Abstract
Nodes of Ranvier are specialized axonal domains formed in response to a glial signal. Recent research advances have revealed that both CNS and PNS nodes form by several overlapping molecular mechanisms. However, the precise nature of these mechanisms and the hierarchy existing between them is considerably different in CNS vs. PNS nodes. Namely, the Schwann cells of the PNS, which directly contact the nodal axolemma, secrete proteins that cluster axonodal components at the edges of the growing myelin segment. In contrast, the formation of CNS nodes, which are not contacted by the myelinating glia, is surprisingly similar to the assembly of the axon initial segment and depends largely on axonal diffusion barriers.
Introduction
Myelin is the vertebrates' solution for fast and energetically efficient axonal conduction. Action potentials generated at the axon initial segment (AIS) are passively transmitted under the myelin sheath and regenerate in the periodic gaps in the myelin called nodes of Ranvier (NOR). Thus, for the myelinated nerve to function, voltage gated sodium and potassium channels (Nav and Kv) must be specifically localized to AIS and NOR where action potentials take place. In terms of neuronal conduction, the physiological consequences of having myelin without nodes would probably be more detrimental than not having myelin at all. It is thus not surprising that several mechanisms have evolved to facilitate and ensure the correct assembly and stabilization of these highly important axonal domains. In this review we aim to provide the reader with an up-to-date model for node formation while emphasizing PNS-CNS differences as well as comparing the formation of the node to that of its evolutionary “ancestor”, the AIS.
For any specialized membrane domain to form, proteins must first be transported to the cell membrane then tethered to a particular site on the membrane where they assemble together by specific protein-protein interactions. Finally, the newly formed protein complex is stabilized by restricting its lateral diffusion. Each of these steps can be achieved by different possible mechanisms. The major components of nodes and initial segments are voltage gated ion channels. All other components, namely cell adhesion molecules (CAMs), extracellular matrix (ECM) proteins and cytoskeletal proteins serve to cluster and stabilize the channels in these crucial axonal domains.
Trafficking of nodal components
Targeting proteins to specific membrane domains is achieved either by selective transport, or random transport followed by specific retention in the correct domain or removal of mislocalized proteins. Retention can be accomplished by anchoring to large molecular scaffolds or by restricting lateral diffusion [1,2]. The ion channels of the AIS and nodes are initially transported to all parts of the neuron and then anchored and immobilized by ankyrin G (ankG). Like numerous other axonal proteins, the channels are endoctyosed from the somatodendritic membrane [3] via two endocytosis signals they possess [4]. Interestingly, ankG itself may be subjected to a retention/exclusion targeting mechanism as it is apparently excluded from the submembranous cytoskeleton of the distal axon [5]. Once the AIS is formed, the high density of membrane proteins and their anchorage to the cytoskeleton creates a diffusion barrier that immobilizes all AIS components, including small lipid molecules, regardless of their binding to the cytoskeleton [6,7].
It was recently shown that during PNS node formation, CAMs are initially assembled from a cell surface pool, whereas the accumulation of Nav and ankG requires vesicular transport from the cell soma [8]. These results are in line with current models for node formation in which, according to the removal-retention model, Nav channels targeting to the axolemma is ankG-dependent, whereas gliomedin-induced CAM clustering requires a mobile surface pool of CAMs. The Surface expression of Nav channels may also depend on the beta1 and beta2 auxiliary subunits [9]. These ankyrin-binding proteins enhance the surface expression of the Nav pore forming alpha subunit [10], and their deficiency results in reduced levels of Nav channels at the optic nerve NOR [11]. Moreover, beta 2 and 4 possibly associate with the alpha subunit prior to its transport to the axolemma, since their AIS and nodal localizations depend on a single extracellular disulfide bond with the alpha subunit [12,13].
Assembly
The scaffold protein ankG was first described as a master regulator for AIS assembly 15 years ago when Bennet and colleagues showed that cerebellar Purkinje cells lack AIS in the absence of ankG [14]. Almost a decade later, ankG was shown to be crucial for NOR formation [15] and remains the only protein whose correct localization is consistently both necessary and sufficient for AIS and NOR assembly [16]. This is not surprising as ankG can bind and cluster most if not all of the known axonal proteins that these domains contain (Table 1), namely Nav and Kv channels, the CAMs neurofascin 186 isoform (NF186) and NrCAM, as well as bIV spectrin, an actin binding scaffold protein. βIV spectrin thus connects the ankG-organized AIS and nodal protein complexes to the submembranous actin cytoskeleton.
Table 1.
Molecular components of nodes and AIS.
