A peroxisomal substrate transporter and β-oxidation provide benzoic acid for the accumulation of benzoylated secondary metabolites during Arabidopsis seed development.
Abstract
Secondary metabolites derived from benzoic acid (BA) are of central importance in the interactions of plants with pests, pathogens, and symbionts and are potentially important in plant development. Peroxisomal β-oxidation has recently been shown to contribute to BA biosynthesis in plants, but not all of the enzymes involved have been defined. In this report, we demonstrate that the peroxisomal ATP-binding cassette transporter COMATOSE is required for the accumulation of benzoylated secondary metabolites in Arabidopsis (Arabidopsis thaliana) seeds, including benzoylated glucosinolates and substituted hydroxybenzoylcholines. The ABNORMAL INFLORESCENCE MERISTEM protein, one of two multifunctional proteins encoded by Arabidopsis, is essential for the accumulation of these compounds, and MULTIFUNCTIONAL PROTEIN2 contributes to the synthesis of substituted hydroxybenzoylcholines. Of the two major 3-ketoacyl coenzyme A thiolases, KAT2 plays the primary role in BA synthesis. Thus, BA biosynthesis in Arabidopsis employs the same core set of β-oxidation enzymes as in the synthesis of indole-3-acetic acid from indole-3-butyric acid.
Many important secondary metabolites in plants are derived from, or incorporate, benzoic acid (BA). These include compounds found in root exudates, inflorescences, stems, and flower volatiles (D’Auria and Gershenzon, 2005). BA is also potentially a precursor for the plant hormone salicylic acid (SA; Wildermuth et al., 2001). In Arabidopsis (Arabidopsis thaliana), benzoylated glucosinolates (BGs) accumulate in seeds, presumably as a deterrent against animal feeding. Thus, BA metabolites are believed to play key roles in the interactions of plants with microbial and animal pests as well as in beneficial relationships such as pollination systems (Boatright et al., 2004). Understanding the pathways and control of BA synthesis in plants, therefore, is very important.
Three different pathways for the synthesis of BA have been proposed for plants (Boatright et al., 2004; Wildermuth, 2006). These begin with the first committed step of the phenylpropanoid pathway, the deamination of Phe by Phe ammonia lyase to produce trans-cinnamic acid (CA). CA can then be oxidized by CoA-independent reactions in the cytosol, or it may be activated with CoA and proceed through one cycle of peroxisomal β-oxidation. Alternatively, BA synthesis may proceed via a third, CoA-dependent but β-oxidation-independent, pathway that combines elements of the first two pathways (Wildermuth, 2006). Recent studies in Petunia hybrida have highlighted the importance of the peroxisomal β-oxidation pathway in the production of BA for incorporation into floral volatile benzenoids. Enzymes identified in this pathway to date are a cinnamate:CoA ligase (PhCNL/PhAAE [for acyl-activating enzyme]) that activates CA (Colquhoun et al., 2012; Klempien et al., 2012), a multifunctional protein (PhMFP) that hydrates and oxidizes the trans-cinnamoyl-CoA (Qualley et al., 2012), and a 3-ketoacyl CoA thiolase (PhKAT1) that cleaves the resultant β-keto thioester (Van Moerkercke et al., 2009).
Seeds of Arabidopsis accumulate appreciable amounts of BGs (Reichelt et al., 2002; Kliebenstein et al., 2007). Thus, while free BA is not detected in Arabidopsis seeds (Ibdah and Pichersky, 2009), the accumulation of BGs and other BA-containing secondary metabolites in Arabidopsis seeds provides a powerful experimental system with which to determine the pathway and potential control of BA synthesis in plants. For example, a peroxisomal acyl-CoA ligase (BZO1, for benzoyloxy glucosinolate) has been identified in Arabidopsis that is closely related to PhCNL1 and is required for BG production in seeds (Kliebenstein et al., 2007). BZO1 has recently been shown to be an AAE with cinnamate:CoA ligase activity (Lee et al., 2012).
To further investigate the requirement for peroxisomal β-oxidation in BA synthesis, and to identify key enzymes involved in Arabidopsis, we analyzed BA-containing secondary metabolites (BGs and substituted hydroxybenzoylated choline esters) of seeds from a suite of β-oxidation mutants covering the key steps of β-oxidation, including substrate import, activation, oxidation, and thiolysis. This work identifies specific isozymes in Arabidopsis that mediate these steps, defines a new role for ABNORMAL INFLORESCENCE MERISTEM (AIM1), and determines a route for the entry of CA into peroxisomes.
RESULTS AND DISCUSSION
Seed from β-oxidation mutants (Supplemental Fig. S1; Supplemental Table S1) was analyzed for glucosinolates, including BGs (Reichelt et al., 2002), and phenolic choline esters such as hydroxycinnamoylcholine and hydroxybenzoylcholine esters (Böttcher et al., 2009). Most of these mutants were analyzed previously for potential roles in fatty acid or auxin metabolism, but the specific functions of the genes involved remain unknown (Wiszniewski et al., 2009). Since glucosinolate profiles vary significantly among different Arabidopsis ecotypes (Kliebenstein et al., 2001, 2007), we analyzed mutants from a single ecotype background, Columbia (Col-0), to permit ready comparison among mutant lines.
