Abstract
Infections of adenovirus type 4 (Ad4) and Ad7 were discovered among previously vaccinated individuals through febrile respiratory illness surveillance at military recruit camps. Genetic analysis was performed on these isolates and a sample of adenovirus isolates from unvaccinated patients. Antigenic regions of the adenovirus hexon gene from 21 vaccinated and 31 unvaccinated patients were sequenced and compared to homologous regions of Ad4 and Ad7 vaccine strains and of other representative hexon sequences archived in GenBank. The phylogenetic distribution of sequences from vaccinated individuals closely resembled those from unvaccinated individuals. The most common Ad7 strain was the Ad7d2 hexon genotype, and the most common Ad4 strain was a genotype nearly identical to the recently discovered Z-G 95-873 Ad4 variant. Near exclusive isolation of Ad4 since 1999 indicates that the Ad4 variant is currently responsible for the vast majority of adenovirus morbidity in military recruit camps. Different ratios of nonsynonymous to synonymous nucleotide substitution rates in known antigenic regions compared to nonantigenic regions indicated positive selection for diversity in the antigenic regions and purifying selection in the nonantigenic regions.
Adenovirus was first discovered in 1953 (12, 22). Today, adenoviruses are classically categorized by their species (formerly subgenera) (A to F), based primarily on differences in their hemagglutination properties, and by their serotype, based upon neutralization with type-specific animal antisera (17). There are currently 51 recognized serotypes of human adenovirus (18).
The classical method for subtyping adenoviral isolates is whole-genome digestion via a stepwise, systematic restriction enzyme analysis (REA) process. Restriction enzyme classification methods use a numbering and lettering system appended to the serotype number to distinguish unique strains (16). The letters “a” through “k” represent restriction enzyme whole-genome electrophoretic banding patterns by the referent enzyme BamHI. An Arabic numeral is added when additional enzymes are used to further distinguish whole genomes.
Intensively studied in the 1950s and 1960s, adenoviruses were found to infect up to ca. 80% of military recruits and lead to hospitalization in up to 20% (9). Adenovirus type 4 (Ad4) and Ad7 were the primary serotypes responsible for this morbidity and together constituted 60% of all hospitalized cases of acute respiratory disease (ARD) among military recruits (9). Live, enteric-coated oral vaccines, which induce immune responses through selective infection of the gastrointestinal tract, were developed first for Ad4 and later for Ad7a (3, 28). These vaccines were shown to be safe and highly effective in the immunization of military trainees (28), and routine administration to recruits began in 1971 (10). The Ad4 and Ad7 vaccines together lowered AV morbidity by 95 to 99% and total ARD morbidity by 50 to 60% during the period of vaccine use (10). However, the production of the vaccines was discontinued in 1996, and the remaining lots were rationed until supplies were exhausted in early 1999 (10). Recently, the U.S. Department of Defense awarded a contract to Barr Laboratories, Inc., for resumed production of the vaccines.
Population-based febrile respiratory illness surveillance was initiated by the Naval Health Research Center in 1996 to document the epidemiology of adenoviruses during and after the period of vaccine loss (10). The surveillance program was originally established at four recruit training camps in the United States to define the burden of adenoviruses (10) but was later expanded to eight sites and included testing for other viral agents (24). This surveillance documented large increases in adenovirus morbidity and several fatal cases after the vaccine was exhausted (2, 10, 25), suggesting that the initial vaccine was efficacious for the majority of circulating pathogenic strains. However, several cases of Ad4 and Ad7 infection were discovered among previously vaccinated individuals, raising the possibility that newly emergent strains of adenovirus had appeared.
Recent research on the evolution of circulating adenoviruses has engendered concern about the efficacy of the old vaccine against current strains. In order to determine the suitability of the original vaccine strains for a new vaccine, a study of strain variation among the circulating Ad4 and Ad7 serotypes was conducted in 1999 (5). The antigenic regions of the hexon gene from prototype, vaccine, community-acquired, and military wild-type strains collected from 1953 to 1997 were sequenced and compared. Whereas the hexon antigens of Ad7 were generally conserved over time, an Ad4 variant (strain Z-G 95-873) with nine amino acid changes in the hexon antigens was found to have been circulating since 1995 (5). These changes were noted to confer decreased neutralization. The ability of this strain to cause infection among vaccinated individuals, however, was not investigated. Another recent study analyzed Ad7 isolates from the United States from the years 1966 to 2000 by whole-genome REA (8). The study noted the appearance of two genome types previously undocumented in North America, Ad7d2 and Ad7h, which indicated a shift in the predominant Ad7 genotype circulating in the United States. The hexon protein of Ad7d2 contains only one unique amino acid substitution, but it has possible antigenic implications.