| Cell adhesion | AIS | CNS NOR | PNS NOR |
|---|---|---|---|
| NF186 [59] | + | + | + |
| NrCAM [59] | + | + | + |
| Navb1-4 [9] | + | + | + |
| Caspr2 [9,60] | + | - | - |
| TAG1 [61] | + | - | - |
| ADAM22 [62] | + | - | - |
| Dystroglycan [63] | - | - | + |
| Ion channels | |||
| Nav1 [16,64] | + | + | + |
| KCNQ2,3 [65,66] | + | + | + |
| Kv1 [60,61] | + | - | - |
| Kv3.1b [67] | - | + | + |
| ECM | |||
| Gliomedin [18] | - | - | + |
| Syndecan-3,4 [68] | - | - | + |
| Collagen XXVII [69] | - | - | + |
| Perlecan [70] | - | - | + |
| Collagen V [71] | - | - | + |
| Brevican [39,72] | + | + | - |
| Versican V2 [73,74] | + | + | - |
| Versican V1 [71] | - | - | + |
| Neurocan [39,75] | -/+ | + | - |
| BralI [54,73] | nd | + | - |
| Phosphacan [72] | nd | + | - |
| Tenascin-R [72,76] | nd | + | - |
| Hyaluronan [73,76] | + | + | - |
| NG2 [77,78] | nd | + | + |
| Scaffolds | |||
| AnkG-scaffold [79] | + | + | + |
| βIVspectrin [79] | + | + | + |
| PSD93 [61] | + | - | - |
| Signaling | |||
| IQCJ-SCHIP-1 [80] | + | + | + |
| CK2 [30,31] | + | + | + |
| FHFs [81-83] | + | + | + |
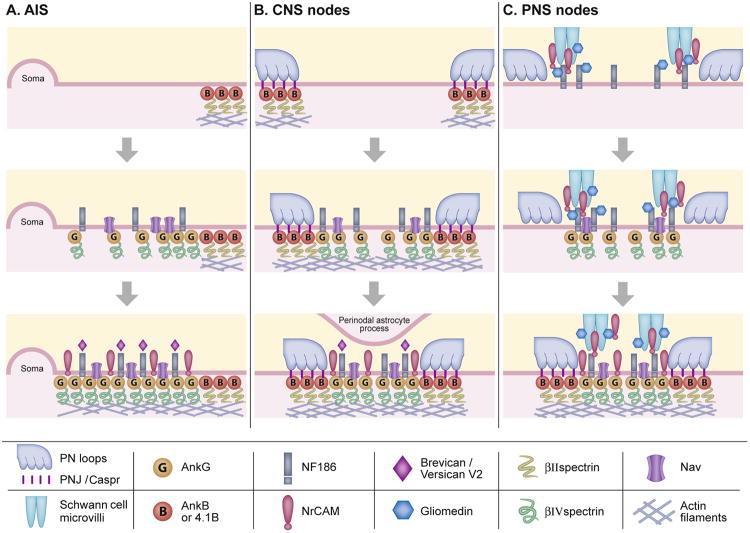
For nodes and AIS to form, ankG has to be tethered to the membrane at specific sites. The specific locations are determined by the neurons alone (AIS) or by the myelinating glial cell, which is the oligodendrocyte in the CNS and the Schwann cell in the PNS. One major difference between CNS and PNS nodes is that PNS nodes are contacted by microvillar processes sent from the Schwann cells that myelinate the flanking internodes. In the CNS, however, the myelinating cells themselves do not contact the nodes which are to some degree contacted by astrocyte processes [17]. The second major difference is the extracellular proteins, mostly ECM, that interact with the nodal axolemma (Table 1). These differences are reflected in the way in which nodes form in the CNS and PNS tissues, as depicted in Figure 1.
Figure 1.
Current models for the formation of nodes and AIS. A-B. AIS and CNS nodes. The assembly of both the AIS and CNS nodes starts by the formation of a diffusion barrier marked by ankB and 4.1B. For nodes, this occurs at the base of the formed paranodal junction. In the adjacent AIS or nodal axolemma, accumulation of ankG results in the clustering of Nav and of NF186. ECM is the last to appear, and together with NF186, is probably required for the stabilization of AIS and nodal complexes. An asctrocyte process may contact CNS nodes late in development, although the exact timing is still to be determined. C. PNS nodes. The assembly of PNS nodes is induced by glial gliomedin and NrCAM that cluster NF186 on the axolemma, resulting in the accumulation of ankG and Nav. Linkage of the forming complex to the submembranous actin cytoskeleton likely occurs earlier in CNS nodes formation [26] and later in AIS and PNS nodes [7,26]. See table 1 for additional components not described here.
PNS nodes
During PNS myelination, heminodal clustering of Nav channels, i.e. their accumulation at the edges of a growing myelin segment, depends on the interaction of axonal NF186 with glial gliomedin and NrCAM [18,19]. Gliomedin incorporates into the nodal ECM where it creates high avidity CAM-binding multimolecular complexes that drive the accumulation of NF186 in the underlying axolemma [20]. AnkG is recruited to NF186 complexes, followed by bIV spectrin and Nav [15,18,21,22]. A recent paper by Zhang et al provides an elegant support for this model showing that initial accumulation of NF186 at heminodes as well as its internodal clearance requires its ectodomain, while its stabilization in mature nodes requires its cytoplasmic ankG binding domain. This is in contrast to the AIS where the ankG binding domain is sufficient for early clustering of NF186 [8].
Flanking the nodes are the paranodal junctions (PNJ) that form between the terminal loops of the glia and the axon. These junctions form after the formation of nodes in the PNS [23]. Nevertheless the PNJ themselves, serving as diffusion barriers that limit the lateral diffusion of membrane proteins, can drive the assembly of PNS nodes in the absence of a functioning heminodal clustering mechanism (e.g. in gliomedin or NrCAM null mice). Consequently, only in the absence of both heminodal and paranodal mechanisms, nodes would not form. Thus in the PNS clustering of nodal components is induced by a glial signal at the heminodes. A second mechanism, which depends on intact paranodal junctions, ensures nodal assembly [19].