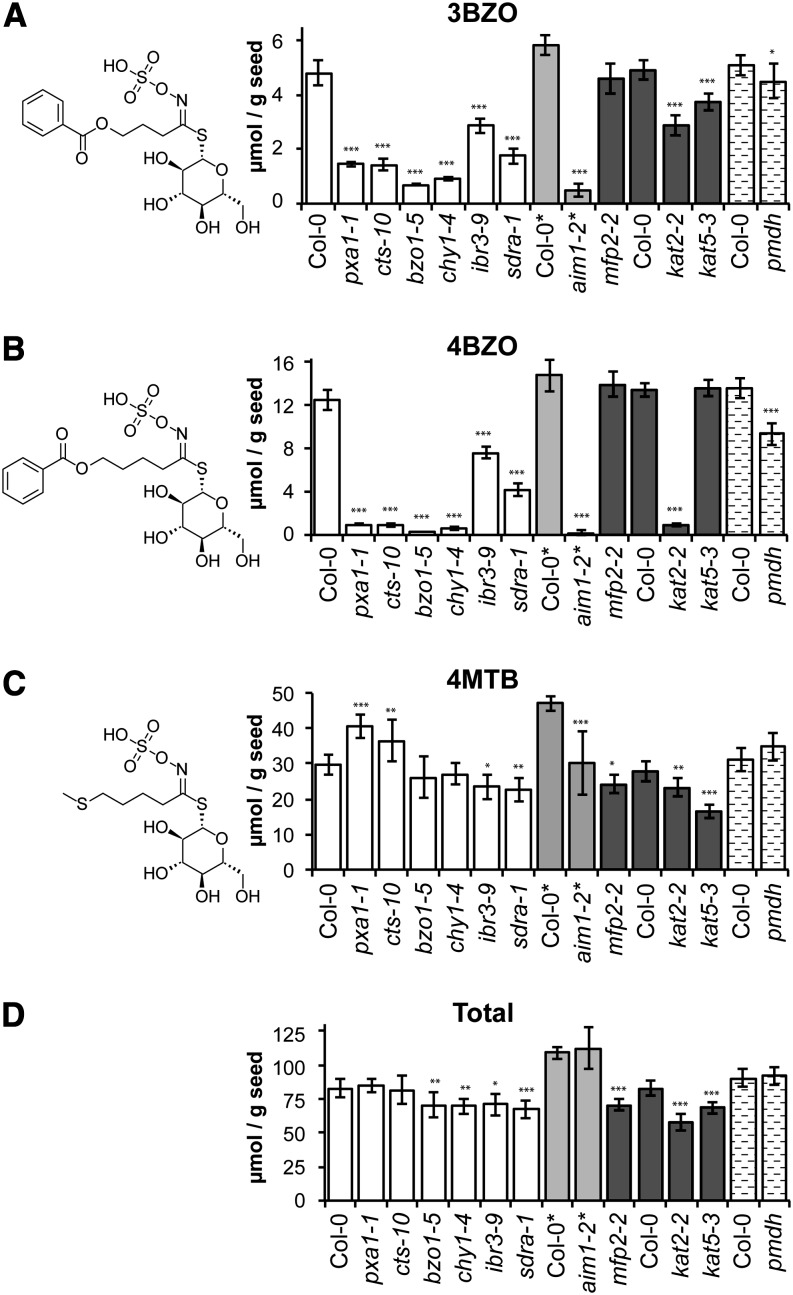
Seeds of wild-type Col-0 plants accumulated significant quantities of 3-benzoyloxypropyl glucosinolate (3BZO) and 4-benzoyloxybutyl glucosinolate (4BZO), while the known BG-deficient mutants bzo1 and chy1 (β-hydroxyisobutyryl-CoA hydrolase; Kliebenstein et al., 2007; Ibdah and Pichersky, 2009) almost completely lacked these compounds (Fig. 1, A and B). The abundance of BGs varied significantly among other β-oxidation mutants. Knockout mutants of the ATP-binding cassette (ABC) transporter COMATOSE/PEROXISOMAL ABC TRANSPORTER1 (CTS/PXA1) and of the multifunctional protein AIM1 accumulated almost no BGs (Fig. 1, A and B). The 3-KETOACYL-COENZYME A THIOLASE2/PEROXISOME DEFECTIVE1 (KAT2/PED1) mutant kat2-2 had greatly reduced 4BZO, and 3BZO was reduced by about 40% compared with the wild type. In contrast, total glucosinolates accumulated to comparable levels in mutants and the wild type (Fig. 1D), and many related glucosinolates, including methylthioalkyl, methylsulfinylalkyl, and hydroxyalkyl glucosinolates, showed only minor variation compared with BGs (Supplemental Table S2; the example of 4-methylthiobutyl glucosinolate is shown in Fig. 1C). Interestingly, the immediate precursor of 4BZO, 4-hydroxybutyl glucosinolate, was present at approximately wild-type levels in most of the mutants that displayed the greatest changes in 4BZO (cts, bzo1-5, chy1-4, and aim1-2) but was almost absent in kat2-2 and was reduced to about 50% of wild-type levels in kat5-3 and mfp2-2 (Supplemental Table S2).
Figure 1.
Analysis of glucosinolate content in seeds of β-oxidation mutants. Structures of specific glucosinolates are depicted. A, 3BZO (CAS no. 80667-69-2). B, 4BZO (CAS no. 75331-11-2). C, 4MTB, 4-Methylthiobutyl glucosinolate (CAS no. 21973-56-8), the most abundant nonbenzoylated glucosinolate. D, Total glucosinolates. Values (μmol g−1 seed) are averages of eight individual parent plants for each genotype. Error bars represent sd (*P < 0.05, **P < 0.01, ***P < 0.001, ANOVA). Plants were grown under long-day conditions, except those marked with asterisks after their name, which were grown in short days. The different shading identifies analyses of mutants and the wild types that were grown in separate batches and processed in separate analytical runs. The full data set including other glucosinolates is provided in Supplemental Table S2, and β-oxidation mutant details are provided in Supplemental Table S1.
