Abstract
Mesenchymal stem cells (MSCs) of human origin have been frequently applied to experimental animal models to evaluate their immunomodulatory functions. MSCs are known to be activated by cytokines from T cells, predominantly by interferon-γ (IFN-γ), in conjunction with other cytokines such as tumor necrosis factor-α (TNF-α) and interlukin-1β. Because IFN-γ is not cross-reactive between human and mouse species, the manner in which human MSCs administered in experimental animals are activated and stimulated to function has been questioned. In the present study, we established MSCs from human adipose tissue. They successfully suppressed the proliferation of not only human peripheral blood mononuclear cells but also mouse splenic T cells. When these human MSCs were stimulated with a culture supernatant of mouse T cells or recombinant murine TNF-α, they expressed cyclooxygenase-2 (COX-2), but not indoleamine 2,3-dioxygenase. The dominant role of COX-2 in suppressing mouse T cell proliferation was validated by the addition of COX-2 inhibitor in the co-culture, wherein the suppressed proliferation was almost completely recovered. In conclusion, human MSCs in a murine environment were activated, at least in part, by TNF-α and mainly used COX-2 as a tool for the suppression of in vitro T cell proliferation. These results should be considered when interpreting results for human MSCs in experimental animals.
Keywords: Mesenchymal stem cells, Humans, Cyclooxygenase-2, Immunomodulation
Introduction
Mesenchymal stem cells (MSCs) are multipotent, non-hematopoietic stromal cells that reside in almost all solid organs, as well as in the bone marrow and adipose tissue. Because of their multipotent capacity for differentiation, they have been targeted for use in tissue engineering strategies for the development of replacements for damaged tissues [1, 2]. MSCs are also characterized by their immunomodulatory function to suppress the proliferation and differentiation of the immune cells involved in both innate and adaptive immunity, such as natural killer cells, dendritic cells, B cells, and T cells (reviewed in De Miguel et al. [3] and Gebler et al. [4]).
Cytokines produced by activated T cells, particularly interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interlukin (IL)-1β, are required for MSC activation, which is essential for MSCs to function as effective immunomodulators [5-7]. That is, MSCs derived from the bone marrow or adipose tissue must first be exposed to proinflammatory cytokines from T cells before they express cyclooxygenase-2 (COX-2) and thus secrete prostaglandin E2 (PGE2) [6, 8], express indoleamine 2,3-dioxygenase (IDO) [9], or induce inducible NO synthase (iNOS) to secrete nitric oxide (NO) [10]. These end products, in conjunction with other secretory molecules such as IL-6, tumor growth factor-β (TGF-β), and IL-10, prevent proper differentiation and maturation of dendritic cells, inhibit plasma cell differentiation of antigen-experienced B cells, and suppress proliferation and cytokine production of T cells. In addition, MSCs express B7-H1 (i.e., PD-L1) on their surface [11], which binds to PD-1 on T cells, and thus suppresses the function of T cells. Activated MSCs also express adhesion molecules such as integrin, intercellular adhesion molecule (ICAM)-1, ICAM-2, and vascular cell adhesion molecule-1, which enable these cells to migrate [12], in addition to the expression of various chemokines (CXCL9, CXCL10, CXCL11, and CXCL12) to recruit T cells [13].
Clinical application of the immunomodulatory functions of MSCs has been demonstrated in the considerably effective management of severe graft-versus-host disease in a 9-year-old patient that was treated with MSCs from his mother [14]. In vivo experiments have also been performed in animal disease models to elucidate the function of human MSCs. For example, human adipose tissue-derived MSCs (hAd-MSCs) have been applied in a trinitrobenzene sulfonate-induced experimental colitis model [15], where hAd-MSCs administered in colitis mice attenuated the disease progression by reducing Th1 cell activation and enhancing Treg production. Moreover, in collagen-induced arthritis, hAd-MSCs reduced the prevalence and severity of the disease [16, 17]. The immunoregulatory function of human MSCs of various origin has been shown in many other animal models, including streptozotocin-induced diabetes [18], fulminant hepatic failure [19], amyotrophic lateral sclerosis [20], Parkinson's disease [21], systemic lupus erythematosus [22], and acute pancreatitis [23]. Such experiments are possible because MSCs are immune-tolerable, and human MSCs are capable of surviving for at least 8 weeks in immunocompetent mice [24].
While in vivo experiments in animal models have been performed, question surrounding the manner in which human MSCs are activated to exert their immunosuppressive effects in experimental animals has arisen, specifically in mice, given that not all murine cytokines are compatible to humans. Particularly, IFN-γ, the most important cytokine for the activation of human MSCs, is not interchangeably effective between human and mouse cells [25]. Thus, the mechanism by which human MSCs are activated in mice could be different from that in humans. To date, this mechanism has not yet been explored. Furthermore, whether human MSCs can suppress in vitro mouse T cell proliferation, which is the basic mechanism for the immunosuppressive effects of MSCs, has not been evaluated. For interpretation of results previous and future animal studies using human MSCs, it is important to understand how these cells are activated within a murine model; therefore, in this study, we evaluated the activation of hAd-MSCs within a murine environment.
Materials and Methods
Mice
Male C57BL/6 mice, 7-12 weeks old, were purchased from Saemtako (Osan, Korea). They were maintained at the animal facility of the Seoul National University College of Medicine. All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee at Seoul National University (SNU-121004-1).