The primary objective of the present study was to determine whether the adenovirus infections among previously vaccinated individuals who became ill between 1996 and 2000 resulted from prototype strains, recently discovered adenovirus variants, or a completely new variant. A 1,500-bp region of the adenovirus hexon capsid protein gene contains seven discrete hypervariable regions (HVR1 to HVR7) that account for >99% of serotype-specific variation and code for the type-specific epitopes on the protein (4, 5). This region of the hexon gene from adenoviruses isolated from vaccinated individuals and unvaccinated individuals was sequenced and compared to the vaccine strains, the Ad4 variant strain, and the other existing Ad4 and Ad7 hexon sequences in GenBank. The results of the present study will help guide future vaccine development initiatives.
MATERIALS AND METHODS
Naval Health Research Center surveillance for febrile respiratory illness.
The Naval Health Research Center has conducted population-based surveillance for febrile respiratory illness among basic training recruits since 1996. Present sites include Fort Jackson (South Carolina), Fort Leonard Wood (Missouri), Great Lakes Naval Recruit Training Command (Illinois), Marine Corps Recruit Depot (California), Fort Benning (Georgia), Lackland Air Force Base (Texas), Parris Island (South Carolina), and Cape May (New Jersey). Weekly rates of febrile respiratory illness, defined by an oral temperature of ≥38°C and either a cough or sore throat, are recorded. Recruits meeting the case definition are asked to permit the collection of a throat swab specimen and to answer a brief questionnaire. Specimens are stored in viral transport medium (Remel, Lenexa, Kans.) and sent in batches with associated data to the Naval Health Research Center Respiratory Disease Laboratory, San Diego, Calif., for analysis. Classic viral culture and isolation are performed using R-Mix shell vials (Diagnostic Hybrids, Athens, Ohio) and A549 (American Type Culture Collection, Manassas, Va.) and RMK (Diagnostic Hybrids) cells. Cultures exhibiting viral growth are processed for identification of the infecting viral pathogen by using immunofluorescence. A random selection of isolates positive for adenovirus are further analyzed by microneutralization with type-specific antisera to determine serotype, as previously described (6, 17).
Viral isolate selection.
Adenovirus-positive samples, negative for other respiratory pathogens, were considered for inclusion in the study. In this manner, adenovirus was likely the causative agent of illness in the symptomatic recruit at the time of collection. The vaccination status of the host (vaccinated or unvaccinated) and serotype (Ad4 or Ad7), as previously determined by microneutralization, were then considered. Vaccinated individuals were defined as those with febrile respiratory illness beginning >10 days after documented receipt of the Ad4 and Ad7 vaccines. The Ad4 sample set consisted of 10 isolates from vaccinated individuals and 30 from unvaccinated individuals and represented six of the eight recruit camps participating in surveillance. The Ad7 sample set consisted of 11 isolates from vaccinated individuals and 1 from an unvaccinated individual. A low incidence of Ad7 in recent years, with the exception of an outbreak at Great Lakes in the Fall of 1997 (25), limited the availability of clinical isolates from unvaccinated individuals for this set. However, additional sequences from GenBank supplemented the unvaccinated Ad7 sequences used in this analysis.
PCR amplification and sequencing.
All selected samples were regrown in A549 cells from original patient specimens. Viral DNA was extracted with the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, Calif.). The early conserved region and serotype-defining hypervariable regions of the adenovirus hexon, corresponding approximately to the first 1,500 bp of the gene, was amplified by PCR with the primers UP and 5L, as previously described (5). The reaction mixture contained 1× PCR Buffer II (Applied Biosystems, Foster City, Calif.), 3 mM MgCl2, 200 μM concentrations of each deoxynucleoside triphosphate, 0.5 μM concentrations of each primer, 2.5 U of Taq polymerase (Applied Biosystems), and 5 μl of DNA template in a total volume of 100 μl. The cycling consisted of an initial denaturation at 94°C for 2 min, followed by 30 cycles of 94°C for 1 min, 45°C for 1 min, and 72°C for 3 min, with a final extension of 72°C for 7 min. The amplicons were purified with QIAquick PCR purification kit (Qiagen).
Purified adenovirus hexon amplicons were cycle sequenced with the Perkin-Elmer 9700 thermocycler (PE Biosystems, Foster City, Calif.), using the Big Dye terminator cycle sequencing kit V3.0 (Applied Biosystems). The sequencing consisted of 25 cycles of 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min. All products were purified by using CentriSep columns (Princeton Separations, Adelphia, N.J.). Direct nucleotide sequencing was performed with an ABI 310 genetic analyzer (Applied Biosystems) according to the manufacturer's protocols. The primers used for sequencing included two PCR primers (UP and 5L), four internal primers (1Lm, 1Rm, 3L, and 3R) as previously described (5), and four novel internal primers (R630, R822, R440, and R674) (Table 1). Contig assembly was performed using the DNAstar software package (DNA Star, Inc., Madison, Wis.). BioEdit (v5.0.9) (11) was used for multiple sequence alignments, DNA translations, and additional sequence analysis.
TABLE 1.