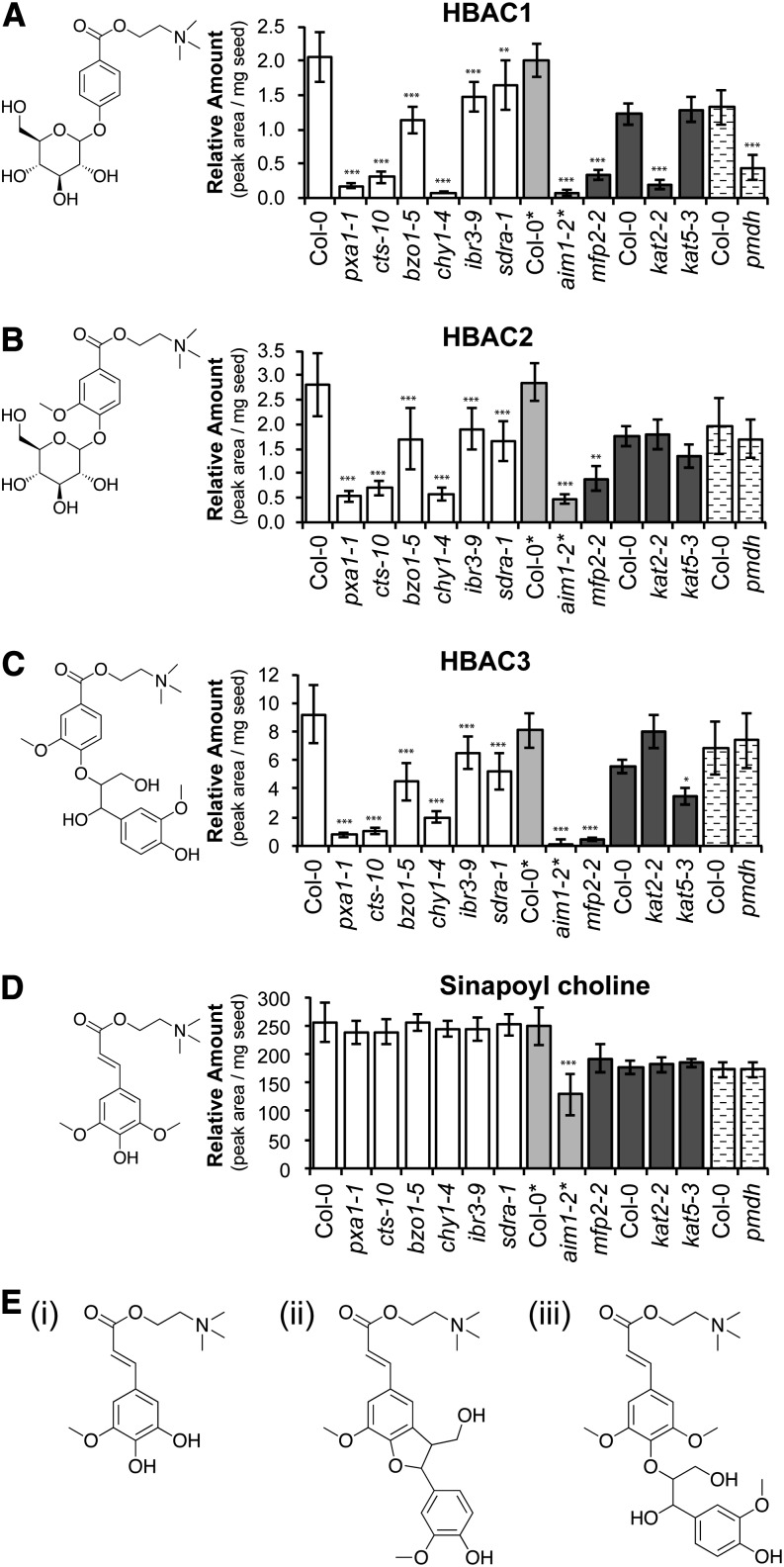
Similar to the results for BGs, the abundance of substituted hydroxybenzoylated choline esters (HBACs) was much lower in cts, aim1, and kat2, but related nonbenzoylated compounds such as sinapoylcholine accumulated normally (Fig. 2; Supplemental Table S3). While the three HBACs (p-hydroxybenzoylcholine-4-O-hexoside [HBAC1], vanilloylcholine-4-O-hexoside [HBAC2], and vanilloylcholine 4-O-8′ coupled to coniferyl alcohol [HBAC3]) that we measured were almost eliminated in cts mutants and aim1-2, only HBAC1 was lost in kat2-2 (Fig. 2, A–C). There did not seem to be a compensatory increase in sinapoyl cholines in the mutants, but this could be due to the already much greater abundance of sinapoylated versus benzoylated choline esters in Col-0 (e.g. note the different y axis scales in Fig. 2). Interestingly, in bzo1-5, HBACs were only reduced to 40% to 50% of wild-type levels, compared with the near-complete loss of BZOs in that mutant. In contrast, mfp2-2 mutants were unaltered in BGs but reduced in HBACs by almost as much as aim1-2 (Figs. 1 and 2). One possible explanation for the varied effects on BA-containing metabolites of these mutations is that the individual BA metabolites accumulate in different cells or at different times during plant or seed development, during which alternative pathways or isozymes might be operational. Thus, loss of a particular protein would not necessarily result in the loss of all BA-containing compounds. Another explanation is that pleiotropic effects in different mutants may lead to differential further metabolism of BGs and HBACs.
Figure 2.
Phenolic choline esters content in seeds of β-oxidation mutants. Compounds were identified based on the data of Böttcher et al. (2009). A to C, BA-containing choline esters HBAC1 (CAS no. 1050631-62-3), HBAC2 (CAS no. 1050631-92-9), and HBAC3 (CAS no. 1050631-96-3). D, Sinapoyl choline (CAS no. 18696-26-9). Structures are provided for the specific metabolites. Values are averages from eight individual parent plants for each genotype. Error bars represent sd (*P < 0.05, **P < 0.01, ***P < 0.001, ANOVA). The data are presented as peak areas (of extracted ion traces) × 10−6 mg−1 seed sample but are not further normalized, as described in “Materials and Methods.” As the analyses are only strictly comparable among samples run in the same batch, different types of shading identify the batches of mutants and wild types grown and analyzed together. Plants were grown under long-day conditions, except those marked with asterisks after their name, which were grown in short days. The full data set including other sinapoyl choline esters is provided in Supplemental Table S3, and β-oxidation mutant details are provided in Supplemental Table S1. E, Structures of other analyzed phenolic choline esters: (i) 5-hydroxyferuloylcholine (CAS no. 1191425-45-2); (ii) feruloylcholine-5-8′ cross-coupled to coniferyl alcohol (CAS no.1050631-58-7); (iii) sinapoylcholine-4-O-8′ coupled to coniferyl alcohol (CAS no. 1050631-54-3).