Isolation and culture of hAd-MSC
MSCs were isolated from freshly excised human fat tissue, obtained from surgical procedures after receiving informed consent. The adipose tissue was washed with an equal volume of phosphate-buffered saline (PBS), minced, and digested for 1 hour at 37℃ with PBS containing 0.2% bovine serum albumin (BSA; Sigma Chemicals Co., St. Louis, MO, USA) and 2 mg/ml type II collagenase (Gibco, Carlsbad, CA, USA). Digested tissue was washed with PBS and centrifuged for 5 minutes at 400 g. The pellet was obtained and filtered through a 100-mm nylon mesh (BD Bioscience, San Jose, CA, USA) to remove cellular debris and then incubated overnight at 37℃ under a 5% humidified CO2 atmosphere in Dulbecco's modified Eagle medium (WelGENE, Seoul, Korea) with 10% FBS (Gibco) or in endothelial cell growth medium-2 (EGM-2; Lonza, Walkersville, MD, USA). After 24 hours, non-adherent cells were removed. Media were changed every 3 days until the cells became confluent. When cultures reached greater than 90% confluence, cells were subcultured or stored in liquid nitrogen. The study protocol was approved by the Institutional Review Board at Seoul National University (SNU-E-1107-017-368).
Immunophenotyping
The hAd-MSCs were washed and suspended in PBS with 0.5% BSA in aliquots of 5×105 cells. The cells were incubated with anti-CD29-APC, anti-CD34-APC, anti-CD44-FITC, anti-CD45-FITC, anti-CD90-PE, anti-CD105-APC, or anti-CD117-PE antibody (all from e-Bioscience, San Diego, CA, USA) for 30 minutes at 4℃ and then washed twice in PBS containing 0.5% BSA. Cells were re-suspended in 200 ml of PBS with 0.5% BSA, and analyzed at 10,000 events per test with a FACSCalibur (BD Biosciences). Data were analyzed with WinMDI 2.8 software (J. Trotter, The Scripps Research Institute, San Diego, CA, USA).
Differentiation of hAd-MSCs
hAd-MSCs of either passage five or six were induced to differentiate to osteoblasts, chondrocytes, or adipocytes using a STEMPRO Differentiation Kit (Invitrogen, Carlsbad, CA, USA) with media changed twice a week. MSCs differentiated to adipocytes were fixed on day 14 of culture with 4% paraformaldehyde, and stained with Oil Red-O stain (Sigma-Aldrich, St. Louis, MO, USA). Those differentiated to osteoblasts were fixed on day 28 of culture in the same fixative and stained with Alizarin Red S (Sigma-Aldrich). In the case of chondrocytes, cells were fixed on day 21 and stained with Alcian blue (Sigma-Aldrich).
Cell preparation and proliferation assay
Human peripheral blood mononuclear cells (PBMCs) were isolated with a Ficoll-Paque PLUS density gradient for heparinized samples that were obtained from healthy donors after receiving informed consent. Cells were cultured in RPMI-1640 medium (Gibco) supplemented with penicillin/streptomycin (Gibco), and 10% fetal bovine serum (FBS; Gibco). Human T cells and mouse splenic T cells were isolated according to the manufacturer's instructions by negative selection using a human Pan T cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and a mouse Pan T cell Isolation Kit (Miltenyi Biotec), respectively. Cells were cultured in RPMI-1640 medium (WelGENE) with 10% (v/v) FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin (Gibco).
PBMC and human T cells were seeded in 96-well plates at a density of 2×105 cells/well and stimulated with concanavalin A (ConA; 5 µg/ml, Sigma-Aldrich), with phorbol myristate acetate (PMA; 100 ng/ml, Sigma-Aldrich) and ionomycin (I; 500 ng/ml, Sigma-Aldrich), or with Dynabeads human T-Activator CD3/CD28 (bead-to-cell ratio of 1:1, Life Technologies AS, Oslo, Norway).
Mouse splenic T cells were seeded in 96-well plates at a density of 2×105 cells/well and activated with anti-CD3ε (1 µg/ml, clone 1.45-2C11, BD Biosciences) and anti-CD28 (2 µg/ml, clone 37.51, BD Biosciences) antibodies, with ConA (5 µg/ml), or with PMA/I (100 ng/ml and 500 ng/ml, respectively).
To evaluate the suppressive effects, cells were co-cultured with 2×104 hAd-MSCs for 2 days. One microcurie per well of 3[H]-thymidine (Amersham Pharmacia Biotech, Oslo, Norway) was added to the cultures and they were then incubated for an additional 18 hours. The cells were harvested on glass-fiber filters using a cell harvester (INOTECH, Dottikon, Switzerland). Radioactivity was counted with a scintillation β-counter (MicroBeta Trilux, PerkinElmer, Waltham, MA, USA). All samples were prepared in triplicate. If necessary, an inhibitor of COX-2 (0.5 mM, NS-398, Sigma-Aldrich), of IDO (0.5 mM, 1-methyl-DL-tryptophan [1-MT]; Sigma-Aldrich), or of iNOS (Nω-nitro-L-arginine methyl ester hydrochloride [L-NAME], 1 mM, Sigma-Aldrich) was added to the co-culture.
