Abstract
Placental morphology and cellular arrangement are altered in maternal diseases such as preeclampsia (PE) in which oxygen delivery from the mother to the fetus is greatly disturbed, ultimately resulting in cellular oxidative stress. The present study was conducted at the Department of Anatomy and included 112 placentas (56 each from mothers with and without PE [controls]) collected at the Department of Obstetrics and Gynecology. A histological study was performed using hematoxylin and eosin staining. The morphology of stem and terminal villi (TV) was studied, and the surface area and diameter of TV and capillaries were measured. The gross placental morphometrical study revealed that the mean placental weight, thickness, diameter, and surface area were significantly lower in placentas with PE than in controls. The histomorphometrical findings of the villous surface area and diameter were lower in placentas with PE, whereas the TV density was higher in placentas with PE than in controls, and the differences were significant (P<0.0001). In these TV, the diameter and density of fetal blood vessels of placentas with PE were significantly lower than those of controls (P<0.05). In conclusion, the both morphological and histological changes in PE placentas are indicative of the pathogenesis of maternal and fetal morbidity and mortality in women with PE. The observed and comparative histomorphometrical changes indicate a decline in all aspects of the PE placenta, except the number of TV.
Keywords: Pre-eclampsia, Placenta, Terminal villi, Stem villi, Histomorphometry
Introduction
The placenta is an ephemeral organ interposed between the mother and fetus and is vital for the survival of the fetus [1]. Fetal growth depends on the proper development and function of the placenta, which serves to maintain maternofetal interference for the exchange of blood gases, nutrients, and waste [2]. The architecture of the placenta is altered in many maternal diseases such as diabetes mellitus [3], hypertension [4-6], preeclampsia (PE) [7, 8], and eclampsia [9]. Although the placenta is a vital organ, its systemic study has been neglected; however, in recent times, it has evoked great interest, and much work is being conducted to understand the unique biological status of this complex organ [10]. Placental examination has clinical value in cases of PE and intrauterine growth retardation (IUGR), both of which are associated with high perinatal morbidity and mortality accompanied with gross pathological changes in the placenta.
PE is unique pregnancy-related disease that affects 5-7% pregnancies worldwide [11]. It is associated with hypertension and proteinuria. The primary cause of PE is the widespread apoptosis of cytotrophoblast cells [12, 13]. The invasion of uterine spiral arterioles by trophoblasts is limited to the superficial portions of the decidua, and 30-50% of these arterioles in the placental bed escape trophoblast remodeling [14, 15]. The mean luminal diameter of uterine spiral arterioles in women with PE is less than one-third of the diameter of similar vessels from uncomplicated pregnancies [16]. Consequently, uteroplacental perfusion reduces, and the placenta becomes ischemic as gestation progresses [17, 18]. This causes fetal hypoxia as well as morphological and histological changes in the placenta, leading to PE or PE-associated IUGR, which contributes to premature delivery and fetal death [19, 20].
The objective of the present study is to compare morphological and histomorphometrical changes in placentas from mothers with PE with those in placentas from normotensive mothers, in relation to the surface area and diameter of the terminal villi (TV) and blood vessels.
Materials and Methods
In the present study, 112 placentas were collected at the Department of Obstetrics and Gynecology, Narayana Medical College and General Hospital, Nellore. Informed consent was obtained from each mother, and the study protocol was approved by the Institutional Ethical Committee. Fifty-six placentas were collected from normotensive pregnant patients (controls), and the remaining 56 were obtained from patients whose pregnancies were complicated by PE. PE was defined as a blood pressure >140/90 mm Hg, with protein values ≥300 mg in the 24-hour urine or a protein concentration of 1 g/l on 2 occasions at least 6-hour apart [21]. Inclusion criteria for controls were normal blood pressure and no proteinuria. Exclusion criteria for both the control and PE groups were diabetes mellitus, obesity, severe anemia (hemoglobin<6 g%), and eclampsia or any other systemic or endocrine disorders [7].
Immediately after the delivery, the umbilical cord was clamped close to the placental insertion point; membranes were trimmed and blood coagulants were removed. The placental weight, thickness, diameter [8], and surface area [22] were recorded. For histological studies, full-depth tissue samples were placed in 10% formol-saline solution for 24-48 hours and were subsequently embedded in paraffin. The 5-µm thick sections were stained with hematoxylin and eosin. In all, 200 random sections of TV per placenta were observed microscopically (approximately 11,200 TV for each control and PE). TV were those that had the smallest villi containing capillary loops without any histological artifacts, as observed under a trinocular microscope (CX31, Olympus, Tokyo, Japan) with a 40× objective. In addition to TV, other pathological findings of the placental villi were also noted.