In line with these possibilities, a selection of other β-oxidation mutants exhibited somewhat more selective reductions in BGs or HBACs, suggesting that enzymes auxiliary to the core β-oxidation pathway may also contribute to the production of BA. INDOLE BUTYRIC ACID RESPONSE3 (IBR3) and IBR1/ SDRa (SHORT-CHAIN DEHYDROGENASE/REDUCTASE A) are dehydrogenase-like proteins that are required for the processing of proauxins (Zolman et al., 2007, 2008; Wiszniewski et al., 2009). ibr3-9 and sdra-1 mutants exhibited reduced amounts of BGs and marginally lower HBACs (Figs. 1 and 2). The pmdh1 pmdh2 knockout that lacks PEROXISOMAL MALATE DEHYDROGENASE was reduced in HBAC1 and 4BZO (Figs. 1 and 2). ARABIDOPSIS ALDEHYDE OXIDASE4 (AAO4) contributes to cytosolic BA synthesis by oxidizing benzaldehyde. Seeds of aao4 mutants have previously been shown to exhibit differential reduction in 3BZO versus 4BZO, being significantly reduced in 4BZO, but barely affected in 3BZO (Ibdah et al., 2009).
In addition to the mutants described above, we analyzed available mutants for uncharacterized peroxisomal acyl-activating enzymes (aae1-1, aae5-1, aae11-1, aae12-1, aae17-1, and aae18-1), enoyl-CoA hydratases (echia and echic), 2,4-dienoyl-CoA reductase, and NADPH:quinone oxidoreductase (Supplemental Table S1). AAE11 and AAE12 were of interest as they are the most closely related AAE isozymes to BZO1. None of these mutants exhibited appreciable changes in their profiles of BA-containing compounds in seeds compared with the wild type (Supplemental Tables S2 and S3). Acyl-CoA oxidase mutants were not studied because there is no described role for these enzymes in the oxidation of CA.
SA is potentially synthesized in plants via BA (Boatright et al., 2004), although the major pathway for its synthesis during the plant defense response is via isochorismate synthase rather than the phenylpropanoid pathway (Wildermuth et al., 2001). We analyzed SA in seeds of the mutants and observed that it was present at lower amounts in cts and aim1 (but not kat2) mutants, where it was reduced to 40% to 50% of wild-type levels (Fig. 3; Supplemental Table S3), indicating a possible role for peroxisomal β-oxidation in SA production during seed development.
Figure 3.
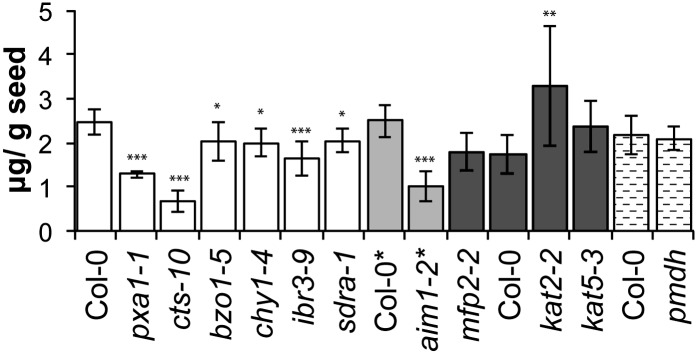
Analysis of SA content in seeds of β-oxidation mutants. Values are μg g−1 seed and the average of seeds collected from eight individual parent plants for each genotype. Error bars represent sd (*P < 0.05, **P < 0.01, ***P < 0.001, ANOVA). Plants were grown under long-day conditions, except those marked with asterisks after their name, which were grown in short days. The different shading identifies analyses of mutants and wild types that were grown in separate batches and processed in separate analytical runs.
That each of the assayed classes of BA-containing secondary metabolites was greatly reduced in cts/pxa1 mutants (Figs. 1 and 2) points to a central role for CTS in the import of CA. CTS is required for the import into peroxisomes of all fatty acids, the auxin precursors indole butyric acid and 2,4-dichlorophenoxybutyric acid, and the jasmonic acid precursor 12-oxophytodieonoic acid (Hayashi et al., 1998; Zolman et al., 2001b; Theodoulou et al., 2005; Dietrich et al., 2009). Our results indicate that CA is also imported into peroxisomes via CTS and activated by BZO1 to produce cinnamoyl-CoA for β-oxidation. Whether benzoyl-CoA is ultimately exported from the peroxisome or is acted upon by a thioesterase to produce BA for export is unknown, although it is generally argued that CoA esters are not exported, since this could deplete the peroxisomal pools of CoA (De Marcos Lousa et al., 2013). Despite the central role of CTS in peroxisomal metabolism, null mutants are viable, suggesting that substrates for any essential β-oxidation processing might be able to enter the peroxisome independently of CTS (Theodoulou et al., 2005; De Marcos Lousa et al., 2013).
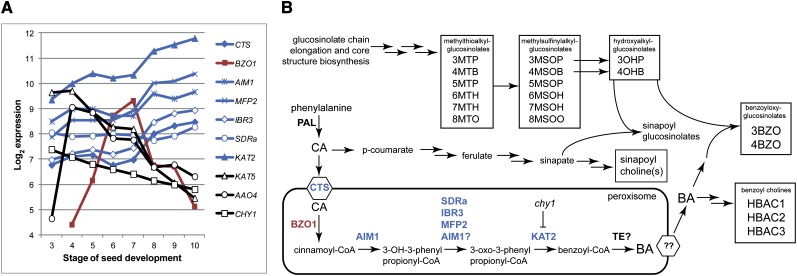
Land plants possess two clades of multifunctional proteins that correspond to the AIM1-like and MFP2-like proteins of Arabidopsis (Arent et al., 2010; Supplemental Fig. S2). Based on mutant phenotypes, AIM1 is required for the efficient processing of auxin and jasmonic acid precursors while MFP2 appears to be largely responsible for the processing of aliphatic fatty acids (Richmond and Bleecker, 1999; Rylott et al., 2006; Delker et al., 2007). The aim1 mutant also has gross anatomical defects and is almost sterile, although some seed can be obtained by growth in short days (Richmond and Bleecker, 1999). Despite aim1 and mfp2 mutants exhibiting very different phenotypes after germination, the aim1 mfp2 double mutant aborts during early embryogenesis, suggesting that the genes provide, at that stage, an essential function (Rylott et al., 2006). AIM1 appears to be required for BA synthesis, as aim1-2 mutants almost completely lacked any of the assayed BA-containing metabolites and were reduced in their amounts of SA. In contrast, mfp2-2 exhibited wild-type levels of BGs and SA but reduced HBACs (Figs. 1–3). AIM1 is apparently specialized for the oxidation of short-chain acyl-CoAs (Arent et al., 2010), including those attached to aromatic and heterocyclic moieties. Another pertinent observation is that the dehydrogenase activity of MFP2 may be more important than its hydratase activity: seedlings of mfp2 knockout mutants have lower l-3-hydroxyacyl-CoA dehydrogenase activity but are unaltered in 2-trans-enoyl-CoA hydratase activity compared with the wild type (Rylott et al., 2006). This implies that other peroxisomal hydratase enzymes can compensate for the loss of this activity in mfp2 mutants. Although our results suggest a degree of functional redundancy between AIM1 and MFP2, their operation does not strictly overlap. Based on these observations, we propose that AIM1 provides essential cinnamoyl-CoA hydratase activity for the β-oxidation pathway of BA synthesis, whereas MFP2, SDRa/IBR1, and IBR3 might substitute functionality at specific stages for the 3-hydroxy-3-phenylpropionyl-CoA dehydrogenase activity (Fig. 4B).