RNA isolation and polymerase chain reaction
Total RNA was purified from hAd-MSCs using Trizol reagent (Invitrogen) according to the manufacturer's instructions. RNA samples were re-suspended in diethyl pyrocarbonate-treated water, quantified, and then stored at -80℃ until used. cDNA was synthesized from 1 µg of RNA using an reverse transcription polymerase chain reaction (RT-PCR) Kit (Intron, Seongnam, Korea). The PCR products after 35 cycles were subjected to electrophoresis on a 2% agarose gel. Bands were visualized under UV light and images were captured using a Multi-Image Light Cabinet (Alpha Innotech Corp., San Leandro, CA, USA). Primers used for RT-PCR are listed in Table 1.
Table 1.
Primer sets used in this study
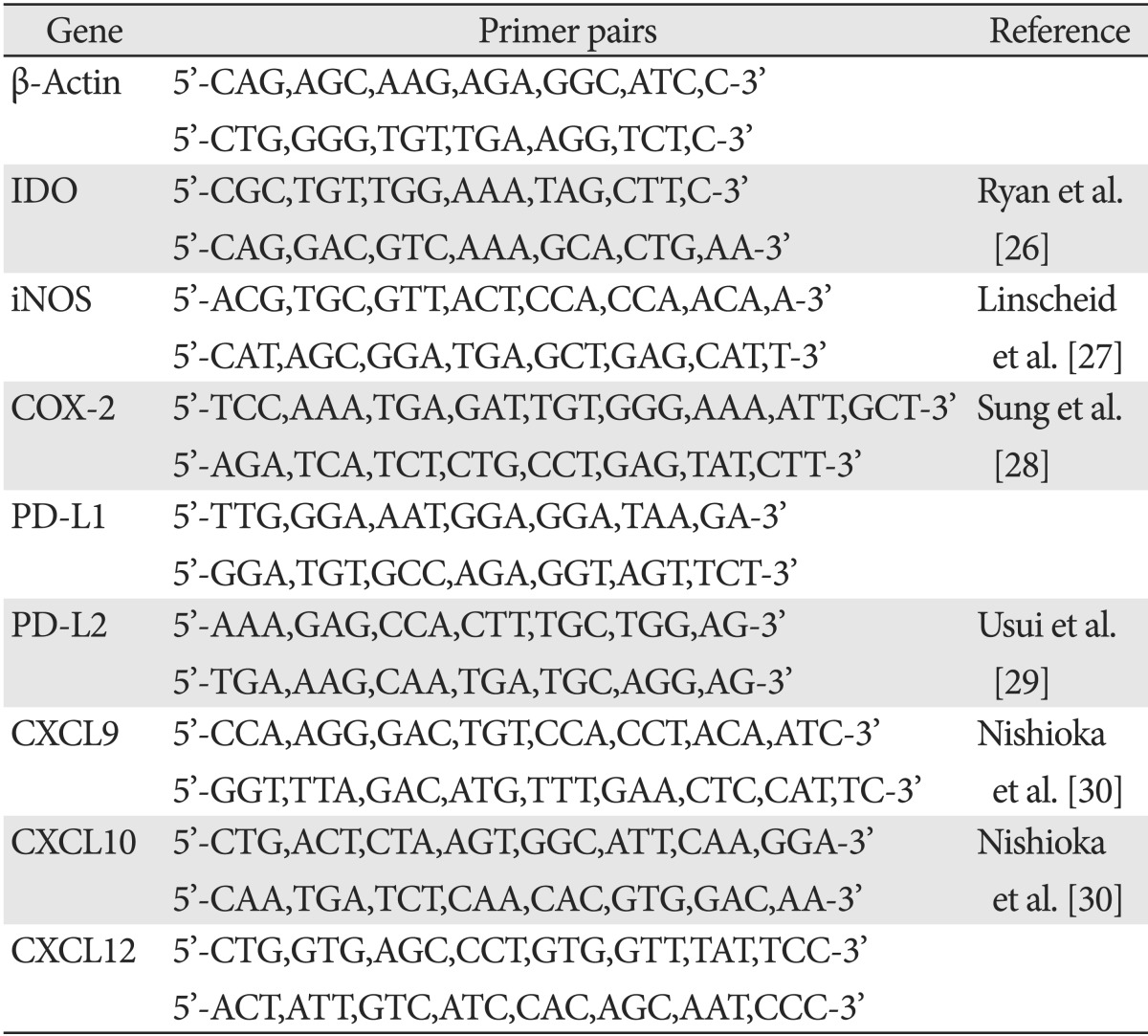
IDO, indoleamine 2,3-dioxygenase; iNOS, inducible NO synthase; COX-2, cyclooxygenase-2.