Histomorphometrical analyses
The following parameters were estimated in TV of the control and PE placentas: 1) Numerical density [23] and diameter [24] of TV and blood vessels were measured using stage, ocular, and reticule micrometers. 2) The surface area [25] of TV in the control and PE groups was measured using the intercept count method with the eyepiece reticule micrometer.
The data were inserted into a computer program, SPSS ver. 10 (SPSS Inc., Chicago, IL, USA). The statistical significance of difference between the 2 groups was evaluated using the Student's paired t-test. Data were presented as mean±SD. A P<0.05 was considered statistically significant.
Results
The mean gestational age was 36.61±1.55 weeks in the control group and 33.79±4.25 weeks in the PE group (P<0.0001). Infants in the PE group had statistically and significantly lower gestational age and birth weight than those in the control group (P<0.0001).
On gross placental morphometrical study, the placentas in the PE group had significantly lower mean placental weights, thicknesses, diameters, and surface areas than those in the control group (Table 1). The fetoplacental index was significantly decreased in the PE group than in the control group (P<0.009).
Table 1.
Macroscopic findings of maternal, neonatal and placental parameters of control and preeclampsia (PE)
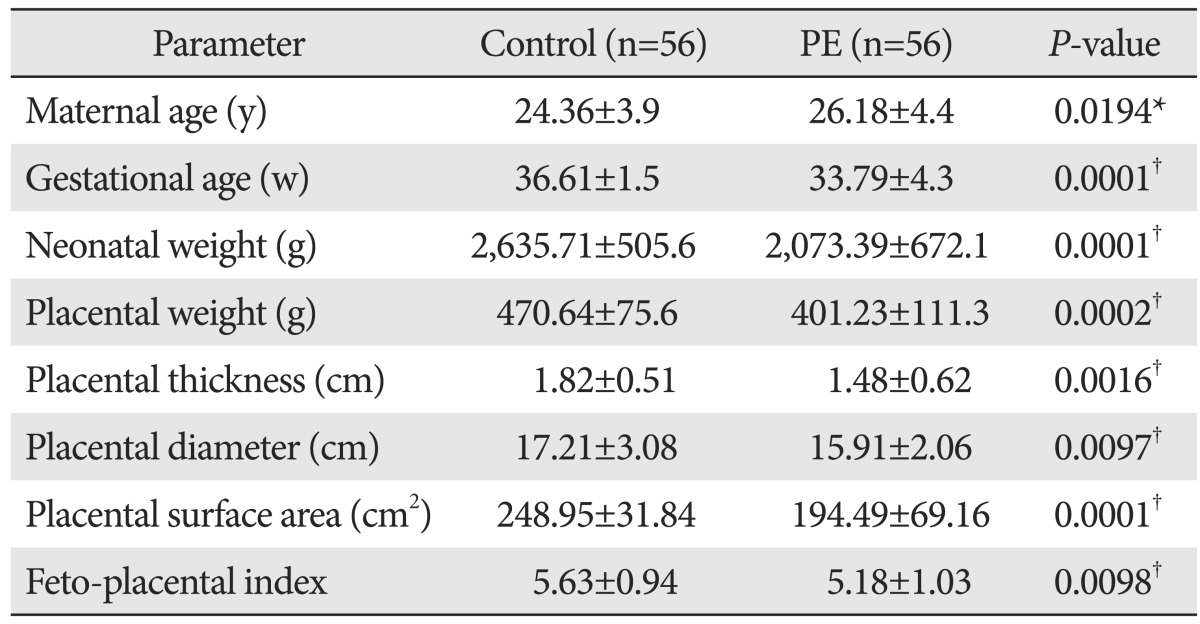
Values are presented as mean±SD. *Significant. †Extremely significant.
