Abstract
Although the epidemiologic evidence that adequate vitamin D nutrition protects against non-melanoma skin cancer (NMSC) is limited, recent evidence that the vitamin D receptor (VDR) is protective is compelling. The role of vitamin D signaling in limiting the proliferation while promoting the differentiation of keratinocytes, the major cell in the epidermis from which NMSC are derived, is well known. However, recent findings that mice lacking the VDR are predisposed to skin cancer has brought to the fore the question of how the VDR is protective. In this review we will look first at the role of vitamin D signaling in regulating the proliferation and differentiation of keratinocytes. We will examine two pathways, β-catenin (CTNNB) and hedgehog (HH), that are regulated by vitamin D signaling and may contribute to the dysregulated proliferation and differentiation in the absence of VDR. We will then examine the failure of VDR deficient keratinocytes to repair DNA damaged by UVB. Finally we will examine the change in long non-coding RNA (LncRNA) expression in VDR null keratinocytes that in other cells is associated with malignant transformation, a potential newly appreciated mechanism by which vitamin D signaling is protective against NMSC.
Keywords: vitamin D receptor (VDR), β-catenin (CTNNB), hedgehog (HH), DNA damage repair (DDR), long non coding RNA (LncRNA)
1. Introduction
Skin cancer is by far the most common cancer afflicting humankind. Over 1 million skin cancers occur annually in the United States, 80% of which are basal cell carcinomas (BCC), 16% squamous cell carcinomas (SCC), and 4% melanomas [1]. Most can be cured by surgery, but this can be disfiguring and costly. For other types of tumors epidemiologic evidence linking adequate vitamin D levels from diet and/or sun exposure to cancer prevention, especially colon cancer prevention, is strong [2,3,4,5,6]. However, such epidemiologic evidence is lacking for skin cancers [7,8,9], and some studies have even demonstrated a positive correlation between 25OHD levels (the marker of vitamin D status) and BCC [10]. This apparent paradox between the impact of vitamin D on non-epidermal vs. epidermal malignancies is likely due to the role of ultraviolet B radiation (UVB) as the major etiologic agent for skin cancers. UVB is also the principal means by which the body obtains vitamin D. Sun avoidance may reduce one’s risk of developing skin cancer, but this practice frequently results in suboptimal levels of vitamin D in the body, not to mention the epidermis. A cost benefit analysis by Lucas et al. [11] indicates that sun avoidance and vitamin D deficiency increase the global disease burden, so this is not a good trade off. Furthermore, low dose UVB may be protective against skin cancer via the vitamin D signaling mechanisms that will be reviewed in this article, as suggested by the study by Armstrong and Kricker [12] who reported a decrease in the incidence of SCC, BCC, and melanomas in 10 US populations when the solar UV measurement was increased moderately from low levels. High levels increased the incidence. Other studies likely support the possibility that a threshold of UVB exposure exists that could allow adequate production of vitamin D in the skin without increasing the risk of skin cancer development [13,14].
Although at this point the role of vitamin D per se in the prevention of skin cancer remains uncertain, animal studies over the past decade have demonstrated a convincing role for the vitamin D receptor (VDR) in preventing skin cancer. When Zinser et al. [15] treated VDR null mice orally with the carcinogen 7,12-dimethylbenzanthracene (DMBA), they observed that nearly all the VDR null mice developed skin tumors, mostly papillomas, whereas none of the wildtype controls did. These results have been confirmed by other groups including ourselves using topical administration of DMBA/phorbol esters [16] or UVB [17,18]. In the latter case, SCC was the predominant cancer formed [17,18]. Surprisingly, mice lacking the ability to produce 1,25(OH)2D (CYP27B1 null) do not show increased susceptibility to tumor formation following either DMBA [17] or UVB [18]. These results suggest that the VDR independent of its ligand 1,25(OH)2D is exerting a protective effect against tumor formation. As will be demonstrated in this review, this conclusion is only partially true, but reflects an interesting distinction between the keratinocyte and most other cells in that VDR regulation of a number of biologic processes in keratinocytes is not totally dependent on 1,25(OH)2D.
To understand the role that VDR might be playing in its protective actions against chemical and UVB induced skin cancer we have examined three interacting mechanisms. First, vitamin D signaling has a well-established role in inhibiting proliferation and promoting differentiation of keratinocytes, which we have recently reviewed and will not reexamine here [19]. However, in this regard we will review the role of two pathways that contribute to VDR regulation of proliferation and differentiation, namely the β-catenin (CTNNB) and hedgehog (HH) pathways, pathways that when abnormally activated result in epidermal tumor formation [20,21]. Second, we will review the data indicating that the VDR facilitates DNA damage repair (DDR). UVB induces characteristic alterations in DNA (cyclobutane pyrimidine dimers [CPD] and 6,4-photoproducts [6,4-PP]) that if not repaired lead to mutations with the potential for initiating cancer [22]. Finally we will review new data from our laboratory that keratinocytes lacking VDR have altered expression of long non-coding RNAs (LncRNA) in a pattern associated with malignant transformation in other tissues [23].