Results
Establishment of hAd-MSCs
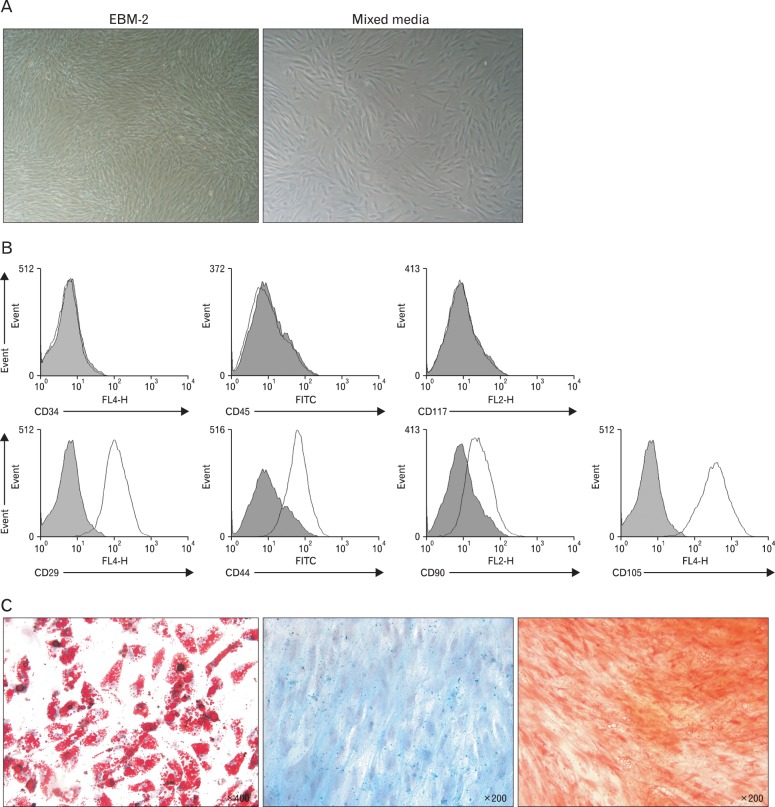
After initial expansion, cells were grown in EGM-2 or in a mixture of EGM-2 and Dulbecco's modified Eagle's medium (DMEM) at a ratio of 1:4. Cells cultured in EGM-2 proliferated faster than those cells in the mixed media, however, cells exhibited a similar spindle-shaped and fibroblast-like appearance, regardless of the culture media in which they were grown (Fig. 1A), and were adherent to the plastic culture surface, typical of human MSCs [31]. During passage 4-8, the cells were subjected to flow cytometric analysis for the identification of stem cell markers which revealed that cells were negative for hematopoietic markers such as CD34, CD45, and CD117 (Fig. 1B, upper panels), and positive for stem cell markers such as CD29, CD44, CD90, and CD105 (Fig. 1B, lower panels). There was no difference in markers expressed by the cells cultured in EGM-2 only and those cultured in the mixed medium (Electronic Supplementary Fig. 1).
Fig. 1.
Establishment of human mesenchymal stem cells (MSCs) from adipose tissue. MSCs were isolated from human fat tissue. (A) On day 3 of culture, cells adhered to the bottom of the culture dish and appeared spindle-shaped. On day 3, cells cultured in EMG-2 media were nearly 90% confluent (left panel), while cells in mixed media were about 50% confluent (right panel). (B) The obtained cells were stained with antibodies against various surface markers, as indicated, and analyzed by flow cytometric analysis. Cell populations were negative for hematopoietic markers (CD34, CD45, and CD117), and positive for stem cell markers (CD29, CD44, CD90, and CD105). (C) Cells were induced to differentiate to adipocytes (left panel), chondrocytes (middle panel), or osteoblasts (right panel) using corresponding differentiation medium, fixed with 4% paraformaldehyde at an appropriate time point, and stained with Oil Red-O, Alcian blue, or Alizarin red S, respectively.
Following the induction of cell differentiation using the commercially available cell-specific culture media, cell differentiation to adipocytes, chondrocytes, or osteoblasts was confirmed using Oil Red-O, Alcian blue, or Alizarin red S, respectively (Fig. 1C). Application of these staining methods to hAd-MSCs before culturing in differentiation medium showed no staining for these cell types (Electronic Supplementary Fig. 2). These results indicate that the cells obtained from adipose tissue were MSCs.
Inhibition of mouse T cell proliferation by hAd-MDC
To confirm the well-known in vitro suppressive effects of MSCs on T cell proliferation, human PBMCs were stimulated with anti-human CD3 and CD28 antibodies and cultured in the presence or absence of hAd-MSCs at a ratio of 1:10 (hAd-MSC:PBMC). The presence of hAd-MSCs in the culture almost completely blocked the proliferation of human PBMCs (Fig. 2A). When mouse splenocytes (Fig. 2B) or isolated splenic T cells (Fig. 2C) were co-cultured with hAd-MSCs in the same way, similar results were obtained. Thus, Ad-MSCs of human origin suppressed the proliferation of mouse T cells, as well as of human PBMCs.
Fig. 2.
Suppressive effects of human adipose tissue-derived mesenchymal stem cells (hAd-MSCs) on the proliferation of T cells. Human peripheral blood mononuclear cells (PBMCs) (A), mouse splenocytes (B), and mouse splenic T cells (C) were stimulated with antibodies against human or mouse CD3 and CD28, respectively, and cultured in the presence or absence of hAd-MSCs (1:10 ratio of MSCs:target cells) for 2 days. 3[H]-thymidine was added to the culture, and radioactivity was determined after 18 hours. The presence of hAd-MSCs markedly suppressed T cell proliferation of both human and mouse cells. Results are representative of three independent experiments. CPM, count per minute. *P<0.001.