Figure 4.
A, Expression of key genes of BA biosynthesis during seed development. Absolute expression values were obtained from the Electronic Fluorescent Pictograph browser (http://bar.utoronto.ca/efp_arabidopsis/cgi-bin/efpWeb.cgi) and log2 transformed. Coexpressed β-oxidation genes are shown in blue; other β-oxidation genes and AAO4 are in black, and BZO1 is in red. B, General model of peroxisomal BA production and its relation to BG production. Metabolites detected and quantified in this study are highlighted in boxes, and β-oxidation enzymes shown to play a role in BA biosynthesis are printed blue or red as in A. Multiple arrows between metabolites indicate multiple enzymatic steps. The scheme is modeled after Lee et al. (2012) and Sønderby et al. (2010).
Land plants contain two defined clades of KAT proteins (Wiszniewski et al., 2012). The KAT2 isozyme in Arabidopsis participates in the processing of all known fatty acids, including storage lipids, 12-oxophytodieonoic acid, indole butyric acid, and 2,4-dichlorophenoxybutyric acid (Hayashi et al., 1998; Germain et al., 2001; Cruz Castillo et al., 2004; Wiszniewski et al., 2009). Accordingly, kat2 seeds require exogenous sugar in order to germinate, and they display resistance to proauxins. In contrast, KAT5 has not yet had a specific function assigned, despite its ubiquity through plant evolution. In this study, the kat5-3 line had wild-type abundance of most of the BA-containing metabolites, whereas kat2-2 was deficient in 3BZO, 4BZO, and HBAC1 (Figs. 1 and 2). The lack of effect of the kat5 mutation suggests that neither the cytosolic nor the peroxisomal variant of KAT5 (Wiszniewski et al., 2012) supports appreciable BA synthesis.
Along with CTS and AIM1, CHY1 was also required to attain normal abundance of BGs and HBACs (Figs. 1 and 2). CHY1 has been shown to act in the catabolism of propionate and isobutyrate (Lucas et al., 2007), chy1 mutants potentially accumulate methacrylyl-CoA (and acrylyl-CoA) that is inhibitory to KAT activity (Zolman et al., 2001a; Lange et al., 2004; Lucas et al., 2007), and KAT enzyme activity was comparable to or lower than kat2 in chy1 seedlings (Lange et al., 2004). Thus, the effect of chy1 mutants on BA production may be indirect (Fig. 4B). Alternatively CHY1 might act directly in BA synthesis as a hydrolase that produces benzaldehyde from cinnamoyl-CoA (Ibdah and Pichersky, 2009). In vitro assays have shown that CHY1 can release cinnamate from cinnamoyl-CoA, but this reaction is not currently proposed to occur during BA synthesis in vivo (Ibdah and Pichersky, 2009).
Publicly available gene expression data accessed via the Electronic Fluorescent Pictograph interface (Winter et al., 2007) shows that CTS, AIM1, MFP2, and KAT2 genes are expressed broadly in all plant tissues and follow a similar pattern of up-regulation in developing seeds (Fig. 4A). This up-regulation occurs at the stage when transient starch reserves are consumed and the accumulation of glucosinolates and oil is most rapid (Siloto et al., 2006). The expression of KAT5 contrasts markedly with this pattern, decreasing throughout seed development, while BZO1 is mainly expressed between stages 5 and 9 of seed development. By way of contrast, the aforementioned AAO4 peaks early in seed development (stages 6–8, corresponding to heart-shaped embryos) and declines steadily thereafter. Moreover, during embryo development, AAO4 is expressed exclusively in the seed coat, whereas BZO1 is only expressed in the developing embryo (http://bar.utoronto.ca/). Differing patterns of expression for key genes of the alternative pathways suggest a shift in their importance during development and support the possibility that compounds such as BGs and HBACs are synthesized at different times from differentially expressed pathways. Indeed, even in the most BG-deficient mutants (bzo1-5, chy1-4, and aim1-2), 3BZO accumulated to a greater extent than 4BZO, even though 4BZO was normally present in greater amounts in Col-0 (Fig. 1).
The dependence on CTS, AIM1, and KAT2 for BA synthesis (Fig. 4B) raises the possibility that some phenotypes of cts, kat2, and aim1 mutants could be explained by the absence of BA or benzoylated products. Each of these mutants has reduced fertility: this is most apparent in aim1, with its abnormal rosettes and largely sterile inflorescences, but cts and kat2 have reduced fecundity due to a higher incidence of unfertilized ovules and increased postzygotic abortion compared with the wild type (Footitt et al., 2007a, 2007b). Exogenously applied jasmonic acid and auxin (indole-3-acetic acid or naphthylacetic acid) are unable to improve fertility in cts mutants, despite the ability of auxins to facilitate stamen elongation, and it was suggested that the primary role of CTS in fertility was in the provision of energy to fuel pollen tube growth (Footitt et al., 2007b). It is not known if other products of β-oxidation play a role in reproductive development. BA may be important as a precursor of SA, which has multiple functions in plants, including seed germination, thermogenesis, and defense (Rivas-San Vicente and Plasencia, 2011). Future research can use the mutants described here to identify new roles for BA or its metabolites in plant development and environmental interactions.
