Abstract
The Wnt/β-catenin signaling pathway is abnormally activated in most colorectal cancers and in a proportion of other neoplasias. This activation initiates or contributes to carcinogenesis by regulating the expression of a large number of genes in tumor cells. The active vitamin D metabolite 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) inhibits Wnt/β-catenin signaling by several mechanisms at different points along the pathway. Additionally, paracrine actions of 1,25(OH)2D3 on stromal cells may also repress this pathway in neighbouring tumor cells. Here we review the molecular basis for the various mechanisms by which 1,25(OH)2D3 antagonizes Wnt/β-catenin signaling, preferentially in human colon carcinoma cells, and the consequences of this inhibition for the phenotype and proliferation rate. The effect of the vitamin D system on Wnt/β-catenin signaling and tumor growth in animal models will also be commented in detail. Finally, we revise existing data on the relation between vitamin D receptor expression and vitamin D status and the expression of Wnt/β-catenin pathway genes and targets in cancer patients.
Keywords: vitamin D; 1α,25-dihydroxyvitamin D3; Wnt; β-catenin; colon cancer; vitamin D receptor
1. Introduction
Vitamin D3 is obtained from the diet or mainly synthesized in the skin upon UV-B solar radiation. Subsequent hydroxylation in the liver, kidney and other tissues renders 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3, calcitriol), the most active vitamin D metabolite. 1,25(OH)2D3 is a pleiotropic hormone with many regulatory effects. In addition to its classical action on intestinal calcium absorption and bone biology, 1,25(OH)2D3 inhibits proliferation, migration and anchorage-independent growth, and promotes differentiation of a variety of cultured cancer cells [1,2,3]. Consistently, numerous studies have shown tumor-suppressive actions (anti-angiogenic, anti-invasive, antimetastatic) in animal models [3], and epidemiological data suggest a protective role of vitamin D against several neoplasias, particularly colorectal cancer [4,5].
1,25(OH)2D3 regulates gene expression via binding to the vitamin D receptor (VDR), a member of the superfamily of nuclear receptors that is expressed in many normal and cancer cell types. VDR heterodimerizes with Retinoid X Receptor (RXR) and acts as a ligand-regulated transcription factor modulating the transcription rate of hundreds of target genes [1,6,7]. VDR also mediates rapid, non-genomic effects of 1,25(OH)2D3 on membrane and cytosolic signaling molecules (ion channels, kinases, phosphatases) [8]. These extra-nuclear effects are sometimes required for the gene regulatory activity of 1,25(OH)2D3/VDR complexes [9].
Human Wnts are a group of 19 secreted proteins that control proliferation, survival, migration, differentiation, and/or lineage decisions in many cell types during development and adult life [10]. To this end, Wnts activate a series of signaling pathways upon binding to specific membrane receptors: the Wnt/β-catenin or canonical pathway and several β-catenin-independent non-canonical pathways. Activation of a particular Wnt pathway depends on the individual Wnt ligand and the repertoire of receptors expressed in the target cell [11,12].
The Wnt/β-catenin pathway controls the intracellular levels of β-catenin. In the absence of Wnt signals, free β-catenin is targeted by a cytoplasmic protein complex known as the β-catenin destruction complex, which promotes its phosphorylation, ubiquitination and degradation by the proteasome. Components of this complex include the tumor suppressors AXIN and APC and the protein kinases CK1 and GSK3β. Wnt binding to membrane heterodimeric receptors (Frizzled and LRP5/6) results in inhibition of destruction complexes and subsequent accumulation of unphosphorylated β-catenin molecules in the cytoplasm, a part of which enters the nucleus and behaves as a co-activator of LEF/TCF transcription factors. β-catenin/LEF/TCF target genes encode proteins that are involved in most if not all cellular processes including proliferation, cell cycle regulation, metabolism, migration, lineage commitment, and differentiation [11,13].
The Wnt/β-catenin pathway is mainly active during embryonic development, although it also contributes to homeostasis in different tissues in adult life. Importantly, its abnormal activation in a series of epithelia is linked to generation or progression of carcinomas of the colon, breast, liver, pancreas and others. In particular, activation of the Wnt/β-catenin pathway is the initial event in a high proportion of colorectal carcinomas [11,14], and recent massive sequencing has shown that over 94% of colon tumors harbor mutations in one or more genes of the pathway [15]. Importantly, alterations in other genes and/or pathways increase the level of activation of Wnt/β-catenin signaling in colon cancer cells. Thus, crosstalk between hepatocyte growth factor (HGF)/c-MET and β-catenin signaling sustains and increases the invasive properties of colorectal cancer cells [16]. Moreover, activation of Wnt signaling in colon cancer stem cells appears to require a co-stimulatory signal involving c-MET activation by stromal-derived HGF [17,18]. Also, mutant K-RAS may enhance β-catenin/TCF-dependent transcription in APC mutant K-RAS-dependent colon cancer cells by a mechanism involving bone morphogenetic protein (BMP)-7 secretion and autocrine signaling, leading to activation of TGF-β-activated kinase (TAK1) [19].
Mutations in the tumor suppressor APC gene are by far the most frequent insult leading to constitutive activation of the Wnt/β-catenin pathway in colorectal cancer. However, in a small percentage of cases, mutations in AXIN or in the CTNNB1/β-catenin proto-oncogene itself have also been found. In addition, 20%–40% of hepatocellular carcinomas present mutations in CTNNB1/β-catenin or AXIN [20,21,22]. By contrast, overproduction of Wnt factors and/or repression of pathway inhibitors (SFRP, DKK-1...) have an important contribution in a proportion of hepatocellular carcinomas and other cancers such as breast or pancreas [22].
The Wnt/β-catenin pathway cannot be considered intrinsically tumorigenic, as it promotes differentiation of some cell types such as osteoblasts, myoblasts and neural precursors [23,24,25,26]. Moreover, Wnt/β-catenin signaling has controversial effects on melanoma [27] and its activation seems to collaborate with B-RAF inhibitors to impede melanoma progression [28]. Why the aberrant activation of this pathway in the colonic epithelium is so important for the initiation and progression of colon cancer is unclear.
Non-canonical Wnt pathways include the so-called planar cell polarity pathway which involves activation of Rho small GTPases and JNK and ROCK kinases, and the calcium pathway with activation of protein kinase C, calmodulin kinase, calcineurin and NFAT transcription factor [29,30]. The role of non-canonical Wnt pathways in cancer is unclear. Several authors suggest that they antagonize the tumorigenic effects of Wnt/β-catenin signaling [31]. Others propose that while this may occur at early stages of carcinogenesis, non-canonical pathways would contribute at later stages [32].
2. Antagonism of Wnt/β-Catenin Signaling by 1,25(OH)2D3
The first evidences of crosstalk between nuclear hormone receptors (NHR) and Wnt/β-catenin signaling appeared the late 1990s [33] and early 2000s [34,35]. Since these initial discoveries, several laboratories have demonstrated functional interactions between both pathways underlying important biological and pathological processes. The nature of this crosstalk is complex and not fully understood, as the functional outcome depends largely upon cellular context [36,37]. In general, β-catenin potentiates NHR activity while liganded NHRs attenuate or even repress β-catenin signaling, although there are important exceptions to this rule. Molecular mechanisms underlying this crosstalk are also abundant and include, but are not limited to, physical interaction between NHRs and β-catenin or TCF/LEF transcription factors. In this review we focus on the crosstalk between VDR and Wnt/β-catenin signaling in cancer, with special emphasis on colon cancer, and the functional outcome of this interaction.