Activation of hAd-MSCs by murine T cell culture supernatant
The aforementioned results also imply that hAd-MSCs were activated with mouse T cell supernatant, to exert suppressive effects on mouse T cell proliferation. When activated, MSCs up-regulate COX-2, iNOS, and/or IDO, through which they suppress T cell proliferation by secreting PGE2, NO, and/or IDO to the surrounding environment [6, 8, 9]. Additionally, they express PD-L1 to suppress T cells by contact [11]. Thus, we determined the expression of these molecules, as well as of chemokines, which are needed for recruitment of T cells. When the hAd-MSCs were treated with culture supernatant from PBMCs activated with various activators, they expressed all the molecules examined except iNOS (Fig. 3A, left panels). Meanwhile, when treated with mouse T cell supernatant, hAd-MSCs expressed immunomodulatory molecules differently depending on the mode of T cell activation, as is shown in the right panels of Fig. 3. Overall, the expression levels of the molecules, when it was expressed, were lower than those treated with the PBMC supernatant. Noticeably, COX-2 was expressed in all cases, but IDO was not expressed in any case. Densitometric values for each band were determined (Fig. 3B).
Fig. 3.
Expression of immunomodulatory molecules in activated human adipose tissue-derived mesenchymal stem cells (hAd-MSCs). (A) Peripheral blood mononuclear cells (PBMCs) or mouse T cells were activated with concanavalin A (ConA), with antibodies against human or mouse CD3 and CD28, or with phorbol myristate acetate/ionomycin (PMA+I) for 3 days, and the supernatants were thus obtained. hAd-MSCs were stimulated with each supernatant for 24 hours and harvested. Total RNA was purified from these cells, and reverse transcription polymerase chain reaction analysis was performed for the molecules as indicated. (B) The average values of the intensities of the polymerase chain reaction bands from three replicate experiments measured by densitometry are shown. Lines at the top of each bar represent the magnitude of standard deviation. COX-2, cyclooxygenase-2; iNOS, inducible NO synthase; IDO, indoleamine 2,3-dioxygenase.
Inhibition of murine T cell proliferation by hAd-MSCs mainly via COX-2 expression
The above results indicate that the suppressive effect of hAd-MSCs upon mouse T cell proliferation may be principally due to PGE2, the product of COX-2. To verify this, NS-398, a COX-2 inhibitor, was added to the co-culture of mouse T cells and hAd-MSCs. With the addition of 0.5 mM NS-398, the suppression of T cell proliferation was almost completely recovered (Fig. 4A, left panel). Meanwhile, inhibitors of IDO (1-MT) and iNOS (L-NAME) did not affect the suppressive effect of hAd-MSCs on mouse T cell proliferation as expected (Fig. 4A, right panel).
Fig. 4.
Restoration of the suppressive effect of human adipose tissue-derived mesenchymal stem cells (hAd-MSCs) by the cyclooxygenase-2 (COX-2) inhibitor NS-398. (A) Mouse splenic T cells were co-cultured with hAd-MSCs in the presence or absence of 0.5 mM NS-398 (left panel), or in the presence or absence of 0.5 mM 1-MT (indoleamine 2,3-dioxygenase [IDO] inhibitor), or 1 mM L-NAME (right panel) for 2 days. 3[H]-thymidine was added to the co-culture, and radioactivity was determined after 18 hours. The presence of hAd-MSCs markedly suppressed the T cell proliferation of mouse T cells, which was almost completely recovered by the addition of the COX-2 inhibitor (left panel), while the addition of the IDO inhibitor or inducible NO synthase inhibitor did not affect the suppressive function of hAd-MSCs (right panel). (B) hAd-MSCs were stimulated with the culture supernatant of mouse T cells or with each recombinant mouse cytokine including tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interlukin (IL)-4, or IL-5 at a concentration of 1 ng/ml. Cells were harvested after 24 hours, total RNA was extracted, and reverse transcription polymerase chain reaction was performed for COX-2 expression. Only the hAd-MSCs stimulated with culture supernatant or TNF-α expressed the molecule. Each experiment was repeated more than three times with representative results depicted here. CPM, count per minute; DMSO, dimethyl sulfoxide, vehicle control; Sup, culture supernatant of mouse T cells; *P<0.01.
The component in the mouse supernatant that stimulated hAd-MSCs was still not known. These cells are known to be critically activated by IFN-γ [32], a cytokine known not to be interchangeable between the human and mouse forms [25]. Thus, we assumed that TNF-α and/or Th2 cytokines, such as IL-4 and IL-5, in the murine supernatant would help activate these cells. To this end, hAd-MSCs were treated with mouse T cell culture supernatant or recombinant mouse cytokines, including TNF-α, IFN-γ, IL-4, and IL-5, and were harvested for RNA preparation. RT-PCR analysis revealed that murine T cell culture supernatant and TNF-α induced COX-2 expression in the hAd-MSCs (Fig. 4B).
Discussion
In the present study, we isolated MSCs from human adipose tissue. The cells were characterized by their adherence to the bottom of the culture dish exhibiting a fibroblast-like appearance; expression of surface markers and absence of hematopoietic markers; and their ability to differentiate into adipocytes, chondrocytes, and osteoblasts-all these characteristics attributed to cells defined as MSCs [31]. These cells, when treated with the culture supernatant of PBMCs, which are presumed to primarily be T cells, expressed COX-2 and IDO, through which the proliferation of PBMCs is suppressed. PD-L1 and PD-L2 were also expressed, which have also been shown to help the suppressive effect of the mesenchymal cells [11].
For the cultivation of hAd-MSCs, we used a mixture of EGM-2 and DMEM culture medium (1:5 ratio), in which the cells exhibited slower proliferation, but the same surface markers to those grown in only EGM-2 medium, and also exhibited the capacity to differentiate to adipocytes, chondrocytes, and osteoblasts. Considering that EGM-2 is expensive, and that mixture with DMEM did not deteriorate the stemness of MSCs, it would be beneficial to use the mixed media for the maintenance of MSC cultures.