CNS nodes
A different sequence of events is observed is the developing CNS node:the PNJ appear first [24], followed by ankG which in turn recruits the nodal CAMs and Nav [25]. Therefore, The primary inductive signal does not appear at the nascent nodes themselves (which lack oligodendrocyte contact), but at the PNJ driving the accumulation of ankG in the adjacent nodal axolemma. In a recent paper Suzuki et al show that similarly to the PNS, CNS nodes form byseveral mechanisms [26]. This paper focuses on three elements known to be involved in node formation: The PNJ, NF186-binding ECM proteins, and the spectrin-based cytoskeleton. By generating mice lacking different pairs of nodal elements, as well as testing the function of mutant NF186 constructs, they unraveled the existence of a hierarchy between the different mechanisms that control node formation. They concluded that the PNJ play the primary role during CNS node formation, while the ECM components, which as they show are the last to appear in the node, play a role in stabilizing rather than assembling the nodes. In addition they show that linkage to the actin-spectrin cytoskeleton contributes to CNS nodal assembly. This is in contrast to the PNS, as mice lacking both paranodal and spectrin-based mechanisms had normal PNS nodes [26]. These results demonstrate that while ECM-based interactions play a primary role in PNS node formation, in the CNS, paranodal and cytoskeletal (rather than ECM-based) mechanisms collaborate to assemble nodes. Notably, in both PNS and CNS, removing the primary assembly mechanisms would not result in nodal loss due to the compensation by the other mechanisms [19,26,27]. Thus, the crucial importance of the NOR for neural function has prompted the evolution of a fail-safe system for assembling nodes, using multiple mechanisms with mutual backup capacities.
AnkG-based mechanisms
Nav1 channels contain an ankyrin binding motif, that binds both ankB, expressed along distal axons, and ankG [28]. This sequence was shown to be sufficient for targeting proteins to AIS [28,29] and NOR [30]. Phosphorylation by the protein kinase CK2 dramatically increases the affinity of this site specifically to ankG [31]. AIS localization of CK2 and ankG are mutually dependent and are both necessary for microtubule stabilization at AIS [32], which in turn is necessary for the preservation of AIS integrity and neuronal polarity [33]. A CK2-ankG mutual dependence makes it hard to conclude whether CK2 is truly necessary for Nav-ankG association in this domain. However, using mutants of full length Nav1.6 alpha subunit, Gasser et al showed that while Nav1.6 interaction with ankG is crucial for its AIS and NOR localization, its phosphorylation by CK2 is not critical but serves to facilitate channel clustering at these domains [30]. They attribute the apparent discrepancy with previous publications to the different reporter protein that were used. In this paper, the use of the full length alpha subunit ratherthan a kv2.1/Nav1.2 chimera also revealed that Nav-ankG interactions are necessaryand sufficient for Nav clustering at both AIS and NOR, as no other protein-protein interaction can substitute for the loss of ankG binding [30]. Interestingly, ankG can associate to the MDCK cell membrane independently of its binding partners E-cadherin and βII spectrin. This membrane association depends on ankG's palmitoylation at Cys-70, which was shown to be crucial for its function as the AIS organizer [34]. As a C70A mutant could localize to AIS in WT but not in ankG-deficient neurons, this may indicate that ankG initially accumulates in the forming AIS via its Cys-70 plamitate modification [34].
NF186
Owing to its capacity to bind both ankG [35] and ECM proteins [20,26], NF186 plays a key role during node formation in both CNS and PNS [36-38]. In contrast, it is mostly involved in stabilization rather than formation of the AIS [39-41]. In the PNS, secretion of glial gliomedin and NrCAM at the nodal gap initiates node assembly [18]. Different binding sites for gliomedin and NrCAM on NF186 probably allow the multimolecular glial complexes to cluster axonal NF186 at heminodes rapidly and efficiently [42].
Can the PNJ-induced mechanism for node formation compensate for NF186 deficiency or is it NF186-dependent as well? The fact that NF186 accumulates at mature nodes in both PNS [19] and CNS [26] ECM mutants implies that NF186 may be involved in the PNJ-mediated node formation. Using neuron-specific ablation of neurofascin (as the glial isoform 155 is necessary for PNJ formation) it was concluded that NF186 is necessary for both CNS and PNS node formation [38]. On the other hand, using a NF155 transgene that recovers PNJ in neurofascin-null animals, it was shown that NF186 was necessary for PNS but not for CNS node formation [36,37]. Finally, Feinberg et al showed that in DRG myelinating cultures, NF186 is not necessary for node formation when the PNJ is intact. The apparent discrepancies between these papers most probably arise from the different models that were studied. In [38], the neuronal specific ablation of neurofascin was only partial. As a result, nodal counting was based on N186-negative nodes, which could result in a bias towards the less stable node population. The finding that in DRG cultures, representing an early developmental stage, NF186 is dispensable, could suggest that NF186 is crucial for early stabilization of the node and not for its assembly, two processes that are hard to distinguish.