2.1. Studies in Cultured Cells
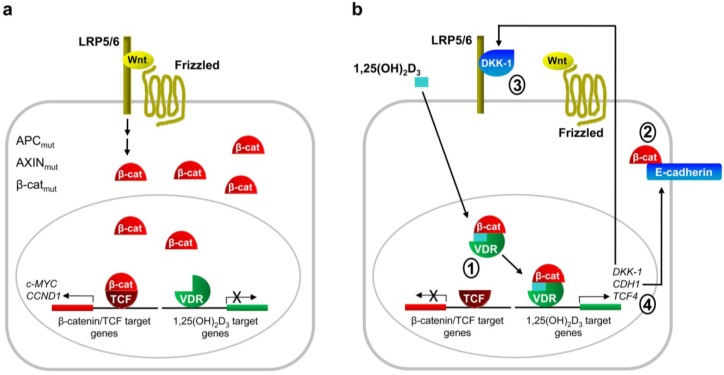
Results from our group showed that 1,25(OH)2D3 antagonizes the Wnt/β-catenin pathway in human colon cancer cells by three main mechanisms (Figure 1). First, it induces VDR/β-catenin interaction, thus reducing the amount of β-catenin bound to TCF [35]. Second, 1,25(OH)2D3 induces E-cadherin expression, leading to β-catenin nuclear export and relocation to the adherens junctions at the plasma membrane [35]. And third, 1,25(OH)2D3 induces the expression of Dickkopf (DKK)-1, an extracellular inhibitor of Wnt signaling [38]. As a result, we have found that 1,25(OH)2D3 inhibits the expression of several β-catenin/TCF target genes such as c-MYC, TCF1, LEF1, AXIN2, PPARδ, CD44, ENC1 and EPHB2 in human colon cancer cells [35,39,40]. These mechanisms of Wnt/β-catenin antagonism are completely dependent on VDR expression, as they are not observed in VDR-negative human colon cancer cells (SW480-R and SW620) or in VDR-positive cells (SW480-ADH) in which VDR expression has been repressed by Snail1 or Snail2 overexpression or by shRNA technology [35,38,39,40,41,42].
Figure 1.
Schematic representation of the mechanisms of Wnt/β-catenin pathway repression by 1,25(OH)2D3 in human colon carcinoma cells. (a) Wnt/β-catenin pathway is activated by mutation of APC, CTNNB1/β-catenin or AXIN genes, or by deregulated signaling from Wnt plasma membrane receptors. These alterations cause accumulation of β-catenin protein in the cytoplasm and nucleus and transcription of its target genes; (b) 1,25(OH)2D3 inhibits β-catenin/TCF transcriptional activity in colon carcinoma cells by several mechanisms. It promotes VDR/β-catenin binding, thus reducing the amount of β-catenin bound to TCF (1); it induces the expression of CDH1 gene coding for E-cadherin, which sequesters β-catenin at the plasma membrane adherens junctions (2); and it enhances the expression of the extracellular Wnt inhibitor DKK-1 (3) and of TCF4 (4). Additional paracrine mechanisms of antagonism have been proposed.
VDR/β-catenin interaction was later confirmed by Shah et al. in other cell types [43]. In addition, these authors characterized the domains responsible for the interaction: the C-terminal region of β-catenin and the activator function-2 domain of VDR. Interestingly, while 1,25(OH)2D3 antagonizes β-catenin/TCF transcriptional activity, β-catenin potentiates that of 1,25(OH)2D3/VDR [35,43,44]. Recently, Egan et al. have reported that wild-type APC enhances VDR/β-catenin interaction and that the VDR ligand lithocholic acid also promotes the interaction, albeit to a lesser extent than 1,25(OH)2D3 [45].
The contribution of E-cadherin induction to Wnt/β-catenin inhibition by 1,25(OH)2D3 seems to be cell-type specific, as the antagonism has also been observed in E-cadherin-negative cell lines such us LS174T [35]. Supporting the relation between E-cadherin induction and Wnt/β-catenin antagonism, we have shown that activation of the RhoA small GTPase and induction of JMJD3 histone demethylase contribute to both the induction of E-cadherin and the inhibition of β-catenin/TCF transcriptional activity by 1,25(OH)2D3 [9,46]. In addition to human colon cancer cells, Xu et al. observed that 1,25(OH)2D3 induces E-cadherin expression and inhibits β-catenin/TCF transcriptional activity in rat Rama-37 mammary epithelial cells [47].
DKK-1 belongs to the Dickkopf gene family that encodes secreted proteins that bind to LRP5/6 and function as extracellular inhibitors of Wnt/β-catenin signaling. DKK-1 binding to LRP5/6 blocks Wnt-Frizzled-LRP5/6 interaction and also induces the formation of a complex with another DKK receptor named Kremen that leads to LRP5/6 endocytosis [48,49]. As most colon tumors have mutations that render a constitutively active Wnt/β-catenin pathway, the importance of DKK-1 induction for Wnt/β-catenin antagonism by 1,25(OH)2D3 is unclear. However, DKK-1 seems to have antitumor effects independent of β-catenin/TCF transcriptional inhibition, as DKK-1 overexpression in APC-mutant colon cancer cells decreases colony formation capacity in vitro and tumor growth in immunodeficient mice [50].
DKK-4 is a weaker inhibitor of Wnt/β-catenin signaling than DKK-1. Surprisingly, we found that 1,25(OH)2D3 inhibits DKK-4 expression in human colon and breast cancer cells and that DKK-4 overexpression in human colon cancer cells increases their migratory, invasive and angiogenic capacities [51]. These results suggest that the inhibition of DKK-4 by 1,25(OH)2D3 may contribute to the antitumor effects of 1,25(OH)2D3 in colon cancer.
The use of 1,25(OH)2D3 in cancer prevention and therapy is restricted by its hypercalcemic effects at therapeutic doses. This has led to the development of several 1,25(OH)2D3 analogues that retain the antitumor actions but lack the hypercalcemic effects of 1,25(OH)2D3. We have found that 1,25(OH)2D3 analogues EB1089, KH1060, MC903, WU515, CD578 and WY1113 inhibit β-catenin/TCF transcriptional activity in a similar or even greater extent than 1,25(OH)2D3 in human colon cancer cells [35,52]. In addition, Xu et al. obtained similar results with the QW and BTW analogues in colon and breast cancer cells [47].
Notably, other authors have reported different mechanisms of crosstalk between 1,25(OH)2D3 and Wnt/β-catenin pathway (Figure 1). Beildeck et al. found that 1,25(OH)2D3 induces the expression of TCF4 in several human colon cancer cell lines by a VDR-dependent indirect mechanism [53]. However, the consequences of TCF4 induction for β-catenin/TCF transcriptional activity are not clear. The authors proposed a model whereby in normal cells, or in tumor cells that do not have nuclear β-catenin, TCF4 upregulation would enhance the repression of β-catenin/TCF target genes. In line with this, it has been reported that TCF4 inhibits growth of colon cancer cells [54]. Thus, the induction of TCF4 expression by 1,25(OH)2D3 may have a protective role in colon cancer.
Kaler et al. have reported a role of tumor stroma in the interplay between Wnt/β-catenin signaling and 1,25(OH)2D3 [55]. They found that tumor cells induce the release of interleukin (IL)-1β by THP-1 macrophages. In turn, macrophage-derived IL-1β inhibits GSK3β activity in colon carcinoma cells, leading to β-catenin protein stabilization and increasing β-catenin/TCF transcriptional activity. This mechanism is repressed by 1,25(OH)2D3 through the inhibition of IL-1β production in THP-1 cells and, if functional in tumor-associated macrophages, it may be another way to interfere with Wnt/β-catenin signaling in vivo.