When hAd-MSCs were stimulated with the PBMC supernatant, COX-2 and IDO were expressed, but not iNOS, consistent with previous reports [13, 26]. However, when these cells were stimulated with mouse supernatant, of the three genes designated as enzymes secreting soluble factors responsible for the immunosuppressive function of MSCs, only COX-2 was expressed (Figs. 3, 4). This finding implies that the manner in which mouse T cell supernatant stimulates hAd-MSCs differs from that of human T cell supernatant. To express IDO, human MSCs must be exposed to IFN-γ. When the PBMC culture media was applied to hAd-MSCs after incubation with an antibody to each cytokine, only that treated with the anti-IFN-γ antibody markedly decreased its capacity to induce the hAd-MSCs to express IDO (Electronic Supplementary Fig. 3A). Again, IFN-γR1-deficient human bone marrow (BM)-MSCs did not express IDO, even though they were co-cultured with PBMCs [32], indicating a critical role of IFN-γ signals for the expression of IDO. Our hAd-MSCs should be presented with murine IFN-γ when co-cultured with activated mouse T cells or when treated with the mouse supernatant; however, they failed to express IDO. This outcome is likely due to the fact that murine IFN-γ is not interchangeable with the human form [25, 33]. That is, even though the receptors of the two species show 53% homology, they hardly bind with IFN-γ from another species. Concordantly, hAd-MSCs expressed IDO when they were treated with human IFN-γ (Electronic Supplementary Fig. 3B), while they did not express IDO, when treated with murine IFN-γ (Fig. 4A).
Without IDO, hAd-MSCs still exerted in vitro suppressive effects upon the proliferation of mouse T cells. Undoubtedly, this effect is, at least in part, attributed to the expression of COX-2. Human BM-MSCs secrete PGE2, the product of COX-2, when cultured in the presence of IFN-γ or TNF-α [34], as was also shown in our results (Electronic Supplementary Fig. 3B). COX-2 was also induced by treatment with mouse supernatant and recombinant murine TNF-α (Fig. 4A). This was as expected, because murine TNF-α binds to both the human TNFαR1 and R2 TNF-α receptors [35]. It is also important to note that, although mouse TNF-α acts on human cells, a higher concentration than that needed for human TNF-α is required to obtain a similar effect [36]. This could explain the tiny COX-2 PCR bands obtained from the mouse supernatant or TNF-α treatment. Meanwhile, considering that the concentration of murine TNF-α that was used (1 ng/ml) is higher than that which would be found in the supernatant of cultured T cells, and that the intensity of the COX-2 band is weaker than that induced by the supernatant (Fig. 4B), it appears that TNF-α is not the sole factor to induce COX-2 in the hAd-MSCs. Other factors, currently unknown, could synergize the action of TNF-α.
In the murine environment, at least in vitro, COX-2 seems to be the main cause of the suppressive effect of hAd-MSCs on mouse T cell proliferation, because the COX-2 inhibitor NS-398 almost completely recovered mouse T cell proliferation (Fig. 4B, left panel), while the inhibitors of IDO (1-MT) or iNO (L-NAME) failed to recover the suppressed proliferation. Whether or not this is also the case in vivo is still unknown. Recent studies have suggested that factors other than IDO and COX-2 function as an immunomodulatory tool for MSCs. For example, increased production of adenosine via increased expression of CD39 on MSCs has reportedly contributed to the suppression of T cells [37]. In mouse MSCs, FAS ligand is expressed, and binds to FAS on T cells, thus leading to T cell death [38]. More importantly, MSCs express IL-10 and TGF-β [3, 4], cytokines that drive regulatory T cell production and reduce Th17 differentiation, both of which result in the diminution of T cell-mediated immune responses [39]. While IL-10 is not interchangeable between human and mouse species, TGF-β is [40]. Thus, it is highly plausible that TGF-β takes part in the immunomodulatory function of hAd-MSCs in experimental animal models. Even though we did not examine the secretion of TGF-β from hAd-MSC treated with mouse supernatant, experimental animal studies have reported both increased Treg and reduced Th17 production with human MSC administration for the treatment of disease models [15, 16, 22, 41].
In conclusion, hAd-MSCs undergo different modes of activation, when they were treated with mouse T cell culture supernatant, compared to when they were stimulated with human PBMC supernatant. However, they still exerted an anti-proliferative effect on mouse T cells in vitro, primarily through COX-2 expression. These results have not previously been reported, and should be considered when interpreting the results of experiments evaluating the effects of hAd-MSCs in experimental animal models. More sophisticated studies are needed to analyze the exact mechanisms of immunomodulation by hAd-MSCs in experimental disease models.
Acknowledgements
This study was supported by a grant from the Seoul National University Hospital Research Fund (04-2010-0330).
Electronic Supplementary Materials
Supplementary data including three figures can be found with this article online at http://www.acbjournal.org/src/sm/acb-46-262-s001.pdf.
Surface marker expression of the cells grown in EBM-2 media or in mixed media (EBM-2:DMEM=1:4). After established in each medium, human adipose tissue-derived cells were stained with antibodies against (A) hematopoietic markers (CD34, CD45, and CD117) and (B) positive markers for stem cells (CD29, CD44, CD90, and CD105). Two groups of cells did not differ with respect to the expression of the explored surface molecules. Data are representative of three independent experiments. FSC, forward scatter; SSC, side scatter.