Histological findings
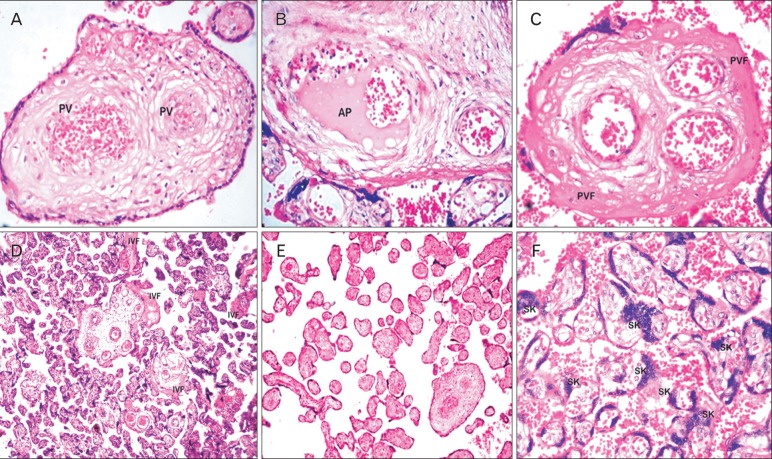
The stem villi of the PE placentas showed numerous arteriosclerotic blood vessels with endothelial degeneration presenting progressive fibrosis, stem villous perivasculitis, and subsequent lumen obliteration (Fig. 1A). These villi had smooth muscle hypertrophy with greatly multiplied numbers of muscle layers in the tunica intima. Stem villi thrombosis seen as atheromatous plaques was observed in the PE placentas (Fig. 1B).
Fig. 1.
Histological sections of stem and terminal villi of preeclamptic placentas stained with hematoxylin and eosin. (A) A section of stem villus showing perivasculitis (PV) of fetal vessels. (B) A section of stem villus showing thrombosis and atheromatous plaque (AP) formation in fetal vessels. (C) A section of stem villus showing perivillous fibrin (PVF) deposition. (D) A placental section showing intervillous fibrin (IVF) deposition. (E) Avascular terminal villi of preeclampsia. (F) Placental villi showing the clusters or sprouts of syncytiotrophoblast, that is, syncytial knots (SK) (A-C, ×1,000; D-F, ×400).
Perivillous fibrin (Fig. 1C) and intervillous fibrin deposition (Fig. 1D), which also extended to the intervillous bridges, were observed in the PE cases. The number and structure of TV specifically varied in PE. The total numbers of TV were significantly lesser, indicating distal villous hypoplasia. The paucity of TV was probably because the capillary, which initiates villous sprouting in the placental core, had not been established.
Numerous avascular TV (Fig. 1E) surrounded the arteriosclerotic stem villi, possibly reflecting failure of vascular organization (villitis). TV syncytiotrophoblasts invariably developed clusters and sprouts to form syncytial knots (Fig. 1F); the PE group had significantly more knots than the control group.
Histomorphometrical findings (Table 2) indicated that, compared to the placentas in the control group, the placentas in the PE group had lesser villous surface area and smaller diameter, whereas the density of the TV was significantly higher in PE (P<0.0001). The density of fetal blood vessels in these TV was significantly decreased in the PE group compared to controls (P<0.0001), but the diameters of TV were more or less similar between the groups and were significant (P<0.05).
Table 2.
Histometrical findings of placental terminal villi of controls and preeclampsia (PE)
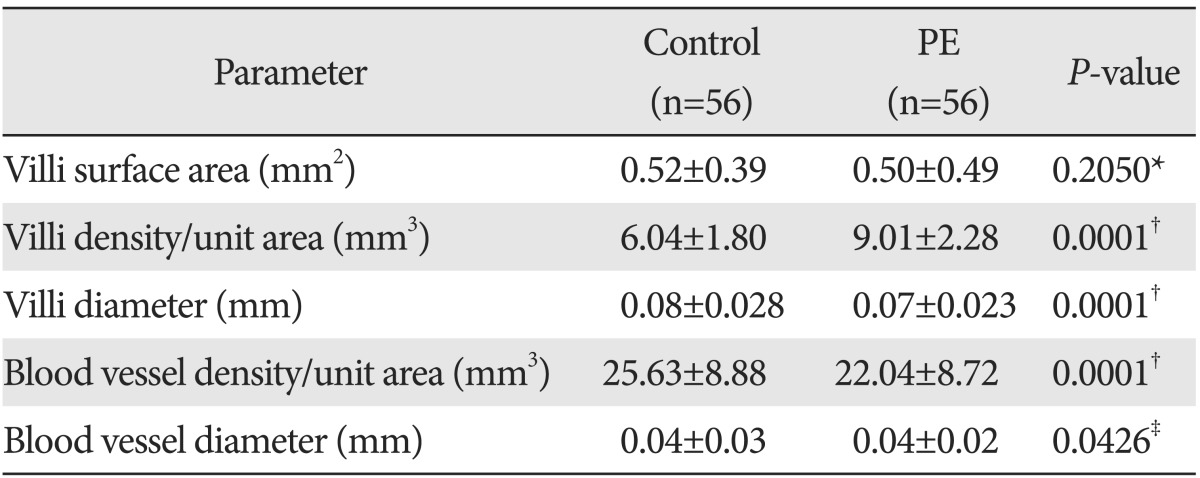
Values are presented as mean±SD. *Not significant. †Extremely significant. ‡Significant.