Interestingly, Meyer et al. have recently identified VDR/RXR and TCF4/β-catenin cistromes using chromatin immunoprecipitation followed by high-throughput DNA sequencing (ChIP-Seq) in a human colon cancer cell line [56]. They found that VDR/RXR co-occupied 1,674 sites upon 1,25(OH)2D3 treatment, most of them distal to transcription start sites. ChIP-Seq analysis also revealed 828 β-catenin and 3,161 TCF4 binding sites: after treatment with 1,25(OH)2D3 these figures decreased slightly for the former, but increased significantly for the latter. Examination of the overlap between TCF4/β-catenin and VDR/RXR cistromes indicates that the two heterodimers colocalize at 74 sites located near a limited set of genes that included c-FOS and c-MYC. These data support a direct action of both complexes at certain gene loci [56].
c-MYC is a key regulator of cell cycle progression and its expression is frequently elevated or deregulated in human cancer [57,58]. In addition, mutational and integrative analyses have emphasized the critical role of this proto-oncogene in colorectal cancer [15]. 1,25(OH)2D3 has been reported to downregulate c-MYC expression by two mechanisms. On the one hand, ligand-activated VDR directly represses c-MYC expression by binding two vitamin D response elements (VDRE) in the c-MYC promoter [59]. On the other, 1,25(OH)2D3 interferes with β-catenin/TCF-induced c-MYC transcription mediated by Wnt responsive elements (WRE) in the proto-oncogene promoter [60]. Interestingly, Salehi-Tabar et al. have recently demonstrated that 1,25(OH)2D3-dependent suppression of β-catenin function in a head and neck squamous cell carcinoma cell line is largely responsible for the inhibition of c-MYC RNA expression [61]. Moreover, they also showed that 1,25(OH)2D3 enhanced the expression of the c-MYC antagonist partner Mad/Mxd1 [61], further contributing to the inhibition of c-MYC target genes.
Although mutations in APC, AXIN or CTNNB1/β-catenin genes in breast cancer are rare, the Wnt/β-catenin pathway may be active in a proportion of these carcinomas according to the accumulation of nuclear β-catenin protein observed, particularly in the triple-negative and basal-like subtypes, which are highly aggressive and of poor prognosis [62,63]. Moreover, recent data indicate that WNT10B induces transcriptionally active β-catenin in human triple-negative breast cancers and predicts survival-outcome of patients with these two types of tumors [64]. Interestingly, 1,25(OH)2D3 regulates the phenotype of cultured human breast cancer cells by modulating the level and localization of cytoskeletal and adhesion proteins. Among them, 1,25(OH)2D3 represses the expression of P-cadherin, smooth muscle α-actin and α6- and β4-integrins that are myoepithelial/basal markers [65]. In line with this, Vdr-deficient mice express abnormally high levels of P-cadherin and smooth muscle α-actin in the mammary gland [65]. These data suggest that 1,25(OH)2D3 may protect against the triple-negative and basal-like breast cancers that are associated with poor prognosis, perhaps via the inhibition of the Wnt/β-catenin pathway.
Remarkably, the antagonism of Wnt/β-catenin signaling by the 1,25(OH)2D3 analogue paricalcitol has also been observed in other cellular systems unrelated to cancer such as vascular smooth muscle cells and renal podocytes [66,67].
2.2. Studies in Animal Models
Data from several types of studies using experimental animals support a protective and therapeutic effect of 1,25(OH)2D3 against several neoplasias [3,68,69].
Human cancer cells injected subcutaneously into immunosuppressed mice (xenografts) are commonly used as an in vivo approach in cancer research. Numerous studies have shown that 1,25(OH)2D3 and many analogues significantly reduce the growth of xenografts generated by tumor cells derived from several types of cancers [68,69]. We found that the 1,25(OH)2D3 analogue EB1089 inhibits the growth of xenografts generated by SW480-ADH human colon cancer cells. Remarkably, this effect is associated with induction of E-cadherin and DKK-1 expression, β-catenin nuclear export and inhibition of the expression of the β-catenin/TCF target gene ENC1 in the xenografts [38,39,41].
Similar results were obtained when chemical carcinogens (azoxymethane or azoxymethane plus dextran sodium sulphate) were used to induce colon tumors in mice or rats. Several studies showed that tumor incidence in these models decreases following treatment with 1,25(OH)2D3 or several analogues [68]. In addition, Bissonnette’s group found that this antitumor action of 1,25(OH)2D3 is accompanied by the induction of E-cadherin expression and the repression of the β-catenin/TCF target genes c-Myc and Ccnd1/Cyclin D1 in the colonic crypts and tumors of these animals [70,71].
Apcmin/+ mice are commonly used animal model for intestinal cancer. They harbour a germ line inactivating mutation in one Apc allele and spontaneously develop multiple intestinal neoplasias at approximately three months of age [72]. This phenotype is due to the spontaneous mutation of the remaining Apc allele (loss of heterozygosity) and the consequent activation of the Wnt/β-catenin pathway. Huerta et al. found that both 1,25(OH)2D3 and the 1,25(OH)2-16-ene-19-nor-24-oxo-D3 analogue reduce tumor load (the sum of all polyp areas) along the entire gastrointestinal tract of Apcmin/+ mice [73]. Xu et al. confirmed these results and showed that treatment with 1,25(OH)2D3 or two of its analogues increases E-cadherin expression, reduces nuclear β-catenin levels and inhibits the expression of the β-catenin/TCF target genes c-Myc and Tcf1 in the small intestine and colon of these mice [74]. However, Irving et al. did not find any effect of two 1,25(OH)2D3 analogues on the growth rate of colonic tumors developed in Apcmin/+ mice treated with the tumor inducer dextran sodium sulphate. As mentioned by the authors, the length of treatment with the 1,25(OH)2D3 analogues or the putative loss of Vdr expression in the tumors might be the cause of the discrepancy with previous studies [75].
Several studies indicate that a Western-style diet that is high in fat and low in calcium and vitamin D increases the incidence of spontaneous intestinal tumors in normal mice and dramatically accelerates tumor formation in Apcmin/+ mice and in other animal models for intestinal cancer [76]. In addition, this effect is reversed if the Western-style diet is supplemented in calcium and vitamin D [76]. Yang et al. used transcriptomic analyses to characterize the changes induced by the Western-style diet in colon epithelial cells of normal mice. They found that Wnt signaling is one of the functional categories that are significantly enriched among the genes whose expression is altered by Western-style diet and reversed to normal by calcium and vitamin D supplementation. These genes include those coding for β-catenin and for the Wnt receptors Frizzled-2 and -10 [76,77]. Similarly, Wang et al. observed that calcium and vitamin D supplementation abrogates the increase of β-catenin/TCF transcriptional activity and of Frizzled-5 and Ephb2 expression promoted by Western-style diet in intestinal villi and colon crypt cells of normal mice [78].
Genetically-modified mice have also been used to analyze the effects of VDR on carcinogenesis. Vdr-deficient mice do not show an increase in spontaneous cancer incidence but are more predisposed to oncogene- or carcinogen-induced breast and skin cancer and leukemia [79]. In the distal colon, these animals display hyperproliferation and an increased rate of DNA damage by oxidative stress [80,81]. Thus, two teams attempted to decipher the effects of Vdr gene deletion on intestinal tumorigenesis by generating Apcmin/+Vdr−/− mice [40,82]. No differences were found in the number of small intestinal and colonic tumors. However, significantly increased tumor load and number of colonic aberrant crypt foci (premalignant lesions) were observed in Apcmin/+Vdr−/− mice as compared to Apcmin/+Vdr+/+. Remarkably, the lesions of Apcmin/+Vdr−/− mice showed higher β-catenin nuclear levels and expression of its targets genes Ccnd1/Cyclin D1 and Lef1 than those of Apcmin/+Vdr+/+ mice, suggesting that Vdr deletion promotes intestinal tumor growth through the activation of the Wnt/β-catenin pathway [40,82].
2.3. Studies in Patients
Garland and Garland were the first to suggest that vitamin D deficiency may explain the higher colon cancer mortality rates found in latitudes with low solar radiation in USA [83]. Since then, several epidemiological studies have shown that incidence and/or risk of mortality of several types of cancer (particularly colon and breast) increases in people less exposed to sunlight or UV-B radiation, with low vitamin D dietary intake, or with low 25-hydroxyvitamin D serum levels [3,84]. Few cancer intervention clinical trials using vitamin D compounds for prevention or treatment have been completed in humans. Furthermore, the results obtained have been inconclusive, inconsistent or contradictory [3,85]. Thus, to establish a cause-effect relationship between vitamin D and cancer, it is necessary to perform new large-scale and well-designed clinical studies with cancer as the primary pre-specified outcome [3,85].