2. Vitamin D Regulation of Proliferation and Differentiation: Role of the CTNNB and HH Pathways
The epidermis and hair follicles of VDR null mice show increased proliferation and disruption of differentiation beginning during the first catagen [24]. This leads to total alopecia after several months [24] and disrupted barrier formation in the epidermis [25]. Deleting the VDR in keratinocytes in vitro increases proliferation, decreases apoptosis, and alters the morphology of the keratinocyte from the normal cuboidal form with tight intercellular junctions to a loosely aggregated collection of fibroblast like cells suggesting epithelial/mesenchymal transformation [26] (Figure 1).
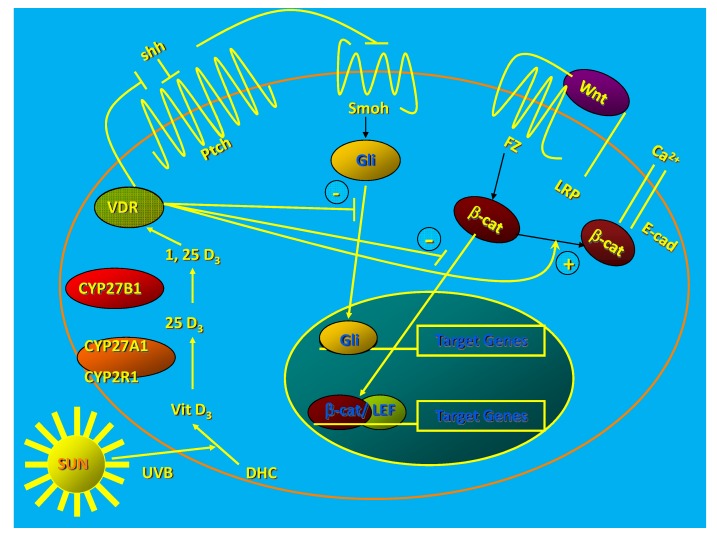
Figure 1.
1,25(OH)2D/VDR regulation of HH and CTNNB pathways in keratinocytes. The keratinocyte expresses both the VDR and the enzymatic machinery to produce its own 125(OH)2D from the vitamin D made from 7-dehydrocholesterol following UVB exposure. The VDR with or without its ligand suppresses Shh expression, may have a direct inhibitory action on Gli transcriptional activity (postulated, not demonstrated), binds CTNNB and induces E-cadherin expression reducing the amount of CTNNB available for binding to its nuclear transcription factor partners like LEF1. These actions block the transcriptional activity of both Gli and CTNNB, reducing their proliferative actions and limiting their ability to induce tumors in the skin. (Adapted from [27]).
Calcium-induced expression of differentiation markers and formation of the E-cadherin/catenin complex (adherens junctions) critical for the differentiation process is prevented by VDR deletion [26]. Acute UVB exposure in vivo stimulates the proliferation of the VDR null epidermis more than that of their wildtype littermates [18], and this hyperproliferation persists longer than that in the wildtype littermates such that there is an almost 3-fold increase in epidermal thickness in the VDR null mice compared to wildtype littermates by 48 h following UVB exposure.
