23 inhibits internalization of the transferrin receptor by perturbing the interaction between tyrosine motifs and the medium chain subunit of the AP-2 adaptor complex. J Biol Chem 278: 12022–12028 [DOI] [PubMed] [Google Scholar]
- Bauer Z, Gómez-Gómez L, Boller T, Felix G. (2001) Sensitivity of different ecotypes and mutants of Arabidopsis thaliana toward the bacterial elicitor flagellin correlates with the presence of receptor-binding sites. J Biol Chem 276: 45669–45676 [DOI] [PubMed] [Google Scholar]
- Beck M, Zhou J, Faulkner C, MacLean D, Robatzek S. (2012) Spatio-temporal cellular dynamics of the Arabidopsis flagellin receptor reveal activation status-dependent endosomal sorting. Plant Cell 24: 4205–4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block A, Alfano JR. (2011) Plant targets for Pseudomonas syringae type III effectors: virulence targets or guarded decoys? Curr Opin Microbiol 14: 39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G. (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Chandra S, Cessna SG, Yahraus T, Devine R, Low PS. (2000) Homologous and heterologous desensitization and synergy in pathways leading to the soybean oxidative burst. Planta 211: 736–742 [DOI] [PubMed] [Google Scholar]
- Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. (2006) The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 18: 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Shan L, He P, de Vries S, Kemmerling B. (2009) One for all: the receptor-associated kinase BAK1. Trends Plant Sci 14: 535–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, Felix G, Boller T. (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Choi SW, Tamaki T, Ebine K, Uemura T, Ueda T, Nakano A. (2013) RABA members act in distinct steps of subcellular trafficking of the Flagellin Sensing2 receptor. Plant Cell 25: 1174–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoux C, Galletti R, Mammarella N, Gopalan S, Werck D, De Lorenzo G, Ferrari S, Ausubel FM, Dewdney J. (2008) Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol Plant 1: 423–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, Friml J. (2007) Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol 17: 520–527 [DOI] [PubMed] [Google Scholar]
- Dunning FM, Sun W, Jansen KL, Helft L, Bent AF. (2007) Identification and mutational analysis of Arabidopsis FLS2 leucine-rich repeat domain residues that contribute to flagellin perception. Plant Cell 19: 3297–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emans N, Zimmermann S, Fischer R. (2002) Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell 14: 71–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Baureithel K, Boller T. (1998) Desensitization of the perception system for chitin fragments in tomato cells. Plant Physiol 117: 643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18: 265–276 [DOI] [PubMed] [Google Scholar]
- Flury P, Klauser D, Schulze B, Boller T, Bartels S. (2013) The anticipation of danger: microbe-associated molecular pattern perception enhances AtPep-triggered oxidative burst. Plant Physiol 161: 2023–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Hyman DL, Wang X, Schumacher K, Chory J. (2007) Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev 21: 1598–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Robatzek S. (2008) Plant receptors go endosomal: a moving view on signal transduction. Plant Physiol 147: 1565–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göhre V, Spallek T, Häweker H, Mersmann S, Mentzel T, Boller T, de Torres M, Mansfield JW, Robatzek S. (2008) Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr Biol 18: 1824–1832 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Bauer Z, Boller T. (2001) Both the extracellular leucine-rich repeat domain and the kinase activity of FSL2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell 13: 1155–1163 [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L, Felix G, Boller T. (1999) A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J 18: 277–284 [DOI] [PubMed] [Google Scholar]
- Granado J, Felix G, Boller T. (1995) Perception of fungal sterols in plants (subnanomolar concentrations of ergosterol elicit extracellular alkalinization in tomato cells). Plant Physiol 107: 485–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann DR, Rathjen JP. (2007) Early events in the pathogenicity of Pseudomonas syringae on Nicotiana benthamiana. Plant J 49: 607–618 [DOI] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 104: 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani NG, Di Rubbo S, Mylle E, Van den Begin J, Schneider-Pizoń J, Hniliková J, Šíša M, Buyst D, Vilarrasa-Blasi J, Szatmári AM, et al. (2012) Fluorescent castasterone reveals BRI1 signaling from the plasma membrane. Nat Chem Biol 8: 583–589 [DOI] [PubMed] [Google Scholar]