At the molecular level, Ahearn et al. conducted a randomized, double-blinded, placebo-controlled, 2 × 2 factorial clinical trial to analyze the effect that daily supplementation of sporadic colorectal adenoma patients with vitamin D (800 IU) and/or elemental calcium (2 g) for 6 months has on the expression of APC, E-cadherin and β-catenin in crypts of the normal appearing rectal mucosa [86]. Vitamin D or calcium supplementation increased the expression of APC and reduced that of β-catenin in the differentiation zone of the crypt (the upper 40% of the crypt), which led to an increased APC/β-catenin ratio. The supplementation only with vitamin D also induced E-cadherin expression in the same region. These results support those found in cultured cells and animal models and indicate that vitamin D can modify the expression of genes related to Wnt/β-catenin signaling in humans in directions hypothesized to inhibit colon cancer [86].
VDR is expressed in the normal colon and upregulated at early stages of colon tumorigenesis (polyps, adenomas), whereas it decreases at advanced stages (carcinomas) [87,88,89,90]. Accordingly, VDR expression is associated with high tumor differentiation, absence of node involvement, and good prognosis in colon cancer [91,92]. These data suggest that advanced colon tumors with low VDR expression will probably be unresponsive to therapy with 1,25(OH)2D3 or its analogues. Our group found that the transcription factors Snail1 and Snail2 repress VDR expression and block the antitumoral actions of 1,25(OH)2D3 in cultured colon cancer cells and in xenografted mice, including the inhibition of Wnt/β-catenin signaling. In addition, VDR RNA expression in colon tumors inversely correlates with that of SNAIL1 and SNAIL2, suggesting that these transcription factors are responsible for the downregulation of VDR found in colon cancer [39,41,42,84,93,94]. Interestingly, Snail1 protein levels and activity are induced by canonical Wnt signaling [95,96,97,98], which may constitute a mechanism to bypass the inhibitory effect of 1,25(OH)2D3 on this pathway. In addition to colon cancer, VDR downregulation by Snail factors has been also observed in osteoblasts, osteosarcoma, breast cancer and renal cells [99,100,101,102].
E-cadherin expression is lost during the progression of several types of carcinomas and its downregulation is frequently associated with the acquisition of invasive and metastatic properties by tumor cells. Thus, it is considered an invasion-suppressor gene [103,104]. According to data from cultured cells and animal models, we found a significant direct correlation between VDR and CDH1/E-cadherin RNA levels in human colon tumors, suggesting that VDR/1,25(OH)2D3 may contribute to the restoration of normal E-cadherin levels in human colon cancer [93,105].
Our group reported that DKK-1 expression is frequently downregulated in human colon cancer, which suggests that DKK-1 may act as a tumor suppressor gene in this neoplasia [106]. DKK-1 downregulation is due to promoter methylation in 24% of advanced colon tumors (Dukes’ stages C and D) [50]. Thus, the induction of DKK-1 expression by 1,25(OH)2D3 found in cultured cells and animal models may contribute to restore DKK-1 expression and antitumor effects in human colon cancer. Accordingly, we found a significant direct correlation between VDR and DKK-1 RNA expression in tumor biopsies from colon cancer patients [38], and Rawson et al. have recently reported that dietary vitamin D intake was negatively associated with DKK-1 promoter methylation in a large cohort of human colorectal cancer patients [107]. These results suggest that the extracellular inhibition of Wnt/β-catenin signaling may contribute to the effect of 1,25(OH)2D3 against colon carcinogenesis.
We and others found that DKK-4 is overexpressed in human colon tumors and in colon samples from patients with inflammatory bowel disease [51,108,109]. Interestingly, our group also observed a significant inverse correlation between VDR and DKK-4 RNA levels in human colorectal tumors, suggesting that the regulation of DKK-4 observed in cell lines also occurs in patients [51].
3. Cooperation Between VDR and Wnt/β-Catenin Signaling
Although 1,25(OH)2D3 inhibits β-catenin/TCF transcriptional activity in colon and other cancer cells, the upregulation of the Wnt/β-catenin pathway by either ligand-activated or unliganded VDR has been described in osteoblasts and keratinocytes, where it promotes bone formation and hair follicle differentiation, respectively. Thus, the interplay between Wnt/β-catenin pathway and 1,25(OH)2D3/VDR seems to depend on the cell or tissue type.
Wnt signaling promotes the differentiation of bone marrow-derived mesenchymal stem cells to bone while it represses their differentiation to other cell types, such as adipocytes [110,111]. Some 1,25(OH)2D3 effects in bone are similar to those of Wnt, suggesting a crosstalk between both pathways. Indeed, 1,25(OH)2D3 induces the expression of the Wnt co-receptor Lrp5 in mouse osteoblasts [112,113], while represses that of the Wnt inhibitors Dkk-1 and Sfrp2 in mouse bone marrow-derived mesenchymal stem cells [114]. These effects support a role of 1,25(OH)2D3 stimulating Wnt signaling in normal bone. However, the interplay between these two pathways seems to change in tumor tissue, as it has been reported that 1,25(OH)2D3 inhibits Wnt/β-catenin signaling in SaOS2 osteosarcoma cells [102].
Ligand-independent actions of VDR on Wnt canonical signaling have also been reported. In the skin, absence of Vdr, but not of 1,25(OH)2D3, results in alopecia in mice [115], and two independent groups have demonstrated that this is due, at least in part, to impaired Wnt/β-catenin signaling in keratinocytes [116,117]. In this regard, Pálmer et al. have shown that VDR is a Wnt effector and that β-catenin behaves as a VDR co-activator in the skin to induce transcription of genes associated with differentiation of hair follicle lineages [117]. Although this effect is largely 1,25(OH)2D3-independent, it is enhanced by the hormone. More recently, Luderer et al. have reported a direct interaction between VDR and LEF1 that is independent of both ligand and β-catenin, and that is required for normal canonical Wnt signaling in keratinocytes [118]. Interestingly, Lef1 knock-out mice develop alopecia at an early age [119] and transgenic mice expressing a dominant negative Lef1 in keratinocytes also show a phenotype that resembles that of Vdr−/− mice [120]. Therefore, although the in vivo significance of the VDR-LEF1 interaction is not yet clear, it may contribute to at least some of the ligand-independent effects of VDR in the skin. Similarly to what happens in bone, this interplay seems to change in the tumoral context. The development of trichofolliculomas (benign hair follicle tumors) induced by prolonged activation of β-catenin in the skin is inhibited by the 1,25(OH)2D3 analogue EB1089 [117]. In addition, undifferentiated tumors resembling basal cell carcinomas instead of trichofolliculomas are developed by β-catenin activation in Vdr−/− mice [117]. Accordingly, keratinocytes lacking VDR present decreased E-cadherin expression, increased β-catenin/TCF transcriptional activity, and a higher proliferation rate; whereas VDR overexpression or 1,25(OH)2D3 treatment has the opposite effect [121,122]. These results suggest that 1,25(OH)2D3/VDR suppresses epidermal tumor formation by limiting the hyperproliferative actions of β-catenin in the skin. Surprisingly, epidermal β-catenin ablation cannot reduce the increased number of UVB-induced tumors developed by epidermis-specific Vdr knock-out mice, suggesting that VDR has β-catenin-independent anticancer functions in this model [122].
4. Conclusions
Evidence from a wide series of biological systems shows that inhibition of Wnt/β-catenin pathway is one of the mechanisms of vitamin D action. Wnt/β-catenin signaling has many important regulatory effects in the organism and thus, it is conceivable that the role of vitamin D is the control of an adequate level of activation of the route in each tissue and developmental stage. This most probably includes the antagonism of Wnt/β-catenin signaling in colorectal and possibly other carcinomas in which this pathway has a crucial oncogenic action. The finding that the repressive action of vitamin D takes places at different levels of the Wnt/β-catenin pathway, by distinct mechanisms, and in several cell types reinforces the importance of this regulatory action.