Adenovirus hexon and fiber gene primers
| Primer | Gene | Sequence |
|---|---|---|
| R630 | Hexon | 5′-ATTCTAATAGGCAGCCCATCAG-3′ |
| R822 | Hexon | 5′-CCACCTTCTTTGTTGGTAGGC-3′ |
| R440 | Hexon | 5′-CAAAGAATGTGCTGGCCA-3′ |
| R674 | Hexon | 5′-CGGCATAAATGGGCTTGT-3′ |
| AV5HF | Hexon | 5′-GGCTACCCCTTCGATGATGCC-3′ |
| AV5HR | Hexon | 5′-GCGTTGTAGGCAGTGCCAGAG-3′ |
| AdFIB-5F1 | Fiber | 5′-TATTGCAGCTTCCTCCTGGCTGCA-3′ |
| AdFIB-5R1 | Fiber | 5′-CGGCCTCGTCCAGAGAGAGGCCGT-3′ |
Additional sequences for comparison.
All available Ad4 and Ad7 sequences containing the first 1,500 bp of the hexon gene in GenBank were added to our sequences for analysis. Four Ad4 sequences were added: the vaccine strain (AF065063) (5), the RI-67 prototype (AF065062) (5), the newly discovered Z-G 95-873 variant (AF065064) (5), and a strain isolated in Korea (AF542122). Ten Ad7 sequences were added: the vaccine strain (AF065067) (5), three versions of the Gomen Ad7 prototype (AF065065, Z48571, and X76551) (5, 15, 20), two versions of the S-1058 Ad7a prototype (AF065066 and AF053085) (5), the Kn T96-0620 7a strain from a patient with a fatal case of ARD (AF065068) (5), the Ad7d2 prototype strain as defined by REA (AF321311) (8), an isolate from Japan (AF053086), and an isolate from China (AF515814).
Analysis.
Phylogenetic trees were calculated with PAUP version 4.0 b9 (26) for all nucleotide and predicted amino acid sequences by neighbor-joining and parsimony methods. Sequences were analyzed with reference to the trees to reveal character states relevant to phylogenetic branching. Synonymous substitution rates, defined as the number of synonymous substitutions per 1,000 synonymous sites, and nonsynonymous substitution rates, defined as the number of nonsynonymous substitutions per nonsynonymous site, were computed for all pairwise sequence comparisons within the hypervariable and conserved regions of each serotype by using MEGA, version 2.1 (13). These values were used to detect differential evolutionary selective pressure between hypervariable and conserved regions of the hexon.
GenBank accession numbers.
Novel nucleotide sequences were submitted to the GenBank database. The accession numbers for submitted Ad4 sequences included AY337237, AY337238, AY337239, AY337240, AY337241, AY337242, AY337243, AY337244, AY337245, AY337246, AY337247, AY337248, AY337249, AY337250, AY337251, and AY337252 (for Ad4 isolates) and AY337253, AY337254, AY337255, AY337256, AY337257, and AY337258 (for Ad7 isolates).
RESULTS
Surveillance serotype distribution.
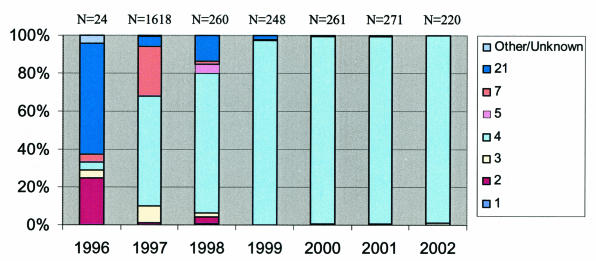
The proportional distribution of adenovirus serotypes isolated from military recruit camps from 1996 to 2002 differed markedly (Fig. 1). In 1996, during the last year of routine vaccine administration, Ad21 constituted the majority (58%, 14 of 24) of adenovirus morbidity, whereas Ad4 and Ad7 constituted only 4% each. Upon tapered use of the vaccine in 1997, however, Ad4 and Ad7 morbidity rose to 58% (939 of 1,618) and 26% (427 of 1,618), respectively, with other adenovirus serotypes constituting the remaining 16%. In 1998, Ad7 virtually disappeared, whereas Ad4 increased to 73% (191 of 260). The vaccine was completely depleted in 1999, with Ad4 responsible for 98% (242 of 248) of adenovirus morbidity. Ad4 remained at least at this proportion for the remainder of the analysis period with negligible representation from other serotypes.
FIG. 1.
Proportional distribution of adenovirus serotypes from febrile respiratory surveillance in military recruit camps from 1996 to 2002.
Phylogeny and mutational characteristics.