The paranodal junction
How do the PNJ drive the accumulation of ankG in the developing node? The role of the transverse bands, intercellular densities at the paranodal junction, is unknown. Recently it was shown that in contrast to previous hypotheses, this structure does not create an extracellular seal [43]. However, using electron tomography on thin sections of mouse corpus callosum, Nans et al identified an extensive network of filamentous linkers in the paranodal axoplasm. These linkers interconnected the three cytoskeletal systems of the axon to one another as well as to the transverse bands, making the PNJ a center of cytoskeletal connections. In addition they revealed that various membranous organelles, including transport vesicles, were tethered to the PNJ by short filaments. These observations support a role for the PNJ as a diffusion barrier fencing the NOR, and suggest an additional role in targeted trafficking of proteins to the nodes [44]. Additional support for a role for cytoskeletal connections in the paranodes comes from two different papers showing that the association of caspr and the actin-spectrin cytoskeleton via protein 4.1B is necessary for an efficient paranodal membrane barrier [45,46].
In addition to their role in creating diffusion barriers, cytoskeletal structures may promote node formation in various other ways. One such mechanism has recently been introduced for AIS formation by Galiano et al. Searching for cellular cues that localize ankG at the forming AIS, these authors found that in young unmyelinated neurons, a submembranous cytoskeleton consisting of ankB, αII-Spectrin, and βII-Spectrin defines an intra-axonal boundary that precedes and localizes ankG at the developing AIS [5]. Moreover 4.1B, another spectrin binding protein, was shown to be involved in the segregation between the AIS and the first myelin segment [47]. These cytoskeletal components are also present in PNJ [48]. It would thus be interesting to test whether the paranodal localization of these proteins precedes nodal ankG. If this were the case, then CNS nodes would form very similarly to the AIS, differing only in the initiating signal, i.e., an axoglial contact vs. a cell-autonomous process.
Maintenance
AnkG appears to play a major role not only in the assembly but also in the maintenance of AIS and nodes. Knock down of ankG in fully polarized neurons resulted in AIS disintegration, as well as loss of neuronal polarity [49]. In the AIS, NF186 and NrCAM accumulate late and depend on ankG for their localization [50]. Although both CAMs are probably dispensable for the assembly of AIS and CNS nodes [37,39], NF186 is critical for the maintenance of the AIS and PNS nodes [8,41], but not for the maintenance of CNS nodes [41]. The authors also show that the NF186 turnover rate is much slower at the node than at AIS, which suggests that nodes are much less plastic than the AIS, which emerges as a highly plastic domain [51,52]. Interestingly, the authors note that the stabilization of the AIS requires NF186 as a linker between extracellular proteins (e.g. brevican) and the axolemma, similarly to its role at the NOR.
In the CNS, perinodal ECM components accumulate after the nodes had assembled, and thus probably play a stabilizing role [26]. This highly variable and redundant ECM always contains BralI which is a brain specific link protein that stabilize the binding of lecticans and hyaluronic acid [53]. In BralI KO mice, changes in the nodal ECM (e.g. brevican, versicanV2 and hyaluronan are missing), do not result in changes in Nav channel clustering at the node. However, BralI mice exhibit slower salutatory conduction [54], probably since the BralI-associated ECM serves as an extracellular ion pool that facilitates nodal action potentials. Thus the nodal ECM, at least in the CNS, is crucial for maintaining functional nodes. These results also emphasize the importance of conducting physiological tests when dealing with nodal mutants, as there is often much discrepancy between histological and physiological observations. Although the mechanisms operating to stabilize PNS nodes are still under investigation, it seems that they constitute very stable domains similarly to their CNS counterparts [8]. Given the analogy between CNS and PNS nodes, it is likely that additional “late-arriving” ECM components that bind NF186 are involved in stabilization and maintenance of PNS nodes.
Concluding remarks
The picture emerging from recent studies indicate that the AIS and CNS nodes probably form by similar mechanisms (Figure 1). In both cases, accumulation of ankG is induced by an intra axonal barrier formed autonomously or by axoglial contact, respectively. The extremely long and large caliber axons of the PNS probably require a more rapid and robust nodal assembly, thus in the PNS, ankG-Nav complex formation is catalyzed by a gliomedin-NrCAM glial signal that binds axonal NF186. Interestingly, in both CNS nodes and AIS, although not crucial for their formation, NF186-ECM interactions are essential for the stabilization and functionality of these domains. It is thus plausible that these NF186-binding ECM proteins are subjected a spatial and/or temporal regulation, possibly by matrix proteinases.
Node formation requires the cooperation between neurons and glia for the formation of diffusion barriers as well as clustering of membrane proteins in a specific time and place. It is thus tempting to speculate that these two intimately connected cells may communicate on additional levels. Vesicle exchange between cells was shown to be a means of sharing proteins, microRNAs, mRNAs and ribosomes between neighboring cells [55]. Moreover, transfer of ribosomes from Schwann cells to injured axons has been recently described [56-58], raising the possibility that the formation and maintenance of remote peripheral nodes may be aided by supplies from the ensheathing Schwann cells.
Highlights.
Nodes of Ranvier form by several overlapping mechanisms.
Node assembly is induced differently by the myelinating glia of the PNS and CNS.
Axonal barriers drive the assembly of both CNS nodes and the AIS.