Acknowledgments
We thank Robin Rycroft for his valuable assistance in the preparation of the English manuscript. The work in the authors’ laboratory is supported by Ministerio de Economía y Competitividad of Spain (SAF2010-18302, BFU2010-19659), Fondo Europeo de Desarrollo Regional-Instituto de Salud Carlos III (RD12/0036/0021) and Comunidad de Madrid (S2010/BMD-2344, Colomics2).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fleet J.C., DeSmet M., Johnson R., Li Y. Vitamin D and cancer: A review of molecular mechanisms. Biochem. J. 2012;441:61–76. doi: 10.1042/BJ20110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira F., Larriba M.J., Muñoz A. Vitamin D and colon cancer. Endocr. Relat. Cancer. 2012;19:R51–R71. doi: 10.1530/ERC-11-0388. [DOI] [PubMed] [Google Scholar]
- 3.Leyssens C., Verlinden L., Verstuyf A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr. Relat. Cancer. 2013;20:R31–R47. doi: 10.1530/ERC-12-0381. [DOI] [PubMed] [Google Scholar]
- 4.IARC . IARC Working Group Reports Vol. 5. International Agency for Research on Cancer; Lyon, France: 2008. Vitamin D and cancer. [Google Scholar]
- 5.Giovannucci E. Epidemiology of vitamin D and colorectal cancer. Anticancer Agents Med. Chem. 2013;13:11–19. doi: 10.2174/187152013804487254. [DOI] [PubMed] [Google Scholar]
- 6.Haussler M.R., Whitfield G.K., Kaneko I., Haussler C.A., Hsieh D., Hsieh J.-C., Jurutka P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2012;92:77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 7.Carlberg C., Campbell M.J. Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor. Steroids. 2013;78:127–136. doi: 10.1016/j.steroids.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haussler M.R., Jurutka P.W., Mizwicki M., Norman A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011;25:543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Ordóñez-Morán P., Larriba M.J., Pálmer H.G., Valero R.A., Barbáchano A., Duñach M., García de Herreros A., Villalobos C., Berciano M.T., Lafarga M., et al. RhoA-ROCK and p38MAPK-MSK1 mediate vitamin D effects on gene expression, phenotype, and Wnt pathway in colon cancer cells. J. Cell Biol. 2008;183:697–710. doi: 10.1083/jcb.200803020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willert K., Nusse R. Wnt proteins. Cold Spring Harb. Perspect. Biol. 2012;4:a007864. doi: 10.1101/cshperspect.a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Niehrs C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 13.Cadigan K.M., Waterman M.L. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 2012;4:a007906. doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polakis P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 2012;4:a008052. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasola A., Fassetta M., de Bacco F., D’Alessandro L., Gramaglia D., di Renzo M.F., Comoglio P.M. A positive feedback loop between hepatocyte growth factor receptor and β-catenin sustains colorectal cancer cell invasive growth. Oncogene. 2007;26:1078–1087. doi: 10.1038/sj.onc.1209859. [DOI] [PubMed] [Google Scholar]
- 17.Korkaya H., Wicha M.S. Cancer stem cells: Nature versus nurture. Nat. Cell Biol. 2010;12:419–421. doi: 10.1038/ncb0510-419. [DOI] [PubMed] [Google Scholar]
- 18.Vermeulen L., de Sousa E Melo F., van der Heijden M., Cameron K., de Jong J.H., Borovski T., Tuynman J.B., Todaro M., Merz C., Rodermond H., et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 19.Singh A., Sweeney M.F., Yu M., Burger A., Greninger P., Benes C., Haber D.A., Settleman J. TAK1 inhibition promotes apoptosis in KRAS-dependent colon cancers. Cell. 2012;148:639–650. doi: 10.1016/j.cell.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zucman-Rossi J., Jeannot E., Van Nhieu J.T., Scoazec J.-Y., Guettier C., Rebouissou S., Bacq Y., Leteurtre E., Paradis V., Michalak S., et al. Genotype-phenotype correlation in hepatocellular adenoma: New classification and relationship with HCC. Hepatology. 2006;43:515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 21.Austinat M., Dunsch R., Wittekind C., Tannapfel A., Gebhardt R., Gaunitz F. Correlation between β-catenin mutations and expression of Wnt-signaling target genes in hepatocellular carcinoma. Mol. Cancer. 2008 doi: 10.1186/1476-4598-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White B.D., Chien A.J., Dawson D.W. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219–232. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prakash N., Wurst W. A Wnt signal regulates stem cell fate and differentiation in vivo. Neurodegener. Dis. 2007;4:333–338. doi: 10.1159/000101891. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka S., Terada K., Nohno T. Canonical Wnt signaling is involved in switching from cell proliferation to myogenic differentiation of mouse myoblast cells. J. Mol. Signal. 2011 doi: 10.1186/1750-2187-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regard J.B., Zhong Z., Williams B.O., Yang Y. Wnt signaling in bone development and disease: Making stronger bone with Wnts. Cold Spring Harb. Perspect. Biol. 2012;4:a007997. doi: 10.1101/cshperspect.a007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma A.R., Choi B.S., Park J.M., Lee D.H., Lee J.E., Kim H.S., Yoon J.K., Song D.K., Nam J.S., Lee S.S. Rspo 1 promotes osteoblast differentiation via Wnt signaling pathway. Indian J. Biochem. Biophys. 2013;50:19–25. [PubMed] [Google Scholar]
- 27.Webster M.R., Weeraratna A.T. A Wnt-er migration: The confusing role of β-catenin in melanoma metastasis. Sci. Signal. 2013 doi: 10.1126/scisignal.2004114. [DOI] [PubMed] [Google Scholar]
- 28.Biechele T.L., Kulikauskas R.M., Toroni R.A., Lucero O.M., Swift R.D., James R.G., Robin N.C., Dawson D.W., Moon R.T., Chien A.J. Wnt/β-catenin signaling and AXIN1 regulate apoptosis triggered by inhibition of the mutant kinase BRAFV600E in human melanoma. Sci. Signal. 2012 doi: 10.1126/scisignal.2002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De A. Wnt/Ca2+ signaling pathway: A brief overview. Acta Biochim. Biophys. Sin. 2011;43:745–756. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- 30.Gao B. Wnt regulation of planar cell polarity (PCP) Curr. Top. Dev. Biol. 2012;101:263–295. doi: 10.1016/B978-0-12-394592-1.00008-9. [DOI] [PubMed] [Google Scholar]
- 31.Ying J., Li H., Yu J., Ng K.M., Poon F.F., Wong S.C., Chan A.T., Sung J.J., Tao Q. WNT5A exhibits tumor-suppressive activity through antagonizing the Wnt/β-catenin signaling, and is frequently methylated in colorectal cancer. Clin. Cancer Res. 2008;14:55–61. doi: 10.1158/1078-0432.CCR-07-1644. [DOI] [PubMed] [Google Scholar]
- 32.Sugimura R., Li L. Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth Defects Res. C Embryo Today. 2010;90:243–256. doi: 10.1002/bdrc.20195. [DOI] [PubMed] [Google Scholar]
- 33.Easwaran V., Pishvaian M., Salimuddin, Byers S. Cross-regulation of β-catenin-LEF/TCF and retinoid signaling pathways. Curr. Biol. 1999;9:1415–1418. doi: 10.1016/s0960-9822(00)80088-3. [DOI] [PubMed] [Google Scholar]
- 34.Truica C.I., Byers S., Gelmann E.P. β-Catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000;60:4709–4713. [PubMed] [Google Scholar]
- 35.Pálmer H.G., González-Sancho J.M., Espada J., Berciano M.T., Puig I., Baulida J., Quintanilla M., Cano A., García de Herreros A., Lafarga M., et al. Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J. Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulholland D.J., Dedhar S., Coetzee G.A., Nelson C.C. Interaction of nuclear receptors with the Wnt/β-catenin/Tcf signaling axis: Wnt you like to know? Endocr. Rev. 2005;26:898–915. doi: 10.1210/er.2003-0034. [DOI] [PubMed] [Google Scholar]