Discussion
Pregnancies complicated by PE are reflected in the placenta both macroscopically and microscopically. Although the placenta adapts well to the hypoxic condition in PE, the compensatory changes that occur are insufficient. These compensatory changes cause maldevelopment and inadequate placental mass, causing placental dysfunction that leads to oxidative stress and chronic fetal hypoxemia [26].
In the present study comparing preeclamptic placentas to control placentas, the mean placental weight, thickness, diameter, and surface area were decreased and were found to be more significant. The macroscopic changes found in our study are analogous to the findings of other PE cases in the literature [27]. The gross reduction of the PE placenta impedes normal placentation and pathologically results in massive microscopic changes in the placenta.
In contrast to the histomorphometrical findings in the normal placentas, changes in the PE placentas cause functional disturbance, which is the result of the oxidative stress/hypoxic conditions due to PE. Stereological assessment in the PE cases revealed that the average number of TV reported, as indicated by villi density, was significantly increased (P<0.0001). The surface areas, villi diameters, and blood vessel densities of the placentas in PE cases were lesser than those of controls. Although the diameter of blood vessels between the two groups did not show much difference, the difference was statistically significant (P<0.05).
In normal placentation, during the first and early second trimesters, the villus growth and arborization are regulated, which are necessary for fetal well being [28, 29].
The cytotrophoblast cells invade into the uterine spiral arteries and transform them from small-caliber resistance vessels into high-caliber capacitance vessels capable of providing enhanced placental perfusion adequate for the growing fetus. For this transformation, a certain amount of hypoxia is needed to stimulate placental blood vessel formation. Until approximately 10 weeks of gestation, the embryo exists in a hypoxic environment with nutrients provided by the endometrial glands [30]. However, prolonged durations of hypoxia or oxidative stress leads to poor placental perfusion, which is the underlying pathogenesis of PE.
In PE, invasion of the uterine spiral arteries is limited to the proximal decidua, and 30-50% of the spiral arteries of the placental bed escape endovascular trophoblast remodeling. Persistence of muscular and elastic tissues of the media of spiral arteries, fail to dilate and remain responsive to vasomotor influences that lead to high resistance low flow choriodecidual circulation [31, 32].
The reduction in the vascular dimensions is constantly accompanied by a significant impact on the lumen of the arteriole with changes in its muscular wall [33]. Thus, the average diameter of the blood vessels, which normally expands to 4 times their original size, is greatly decreased in PE.
In the present study, significant reduction in the blood vessel density in TV of placentas in PE cases is caused by fibrinization and the stenosis and atherosis of the stem villi. The decreased blood vessel lumen found in the stem villi and mature intermediate villi ultimately fail to replicate and establish a network into the TV. This results in the complete absence of capillaries in the TV in most vicinities of the placenta, leading to the formation of avascular villitis. Consequently, the resultant decreased perfusion causes oxidative stress [34].
In the present study, we believe that the increased villi density and decreased villi diameter in PE cases may have occurred because of the continuous sprouting of the intermediate villi into TV in order to compensate for the placental maldevelopment and dysfunction. Although the number of villi increased, their diameter decreased because vasculature could not be established owing to unsustained normal villi size as noted in the normal placenta. Further, fetoplacental vascular development is an evidence for the anatomical strategy, which substantially affects the placental villi [35, 36]. Accelerated villous branching, numerous syncytial knots, and villitis are the related findings of reduced perfusion [37, 38]. According to Burton et al. [34], generation of reactive oxygen species under oxidative stress could be the major reason of abnormal vascular remodeling and production of increased syncytial knots. In our previous study [7], we reported similar findings with the oxidative stress injury disrupting syncytiotrophoblast arrangement and resulting in increased vasculosyncytial membrane thickness and syncytial knot density.