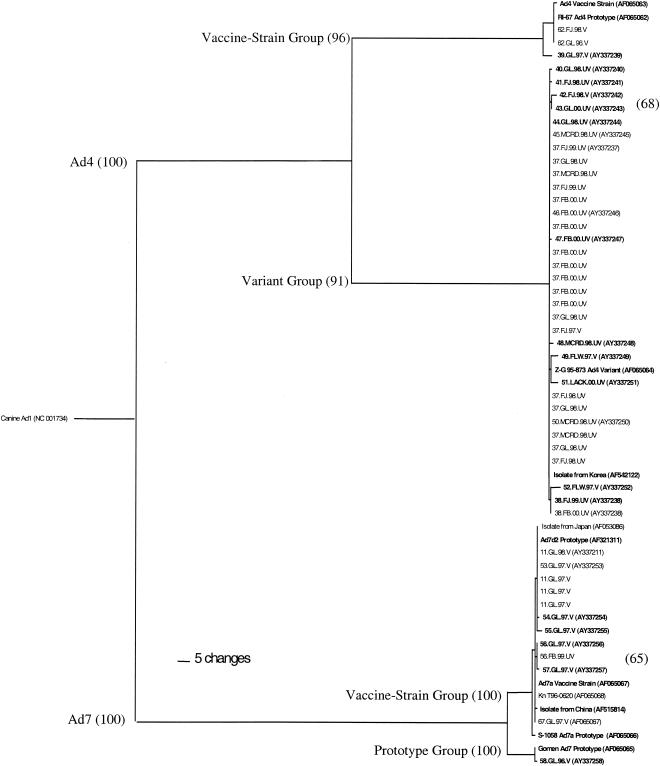
Phylogenetic analysis revealed a branching of the Ad7 sequences into two predominant groups. One group (Fig. 2, prototype group) contained the three Gomen prototype sequences (only AF065065 represented on the tree) and one military sample (58.GL.96.V). The other group (Fig. 2, vaccine-strain group) contained the Ad7a vaccine strain, S-1058 Ad7a prototype, Ad7d2 prototype, one sequence from an unvaccinated military patient (56.FB.99.UV), ten sequences from vaccinated military patients, and three other sequences from GenBank.
FIG. 2.
Ad4 and Ad7 hexon maximum-parsimony protein tree with canine Ad1 root. Isolates sequenced in the present study are labeled according to the following format: last two digits of accession number of identical sequence.location of isolation.year of isolation.vaccination status. FB, Fort Benning; FJ, Fort Jackson; FLW, Fort Leonard Wood; LACK, Lackland; GL, Great Lakes; MCRD, Marine Corps Recruit Depot; V, vaccinated; UV, unvaccinated. Full accession numbers appear once in parentheses. Accession numbers in boldface correspond to those in Fig. 3. Multiple accession numbers among identical protein sequences are due to DNA sequences with silent mutations. Bootstrap values greater than 50 (of 100 total bootstraps) are shown. Rescaled consistency index = 0.97.
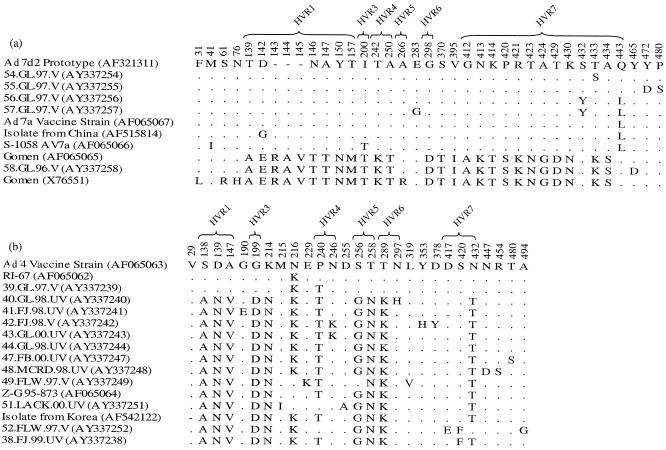
The Ad7 vaccine-strain group was distinguished from the prototype group by 25 coding differences (Fig. 3a). The vaccine strain was identical to only one military strain (67.GL.97.V), which was from a vaccinated individual. The other 10 military isolate sequences in this group differed by at least one amino acid. Seven of these contained an L443Q (HVR7) substitution, which was shared with the Ad7d2 strain and the prototype group (Fig. 3a). Of the 11 samples from vaccinated military patients, 7 carried L443Q.
FIG. 3.
Amino acid polymorphisms within aligned Ad7 (a) and Ad4 (b) sequence sets. Conserved positions are omitted, and residues identical to first sequences are represented by a period. Numbers refer to the amino acid position according to alignment with the Gomen prototype (AF065065) (a) and the Ad4 vaccine strain (AF065063) (b). Isolates correspond to those in boldface in Fig. 2.