Collectively, these results highlight the importance of the β-oxidation pathway for BA production in Arabidopsis. We have shown that the accumulation of BGs and HBACs in seeds depends on CTS, AIM1, and KAT2, which mediate the transport into peroxisomes of CA and its subsequent oxidation and thiolysis. These core β-oxidation enzymes are also required for the conversion of indole-3-butyric acid to indole-3-acetic acid (Hu et al., 2012). Our discovery that BA production in seeds is absolutely dependent on AIM1 and CTS establishes new, specific functions for these proteins.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The mutants used in this study and their sources are summarized in Supplemental Table S1. Arabidopsis (Arabidopsis thaliana) encodes a single peroxisomal ABC transporter that has been identified variously as CTS, PXA1, PED3, and ACETATE NON-UTILIZING2 and by its systematic name of AtABCD1 (Verrier et al., 2008); we refer to it here as CTS. To restrict the analysis to mutants in a single ecotype (Col-0), new transfer DNA insertion alleles for AAE11, CTS, AIM1, and KAT5 were identified from publicly available collections (Supplemental Fig. S1; Supplemental Table S1). Oligonucleotides used for mutant screening are given in Supplemental Table S1. Parent plants of seeds subject to metabolite analysis were grown at the same time in peat-vermiculite soil mix under long-day (16 h of light/8 h of dark, 21°C, 120 μmol m−2 s−1) conditions, and the seeds were allowed to dry on the plants prior to collection for analysis. aim1-2 seeds and a corresponding wild-type control were obtained by growth in short days (8 h of light/16 h of dark), as described previously for aim1-1 (Richmond and Bleecker, 1999).
Analysis of Glucosinolates
Approximately 10 mg of Arabidopsis seeds was extracted with 1 mL of 80% methanol solution containing 0.05 mm intact 4-hydroxybenzyl glucosinolate as an internal standard. Samples were homogenized with a paint shaker. After centrifugation, supernatants were loaded onto DEAE Sephadex A 25 columns (flow through was collected for further metabolite analysis [phenolic choline esters]; see below) and treated with arylsulfatase for desulfation (Sigma-Aldrich). The eluted desulfoglucosinolates were separated using HPLC (Agilent 1100 HPLC system; Agilent Technologies) on a reverse-phase C-18 column (Nucleodur Sphinx RP; 250 × 4.6 mm, 5 µm; Macherey-Nagel) with a water (A)-acetonitrile (B) gradient (0–1 min, 1.5% B; 1–6 min, 1.5%–5% B; 6–8 min, 5%–7% B; 8–18 min, 7%–21% B; 18–23 min, 21%–29% B; 23–30 min, 29%–43% B; 30–30.1 min, 43%–100% B; 30.1–33 min, 100% B; and 33.1–38 min, 1.5% B; flow, 1.0 mL min−1). Detection was performed with a photodiode array detector, and peaks were integrated at 229 nm. We used the following response factors (aliphatic glucosinolates, 2.0; indole glucosinolates, 0.5) for quantification of individual glucosinolates (Burow et al., 2006).
Analysis of Phenolic Choline Esters
Around 10 mg of Arabidopsis seeds was extracted in 1 mL of 80% methanol (v/v). After centrifugation, extracts were loaded onto DEAE Sephadex A 25 columns, and the flow-through fraction was collected for phenolic choline ester analysis. Extracts were analyzed by liquid chromatography-mass spectrometry using a Bruker Esquire 6000 ion-trap mass spectrometer (Bruker Daltonics) operated in positive ionization mode in the mass-to-charge ratio (m/z) range 60 to 1,400 (capillary exit voltage, +117 V; capillary voltage, +4,000 V; nebulizer pressure, 35 pounds per square inch [p.s.i.]; drying gas, 11 L min−1; gas temperature, 330°C) coupled to an Agilent 1100 series HPLC device (Agilent Technologies). Elution was accomplished using a Nucleodur Sphinx RP column (250 × 4.6 mm, 5 µm; Macherey-Nagel). Mobile phases were 0.2% (v/v) formic acid (A) and acetonitrile (B), starting with 100% A for 5 min, followed by a gradient to 45% B in 15 min. For relative quantification, peak areas of the respective extracted ion traces for different compounds were extracted as follows and divided by the sample weight: sinapoyl choline, m/z 310; HBAC1, m/z 386; HBAC2, m/z 416; HBAC3, m/z 450; sinapoylcholine-4-O-8′ coupled to coniferyl alcohol (Chemistry Abstracts Service no. 1050631-54-3), m/z 506; feruloylcholin-5-8′ coupled to coniferyl alcohol (CAS no. 1050631-58-7), m/z 458; 5-hydroxyferuloylcholine (CAS no. 1191425-45-2), m/z 296. The compounds were identified based on comparison of mass spectra with those in the literature (Böttcher et al., 2009).
Analysis of SA
A 10-mg sample of Arabidopsis seeds was extracted with 1 mL of methanol containing 40 ng of [3,4,5,6-2H4]-salicylic acid (D4-SA; Sigma-Aldrich). The homogenate was mixed for 30 min and centrifuged at 14,000 rpm for 20 min at 4°C. The supernatant was collected. The homogenate was reextracted with 500 µL of methanol, mixed, and centrifuged, and the supernatants were pooled. Chromatography was performed on an Agilent 1200 HPLC system (Agilent Technologies). Separation was achieved on a Zorbax Eclipse XDB-C18 column (50 × 4.6 mm, 1.8 µm; Agilent Technologies). Formic acid (0.05%) in water and acetonitrile were employed as mobile phases A and B, respectively. The elution profile was as follows: 0 to 0.5 min, 5% B; 0.5 to 9.5 min, 5% to 42% B; 9.5 to 9.51 min, 42% to 100% B; 9.51 to 12 min, 100% B; and 12.1 to 15 min, 5% B. The mobile phase flow rate was 1.1 mL min−1. The column temperature was maintained at 25°C. An API 5000 tandem mass spectrometer (Applied Biosystems) equipped with a Turbospray ion source was operated in negative ionization mode. The ion spray voltage was maintained at –4,500 eV. The turbo gas temperature was set at 700°C. Nebulizing gas was set at 60 p.s.i., curtain gas at 25 p.s.i., heating gas at 60 p.s.i., and collision gas at 7 p.s.i. Multiple reaction monitoring was used to monitor analyte parent ion→product ion: m/z 136.9→93.0 (collision energy, −22 V; declustering potential, −35 V) for SA; m/z 140.9→97.0 (collision energy, −22 V; declustering potential, −35 V) for D4-SA. Both Q1 and Q3 quadrupoles were maintained at unit resolution. Analyst 1.5 software (Applied Biosystems) was used for data acquisition and processing. SA was quantified relative to the signal of D4-SA.