Confluent human adipose tissue-derived mesenchymal stem cells (hAd-MSCs) were fixed with 4% paraformaldehyde and were stained for adipocytes (D; Oil Red-O), chondrocytes (E; Alcian blue), or osteoblasts (F; Alizarin red S) before the induction of cell differentiation (A-C). Minimal staining for each cell type was observed. Photos in the lower panels are from Fig. 1C for comparison.
Expression of immunomodulatory enzymes of human adipose tissue-derived mesenchymal stem cells (hAd-MSCs). (A) hAd-MSCs were stimulated with peripheral blood mononuclear cell (PBMC) supernatant that was pretreated with an antibody against a specific cytokine, as indicated in the Figure for 24 hours. Cells were harvested, total RNA was extracted, and reverse transcription polymerase chain reaction was performed for indoleamine 2,3-dioxygenase (IDO) expression. The supernatant pretreated with antibody against interferon-γ (IFN-γ) failed to induce the cells to express IDO, while others did not. (B) hAd-MSCs were stimulated with human T cell culture supernatant or with 1 ng/ml of each recombinant human cytokine as specified in the Figure. TNF-α, tumor necrosis factor-α; IL, interleukin; Sup, culture supernatant of human T cells; COX-2, cyclooxygenase-2; iNOS, inducible NO synthase.
References
- 1.Maumus M, Guérit D, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther. 2011;2:14. doi: 10.1186/scrt55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoltz JF, Huselstein C, Schiavi J, Li YY, Bensoussan D, Decot V, De Isla N. Human stem cells and articular cartilage tissue engineering. Curr Pharm Biotechnol. 2012;13:2682–2691. doi: 10.2174/138920112804724846. [DOI] [PubMed] [Google Scholar]
- 3.De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 4.Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18:128–134. doi: 10.1016/j.molmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 5.DelaRosa O, Lombardo E, Beraza A, Mancheño-Corvo P, Ramirez C, Menta R, Rico L, Camarillo E, García L, Abad JL, Trigueros C, Delgado M, Büscher D. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009;15:2795–2806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- 6.English K, Barry FP, Field-Corbett CP, Mahon BP. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett. 2007;110:91–100. doi: 10.1016/j.imlet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Kang JW, Kang KS, Koo HC, Park JR, Choi EW, Park YH. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008;17:681–693. doi: 10.1089/scd.2007.0153. [DOI] [PubMed] [Google Scholar]
- 9.Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 10.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 11.Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, Shen B, Yin S, Liu W, Cui L, Li N. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846–857. doi: 10.1038/cr.2008.80. [DOI] [PubMed] [Google Scholar]
- 12.Xu G, Zhang L, Ren G, Yuan Z, Zhang Y, Zhao RC, Shi Y. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007;17:240–248. doi: 10.1038/cr.2007.4. [DOI] [PubMed] [Google Scholar]
- 13.Ren G, Su J, Zhang L, Zhao X, Ling W, L'Huillie A, Zhang J, Lu Y, Roberts AI, Ji W, Zhang H, Rabson AB, Shi Y. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 14.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 15.González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 16.González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 17.Zhou B, Yuan J, Zhou Y, Ghawji M, Jr, Deng YP, Lee AJ, Nair U, Kang AH, Brand DD, Yoo TJ. Administering human adipose-derived mesenchymal stem cells to prevent and treat experimental arthritis. Clin Immunol. 2011;141:328–337. doi: 10.1016/j.clim.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vercelli A, Mereuta OM, Garbossa D, Muraca G, Mareschi K, Rustichelli D, Ferrero I, Mazzini L, Madon E, Fagioli F. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2008;31:395–405. doi: 10.1016/j.nbd.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Park HJ, Lee PH, Bang OY, Lee G, Ahn YH. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson's disease. J Neurochem. 2008;107:141–151. doi: 10.1111/j.1471-4159.2008.05589.x. [DOI] [PubMed] [Google Scholar]
- 22.Gu Z, Akiyama K, Ma X, Zhang H, Feng X, Yao G, Hou Y, Lu L, Gilkeson GS, Silver RM, Zeng X, Shi S, Sun L. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus. 2010;19:1502–1514. doi: 10.1177/0961203310373782. [DOI] [PubMed] [Google Scholar]
- 23.Jung KH, Song SU, Yi T, Jeon MS, Hong SW, Zheng HM, Lee HS, Choi MJ, Lee DH, Hong SS. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011;140:998–1008. doi: 10.1053/j.gastro.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 24.Niemeyer P, Vohrer J, Schmal H, Kasten P, Fellenberg J, Suedkamp NP, Mehlhorn AT. Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy. 2008;10:784–795. doi: 10.1080/14653240802419302. [DOI] [PubMed] [Google Scholar]