In summary, placental architecture is altered in many maternal diseases such as PE and eclampsia. Placental morphology and cellular arrangement are important for oxygen delivery from the mother to the fetus. Placental weight in women with PE is directly proportional to neonatal birth weight. Moreover, gestational age is the most important factor affecting maternal and perinatal outcomes. The present study showed that both morphological and histological changes in the PE placenta such as decreased weight, thickness, and diameter, and increased TV density and decreased blood vessel density are the pathogenesis involved in maternal and fetal morbidity and mortality in women with PE.
Acknowledgements
We wish to thank the staff of the Obstetrics and Gynecology Department for their helping in patient enrollment and placental tissue collection, and Mr. Robert, histochemistry technician of the Anatomy Department for his valuable help in histological sectioning. We also wish to acknowledge the support from Dr. NTR University of Health Sciences, Vijayawada, Andhra Pradesh, India.
References
- 1.Mardi K, Sharma J. Histopathological evaluation of placentas in IUGR pregnancies. Indian J Pathol Microbiol. 2003;46:551–554. [PubMed] [Google Scholar]
- 2.Vogel P. The current molecular phylogeny of Eutherian mammals challenges previous interpretations of placental evolution. Placenta. 2005;26:591–596. doi: 10.1016/j.placenta.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Pardo F, Arroyo P, Salomón C, Westermeier F, Guzmán-Gutiérrez E, Leiva A, Sobrevia L. Gestational diabetes mellitus and the role of adenosine in the human placental endothelium and central nervous system. J Diabetes Metab. 2012;S2:010. [Google Scholar]
- 4.Barker DJ, Bagby SP, Hanson MA. Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:700–707. doi: 10.1038/ncpneph0344. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. Int J Dev Biol. 2010;54:525–530. doi: 10.1387/ijdb.082760db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhananjay BS, Dayananda G, Sendilkumaran D, Murthy N. A study of factors affecting perinatal mortality in eclampsia. J Physiol Biomed Sci. 2009;22:2–5. [Google Scholar]
- 7.Sankar KD, Bhanu PS, Kiran S, Ramakrishna BA, Shanthi V. Vasculosyncytial membrane in relation to syncytial knots complicates the placenta in preeclampsia: a histomorphometrical study. Anat Cell Biol. 2012;45:86–91. doi: 10.5115/acb.2012.45.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishwara S, Ara S, Rayhan KA, Begum M. Morphological changes of placenta in preeclampsia. Bangladesh J Anat. 2009;7:49–54. [Google Scholar]
- 9.Akhlaq M, Nagi AH, Yousaf AW. Placental morphology in pre-eclampsia and eclampsia and the likely role of NK cells. Indian J Pathol Microbiol. 2012;55:17–21. doi: 10.4103/0377-4929.94848. [DOI] [PubMed] [Google Scholar]
- 10.Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev. 2006;27:141–169. doi: 10.1210/er.2005-0011. [DOI] [PubMed] [Google Scholar]
- 11.Bdolah Y, Karumanchi SA, Sachs BP. Recent advances in understanding of preeclampsia. Croat Med J. 2005;46:728–736. [PubMed] [Google Scholar]
- 12.Huppertz B. Placental villous trophoblast: the altered balance between proliferation and apoptosis triggers pre-eclampsia. J Reprod Med Endocrinol. 2006;3:103–108. [Google Scholar]
- 13.Crocker IP, Cooper S, Ong SC, Baker PN. Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. Am J Pathol. 2003;162:637–643. doi: 10.1016/S0002-9440(10)63857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dash PR, Whitley GS, Ayling LJ, Johnstone AP, Cartwright JE. Trophoblast apoptosis is inhibited by hepatocyte growth factor through the Akt and beta-catenin mediated up-regulation of inducible nitric oxide synthase. Cell Signal. 2005;17:571–580. doi: 10.1016/j.cellsig.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 16.Cartwright JE, Fraser R, Leslie K, Wallace AE, James JL. Remodelling at the maternal-fetal interface: relevance to human pregnancy disorders. Reproduction. 2010;140:803–813. doi: 10.1530/REP-10-0294. [DOI] [PubMed] [Google Scholar]