Ad4 also divided into two main phylogenetic lineages. The first lineage (Fig. 2, vaccine-strain group) contained the Ad4 vaccine strain, the RI-67 prototype, and three military samples from 1998 and earlier (62.GL.96.V, 62.FJ.98.V, and 39.GL.97.V). The second group (Fig. 2, variant group) contained the Z-G 95-873 variant, an isolate from Korea (AF542122), and the remaining 33 military isolates. All 3 military sample sequences in the vaccine-strain group were from vaccinated individuals compared to 4 of 33 in the variant group. The variant strain comprised 91.7% (33 of 36; 95% confidence interval, 78.2 to 97.1%) of all Ad4 samples sequenced since 1996 and 100% (16 of 16; 95% confidence interval, 80.6 to 100%) since 1999.
The variant group was distinguished from the Ad4 vaccine-strain group by 10 amino acid substitutions (Fig. 3b). Of the 33 military samples in the variant group, 22 had identical amino acid sequences (Fig. 3b, sequence AY337244). Two exceptions (49.FLW.97.V and 51.LACK.00.NV) contained a nonconservative K216N substitution, as defined by differences in size and charge (4), which they shared only with the Z-G 95-873 variant and the vaccine strain (Fig. 3b).
Synonymous versus nonsynonymous substitutions.
Pairwise comparison of conserved regions within each serotype yielded significantly greater mean synonymous substitution rates than mean nonsynonymous substitution rates (Table 2). In contrast, pairwise comparison of hypervariable regions within each serotype yielded greater mean nonsynonymous substitution rates than mean synonymous substitution rates. The differences between the means in the hypervariable regions were not significant; however, 103 of 231 independent pairwise comparisons among the Ad7 sequences had significantly greater nonsynonymous substitution rates, whereas only 10 had significantly greater synonymous substitution rates. Likewise, with the Ad4 sequences 319 of 780 comparisons had significantly greater nonsynonymous substitution rates, and 26 had significantly greater synonymous substitution rates. The large number of nonsignificant pairwise comparisons was primarily the result of a high level of sequence redundancy in our data set.
TABLE 2.
Mean synonymous and nonsynonymous substitution rates in conserved and hypervariable regions of Ad7 and Ad4
| Serotype | Mean substitution ratea ± SD
|
|||
|---|---|---|---|---|
| Conserved regions
|
Hypervariable regions
|
|||
| Synonymous | Nonsynonymous | Synonymous | Nonsynonymous | |
| Ad7 | 39.9 ± 6.4 | 1.7 ± 0.6* | 20.6 ± 8.8 | 28.9 ± 5.6 |
| Ad4 | 17.6 ± 3.6 | 1.2 ± 0.4* | 4.0 ± 2.5 | 8.7 ± 2.3 |
Rates were determined through pairwise sequence comparison and are expressed as synonymous substitutions per 1,000 synonymous sites and nonsynonymous substitutions per 1,000 nonsynonymous sites. *, significant differences between synonymous and nonsynonymous substitution rates.
Atypical strains.
Four samples (three vaccinated and one unvaccinated), all isolated in 1998 and initially serotyped as Ad4, yielded mostly unreadable nucleotide chromatographs, but short portions of readable fragments suggested the strains were Ad5. Primers Ad5HF and Ad5HR were designed from the clean hexon segments, and primers AdFIB-5F1 and AdFIB-5R1 (Table 1) were designed from existing Ad5 sequences in GenBank. Both primer sets were used in sequencing, and the resulting sequences showed 100% identity (288 of 288 and 274 of 274, respectively) with Ad5 in GenBank (versions 2.2.5 and 2.2.6). Two further confirmatory tests included a type-specific PCR assay (19) and a second microneutralization analysis, both of which verified the Ad5 diagnoses and showed no evidence of Ad4 coinfection.
DISCUSSION
Infection with mutant adenovirus strains constitutes only one of many possible reasons for vaccine failure. Improper storage or administration of vaccine, asymptomatic carriage of adenovirus that is not the cause of acute illness, or imperfect vaccine efficacy are all viable explanations for the isolation of adenoviruses among vaccinated individuals. With the development of a new adenovirus vaccine under way, however, it is vital to address the hypothesis of strain variation with these uniquely available isolates. Although it may be impossible to determine whether one mutation or set of mutations was responsible for infection, consideration of these changes among circulating viral strains is an important step.
The major phylogenetic bifurcation within Ad7 hexon sequences was previously shown (5, 8, 14) and provided genetic evidence for a distinction originally seen only at a phenotypic level by cross-neutralization and REA (5). An eightfold reduction in neutralization of the Gomen Ad7 prototype compared to the S-1050 Ad7a strain with Ad7a antiserum was also previously shown (5). This neutralizing difference may have contributed to a breakthrough of the sole strain from a vaccinated patient in the prototype group (58.GL.96.V; Fig. 2), but the low number of prototype-like strains isolated in the present study indicated that they are not currently a major epidemiological threat in recruit camps.