Secreted glial molecules drive the assembly of PNS nodes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted fo publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jensen CS, Rasmussen HB, Misonou H. Neuronal trafficking of voltage-gated potassium channels. Molecular and cellular neurosciences. 2011;48:288–297. doi: 10.1016/j.mcn.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Lasiecka ZM, Winckler B. Mechanisms of polarized membrane trafficking in neurons -- focusing in on endosomes. Molecular and cellular neurosciences. 2011;48:278–287. doi: 10.1016/j.mcn.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winckler B, Mellman I. Trafficking guidance receptors. Cold Spring Harbor perspectives in biology. 2010;2:a001826. doi: 10.1101/cshperspect.a001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leterrier C, Brachet A, Dargent B, Vacher H. Determinants of voltage-gated sodium channel clustering in neurons. Seminars in cell & developmental biology. 2011;22:171–177. doi: 10.1016/j.semcdb.2010.09.014. [DOI] [PubMed] [Google Scholar]
- **5.Galiano MR, Jha S, Ho TS, Zhang C, Ogawa Y, Chang KJ, Stankewich MC, Mohler PJ, Rasband MN. A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell. 2012;149:1125–1139. doi: 10.1016/j.cell.2012.03.039. This study identified an intra-axonal boundary based on ankB,bII spectrin and aII spectrin in the distal axon that precedes the accumulation of ankG at AIS and determines axon specification independently of ankG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakada C, Ritchie K, Oba Y, Nakamura M, Hotta Y, Iino R, Kasai RS, Yamaguchi K, Fujiwara T, Kusumi A. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nature cell biology. 2003;5:626–632. doi: 10.1038/ncb1009. [DOI] [PubMed] [Google Scholar]
- *7.Brachet A, Leterrier C, Irondelle M, Fache MP, Racine V, Sibarita JB, Choquet D, Dargent B. Ankyrin G restricts ion channel diffusion at the axonal initial segment before the establishment of the diffusion barrier. The Journal of cell biology. 2010;191:383–395. doi: 10.1083/jcb.201003042. Using surface diffusion measurement of different Kv-Nav reporter proteins expressed in hippocampal neurons this study shows that the initial immobilization of Nav at AIS is ankG-dependent but does not require an AIS diffusion barrier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **8.Zhang Y, Bekku Y, Dzhashiashvili Y, Armenti S, Meng X, Sasaki Y, Milbrandt J, Salzer JL. Assembly and maintenance of nodes of ranvier rely on distinct sources of proteins and targeting mechanisms. Neuron. 2012;73:92–107. doi: 10.1016/j.neuron.2011.10.016. This study nicely shows that during node formation, CAMs assemble from a mobile surface pool, whereas the nodal accumulation of Nav and ankG requires axonal protein transport. Moreover it shows that heminodal clustering of NF186 depends on its ectodomain while its accumulation in mature nodes requires its cytoplasmic domain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patino GA, Isom LL. Electrophysiology and beyond: multiple roles of Na+ channel beta subunits in development and disease. Neuroscience letters. 2010;486:53–59. doi: 10.1016/j.neulet.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isom LL, Scheuer T, Brownstein AB, Ragsdale DS, Murphy BJ, Catterall WA. Functional co-expression of the beta 1 and type IIA alpha subunits of sodium channels in a mammalian cell line. The Journal of biological chemistry. 1995;270:3306–3312. doi: 10.1074/jbc.270.7.3306. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Bharucha V, Chen Y, Westenbroek RE, Brown A, Malhotra JD, Jones D, Avery C, Gillespie PJ, 3rd, Kazen-Gillespie KA, et al. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel beta 2-subunits. Proceedings of the National Academy of Sciences of the United States of America; 2002. pp. 17072–17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Calhoun JD, Zhang Y, Lopez-Santiago L, Zhou N, Davis TH, Salzer JL, Isom LL. Identification of the cysteine residue responsible for disulfide linkage of Na+ channel alpha and beta2 subunits. The Journal of biological chemistry. 2012;287:39061–39069. doi: 10.1074/jbc.M112.397646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buffington SA, Rasband MN. Na+ channel-dependent recruitment of navbeta4 to axon initial segments and nodes of ranvier. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:6191–6202. doi: 10.1523/JNEUROSCI.4051-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. The Journal of cell biology. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzhashiashvili Y, Zhang Y, Galinska J, Lam I, Grumet M, Salzer JL. Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. The Journal of cell biology. 2007;177:857–870. doi: 10.1083/jcb.200612012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leterrier C, Brachet A, Fache MP, Dargent B. Voltage-gated sodium channel organization in neurons: protein interactions and trafficking pathways. Neuroscience letters. 2010;486:92–100. doi: 10.1016/j.neulet.2010.08.079. [DOI] [PubMed] [Google Scholar]
- 17.Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nature reviews Neuroscience. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- 18.Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, Spiegel I, Bermingham JR, Jr, Peles E. Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron. 2005;47:215–229. doi: 10.1016/j.neuron.2005.06.026. [DOI] [PubMed] [Google Scholar]