Statistical Analysis
Within each analysis batch, metabolite abundances in mutants were compared with those in Col-0 using one-way ANOVA. P values were derived from post hoc tests using Dunnett’s adjustment for multiple comparisons. Statistical analysis was done using SAS (www.sas.com).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Isolation of aae11 and new aim1, cts, and kat5 alleles.
Supplemental Figure S2. Phylogenetic analysis of plant multifunctional proteins.
Supplemental Table S1. Oligonucleotide sequences and transfer DNA lines.
Supplemental Table S2. Glucosinolate content of Arabidopsis β-oxidation mutant seeds.
Supplemental Table S3. Phenolic choline ester content and SA content of Arabidopsis β-oxidation mutant seeds.
Acknowledgments
We thank Beate Rothe for technical assistance in the extraction of samples.
Glossary
- BA
benzoic acid
- CA
trans-cinnamic acid
- BG
benzoylated glucosinolate
- Col-0
Columbia
- 3BZO
3-benzoyloxypropyl glucosinolate
- 4BZO
4-benzoyloxybutyl glucosinolate
- ABC
ATP-binding cassette
- HBAC
hydroxybenzoylated choline ester
- SA
salicylic acid
- m/z
mass-to-charge ratio
- p.s.i.
pounds per square inch
References
- Arent S, Christensen CE, Pye VE, Nørgaard A, Henriksen A. (2010) The multifunctional protein in peroxisomal beta-oxidation: structure and substrate specificity of the Arabidopsis thaliana protein MFP2. J Biol Chem 285: 24066–24077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatright J, Negre F, Chen X, Kish CM, Wood B, Peel G, Orlova I, Gang D, Rhodes D, Dudareva N. (2004) Understanding in vivo benzenoid metabolism in petunia petal tissue. Plant Physiol 135: 1993–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher C, von Roepenack-Lahaye E, Schmidt J, Clemens S, Scheel D. (2009) Analysis of phenolic choline esters from seeds of Arabidopsis thaliana and Brassica napus by capillary liquid chromatography/electrospray-tandem mass spectrometry. J Mass Spectrom 44: 466–476 [DOI] [PubMed] [Google Scholar]
- Burow M, Müller R, Gershenzon J, Wittstock U. (2006) Altered glucosinolate hydrolysis in genetically engineered Arabidopsis thaliana and its influence on the larval development of Spodoptera littoralis. J Chem Ecol 32: 2333–2349 [DOI] [PubMed] [Google Scholar]
- Colquhoun TA, Marciniak DM, Wedde AE, Kim JY, Schwieterman ML, Levin LA, Van Moerkercke A, Schuurink RC, Clark DG. (2012) A peroxisomally localized acyl-activating enzyme is required for volatile benzenoid formation in a Petunia × hybrida cv. ‘Mitchell Diploid’ flower. J Exp Bot 63: 4821–4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Castillo M, Martínez C, Buchala A, Métraux JP, León J. (2004) Gene-specific involvement of β-oxidation in wound-activated responses in Arabidopsis. Plant Physiol 135: 85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Auria JC, Gershenzon J. (2005) The secondary metabolism of Arabidopsis thaliana: growing like a weed. Curr Opin Plant Biol 8: 308–316 [DOI] [PubMed] [Google Scholar]
- Delker C, Zolman BK, Miersch O, Wasternack C. (2007) Jasmonate biosynthesis in Arabidopsis thaliana requires peroxisomal beta-oxidation enzymes: additional proof by properties of pex6 and aim1. Phytochemistry 68: 1642–1650 [DOI] [PubMed] [Google Scholar]
- De Marcos Lousa C, van Roermund CW, Postis VL, Dietrich D, Kerr ID, Wanders RJ, Baldwin SA, Baker A, Theodoulou FL. (2013) Intrinsic acyl-CoA thioesterase activity of a peroxisomal ATP binding cassette transporter is required for transport and metabolism of fatty acids. Proc Natl Acad Sci USA 110: 1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich D, Schmuths H, De Marcos Lousa C, Baldwin JM, Baldwin SA, Baker A, Theodoulou FL, Holdsworth MJ. (2009) Mutations in the Arabidopsis peroxisomal ABC transporter COMATOSE allow differentiation between multiple functions in planta: insights from an allelic series. Mol Biol Cell 20: 530–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt S, Cornah JE, Pracharoenwattana I, Bryce JH, Smith SM. (2007a) The Arabidopsis 3-ketoacyl-CoA thiolase-2 (kat2-1) mutant exhibits increased flowering but reduced reproductive success. J Exp Bot 58: 2959–2968 [DOI] [PubMed] [Google Scholar]
- Footitt S, Dietrich D, Fait A, Fernie AR, Holdsworth MJ, Baker A, Theodoulou FL. (2007b) The COMATOSE ATP-binding cassette transporter is required for full fertility in Arabidopsis. Plant Physiol 144: 1467–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain V, Rylott EL, Larson TR, Sherson SM, Bechtold N, Carde JP, Bryce JH, Graham IA, Smith SM. (2001) Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid beta-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J 28: 1–12 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Toriyama K, Kondo M, Nishimura M. (1998) 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid β-oxidation. Plant Cell 10: 183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Baker A, Bartel B, Linka N, Mullen RT, Reumann S, Zolman BK. (2012) Plant peroxisomes: biogenesis and function. Plant Cell 24: 2279–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibdah M, Chen YT, Wilkerson CG, Pichersky E. (2009) An aldehyde oxidase in developing seeds of Arabidopsis converts benzaldehyde to benzoic acid. Plant Physiol 150: 416–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibdah M, Pichersky E. (2009) Arabidopsis Chy1 null mutants are deficient in benzoic acid-containing glucosinolates in the seeds. Plant Biol (Stuttg) 11: 574–581 [DOI] [PubMed] [Google Scholar]