A recent REA study on the 1997 Great Lakes outbreak showed >70% of the samples were of the Ad7d2 genome type (8). It was also found in the same study, as elsewhere (14), that an L443Q hexon substitution in HVR7 is consistently linked with Ad7d and Ad7d2, as distinct from Ad7a, Ad7b, Ad7c, Ad7g, and Ad7h. Of 10 samples in our study from the same outbreak but from vaccinated individuals, 7 also contained the L443Q substitution, indicating that these samples are most likely of the Ad7d2 genome type. The substitution is nonconservative and dramatically affects the hydropathic—and probably structural—characteristics of the protein by transforming the nine-residue surrounding region in HVR7 from a secondary-structure conformation that exposes primarily hydrophobic amino acid residues to a conformation that exposes primarily hydrophilic residues (14). It has therefore been suggested that this substitution imparts antigenic implications (8), which may explain the high number of Ad7s of this type isolated from vaccinated individuals in our study. In contrast, however, evidence showing a rapid decrease in AV-associated illness after introduction of the vaccine in this outbreak (25) strongly suggests that the strain is at least partially susceptible to vaccine-induced immunity. Cross-neutralization data between Ad7a and a strain containing the L443Q substitution would provide more evidence of the antigenic implications.
Several other substitutions occurring among the Ad7s may have contributed to infection in vaccinated individuals. The nonconservative S432Y substitution in samples 56.GL.97.V and 57.GL.97.V (Fig. 3a) resided in HVR7 and may have directly altered the epitope. In addition, isolated nonconservative substitutions in nearby conserved regions may have indirectly affected the antigens. The hexon epitopes are known to be conformational, since only the protein in its native trimeric form and not linear hexon peptides or heat-denatured monomeric proteins neutralize in vitro (4). Therefore, a change in a structural region, such as the P480S substitution (Fig. 3a, 55.GL.97.V) in the conserved region at the base of the loop containing HVR7 (1, 4), for example, may drastically affect protein folding in the antigenic regions.
Given the predominance of Ad4 among cases of respiratory illness in recent years, as well as the predominance of the variant genotype within Ad4 (Fig. 1), it appears that this single strain was responsible for nearly all adenovirus-associated respiratory illness in military recruit camps since 1999. The continued increase in the dominance of this strain after vaccine cessation suggests that its proliferation was not primarily a result of vaccine use. It is difficult to say how well the prior vaccine strain will clinically protect against this variant, since the majority of the variant isolates were isolated after vaccine use was drastically reduced. However, it was recently shown in an in vitro cross-neutralization analysis that the Z-G 95-873 variant has a fourfold reduction in neutralization in comparison with the Ad4 RI-67 prototype strain (5), a strain similar in genotype to the vaccine strain. The majority (32 of 35) of genotypes in the variant group differed from the Z-G 95-873 variant, as well as the vaccine strain, by the N216K amino acid substitution (Fig. 3b, sequence AY337247). If this substitution changes the epitope at all, it would render it further unrecognizable by vaccine-induced antibodies; however, it is unknown whether the substitution would have such an effect, given its nonconservative character in a conserved region. Nevertheless, due to the current prevalence of the Ad4 variant with the N216K mutation, any new vaccine should be optimized for effectiveness against this strain.
As with Ad7, several isolated substitutions in the Ad4 set have the potential to alter the epitope. Three nonconservative amino acid changes (Fig. 3b, P240T in sample 39.GL.97.V, N246K, and S420F) resided in antigenic regions. Nonidentical nucleotide mutations code for the N246K substitution, which suggests convergent evolution at this site, perhaps driven by selection. However, given that only one sample from a vaccinated individual was found with each mutation, our data do not conclusively show their clinical effects.
Nucleotide sites are classified as either nonsynonymous or synonymous depending on whether or not, respectively, a mutation at the site will lead to an amino acid substitution. The first and second nucleotide sites in a codon are largely nonsynonymous, and the third site, due to redundancy in the genetic code, is largely synonymous. The degree to which a site is classified as either synonymous or nonsynonymous is determined by accounting for the coding imparted by all possible mutations. Within a gene region, the number of nonsynonymous substitutions per nonsynonymous site relative to synonymous substitutions per synonymous site indicates the type of evolutionary selective pressure on the region. Equal rates of nonsynonymous and synonymous mutations indicate neutral drift, an excess of synonymous substitution rates indicates conservative evolutionary pressure (purifying selection), and an excess of nonsynonymous substitution rates indicates selection for diversity (diversifying selection). Evidence of diversifying selection is often found in protein regions that benefit from variation such as surface antigens of pathogens (7) and in immunoglobulin variable regions (27). In our study, the nonsynonymous substitution rates in the antigenic (hypervariable) regions of the sequences suggest that these regions are affected by diversifying selection. In contrast, the nonantigenic (conserved) regions show strong purifying selection, as is expected from regions with a structural function. Given the propensity for variation in the hypervariable regions, one would expect that the selective pressure of vaccination efforts might eventually allow the virus to evolve resistance to vaccine-induced immune responses. Antigenic cross-neutralization analysis between new vaccine strains and current circulating strains should be closely monitored.