- 17.Zigić Z, Marković S, Grbesa D, Ramić S, Halilović A. Quantitative research of capillaries in terminal villi of mature placentae. Bosn J Basic Med Sci. 2010;10:147–152. doi: 10.17305/bjbms.2010.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill JS, Salafia CM, Grebenkov D, Vvedensky DD. Modeling oxygen transport in human placental terminal villi. J Theor Biol. 2011;291:33–41. doi: 10.1016/j.jtbi.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: multiple approaches for a multifactorial disease. Dis Model Mech. 2012;5:9–18. doi: 10.1242/dmm.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilie R, Ilie C, Enătescu I, Bernad E, Frandes CD, Herbeck R. Histological modifications of the feto-placental interface in pregnancy induced hypertension. J Pediatr. 2011;14:55–56. [Google Scholar]
- 21.ACOG Committee on Obstetric Practice. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;77:67–75. [PubMed] [Google Scholar]
- 22.Sivarao S, Vidyadaran MK, Jammal AB, Zainab S, Goh YM, Ramesh KN. Weight, volume and surface area of placenta of normal pregnant women and their relation to maternal and neonatal parameters in Malay, Chinese and Indian ethnic groups. Placenta. 2002;23:691–696. doi: 10.1053/plac.2002.0817. [DOI] [PubMed] [Google Scholar]
- 23.Elias H, Henning A. Stereology of the human renal glomerulus. In: Weibel ER, Elias H, editors. Quantitative Methods in Morphology. Berlin: Springer-Verlag; 1967. pp. 155–158. [Google Scholar]
- 24.Palkovts M, Fischen J. Karyometric investigations. Ch III. Budapest: Akademiai; 1968. p. 75. [Google Scholar]
- 25.Elias H, Hyde DM. An elementary introduction to stereology (quantitative microscopy) Am J Anat. 1980;159:412–446. doi: 10.1002/aja.1001590407. [DOI] [PubMed] [Google Scholar]
- 26.Myatt L. Role of placenta in preeclampsia. Endocrine. 2002;19:103–111. doi: 10.1385/ENDO:19:1:103. [DOI] [PubMed] [Google Scholar]
- 27.Bokhari ZH, Khalid A, Tazeen N, Bukhari MH. Histomorphometric study of maternal side of placenta in preeclampsia. Ann King Edward Med Univ. 2010;16:209–214. [Google Scholar]
- 28.Kaufmann P, Burton G. Anatomy and genesis of the placenta. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 441–483. [Google Scholar]
- 29.Mayhew TM. Villous trophoblast of human placenta: a coherent view of its turnover, repair and contributions to villous development and maturation. Histol Histopathol. 2001;16:1213–1224. doi: 10.14670/HH-16.1213. [DOI] [PubMed] [Google Scholar]
- 30.Burton GJ, Jauniaux E, Charnock-Jones DS. The influence of the intrauterine environment on human placental development. Int J Dev Biol. 2010;54:303–312. doi: 10.1387/ijdb.082764gb. [DOI] [PubMed] [Google Scholar]
- 31.Saeed I, Iqbal I, Sarfaraz R, Qamar K, Butt SA, Shaukat S. Histomorphological changes in placentae of preeclamtic mothers with reference to vasculosnycytial membrane thickness and syncytial knot formation. J Rawalpindi Med Coll. 2012;16:51–54. [Google Scholar]
- 32.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Peng M, Yu L, Ding YL, Zhou CJ. Trophoblast cells invaing the placenta bed and change of spiral arteries and microvessels in pre-eclampsia. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33:121–129. [PubMed] [Google Scholar]
- 34.Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benirschke K, Kaufmann P. Pathology of the human placenta. 4th ed. New York: Springer Verlag; 2000. [Google Scholar]
- 36.Mayhew TM. Fetoplacental angiogenesis during gestation is biphasic, longitudinal and occurs by proliferation and remodelling of vascular endothelial cells. Placenta. 2002;23:742–750. doi: 10.1016/s0143-4004(02)90865-9. [DOI] [PubMed] [Google Scholar]
- 37.Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol. 2008;61:1254–1260. doi: 10.1136/jcp.2008.055236. [DOI] [PubMed] [Google Scholar]
- 38.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92:35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]