- Klempien A, Kaminaga Y, Qualley A, Nagegowda DA, Widhalm JR, Orlova I, Shasany AK, Taguchi G, Kish CM, Cooper BR, et al. (2012) Contribution of CoA ligases to benzenoid biosynthesis in petunia flowers. Plant Cell 24: 2015–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, D’Auria JC, Behere AS, Kim JH, Gunderson KL, Breen JN, Lee G, Gershenzon J, Last RL, Jander G. (2007) Characterization of seed-specific benzoyloxyglucosinolate mutations in Arabidopsis thaliana. Plant J 51: 1062–1076 [DOI] [PubMed] [Google Scholar]
- Kliebenstein DJ, Kroymann J, Brown P, Figuth A, Pedersen D, Gershenzon J, Mitchell-Olds T. (2001) Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol 126: 811–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange PR, Eastmond PJ, Madagan K, Graham IA. (2004) An Arabidopsis mutant disrupted in valine catabolism is also compromised in peroxisomal fatty acid beta-oxidation. FEBS Lett 571: 147–153 [DOI] [PubMed] [Google Scholar]
- Lee S, Kaminaga Y, Cooper B, Pichersky E, Dudareva N, Chapple C. (2012) Benzoylation and sinapoylation of glucosinolate R-groups in Arabidopsis. Plant J 72: 411–422 [DOI] [PubMed] [Google Scholar]
- Lucas KA, Filley JR, Erb JM, Graybill ER, Hawes JW. (2007) Peroxisomal metabolism of propionic acid and isobutyric acid in plants. J Biol Chem 282: 24980–24989 [DOI] [PubMed] [Google Scholar]
- Qualley AV, Widhalm JR, Adebesin F, Kish CM, Dudareva N. (2012) Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. Proc Natl Acad Sci USA 109: 16383–16388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt M, Brown PD, Schneider B, Oldham NJ, Stauber E, Tokuhisa J, Kliebenstein DJ, Mitchell-Olds T, Gershenzon J. (2002) Benzoic acid glucosinolate esters and other glucosinolates from Arabidopsis thaliana. Phytochemistry 59: 663–671 [DOI] [PubMed] [Google Scholar]
- Richmond TA, Bleecker AB. (1999) A defect in beta-oxidation causes abnormal inflorescence development in Arabidopsis. Plant Cell 11: 1911–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-San Vicente M, Plasencia J. (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62: 3321–3338 [DOI] [PubMed] [Google Scholar]
- Rylott EL, Eastmond PJ, Gilday AD, Slocombe SP, Larson TR, Baker A, Graham IA. (2006) The Arabidopsis thaliana multifunctional protein gene (MFP2) of peroxisomal beta-oxidation is essential for seedling establishment. Plant J 45: 930–941 [DOI] [PubMed] [Google Scholar]
- Siloto RM, Findlay K, Lopez-Villalobos A, Yeung EC, Nykiforuk CL, Moloney MM. (2006) The accumulation of oleosins determines the size of seed oilbodies in Arabidopsis. Plant Cell 18: 1961–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønderby IE, Geu-Flores F, Halkier BA. (2010) Biosynthesis of glucosinolates: gene discovery and beyond. Trends Plant Sci 15: 283–290 [DOI] [PubMed] [Google Scholar]
- Theodoulou FL, Job K, Slocombe SP, Footitt S, Holdsworth M, Baker A, Larson TR, Graham IA. (2005) Jasmonic acid levels are reduced in COMATOSE ATP-binding cassette transporter mutants: implications for transport of jasmonate precursors into peroxisomes. Plant Physiol 137: 835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Moerkercke A, Schauvinhold I, Pichersky E, Haring MA, Schuurink RC. (2009) A plant thiolase involved in benzoic acid biosynthesis and volatile benzenoid production. Plant J 60: 292–302 [DOI] [PubMed] [Google Scholar]
- Verrier PJ, Bird D, Burla B, Dassa E, Forestier C, Geisler M, Klein M, Kolukisaoglu U, Lee Y, Martinoia E, et al. (2008) Plant ABC proteins: a unified nomenclature and updated inventory. Trends Plant Sci 13: 151–159 [DOI] [PubMed] [Google Scholar]
- Wildermuth MC. (2006) Variations on a theme: synthesis and modification of plant benzoic acids. Curr Opin Plant Biol 9: 288–296 [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565 [DOI] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiszniewski AAG, Smith SM, Bussell JD. (2012) Conservation of two lineages of peroxisomal (type I) 3-ketoacyl-CoA thiolases in land plants, specialization of the genes in Brassicaceae, and characterization of their expression in Arabidopsis thaliana. J Exp Bot 63: 6093–6103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiszniewski AAG, Zhou W, Smith SM, Bussell JD. (2009) Identification of two Arabidopsis genes encoding a peroxisomal oxidoreductase-like protein and an acyl-CoA synthetase-like protein that are required for responses to pro-auxins. Plant Mol Biol 69: 503–515 [DOI] [PubMed] [Google Scholar]
- Zolman BK, Martinez N, Millius A, Adham AR, Bartel B. (2008) Identification and characterization of Arabidopsis indole-3-butyric acid response mutants defective in novel peroxisomal enzymes. Genetics 180: 237–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Monroe-Augustus M, Thompson B, Hawes JW, Krukenberg KA, Matsuda SP, Bartel B. (2001a) chy1, an Arabidopsis mutant with impaired beta-oxidation, is defective in a peroxisomal beta-hydroxyisobutyryl-CoA hydrolase. J Biol Chem 276: 31037–31046 [DOI] [PubMed] [Google Scholar]
- Zolman BK, Nyberg M, Bartel B. (2007) IBR3, a novel peroxisomal acyl-CoA dehydrogenase-like protein required for indole-3-butyric acid response. Plant Mol Biol 64: 59–72 [DOI] [PubMed] [Google Scholar]
- Zolman BK, Silva ID, Bartel B. (2001b) The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid β-oxidation. Plant Physiol 127: 1266–1278 [PMC free article] [PubMed] [Google Scholar]