A recent study of the adenovirus hexon (23) proposed a reassignment of the hypervariable regions based on stretches of diversity in an alignment of 40 partial hexon amino acid sequences from both human and nonhuman adenoviruses. The hypervariable regions as presented in the alignment in the study generally overlap with the assignments as previously defined for Ad2 (4) but not with those previously defined for Ad4 and Ad7 (5). An analysis of mutation rates in the reassigned hypervariable regions of the sequences used in our study shows higher synonymous than nonsynonymous substitution rates in the hypervariable regions (data not shown), which is the opposite of what was found with the previous assignments. Additional support for the previous region assignments is provided by a visual inspection of the alignment used to define the new assignments. This reveals that definite motifs in a subset of sequences, including that of Ad2, are shifted out of phase with those of Ad4, Ad7, and several others. Such a shift would affect identity scores assigned to each region and hence the placement of hypervariable regions in the alignment. Our data therefore support the hypervariable positions as previously defined for Ad4 and Ad7.
Initial use of the Ad4 vaccine in the 1960s was associated with Ad7 and Ad21 emerging as the chief etiological agents of ARD in military recruit camps. At the time, this constituted the first outbreak of Ad21 in the Western hemisphere (21). The isolation of three of the four Ad5s from vaccinated individuals indicates that this serotype may have similarly filled an evolutionary niche left open during the period of Ad4 and Ad7 vaccine use, but to a much smaller extent. Recent evidence shows that Ad5 manifests a lower binding affinity and replicates more slowly in A549 cells relative to other adenoviruses in subgenus A, B (includes Ad7), D, and E (includes Ad4) (18). The A549-mediated growth typically used in the serological diagnosis of AV may fail to produce an Ad5 titer sufficient for detection by microneutralization. The epidemiological impact of Ad5 on military recruit populations during the period of vaccine use may therefore have been underestimated. Molecular surveillance should be used to monitor for the possible emergence of Ad21, Ad5, or other serotypes upon reinstitution of the Ad4 and Ad7 vaccines.
Although the present study cannot definitively explain the reason for past vaccine failures, it shows the distribution of strains with possible partial resistance to the previous vaccines. Given the predominance of the Ad4 variant in recent years, an effective vaccine against it may significantly reduce adenovirus-associated respiratory infection in the U.S. military. Although Ad7 is not circulating at present, protection against the Ad7d2 strain would prevent its opportunistic proliferation upon controlling Ad4. Other serotypes have not shown Ad7's capacity to fill the Ad4 niche but, with diversifying selection acting on the hexon epitopes, continued surveillance for newly emergent strains should be conducted.
Acknowledgments
This represents report 03-22 and was supported by the U.S. Department of Defense under Work Unit 61102A-M0101-6609.
The views expressed here are those of the authors and do not reflect the official policy or position of the Department of the Navy, the Department of Defense, or the U.S. Government. The work is approved for public release (distribution unlimited). This research has been conducted in compliance with all applicable federal regulations governing the protection of human subjects in research under protocol 32267.
We thank Anthony W. Hawksworth of the Department of Defense Center for Deployment Health Research for organization and analysis of febrile illness surveillance data. We also thank Leta K. Crawford-Miksza and David P. Schnurr of the Viral and Rickettsial Disease Laboratory, Division of Communicable Disease Control, California State Department of Health Services, for adenovirus antisera and expert consultation.
REFERENCES
- 1.Athappilly, F. K., R. Murali, J. J. Rux, Z. Cai, and R. M. Burnett. 1994. The refined crystal structure of hexon, the major coat protein of adenovirus type 2, at 2.9 Å resolution. J. Mol. Biol. 242:430-455. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2001. Two fatal cases of adenovirus-related illness in previously healthy young adults-Illinois, 2000. Morb. Mortal. Wkly. Rep. 50:553-555. [PubMed] [Google Scholar]
- 3.Chanock, R. M., W. Ludwig, R. J. Heubner, T. R. Cate, and L. W. Chu. 1966. Immunization by selective infection with type 4 adenovirus grown in human diploid tissue cultures. I. Safety and lack of oncogenicity and tests for potency in volunteers. JAMA 195:445-452. [PubMed] [Google Scholar]