2.1. Role of CTNNB Pathway
CTNNB has a dual role in keratinocyte proliferation and differentiation. CTNNB is activated when Wnt ligands bind to their seven-transmembrane Frizzled receptors and an LRP5 or LRP6 co-receptor leading to phosphorylation of disheveled (Dvl), disruption of the axin/APC complex and inhibition of the kinase activity of glycogen synthase kinase 3β (GSK-3β). In the absence of wnt, phosphorylation by GSK-3β of the serine(s) within exon 3 of CTNNB results in its degradation by the E3 ubiquitin ligase. Wnt prevents this degradation and enables CTNNB to enter the nucleus where it binds to transcription factors of the T-cell factor (TCF) and lymphoid enhancer factor (LEF) families to promote expression of genes such as cyclin D1 and c-Myc [28] important for proliferation. However, CTNNB also forms part of the adherens junction complex with E-cadherin where it plays an important role in keratinocyte differentiation [29]. We have found that knockdown of VDR in keratinocytes reduces E-cadherin expression and formation of the CTNNB/E-cadherin membrane complex resulting in increased CTNNB transcriptional activity, whereas 1,25(OH)2D administration has the opposite effect [30]. This was associated with increased (with VDR knockdown) or decreased (with 1,25(OH)2D administration) keratinocyte proliferation and cyclin D1 expression, respectively. Calcium induces the tyrosine phosphorylation of E-cadherin, promoting the binding of CTNNB and other catenins to E-cadherin to form the adherens junction complex making CTNNB less available for transcriptional activity [29,31]. The calcium sensing receptor (CaSR) is required for this response to calcium [32], and it is of interest that mice lacking both the CaSR and VDR in their keratinocytes develop epidermal tumors spontaneously [33]. Lack of CTNNB might be expected to reduce tumor formation in VDR null mouse skin, but when we bred CTNNB null mice with VDR null mice we did not see a reduction in tumor formation [34]. However, mice in which CTNNB signaling is disrupted develop alopecia, altered epidermal differentiation, and tumors similar to that seen in mice lacking VDR [35,36] suggesting a biphasic effect of CTNNB on keratinocyte proliferation and differentiation. On the other hand over expression and/or activating mutations in the CTNNB pathway lead to pilomatricomas or trichofolliculomas (hair follicle tumors) [20,37], and when mice with activating mutations of CTNNB are bred with mice lacking VDR, BCC develop [38]. These studies reflect the complexity of CTNNB/VDR interactions in regulating epidermal and hair follicle proliferation and differentiation. VDR/CTNNB interactions can be positive or negative, depending on the gene/cell/function being evaluated, but in the epidermis in the absence of VDR, the unchecked activity of CTNNB appears to be proliferative and inhibitory of differentiation.
2.2. Role of HH Pathway
Nearly all BCCs have mutations in patched 1 (Ptch 1) or other alterations in HH signaling [21,39]. Ptch 1 is the membrane receptor for sonic hedgehog (Shh), which in the basal state inhibits the function of smoothened (Smo), also in the membrane. In the presence of Shh this inhibition of Smo is lost resulting in the activation of a family of Gli transcription factors. These Gli factors in the basal state are maintained in the cytoplasm bound to Suppressor of fused (Sufu), but with the activation of Smo these factors are released from Sufu, enter the nucleus, and promote HH signaling [40,41]. These transcription factors increase the expression of components of the HH pathway, the anti-apoptotic factor bcl2, cyclins D1 and D2, E2F1, cdc45 while suppressing genes associated with keratinocyte differentiation including VDR [42,43,44,45,46].
VDR null mice overexpress elements of the HH signaling pathway in the epidermis and epidermal portion (utricles) of the dysplastic hair follicles [18]. Moreover, the tumors following either DMBA or UVB treatment express elements of the HH signaling pathway at higher levels than that of adjacent normal skin [18]. Shh, Ptch1, Gli1 and Gli2 have consensus sequences for vitamin D response elements (VDRE) in their promoters [38]. 1,25(OH)2D3 inhibits the expression of all elements of the HH pathway in normal skin, and this suppression requires VDR [18]. Surprisingly, vitamin D itself may suppress HH signaling, as it binds to and inhibits Smo directly [47,48]. However, the relative role of this mechanism vs. that of the genomic suppression of the HH pathway by 1,25(OH)2D and VDR is not clear.
The CTNNB and HH pathways interact [31,38]. Evaluating the induction of genes in a mouse model of BCC with a constitutively active Smo in keratinocytes, two groups [49,50] found a rapid increase in genes of the wnt/CTNNB pathway. When this pathway was inhibited as with Dkk1 overexpression or deletion of CTNNB, BCC did not develop. Moreover, in a series of human BCC both HH and CTNNB pathway constituents were over expressed [49]. Putative CTNNB/LEF1 response elements have been found in a number of HH pathway genes [38], and in mice with an activated CTNNB, Shh expression is increased [51].
3. Vitamin D Regulation of the DNA Damage Response (DDR)
The major cause of skin cancer is attributed to UVB with a spectrum between 280–320 nm [52]. The ozone layer protects us from UV wavelengths shorter than 280 nm (UVC). UV wavelengths longer than 320 nm (UVA), the major component of sunlight, can cause oxidative DNA damage that is potentially mutagenic [53]. UVB induced DNA damage includes the formation of cyclobutane pyrimidine dimers (CPD) and pyrimidine (6,4) pyrimidone photoproducts (6,4PP). If these lesions are not repaired C to T or CC to TT mutations result, the UVB “signature” lesion [54]. Actinic keratoses, the precursor lesion to SCC, as well as SCC and BCC contain these mutations in genes such as p53 [22,55,56,57,58]. Preventing UVB induced DNA damage from producing DNA mutations is the role of DDR operating through mechanisms involving damage recognition, repair and signal transduction. Nucleotide excision repair (NER) is the principal means by which UVB damage is repaired. By removing DNA damage before DNA replication begins NER can eliminate DNA damage that would otherwise result in mutations that get incorporated into the DNA during replication [59,60] (Figure 2).