- 25.Hemmi S, Peghini P, Metzler M, Merlin G, Dembic Z, Aguet M. Cloning of murine interferon gamma receptor cDNA: expression in human cells mediates high-affinity binding but is not sufficient to confer sensitivity to murine interferon gamma. Proc Natl Acad Sci U S A. 1989;86:9901–9905. doi: 10.1073/pnas.86.24.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linscheid P, Seboek D, Zulewski H, Scherberich A, Blau N, Keller U, Müller B. Cytokine-induced metabolic effects in human adipocytes are independent of endogenous nitric oxide. Am J Physiol Endocrinol Metab. 2006;290:E1068–E1077. doi: 10.1152/ajpendo.00374.2005. [DOI] [PubMed] [Google Scholar]
- 28.Sung JJ, Leung WK, Go MY, To KF, Cheng AS, Ng EK, Chan FK. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am J Pathol. 2000;157:729–735. doi: 10.1016/S0002-9440(10)64586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Usui Y, Okunuki Y, Hattori T, Takeuchi M, Kezuka T, Goto H, Usui M. Expression of costimulatory molecules on human retinoblastoma cells Y-79: functional expression of CD40 and B7H1. Invest Ophthalmol Vis Sci. 2006;47:4607–4613. doi: 10.1167/iovs.06-0181. [DOI] [PubMed] [Google Scholar]
- 30.Nishioka Y, Manabe K, Kishi J, Wang W, Inayama M, Azuma M, Sone S. CXCL9 and 11 in patients with pulmonary sarcoidosis: a role of alveolar macrophages. Clin Exp Immunol. 2007;149:317–326. doi: 10.1111/j.1365-2249.2007.03423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dominici M, Le Blanc, K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 32.Gieseke F, Schütt B, Viebahn S, Koscielniak E, Friedrich W, Handgretinger R, Müller I. Human multipotent mesenchymal stromal cells inhibit proliferation of PBMCs independently of IFNgammaR1 signaling and IDO expression. Blood. 2007;110:2197–2200. doi: 10.1182/blood-2007-04-083162. [DOI] [PubMed] [Google Scholar]
- 33.Lembo D, Ricciardi-Castagnoli P, Alber G, Ozmen L, Landolfo S, Blüthmann H, Dembic Z, Kotenko SV, Cook JR, Pestka S, Garotta G. Mouse macrophages carrying both subunits of the human interferon-gamma (IFN-gamma) receptor respond to human IFN-gamma but do not acquire full protection against viral cytopathic effect. J Biol Chem. 1996;271:32659–32666. doi: 10.1074/jbc.271.51.32659. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 35.Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, Ambrose C, Tschopp J, Schneider P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006;281:13964–13971. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- 36.Fransen L, Ruysschaert MR, Van der Heyden J, Fiers W. Recombinant tumor necrosis factor: species specificity for a variety of human and murine transformed cell lines. Cell Immunol. 1986;100:260–267. doi: 10.1016/0008-8749(86)90025-0. [DOI] [PubMed] [Google Scholar]
- 37.Saldanha-Araujo F, Ferreira FI, Palma PV, Araujo AG, Queiroz RH, Covas DT, Zago MA, Panepucci RA. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 2011;7:66–74. doi: 10.1016/j.scr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanidziar D, Koulmanda M. Inflammation and the balance of Treg and Th17 cells in transplant rejection and tolerance. Curr Opin Organ Transplant. 2010;15:411–415. doi: 10.1097/MOT.0b013e32833b7929. [DOI] [PubMed] [Google Scholar]
- 40.Scheerlinck JP. Functional and structural comparison of cytokines in different species. Vet Immunol Immunopathol. 1999;72:39–44. doi: 10.1016/s0165-2427(99)00115-4. [DOI] [PubMed] [Google Scholar]
- 41.Chen M, Su W, Lin X, Guo Z, Wang J, Zhang Q, Brand D, Ryffel B, Huang J, Liu Z, He X, Le AD, Zheng SG. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013;65:1181–1193. doi: 10.1002/art.37894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Surface marker expression of the cells grown in EBM-2 media or in mixed media (EBM-2:DMEM=1:4). After established in each medium, human adipose tissue-derived cells were stained with antibodies against (A) hematopoietic markers (CD34, CD45, and CD117) and (B) positive markers for stem cells (CD29, CD44, CD90, and CD105). Two groups of cells did not differ with respect to the expression of the explored surface molecules. Data are representative of three independent experiments. FSC, forward scatter; SSC, side scatter.
Confluent human adipose tissue-derived mesenchymal stem cells (hAd-MSCs) were fixed with 4% paraformaldehyde and were stained for adipocytes (D; Oil Red-O), chondrocytes (E; Alcian blue), or osteoblasts (F; Alizarin red S) before the induction of cell differentiation (A-C). Minimal staining for each cell type was observed. Photos in the lower panels are from Fig. 1C for comparison.
Expression of immunomodulatory enzymes of human adipose tissue-derived mesenchymal stem cells (hAd-MSCs). (A) hAd-MSCs were stimulated with peripheral blood mononuclear cell (PBMC) supernatant that was pretreated with an antibody against a specific cytokine, as indicated in the Figure for 24 hours. Cells were harvested, total RNA was extracted, and reverse transcription polymerase chain reaction was performed for indoleamine 2,3-dioxygenase (IDO) expression. The supernatant pretreated with antibody against interferon-γ (IFN-γ) failed to induce the cells to express IDO, while others did not. (B) hAd-MSCs were stimulated with human T cell culture supernatant or with 1 ng/ml of each recombinant human cytokine as specified in the Figure. TNF-α, tumor necrosis factor-α; IL, interleukin; Sup, culture supernatant of human T cells; COX-2, cyclooxygenase-2; iNOS, inducible NO synthase.