- **19.Feinberg K, Eshed-Eisenbach Y, Frechter S, Amor V, Salomon D, Sabanay H, Dupree JL, Grumet M, Brophy PJ, Shrager P, et al. A glial signal consisting of gliomedin and NrCAM clusters axonal Na+ channels during the formation of nodes of Ranvier. Neuron. 2010;65:490–502. doi: 10.1016/j.neuron.2010.02.004. This paper shows that PNS nodes form by two cooperating mechanisms: an early heminodal clustering mechanism requires glial gliomedin and NrCAM that bind and cluster axonal NF186, and a later PNJ-mediated clustering acting at mature nodes. These mechanisms are shown do have mutual backup capacities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eshed Y, Feinberg K, Carey DJ, Peles E. Secreted gliomedin is a perinodal matrix component of peripheral nerves. The Journal of cell biology. 2007;177:551–562. doi: 10.1083/jcb.200612139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambert S, Davis JQ, Bennett V. Morphogenesis of the node of Ranvier: co-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:7025–7036. doi: 10.1523/JNEUROSCI.17-18-07025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Ogawa Y, Hedstrom KL, Rasband MN. betaIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. The Journal of cell biology. 2007;176:509–519. doi: 10.1083/jcb.200610128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melendez-Vasquez CV, Rios JC, Zanazzi G, Lambert S, Bretscher A, Salzer JL. Nodes of Ranvier form in association with ezrin-radixin-moesin (ERM)-positive Schwann cell processes. Proceedings of the National Academy of Sciences of the United States of America; 2001. pp. 1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasband MN, Peles E, Trimmer JS, Levinson SR, Lux SE, Shrager P. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:7516–7528. doi: 10.1523/JNEUROSCI.19-17-07516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenkins SM, Bennett V. Developing nodes of Ranvier are defined by ankyrin-G clustering and are independent of paranodal axoglial adhesion. Proceedings of the National Academy of Sciences of the United States of America; 2002. pp. 2303–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **26.Susuki K, Chang KJ, Zollinger DR, Liu Y, Ogawa Y, Eshed-Eisenbach Y, Dours-Zimmermann MT, Oses-Prieto J, Burlingame AL, Seidenbecher C, et al. Three Mechanisms Assemble Central Nervous System Nodes of Ranvier. Neuron. in press By systematically knocking down pairs of different cytoskeletal, ECM and PNJ elements of CNS nodes the authors revealed three mechanisms operating in hierarchy during CNS node formation: a primary PNJ-driven, a secondary cytoskeleton-based, and a third ECM-based mechanism that probably serves to stabilize the assembled nodal complexes. [Google Scholar]
- 27.Pillai AM, Thaxton C, Pribisko AL, Cheng JG, Dupree JL, Bhat MA. Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. Journal of neuroscience research. 2009;87:1773–1793. doi: 10.1002/jnr.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemaillet G, Walker B, Lambert S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. The Journal of biological chemistry. 2003;278:27333–27339. doi: 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- 29.Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache MP, Debanne D, Dargent B. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 2003;300:2091–2094. doi: 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- **30.Gasser A, Ho TS, Cheng X, Chang KJ, Waxman SG, Rasband MN, Dib-Hajj SD. An ankyrinG-binding motif is necessary and sufficient for targeting Nav1.6 sodium channels to axon initial segments and nodes of Ranvier. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:7232–7243. doi: 10.1523/JNEUROSCI.5434-11.2012. This paper shows for the first time that the ankG-binding motif in Nav is both necessary and sufficient for the clustering of these channels in both AIS and nodes of Ranvier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brechet A, Fache MP, Brachet A, Ferracci G, Baude A, Irondelle M, Pereira S, Leterrier C, Dargent B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. The Journal of cell biology. 2008;183:1101–1114. doi: 10.1083/jcb.200805169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez-Ponce D, Munoz A, Garrido JJ. Casein kinase 2 and microtubules control axon initial segment formation. Molecular and cellular neurosciences. 2011;46:222–234. doi: 10.1016/j.mcn.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Tapia M, Wandosell F, Garrido JJ. Impaired function of HDAC6 slows down axonal growth and interferes with axon initial segment development. PloS one. 2010;5:e12908. doi: 10.1371/journal.pone.0012908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.He M, Jenkins P, Bennett V. Cysteine 70 of ankyrin-G is S-palmitoylated and is required for function of ankyrin-G in membrane domain assembly. The Journal of biological chemistry. 2012;287:43995–44005. doi: 10.1074/jbc.M112.417501. This paper shows that plamitoylation of ankG on Cys-70 is necessary for its accumulation at the AIS as well as for its function in lateral membrane biogenesis in epethelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garver TD, Ren Q, Tuvia S, Bennett V. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. The Journal of cell biology. 1997;137:703–714. doi: 10.1083/jcb.137.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, Macklin WB, Meek S, Smith AJ, Cottrell DF, et al. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Zonta B, Tait S, Melrose S, Anderson H, Harroch S, Higginson J, Sherman DL, Brophy PJ. Glial and neuronal isoforms of Neurofascin have distinct roles in the assembly of nodes of Ranvier in the central nervous system. The Journal of cell biology. 2008;181:1169–1177. doi: 10.1083/jcb.200712154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thaxton C, Pillai AM, Pribisko AL, Dupree JL, Bhat MA. Nodes of Ranvier act as barriers to restrict invasion of flanking paranodal domains in myelinated axons. Neuron. 2011;69:244–257. doi: 10.1016/j.neuron.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hedstrom KL, Xu X, Ogawa Y, Frischknecht R, Seidenbecher CI, Shrager P, Rasband MN. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. The Journal of cell biology. 2007;178:875–886. doi: 10.1083/jcb.200705119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kriebel M, Metzger J, Trinks S, Chugh D, Harvey RJ, Harvey K, Volkmer H. The cell adhesion molecule neurofascin stabilizes axo-axonic GABAergic terminals at the axon initial segment. The Journal of biological chemistry. 2011;286:24385–24393. doi: 10.1074/jbc.M110.212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Zonta B, Desmazieres A, Rinaldi A, Tait S, Sherman DL, Nolan MF, Brophy PJ. A critical role for Neurofascin in regulating action potential initiation through maintenance of the axon initial segment. Neuron. 2011;69:945–956. doi: 10.1016/j.neuron.2011.02.021. This paper shows that NF186 is necessary for the maintenance of AIS but not of NOR, however it is not directly responsible for the stabilization of pinceau synapses at the AIS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labasque M, Devaux JJ, Leveque C, Faivre-Sarrailh C. Fibronectin type III-like domains of neurofascin-186 protein mediate gliomedin binding and its clustering at the developing nodes of Ranvier. The Journal of biological chemistry. 2011;286:42426–42434. doi: 10.1074/jbc.M111.266353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shroff S, Mierzwa A, Scherer SS, Peles E, Arevalo JC, Chao MV, Rosenbluth J. Paranodal permeability in “myelin mutants”. Glia. 2011;59:1447–1457. doi: 10.1002/glia.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *44.Nans A, Einheber S, Salzer JL, Stokes DL. Electron tomography of paranodal septate-like junctions and the associated axonal and glial cytoskeletons in the central nervous system. Journal of neuroscience research. 2011;89:310–319. doi: 10.1002/jnr.22561. In this study electron tomography on thin sections of CNS myelinated fibers revealed an extensive network of filamentous linkers connecting the PNJ to the three cytoskeletal systems of the axon as well as to vesicular organnels suppoting a role for the PNJ as diffusion barriers and in targeted trafficking of proteins to the nodes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horresh I, Bar V, Kissil JL, Peles E. Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:2480–2489. doi: 10.1523/JNEUROSCI.5225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cifuentes-Diaz C, Chareyre F, Garcia M, Devaux J, Carnaud M, Levasseur G, Niwa-Kawakita M, Harroch S, Girault JA, Giovannini M, et al. Protein 4.1B contributes to the organization of peripheral myelinated axons. PloS one. 2011;6:e25043. doi: 10.1371/journal.pone.0025043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duflocq A, Chareyre F, Giovannini M, Couraud F, Davenne M. Characterization of the axon initial segment (AIS) of motor neurons and identification of a para-AIS and a juxtapara-AIS, organized by protein 4.1B. BMC biology. 2011;9:66. doi: 10.1186/1741-7007-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogawa Y, Schafer DP, Horresh I, Bar V, Hales K, Yang Y, Susuki K, Peles E, Stankewich MC, Rasband MN. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:5230–5239. doi: 10.1523/JNEUROSCI.0425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. The Journal of cell biology. 2008;183:635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boiko T, Vakulenko M, Ewers H, Yap CC, Norden C, Winckler B. Ankyrin-dependent and -independent mechanisms orchestrate axonal compartmentalization of L1 family members neurofascin and L1/neuron-glia cell adhesion molecule. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:590–603. doi: 10.1523/JNEUROSCI.4302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;465:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuba H, Oichi Y, Ohmori H. Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature. 2010;465:1075–1078. doi: 10.1038/nature09087. [DOI] [PubMed] [Google Scholar]
- 53.Cicanic M, Sykova E, Vargova L. Bral1: “Superglue” for the extracellular matrix in the brain white matter. The international journal of biochemistry & cell biology. 2012;44:596–599. doi: 10.1016/j.biocel.2012.01.009. [DOI] [PubMed] [Google Scholar]
- *54.Bekku Y, Vargova L, Goto Y, Vorisek I, Dmytrenko L, Narasaki M, Ohtsuka A, Fassler R, Ninomiya Y, Sykova E, et al. Bral1: its role in diffusion barrier formation and conduction velocity in the CNS. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:3113–3123. doi: 10.1523/JNEUROSCI.5598-09.2010. In this study, BralI KO results in alterations in the ECM composition of CNS nodes and in slower saltatory conduction which is not associated with reduced Nav immunolaebling at CNS nodes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 56.Court FA, Hendriks WT, MacGillavry HD, Alvarez J, van Minnen J. Schwann cell to axon transfer of ribosomes: toward a novel understanding of the role of glia in the nervous system. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:11024–11029. doi: 10.1523/JNEUROSCI.2429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Twiss JL, Fainzilber M. Ribosomes in axons--scrounging from the neighbors? Trends in cell biology. 2009;19:236–243. doi: 10.1016/j.tcb.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Court FA, Midha R, Cisterna BA, Grochmal J, Shakhbazau A, Hendriks WT, Van Minnen J. Morphological evidence for a transport of ribosomes from Schwann cells to regenerating axons. Glia. 2011;59:1529–1539. doi: 10.1002/glia.21196. [DOI] [PubMed] [Google Scholar]