- 4.Crawford-Miksza, L., and D. P. Schnurr. 1996. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J. Virol. 70:1836-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford-Miksza, L. K., R. N. Nang, and D. P. Schnurr. 1999. Strain variation in adenovirus serotypes 4 and 7a causing acute respiratory disease. J. Clin. Microbiol. 37:1107-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford-Miksza, L. K., and D. P. Schnurr. 1994. Quantitative colorimetric microneutralization assay for characterization of adenoviruses. J. Clin. Microbiol. 32:2331-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endo, T., K. Ikeo, and T. Gojobori. 1996. Large-scale search for genes on which positive selection may operate. Mol. Biol. E vol. 13:685-690. [DOI] [PubMed] [Google Scholar]
- 8.Erdman, D. D., W. Xu, S. I. Gerber, G. C. Gray, D. Schnurr, A. E. Kajon, and L. J. Anderson. 2002. Molecular epidemiology of adenovirus type 7 in the United States, 1966-2000. Emerg. Infect. Dis. 8:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaydos, C. A., and J. C. Gaydos. 1995. Adenovirus vaccines in the U.S. military. Mil. Med. 160:300-304. [PubMed] [Google Scholar]
- 10.Gray, G. C., P. R. Goswami, M. D. Malasig, A. W. Hawksworth, D. H. Trump, M. A. Ryan, D. P. Schnurr, et al. 2000. Adult adenovirus infections: loss of orphaned vaccines precipitates military respiratory disease epidemics. Clin. Infect. Dis. 31:663-670. [DOI] [PubMed] [Google Scholar]
- 11.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 12.Hilleman, M. R., and J. H. Werner. 1954. Recovery of new agent from patients with acute respiratory illness. Proc. Soc. Exp. Biol. Med. 85:183-188. [DOI] [PubMed] [Google Scholar]
- 13.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 14.Li, Q., and G. Wadell. 1999. Genetic variability of hexon loops 1 and 2 between seven genome types of adenovirus serotype 7. Arch. Virol. 144:1739-1749. [DOI] [PubMed] [Google Scholar]
- 15.Li, Q. G., K. Lindman, and G. Wadell. 1997. Hydropathic characteristics of adenovirus hexons. Arch. Virol. 142:1307-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, Q. G., and G. Wadell. 1986. Analysis of 15 different genome types of adenovirus type 7 isolated on five continents. J. Virol. 60:331-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malasig, M. D., P. R. Goswami, L. K. Crawford-Miksza, D. P. Schnurr, and G. C. Gray. 2001. Simplified microneutralization test for serotyping adenovirus isolates. J. Clin. Microbiol. 39:2984-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mei, Y. F., K. Lindman, and G. Wadell. 2002. Human adenoviruses of subgenera B, C, and E with various tropisms differ in both binding to and replication in the epithelial A549 and 293 cells. Virology 295:30-43. [DOI] [PubMed] [Google Scholar]
- 19.Na, B. K., J. H. Kim, G. C. Shin, J. Y. Lee, J. S. Lee, C. Kang, and W. J. Kim. 2002. Detection and typing of respiratory adenoviruses in a single-tube multiplex polymerase chain reaction. J. Med. Virol. 66:512-517. [DOI] [PubMed] [Google Scholar]
- 20.Pring-Akerblom, P., F. E. Trijssenaar, and T. Adrian. 1995. Hexon sequence of adenovirus type 7 and comparison with other serotypes of subgenus B. Res. Virol. 146:383-388. [DOI] [PubMed] [Google Scholar]
- 21.Rose, H. M., T. H. Lamson, and E. L. Buescher. 1970. Adenoviral infection in military recruits. Arch. Environ. Health 21:356-361. [DOI] [PubMed] [Google Scholar]
- 22.Rowe, W. P., R. J. Huebner, L. K. Gilmore, R. H. Parrott, and T. G. Ward. 1953. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. 84:570-573. [DOI] [PubMed] [Google Scholar]
- 23.Rux, J. J., P. R. Kuser, and R. M. Burnett. 2003. Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution x-ray crystallographic, molecular modeling, and sequence-based methods. J. Virol. 77:9553-9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan, M., G. Gray, A. Hawksworth, M. Malasig, M. Hudspeth, and S. Poddar. 2000. The Naval Health Research Center Respiratory Disease Laboratory. Mil. Med. 165:32-34. [PubMed] [Google Scholar]
- 25.Ryan, M. A., G. C. Gray, B. Smith, J. A. McKeehan, A. W. Hawksworth, and M. D. Malasig. 2002. Large epidemic of respiratory illness due to adenovirus types 7 and 3 in healthy young adults. Clin. Infect. Dis. 34:577-582. [DOI] [PubMed] [Google Scholar]
- 26.Swofford, D. L. 1999. PAUP*, version 4.0 b9 ed. Sinnauer Associates, Sunderland, Mass.
- 27.Tanaka, T., and M. Nei. 1989. Positive Darwinian selection observed at the variable-region genes of immunoglobulins. Mol. Biol. E vol. 6:447-459. [DOI] [PubMed] [Google Scholar]
- 28.Top, F. H., Jr., R. A. Grossman, P. J. Bartelloni, H. E. Segal, B. A. Dudding, P. K. Russell, and E. L. Buescher. 1971. Immunization with live types 7 and 4 adenovirus vaccines. I. Safety, infectivity, antigenicity, and potency of adenovirus type 7 vaccine in humans. J. Infect. Dis. 124:148-154. [DOI] [PubMed] [Google Scholar]