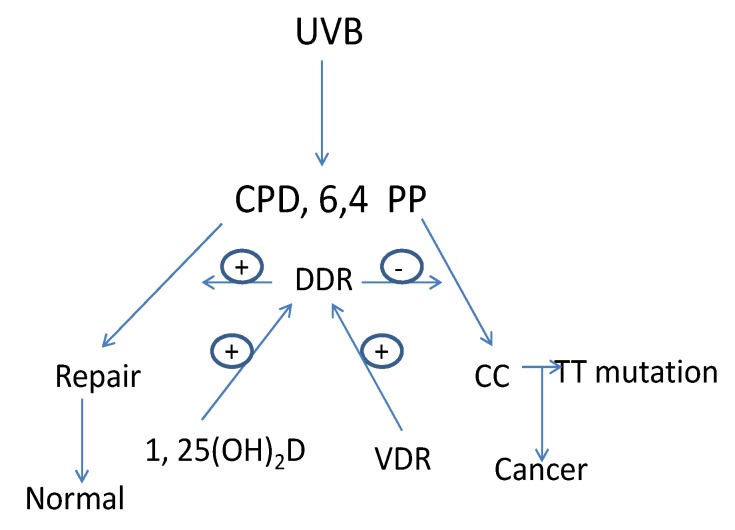
Figure 2.
1,25(OH)2D/VDR regulation of DNA damage repair (DDR) in keratinocytes. UVB causes DNA damage in the form of cyclobutane pyrimidine dimers (CPD) and pyrimidine(6,4)pyrimidone photoproducts (6,4PP). If these DNA lesions are not repaired mutations leading to cancer will result. This is the job of DDR, a multienzyme pathway regulated by VDR and 1,25(OH)2D.
The two major processes used by NER include transcription coupled repair (TCR), involving the repair of genes undergoing active transcription, and global genomic repair (GGR) for the non-transcribed regions of the genome [61,62,63]. Heritable mutations in NER genes occur in several human diseases with increased susceptibility to UVB induced epidermal malignancies such as xeroderma pigmentosum (XP) and Cockayne syndrome (CS) [63]. Identification of the genes mutated in these diseases has assisted substantially in identifying the genes and their protein products critical for DDR.
The epidermis of VDR null mice demonstrates a marked reduction in the clearance of CPDs and 6,4PPs following UVB whether administered in vivo [18] or in vitro [64]. The Mason laboratory [65,66] has demonstrated that 1,25(OH)2D3 topically applied protects the skin from UVB induced photodamage including increased clearance of CPDs, decreased apoptosis, increased survival, and increased expression of p53. These effects do not appear to require genomic actions of VDR, as analogs of 1,25(OH)2D that promote nongenomic actions of the VDR are equally effective. Moreover, using fibroblasts with mutations of the VDR that prevent its genomic actions but not its binding to 1,25(OH)2D, this laboratory demonstrated photoprotective effects comparable to that in normal cells [67]. VDR null cells did not show a protective effect, however [68]. Whether these results will apply in vivo in the epidermis in keratinocytes is not known. On the other hand, Moll et al. [68] found that 1,25(OH)2D induced two genes important for DDR: XPC (xeroderma pigmentosum complementation group C) and DDB2 (damage-specific DNA binding protein 2 also known as XPE). Thus, 1,25(OH)2D may have genomic and non genomic actions to enhance DDR, although in all cases the VDR is required. On the other hand VDR may have 1,25(OH)2D independent actions to promote DDR. Much remains to be investigated in terms of vitamin D signaling and DDR.
4. Vitamin D Regulation of Long Non-Coding RNA (lncRNA) Expression
LncRAs are endogenous cellular RNAs of larger than 200 bases. They account for 80% of the transcriptome [69]. They are spliced and contain polyadenylation signals, much like messenger RNAs [70]. LncRNAs have emerged as master regulators of embryonic pluripotency, differentiation, and body axis patterning, regulating histone modifications and so influencing the epigenetic programs of the transcriptome [70,71]. Of greater relevance to this review is that lncRNAs also regulate cancer development through effects on tumor cell proliferation, evasion of growth suppressors, replicative immortality, angiogenesis, and invasion and metastasis [72,73,74]. As a first step to determining whether lncRNAs play a role in the protective effective of vitamin D signaling in epidermal carcinogenesis we [23] evaluated the profile of lncRNAs in the epidermis of VDR null mice and in keratinocytes lacking VDR.