- 59.Davis JQ, Lambert S, Bennett V. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain-) and NrCAM at nodal axon segments. The Journal of cell biology. 1996;135:1355–1367. doi: 10.1083/jcb.135.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inda MC, DeFelipe J, Munoz A. Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells. Proceedings of the National Academy of Sciences of the United States of America; 2006. pp. 2920–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogawa Y, Horresh I, Trimmer JS, Bredt DS, Peles E, Rasband MN. Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:5731–5739. doi: 10.1523/JNEUROSCI.4431-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogawa Y, Oses-Prieto J, Kim MY, Horresh I, Peles E, Burlingame AL, Trimmer JS, Meijer D, Rasband MN. ADAM22, a Kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:1038–1048. doi: 10.1523/JNEUROSCI.4661-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, et al. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–758. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 64.Rasband MN. Composition, assembly, and maintenance of excitable membrane domains in myelinated axons. Seminars in cell & developmental biology. 2011;22:178–184. doi: 10.1016/j.semcdb.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rasband MN. Clustered K+ channel complexes in axons. Neuroscience letters. 2010;486:101–106. doi: 10.1016/j.neulet.2010.08.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goutebroze L, Carnaud M, Denisenko N, Boutterin MC, Girault JA. Syndecan-3 and syndecan-4 are enriched in Schwann cell perinodal processes. BMC neuroscience. 2003;4:29. doi: 10.1186/1471-2202-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grimal S, Puech S, Wagener R, Venteo S, Carroll P, Fichard-Carroll A. Collagen XXVIII is a distinctive component of the peripheral nervous system nodes of ranvier and surrounds nonmyelinating glial cells. Glia. 2010;58:1977–1987. doi: 10.1002/glia.21066. [DOI] [PubMed] [Google Scholar]
- 70.Bangratz M, Sarrazin N, Devaux J, Zambroni D, Echaniz-Laguna A, Rene F, Boerio D, Davoine CS, Fontaine B, Feltri ML, et al. A mouse model of Schwartz-Jampel syndrome reveals myelinating Schwann cell dysfunction with persistent axonal depolarization in vitro and distal peripheral nerve hyperexcitability when perlecan is lacking. The American journal of pathology. 2012;180:2040–2055. doi: 10.1016/j.ajpath.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Melendez-Vasquez C, Carey DJ, Zanazzi G, Reizes O, Maurel P, Salzer JL. Differential expression of proteoglycans at central and peripheral nodes of Ranvier. Glia. 2005;52:301–308. doi: 10.1002/glia.20245. [DOI] [PubMed] [Google Scholar]
- 72.Bekku Y, Rauch U, Ninomiya Y, Oohashi T. Brevican distinctively assembles extracellular components at the large diameter nodes of Ranvier in the CNS. Journal of neurochemistry. 2009;108:1266–1276. doi: 10.1111/j.1471-4159.2009.05873.x. [DOI] [PubMed] [Google Scholar]
- 73.Oohashi T, Hirakawa S, Bekku Y, Rauch U, Zimmermann DR, Su WD, Ohtsuka A, Murakami T, Ninomiya Y. Bral1, a brain-specific link protein, colocalizing with the versican V2 isoform at the nodes of Ranvier in developing and adult mouse central nervous systems. Molecular and cellular neurosciences. 2002;19:43–57. doi: 10.1006/mcne.2001.1061. [DOI] [PubMed] [Google Scholar]
- 74.Dours-Zimmermann MT, Maurer K, Rauch U, Stoffel W, Fassler R, Zimmermann DR. Versican V2 assembles the extracellular matrix surrounding the nodes of ranvier in the CNS. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:7731–7742. doi: 10.1523/JNEUROSCI.4158-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bekku Y, Oohashi T. Neurocan contributes to the molecular heterogeneity of the perinodal ECM. Archives of histology and cytology. 2010;73:95–102. doi: 10.1679/aohc.73.95. [DOI] [PubMed] [Google Scholar]
- 76.Bruckner G, Szeoke S, Pavlica S, Grosche J, Kacza J. Axon initial segment ensheathed by extracellular matrix in perineuronal nets. Neuroscience. 2006;138:365–375. doi: 10.1016/j.neuroscience.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 77.Butt AM, Duncan A, Hornby MF, Kirvell SL, Hunter A, Levine JM, Berry M. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- 78.Martin S, Levine AK, Chen ZJ, Ughrin Y, Levine JM. Deposition of the NG2 proteoglycan at nodes of Ranvier in the peripheral nervous system. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:8119–8128. doi: 10.1523/JNEUROSCI.21-20-08119.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. The Journal of cell biology. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin PM, Carnaud M, Garcia del Cano G, Irondelle M, Irinopoulou T, Girault JA, Dargent B, Goutebroze L. Schwannomin-interacting protein-1 isoform IQCJ-SCHIP-1 is a late component of nodes of Ranvier and axon initial segments. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:6111–6117. doi: 10.1523/JNEUROSCI.1044-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wittmack EK, Rush AM, Craner MJ, Goldfarb M, Waxman SG, Dib-Hajj SD. Fibroblast growth factor homologous factor 2B: association with Nav1.6 and selective colocalization at nodes of Ranvier of dorsal root axons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:6765–6775. doi: 10.1523/JNEUROSCI.1628-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goldfarb M, Schoorlemmer J, Williams A, Diwakar S, Wang Q, Huang X, Giza J, Tchetchik D, Kelley K, Vega A, et al. Fibroblast growth factor homologous factors control neuronal excitability through modulation of voltage-gated sodium channels. Neuron. 2007;55:449–463. doi: 10.1016/j.neuron.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang C, Hoch EG, Pitt GS. Identification of novel interaction sites that determine specificity between fibroblast growth factor homologous factors and voltage-gated sodium channels. The Journal of biological chemistry. 2011;286:24253–24263. doi: 10.1074/jbc.M111.245803. [DOI] [PMC free article] [PubMed] [Google Scholar]