- Ito E, Fujimoto M, Ebine K, Uemura T, Ueda T, Nakano A. (2012) Dynamic behavior of clathrin in Arabidopsis thaliana unveiled by live imaging. Plant J 69: 204–216 [DOI] [PubMed] [Google Scholar]
- Kadota Y, Fujii S, Ogasawara Y, Maeda Y, Higashi K, Kuchitsu K. (2006) Continuous recognition of the elicitor signal for several hours is prerequisite for induction of cell death and prolonged activation of signaling events in tobacco BY-2 cells. Plant Cell Physiol 47: 1337–1342 [DOI] [PubMed] [Google Scholar]
- Kagan JC. (2012) Signaling organelles of the innate immune system. Cell 151: 1168–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka CA, Bednarek SY. (2008) Comparison of the dynamics and functional redundancy of the Arabidopsis dynamin-related isoforms DRP1A and DRP1C during plant development. Plant Physiol 147: 1590–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korasick DA, McMichael C, Walker KA, Anderson JC, Bednarek SY, Heese A. (2010) Novel functions of Stomatal Cytokinesis-Defective 1 (SCD1) in innate immune responses against bacteria. J Biol Chem 285: 23342–23350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. (2011) Pathogen recognition by the innate immune system. Int Rev Immunol 30: 16–34 [DOI] [PubMed] [Google Scholar]
- Leborgne-Castel N, Lherminier J, Der C, Fromentin J, Houot V, Simon-Plas F. (2008) The plant defense elicitor cryptogein stimulates clathrin-mediated endocytosis correlated with reactive oxygen species production in bright yellow-2 tobacco cells. Plant Physiol 146: 1255–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Bowen CH, Popescu GV, Kang HG, Kato N, Ma S, Dinesh-Kumar S, Snyder M, Popescu SC. (2011) Arabidopsis RTNLB1 and RTNLB2 Reticulon-like proteins regulate intracellular trafficking and activity of the FLS2 immune receptor. Plant Cell 23: 3374–3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre L, Rueter S, Heinstein PF, Low PS. (1993) Characterization of the oligogalacturonide-induced oxidative burst in cultured soybean (Glycine max) cells. Plant Physiol 102: 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem Y, Seri L, Levine A. (2007) Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J 51: 185–197 [DOI] [PubMed] [Google Scholar]
- Li X, Lin H, Zhang W, Zou Y, Zhang J, Tang X, Zhou JM. (2005) Flagellin induces innate immunity in nonhost interactions that is suppressed by Pseudomonas syringae effectors. Proc Natl Acad Sci USA 102: 12990–12995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Lin W, Gao X, Wu S, Cheng C, Avila J, Heese A, Devarenne TP, He P, Shan L. (2011) Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332: 1439–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Tintor N, Mentzel T, Kombrink E, Boller T, Robatzek S, Schulze-Lefert P, Saijo Y. (2009) Uncoupling of sustained MAMP receptor signaling from early outputs in an Arabidopsis endoplasmic reticulum glucosidase II allele. Proc Natl Acad Sci USA 106: 22522–22527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGettrick AF, O’Neill LAJ. (2010) Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr Opin Immunol 22: 20–27 [DOI] [PubMed] [Google Scholar]
- Mersmann S, Bourdais G, Rietz S, Robatzek S. (2010) Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol 154: 391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan J, Zipfel C. (2012) Plant pattern recognition receptor complexes at the plasma membrane. Curr Opin Plant Biol 15: 349–357 [DOI] [PubMed] [Google Scholar]
- Munnik T, Nielsen E. (2011) Green light for polyphosphoinositide signals in plants. Curr Opin Plant Biol 14: 489–497 [DOI] [PubMed] [Google Scholar]
- Navarro L, Zipfel C, Rowland O, Keller I, Robatzek S, Boller T, Jones JDG. (2004) The transcriptional innate immune response to flg22: interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol 135: 1113–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise V, Roux M, Zipfel C. (2009) Recent advances in PAMP-triggered immunity against bacteria: pattern recognition receptors watch over and raise the alarm. Plant Physiol 150: 1638–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntoukakis V, Schwessinger B, Segonzac C, Zipfel C. (2011) Cautionary notes on the use of C-terminal BAK1 fusion proteins for functional studies. Plant Cell 23: 3871–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse TS, Bottrill AR, Jones AME, Peck