- 37.Beildeck M.E., Gelmann E.P., Byers S.W. Cross-regulation of signaling pathways: An example of nuclear hormone receptors and the canonical Wnt pathway. Exp. Cell Res. 2010;316:1763–1772. doi: 10.1016/j.yexcr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aguilera O., Peña C., García J.M., Larriba M.J., Ordóñez-Morán P., Navarro D., Barbáchano A., López de Silanes I., Ballestar E., Fraga M.F., et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1α,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28:1877–1884. doi: 10.1093/carcin/bgm094. [DOI] [PubMed] [Google Scholar]
- 39.Larriba M.J., Valle N., Pálmer H.G., Ordóñez-Morán P., Álvarez-Díaz S., Becker K.F., Gamallo C., García de Herreros A., González-Sancho J.M., Muñoz A. The inhibition of Wnt/β-catenin signalling by 1α,25-dihydroxyvitamin D3 is abrogated by Snail1 in human colon cancer cells. Endocr. Relat. Cancer. 2007;14:141–151. doi: 10.1677/ERC-06-0028. [DOI] [PubMed] [Google Scholar]
- 40.Larriba M.J., Ordóñez-Morán P., Chicote I., Martín-Fernández G., Puig I., Muñoz A., Pálmer H.G. Vitamin D receptor deficiency enhances Wnt/β-catenin signaling and tumor burden in colon cancer. PLoS One. 2011;6:e23524. doi: 10.1371/journal.pone.0023524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pálmer H.G., Larriba M.J., García J.M., Ordóñez-Morán P., Peña C., Peiró S., Puig I., Rodríguez R., de la Fuente R., Bernad A., et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat. Med. 2004;10:917–919. doi: 10.1038/nm1095. [DOI] [PubMed] [Google Scholar]
- 42.Larriba M.J., Martín-Villar E., García J.M., Pereira F., Peña C., García de Herreros A., Bonilla F., Muñoz A. Snail2 cooperates with Snail1 in the repression of vitamin D receptor in colon cancer. Carcinogenesis. 2009;30:1459–1468. doi: 10.1093/carcin/bgp140. [DOI] [PubMed] [Google Scholar]
- 43.Shah S., Islam M.N., Dakshanamurthy S., Rizvi I., Rao M., Herrell R., Zinser G., Valrance M., Aranda A., Moras D., et al. The molecular basis of vitamin D receptor and β-catenin crossregulation. Mol. Cell. 2006;21:799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 44.Byers S.W., Rowlands T., Beildeck M., Bong Y.-S. Mechanism of action of vitamin D and the vitamin D receptor in colorectal cancer prevention and treatment. Rev. Endocr. Metab. Disord. 2012;13:31–38. doi: 10.1007/s11154-011-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egan J.B., Thompson P.A., Vitanov M.V., Bartik L., Jacobs E.T., Haussler M.R., Gerner E.W., Jurutka P.W. Vitamin D receptor ligands, adenomatous polyposis coli, and the vitamin D receptor FokI polymorphism collectively modulate β-catenin activity in colon cancer cells. Mol. Carcinog. 2010;49:337–352. doi: 10.1002/mc.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereira F., Barbáchano A., Silva J., Bonilla F., Campbell M.J., Muñoz A., Larriba M.J. KDM6B/JMJD3 histone demethylase is induced by vitamin D and modulates its effects in colon cancer cells. Hum. Mol. Genet. 2011;20:4655–4665. doi: 10.1093/hmg/ddr399. [DOI] [PubMed] [Google Scholar]
- 47.Xu H., McCann M., Zhang Z., Posner G.H., Bingham V., El-Tanani M., Campbell F.C. Vitamin D receptor modulates the neoplastic phenotype through antagonistic growth regulatory signals. Mol. Carcinog. 2009;48:758–772. doi: 10.1002/mc.20520. [DOI] [PubMed] [Google Scholar]
- 48.Semenov M.V., Tamai K., Brott B.K., Kuhl M., Sokol S., He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 49.Mao B., Wu W., Davidson G., Marhold J., Li M., Mechler B.M., Delius H., Hoppe D., Stannek P., Walter C., et al. Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 50.Aguilera O., Fraga M.F., Ballestar E., Paz M.F., Herranz M., Espada J., García J.M., Muñoz A., Esteller M., González-Sancho J.M. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene. 2006;25:4116–4121. doi: 10.1038/sj.onc.1209439. [DOI] [PubMed] [Google Scholar]
- 51.Pendás-Franco N., García J.M., Peña C., Valle N., Pálmer H.G., Heinaniemi M., Carlberg C., Jiménez B., Bonilla F., Muñoz A., et al. DICKKOPF-4 is induced by TCF/β-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1α,25-dihydroxyvitamin D3. Oncogene. 2008;27:4467–4477. doi: 10.1038/onc.2008.88. [DOI] [PubMed] [Google Scholar]
- 52.Eelen G., Valle N., Sato Y., Rochel N., Verlinden L., De Clercq P., Moras D., Bouillon R., Muñoz A., Verstuyf A. Superagonistic fluorinated vitamin D3 analogs stabilize helix 12 of the vitamin D receptor. Chem. Biol. 2008;15:1029–1034. doi: 10.1016/j.chembiol.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Beildeck M.E., Islam M., Shah S., Welsh J., Byers S.W. Control of TCF-4 expression by VDR and vitamin D in the mouse mammary gland and colorectal cancer cell lines. PLoS One. 2009;4:e7872. doi: 10.1371/journal.pone.0007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang W., Dodge M., Gundapaneni D., Michnoff C., Roth M., Lum L. A genome-wide RNAi screen for Wnt/β-catenin pathway components identifies unexpected roles for TCF transcription factors in cancer. Proc. Natl. Acad. Sci. USA. 2008;105:9697–9702. doi: 10.1073/pnas.0804709105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaler P., Augenlicht L., Klampfer L. Macrophage-derived IL-1β stimulates Wnt signaling and growth of colon cancer cells: A crosstalk interrupted by vitamin D3. Oncogene. 2009;28:3892–3902. doi: 10.1038/onc.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer M.B., Goetsch P.D., Pike J.W. VDR/RXR and TCF4/β-catenin cistromes in colonic cells of colorectal tumor origin: Impact on c-FOS and c-MYC gene expression. Mol. Endocrinol. 2012;26:37–51. doi: 10.1210/me.2011-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eilers M., Eisenman R.N. Myc’s broad reach. Genes Dev. 2008;22:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morrish F., Isern N., Sadilek M., Jeffrey M., Hockenbery D.M. c-Myc activates multiple metabolic networks to generate substrates for cell-cycle entry. Oncogene. 2009;28:2485–2491. doi: 10.1038/onc.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toropainen S., Väisänen S., Heikkinen S., Carlberg C. The down-regulation of the human MYC gene by the nuclear hormone 1α,25-dihydroxyvitamin D3 is associated with cycling of corepressors and histone deacetylases. J. Mol. Biol. 2010;400:284–294. doi: 10.1016/j.jmb.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 60.He T.C., Sparks A.B., Rago C., Hermeking H., Zawel L., da Costa L.T., Morin P.J., Vogelstein B., Kinzler K.W. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 61.Salehi-Tabar R., Nguyen-Yamamoto L., Tavera-Mendoza L.E., Quail T., Dimitrov V., An B.S., Glass L., Goltzman D., White J.H. Vitamin D receptor as a master regulator of the c-MYC/MXD1 network. Proc. Natl. Acad. Sci. USA. 2012;109:18827–18832. doi: 10.1073/pnas.1210037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khramtsov A.I., Khramtsova G.F., Tretiakova M., Huo D., Olopade O.I., Goss K.H. Wnt/β-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am. J. Pathol. 2010;176:2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geyer F.C., Lacroix-Triki M., Savage K., Arnedos M., Lambros M.B., MacKay A., Natrajan R., Reis-Filho J.S. β-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod. Pathol. 2011;24:209–231. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- 64.Wend P., Runke S., Wend K., Anchondo B., Yesayan M., Jardon M., Hardie N., Loddenkemper C., Ulasov I., Lesniak M.S., et al. WNT10B/β-catenin signalling induces HMGA2 and proliferation in metastatic triple-negative breast cancer. MBO Mol. Med. 2013;5:264–279. doi: 10.1002/emmm.201201320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pendás-Franco N., González-Sancho J.M., Suárez Y., Aguilera O., Steinmeyer A., Gamallo C., Berciano M.T., Lafarga M., Muñoz A. Vitamin D regulates the phenotype of human breast cancer cells. Differentiation. 2007;75:193–207. doi: 10.1111/j.1432-0436.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- 66.He W., Kang Y.S., Dai C., Liu Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J. Am. Soc. Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martínez-Moreno J.M., Muñoz-Castañeda J.R., Herencia C., Montes de Oca A., Estepa J.C., Canalejo R., Rodríguez-Ortiz M.E., Pérez-Martínez P., Aguilera-Tejero E., Canalejo A., et al. In vascular smooth muscle cells paricalcitol prevents phosphate-induced Wnt/β-catenin activation. Am. J. Physiol. Renal Physiol. 2012;303:F1136–F1144. doi: 10.1152/ajprenal.00684.2011. [DOI] [PubMed] [Google Scholar]
- 68.Ordóñez-Morán P., Larriba M.J., Pendás-Franco N., Aguilera O., González-Sancho J.M., Muñoz A. Vitamin D and cancer: An update of in vitro and in vivo data. Front. Biosci. 2005;10:2723–2749. doi: 10.2741/1731. [DOI] [PubMed] [Google Scholar]
- 69.Deeb K.K., Trump D.L., Johnson C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 70.Wali R.K., Khare S., Tretiakova M., Cohen G., Nguyen L., Hart J., Wang J., Wen M., Ramaswamy A., Joseph L., et al. Ursodeoxycholic acid and F6-D3 inhibit aberrant crypt proliferation in the rat azoxymethane model of colon cancer: Roles of cyclin D1 and E-cadherin. Cancer Epidemiol. Biomarkers Prev. 2002;11:1653–1662. [PubMed] [Google Scholar]
- 71.Fichera A., Little N., Dougherty U., Mustafi R., Cerda S., Li Y.C., Delgado J., Arora A., Campbell L.K., Joseph L., et al. A vitamin D analogue inhibits colonic carcinogenesis in the AOM/DSS model. J. Surg. Res. 2007;142:239–245. doi: 10.1016/j.jss.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 72.Su L.K., Kinzler K.W., Vogelstein B., Preisinger A.C., Moser A.R., Luongo C., Gould K.A., Dove W.F. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 73.Huerta S., Irwin R.W., Heber D., Go V.L.W., Koeffler H.P., Uskokovic M.R., Harris D.M. 1α,25-(OH)2D3 and its synthetic analogue decrease tumor load in the Apcmin mouse. Cancer Res. 2002;62:741–746. [PubMed] [Google Scholar]
- 74.Xu H., Posner G.H., Stevenson M., Campbell F.C. ApcMIN modulation of vitamin D secosteroid growth control. Carcinogenesis. 2010;31:1434–1441. doi: 10.1093/carcin/bgq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Irving A.A., Halberg R.B., Albrecht D.M., Plum L.A., Krentz K.J., Clipson L., Drinkwater N., Amos-Landgraf J.M., Dove W.F., DeLuca H.F. Supplementation by vitamin D compounds does not affect colonic tumor development in vitamin D sufficient murine models. Arch. Biochem. Biophys. 2011;515:64–71. doi: 10.1016/j.abb.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang K., Yang W., Mariadason J., Velcich A., Lipkin M., Augenlicht L. Dietary components modify gene expression: Implications for carcinogenesis. J. Nutr. 2005;135:2710–2714. doi: 10.1093/jn/135.11.2710. [DOI] [PubMed] [Google Scholar]
- 77.Yang K., Kurihara N., Fan K., Newmark H., Rigas B., Bancroft L., Corner G., Livote E., Lesser M., Edelmann W., et al. Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res. 2008;68:7803–7810. doi: 10.1158/0008-5472.CAN-08-1209. [DOI] [PubMed] [Google Scholar]
- 78.Wang D., Peregrina K., Dhima E., Lin E.Y., Mariadason J.M., Augenlicht L.H. Paneth cell marker expression in intestinal villi and colon crypts characterizes dietary induced risk for mouse sporadic intestinal cancer. Proc. Natl. Acad. Sci. USA. 2011;108:10272–10277. doi: 10.1073/pnas.1017668108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bouillon R., Carmeliet G., Verlinden L., van Etten E., Verstuyf A., Luderer H.F., Lieben L., Mathieu C., Demay M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kállay E., Pietschmann P., Toyokuni S., Bajna E., Hahn P., Mazzucco K., Bieglmayer C., Kato S., Cross H.S. Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis. 2001;22:1429–1435. doi: 10.1093/carcin/22.9.1429. [DOI] [PubMed] [Google Scholar]
- 81.Kállay E., Bareis P., Bajna E., Kriwanek S., Bonner E., Toyokuni S., Cross H.S. Vitamin D receptor activity and prevention of colonic hyperproliferation and oxidative stress. Food Chem. Toxicol. 2002;40:1191–1196. doi: 10.1016/S0278-6915(02)00030-3. [DOI] [PubMed] [Google Scholar]
- 82.Zheng W., Wong K.E., Zhang Z., Dougherty U., Mustafi R., Kong J., Deb D.K., Zheng H., Bissonnette M., Li Y.C. Inactivation of the vitamin D receptor in APCmin/+ mice reveals a critical role for the vitamin D receptor in intestinal tumor growth. Int. J. Cancer. 2012;130:10–19. doi: 10.1002/ijc.25992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garland C.F., Garland F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980;9:65–71. doi: 10.1093/ije/9.1.65. [DOI] [PubMed] [Google Scholar]
- 84.Stubbins R.E., Hakeem A., Núñez N.P. Using components of the vitamin D pathway to prevent and treat colon cancer. Nutr. Rev. 2012;70:721–729. doi: 10.1111/j.1753-4887.2012.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Plum L.A., DeLuca H.F. Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 2010;9:941–955. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 86.Ahearn T.U., Shaukat A., Flanders W.D., Rutherford R.E., Bostick R.M. A randomized clinical trial of the effects of supplemental calcium and vitamin D3 on the APC/β-catenin pathway in the normal mucosa of colorectal adenoma patients. Cancer Prev. Res. 2012;5:1247–1256. doi: 10.1158/1940-6207.CAPR-12-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cross H.S., Kállay E., Khorchide M., Lechner D. Regulation of extrarenal synthesis of 1,25-dihydroxyvitamin D3-relevance for colonic cancer prevention and therapy. Mol. Aspects Med. 2003;24:459–465. doi: 10.1016/S0098-2997(03)00041-4. [DOI] [PubMed] [Google Scholar]
- 88.Larriba M.J., Muñoz A. SNAIL vs. vitamin D receptor expression in colon cancer: Therapeutics implications. Br. J. Cancer. 2005;92:985–989. doi: 10.1038/sj.bjc.6602484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matusiak D., Murillo G., Carroll R.E., Mehta R.G., Benya R.V. Expression of vitamin D receptor and 25-hydroxyvitamin D3-1α-hydroxylase in normal and malignant human colon. Cancer Epidemiol. Biomarkers Prev. 2005;14:2370–2376. doi: 10.1158/1055-9965.EPI-05-0257. [DOI] [PubMed] [Google Scholar]
- 90.Anderson M.G., Nakane M., Ruan X., Kroeger P.E., Wu-Wong J.R. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother. Pharmacol. 2006;57:234–240. doi: 10.1007/s00280-005-0059-7. [DOI] [PubMed] [Google Scholar]
- 91.Cross H.S., Bajna E., Bises G., Genser D., Kállay E., Pötzi R., Wenzl E., Wrba F., Roka R., Peterlik M. Vitamin D receptor and cytokeratin expression may be progression indicators in human colon cancer. Anticancer Res. 1996;16:2333–2337. [PubMed] [Google Scholar]
- 92.Evans S.R.T., Nolla J., Hanfelt J., Shabahang M., Nauta R.J., Shchepotin I.B. Vitamin D receptor expression as a predictive marker of biological behavior in human colorectal cancer. Clin. Cancer Res. 1998;4:1591–1595. [PubMed] [Google Scholar]