SC. (2007) Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J 51: 931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MH, Wang X, Wu X, Zhao Y, Clouse SD, Huber SC. (2010) Autophosphorylation of Tyr-610 in the receptor kinase BAK1 plays a role in brassinosteroid signaling and basal defense gene expression. Proc Natl Acad Sci USA 107: 17827–17832 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Oh MH, Wu X, Clouse SD, Huber SC. (2011) Functional importance of BAK1 tyrosine phosphorylation in vivo. Plant Signal Behav 6: 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ortiz-Zapater E, Soriano-Ortega E, Marcote MJ, Ortiz-Masiá D, Aniento F. (2006) Trafficking of the human transferrin receptor in plant cells: effects of tyrphostin A23 and brefeldin A. Plant J 48: 757–770 [DOI] [PubMed] [Google Scholar]
- Robatzek S. (2007) Vesicle trafficking in plant immune responses. Cell Microbiol 9: 1–8 [DOI] [PubMed] [Google Scholar]
- Robatzek S, Chinchilla D, Boller T. (2006) Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev 20: 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S, Wirthmueller L. (2013) Mapping FLS2 function to structure: LRRs, kinase and its working bits. Protoplasma 250: 671–681 [DOI] [PubMed] [Google Scholar]
- Saijo Y. (2010) ER quality control of immune receptors and regulators in plants. Cell Microbiol 12: 716–724 [DOI] [PubMed] [Google Scholar]
- Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, Boller T, Felix G, Chinchilla D. (2010) Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem 285: 9444–9451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer A, Kazanaviciute V, Scheikl E, Teige M, Doczi R, Hirt H, Schwanninger M, Kant M, Schuurink R, Mauch F, et al. (2007) The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis. Plant Cell 19: 2213–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B, Zipfel C. (2008) News from the frontline: recent insights into PAMP-triggered immunity in plants. Curr Opin Plant Biol 11: 389–395 [DOI] [PubMed] [Google Scholar]
- Scita G, Di Fiore PP. (2010) The endocytic matrix. Nature 463: 464–473 [DOI] [PubMed] [Google Scholar]
- Serrano M, Robatzek S, Torres M, Kombrink E, Somssich IE, Robinson M, Schulze-Lefert P. (2007) Chemical interference of pathogen-associated molecular pattern-triggered immune responses in Arabidopsis reveals a potential role for fatty-acid synthase type II complex-derived lipid signals. J Biol Chem 282: 6803–6811 [DOI] [PubMed] [Google Scholar]
- Shan L, He P, Li J, Heese A, Peck SC, Nürnberger T, Martin GB, Sheen J. (2008) Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe 4: 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. (2009) Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol 10: 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Cao Y, Jansen Labby K, Bittel P, Boller T, Bent AF. (2012) Probing the Arabidopsis flagellin receptor: FLS2-FLS2 association and the contributions of specific domains to signaling function. Plant Cell 24: 1096–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tena G, Boudsocq M, Sheen J. (2011) Protein kinase signaling networks in plant innate immunity. Curr Opin Plant Biol 14: 519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA. (2010) ROS in biotic interactions. Physiol Plant 138: 414–429 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG. (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo M, Ichimura K, Casais C, Shirasu K. (2008) Negative regulation of PAMP-triggered immunity by an E3 ubiquitin ligase triplet in Arabidopsis. Curr Biol 18: 1396–1401 [DOI] [PubMed] [Google Scholar]
- Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L. (2004) Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16: 672–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cai Y, Miao Y, Lam SK, Jiang L. (2009) Wortmannin induces homotypic fusion of plant prevacuolar compartments. J Exp Bot 60: 3075–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, He SY. (2010) A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol 153: 1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li W, Xiang T, Liu Z, Laluk K, Ding X, Zou Y, Gao M, Zhang X, Chen S, et al. (2010) Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 7: 290–301 [DOI] [PubMed] [Google Scholar]
- Zhang J, Shao F, Li Y, Cui H, Chen L, Li H, Zou Y, Long C, Lan L, Chai J, et al. (2007) A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 1: 175–185 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G. (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JDG, Felix G, Boller T. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767 [DOI] [PubMed] [Google Scholar]