- 93.Peña C., García J.M., Silva J., García V., Rodríguez R., Alonso I., Millán I., Salas C., García de Herreros A., Muñoz A., et al. E-cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in colon cancer: Clinicopathological correlations. Hum. Mol. Genet. 2005;14:3361–3370. doi: 10.1093/hmg/ddi366. [DOI] [PubMed] [Google Scholar]
- 94.Larriba M.J., Bonilla F., Muñoz A. The transcription factors Snail1 and Snail2 repress vitamin D receptor during colon cancer progression. J. Steroid Biochem. Mol. Biol. 2010;121:106–109. doi: 10.1016/j.jsbmb.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 95.Yook J.I., Li X.-Y., Ota I., Fearon E.R., Weiss S.J. Wnt-dependent regulation of the E-cadherin repressor Snail. J. Biol. Chem. 2005;280:11740–11748. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]
- 96.Yook J.I., Li X.-Y., Ota I., Hu C., Kim H.S., Kim N.H., Cha S.Y., Ryu J.K., Choi Y.J., Kim J., et al. A Wnt-Axin2-GSK3β cascade regulates Snail1 activity in breast cancer cells. Nat. Cell Biol. 2006;8:1398–1406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- 97.Ten Berge D., Koole W., Fuerer C., Fish M., Eroglu E., Nusse R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu Z.-Q., Brabletz T., Fearon E., Willis A.L., Hu C.Y., Li X.-Y., Weiss S.J. Canonical Wnt suppressor, Axin2, promotes colon carcinoma oncogenic activity. Proc. Natl. Acad. Sci. USA. 2012;109:11312–11317. doi: 10.1073/pnas.1203015109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mittal M.K., Myers J.N., Misra S., Bailey C.K., Chaudhuri G. In vivo binding to and functional repression of the VDR gene promoter by SLUG in human breast cells. Biochem. Biophys. Res. Commun. 2008;372:30–34. doi: 10.1016/j.bbrc.2008.04.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Frutos C.A., Dacquin R., Vega S., Jurdic P., Machuca-Gayet I., Nieto M.A. Snail1 controls bone mass by regulating Runx2 and VDR expression during osteoblast differentiation. EMBO J. 2009;28:686–696. doi: 10.1038/emboj.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bai S., Wang H., Shen J., Zhou R., Bushinsky D.A., Favus M.J. Elevated vitamin D receptor levels in genetic hypercalciuric stone-forming rats are associated with downregulation of Snail. J. Bone Miner. Res. 2010;25:830–840. doi: 10.1359/jbmr.091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang H., Zhang Y., Zhou Z., Jiang X., Shen A. Snail-1 regulates VDR signaling and inhibits 1,25(OH)-D3 action in osteosarcoma. Eur. J. Pharmacol. 2011;670:341–346. doi: 10.1016/j.ejphar.2011.09.160. [DOI] [PubMed] [Google Scholar]
- 103.Jeanes A., Gottardi C.J., Yap A.S. Cadherins and cancer: How does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heuberger J., Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb. Perspect. Biol. 2010;2:a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peña C., García J.M., García V., Silva J., Domínguez G., Rodríguez R., Maximiano C., García de Herreros A., Muñoz A., Bonilla F. The expression levels of the transcriptional regulators p300 and CtBP modulate the correlations between SNAIL, ZEB1, E-cadherin and vitamin D receptor in human colon carcinomas. Int. J. Cancer. 2006;119:2098–2104. doi: 10.1002/ijc.22083. [DOI] [PubMed] [Google Scholar]
- 106.González-Sancho J.M., Aguilera O., García J.M., Pendás-Franco N., Peña C., Cal S., García de Herreros A., Bonilla F., Muñoz A. The Wnt antagonist DICKKOPF-1 gene is a downstream target of β-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005;24:1098–1103. doi: 10.1038/sj.onc.1208303. [DOI] [PubMed] [Google Scholar]
- 107.Rawson J.B., Sun Z., Dicks E., Daftary D., Parfrey P.S., Green R.C., Gallinger S., McLaughlin J.R., Wang P.P., Knight J.A., et al. Vitamin D intake is negatively associated with promoter methylation of the Wnt antagonist gene DKK1 in a large group of colorectal cancer patients. Nutr. Cancer. 2012;64:919–928. doi: 10.1080/01635581.2012.711418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.You J., Nguyen A.V., Albers C.G., Lin F., Holcombe R.F. Wnt pathway-related gene expression in inflammatory bowel disease. Dig. Dis. Sci. 2008;53:1013–1019. doi: 10.1007/s10620-007-9973-3. [DOI] [PubMed] [Google Scholar]
- 109.Matsui A., Yamaguchi T., Maekawa S., Miyazaki C., Takano S., Uetake T., Inoue T., Otaka M., Otsuka H., Sato T., et al. DICKKOPF-4 and -2 genes are upregulated in human colorectal cancer. Cancer Sci. 2009;100:1923–1930. doi: 10.1111/j.1349-7006.2009.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kennell J.A., MacDougald O.A. Wnt signaling inhibits adipogenesis through β-catenin-dependent and -independent mechanisms. J. Biol. Chem. 2005;280:24004–24010. doi: 10.1074/jbc.M501080200. [DOI] [PubMed] [Google Scholar]
- 111.Kang S., Bennett C.N., Gerin I., Rapp L.A., Hankenson K.D., Macdougald O.A. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-bindingprotein α and peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 112.Fretz J.A., Zella L.A., Kim S., Shevde N.K., Pike J.W. 1,25-Dihydroxyvitamin D3 regulates the expression of low-density lipoprotein receptor-related protein 5 via deoxyribonucleic acid sequence elements located downstream of the start site of transcription. Mol. Endocrinol. 2006;20:2215–2230. doi: 10.1210/me.2006-0102. [DOI] [PubMed] [Google Scholar]
- 113.Fretz J.A., Zella L.A., Kim S., Shevde N.K., Pike J.W. 1,25-Dihydroxyvitamin D3 induces expression of the Wnt signaling co-regulator LRP5 via regulatory elements located significantly downstream of the gene’s transcriptional start site. J. Steroid Biochem. Mol. Biol. 2007;103:440–445. doi: 10.1016/j.jsbmb.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cianferotti L., Demay M.B. VDR-mediated inhibition of DKK1 and SFRP2 suppresses adipogenic differentiation of murine bone marrow stromal cells. J. Cell Biochem. 2007;101:80–88. doi: 10.1002/jcb.21151. [DOI] [PubMed] [Google Scholar]
- 115.Sakai Y., Kishimoto J., Demay M.B. Metabolic and cellular analysis of alopecia in vitamin D receptor knockout mice. J. Clin. Invest. 2001;107:961–966. doi: 10.1172/JCI11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cianferotti L., Cox M., Skorija K., Demay M.B. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc. Natl. Acad. Sci. USA. 2007;104:9428–9433. doi: 10.1073/pnas.0702884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pálmer H.G., Anjos-Afonso F., Carmeliet G., Takeda H., Watt F.M. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS One. 2008;3:e1483. doi: 10.1371/journal.pone.0001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Luderer H.F., Gori F., Demay M.B. Lymphoid enhancer-binding factor-1 (LEF1) interacts with the DNA-binding domain of the vitamin D receptor. J. Biol. Chem. 2011;286:18444–18451. doi: 10.1074/jbc.M110.188219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Genderen C., Okamura R.M., Farinas I., Quo R.G., Parslow T.G., Bruhn L., Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 120.Niemann C., Owens D.M., Hülsken J., Birchmeier W., Watt F.M. Expression of ΔNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development. 2002;129:95–109. doi: 10.1242/dev.129.1.95. [DOI] [PubMed] [Google Scholar]
- 121.Bikle D.D. The vitamin D receptor: A tumor suppressor in skin. Discov. Med. 2011;11:7–17. [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang Y.J., Teichert A.E., Fong F., Oda Y., Bikle D.D. 1α,25(OH)2-Dihydroxyvitamin D3/VDR protects the skin from UVB-induced tumor formation by interacting with the β-catenin pathway. J. Steroid Biochem. Mol. Biol. 2012;136:229–232. doi: 10.1016/j.jsbmb.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]