We found that H19, HOTTIP and Nespas are significantly and consistently increased in both cultured keratinocytes and epidermis following VDR deletion as were Air, HOTAIR, Malat1 and SRA. These lncRNAs are known to be oncogenic [74]. H19 is normally expressed during fetal development, but is reexpressed in adult tumors, and is essential for human tumor growth [75,76,77]. HOTTIP (HOXA transcript at the distal tip) is expressed from the 5' end of the HoxA locus and drives histone H3 lysine 4 trimethylation and gene transcription of HoxA distal genes through the recruitment of the WDR5/MLL complex [78]. On the other hand, the 7 lncRNAs that decreased after VDR deletion in vivo or in vitro included lincRNA-p21 and Kcnq1ot1, two well-characterized tumor suppressors [74,79]. LincRNA-p21 is a direct p53 target gene residing next to the p21 gene, which is up-regulated upon DNA damage in different tumor models [80]. LincRNA-p21 exerts its tumor suppressor function via association with hnRNP, a well-known RNA binding protein and itself is a tumor suppressor [81]. Kcnq1ot1 localizes in the nucleus, interacting with chromatin and also with G9a (a H3K9- and H3K27-specific histone methyltransferase) and Ezh2 (histone-lysine N-methyltransferase), resulting in cluster-wide repressive histone marks, gene silencing and DNA methylation of CpG islands. Hence it exerts its tumor suppressor effect via epigenetic gene silencing [82]. Together, our results indicate that part of the protective effect of VDR against epidermal carcinogenesis is due to reducing the expression levels of oncogenic lncRNAs while upregulating tumor suppressor lncRNAs.
5. Conclusions
VDR protects against epidermal carcinogenesis. We have reviewed three modes by which this protective action is mediated. First VDR decreases the proliferation and increases the differentiation of keratinocytes in the skin. At least two pathways are involved with regulation, the CTNNB and HH pathways. Lack of VDR increases the activity of these pathways resulting in increased proliferation and decreased differentiation. Second, VDR is required for full activity of DDR. In the absence of VDR DDR is impaired leading to accumulation of mutations that lead to malignant transformation. Third, VDR regulates the expression of oncogenic and tumor suppressing lncRNAs in keratinocytes. The lack of VDR shifts the profile of these lncRNAs to one predisposing to malignancy. Whether these three modes of VDR actions interact with each other remains unknown and is currently under investigation. Additionally, although for a number of these actions VDR does not appear to require its ligand 1,25(OH)2D, for other actions 1,25(OH)2D is clearly involved. Moreover, not all of the actions of 1,25(OH)2D/VDR seem to involve a genomic effect, although many clearly do. Thus although the role of VDR in protecting us from skin cancer is established, we still have much to learn about the precise mechanisms by which it does so.
Acknowledgments
The authors acknowledge the administrative assistance of Aaminah Khan and Vicky Lee, the technical support of Alicia Menendez, Chak Frankie Fong, and Sally Pennypacker, the contributions of collaborators and former postdoctoral fellows Dennis Oh, Yuko Oda, and Arnaud Teichert, and the financial support from DOD CA110338, NIH AR050023, and VA Merit Review to DDB.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Greenlee R.T., Hill-Harmon M.B., Murray T., Thun M. Cancer statistics 2001. CA Cancer J. Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Garland C., Barrett-Connor E., Rossof A.H., Shekelle R.B., Criqui M.H., Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: A 19-year prospective study in men. Lancet. 1985;1:307–309. doi: 10.1016/s0140-6736(85)91082-7. [DOI] [PubMed] [Google Scholar]
- 3.Bostick R.M., Potter J.D., Sellers T.A., McKenzie D.R., Kushi L.H., Folsom A.R. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women’s Health Study. Am. J. Epidemiol. 1993;137:1302–1317. doi: 10.1093/oxfordjournals.aje.a116640. [DOI] [PubMed] [Google Scholar]
- 4.Kearney J., Giovannucci E., Rimm E.B., Ascherio A., Stampfer M.J., Colditz G.A., Wing A., Kampman E., Willett W.C. Calcium, vitamin D, and dairy foods and the occurrence of colon cancer in men. Am. J. Epidemiol. 1996;143:907–917. doi: 10.1093/oxfordjournals.aje.a008834. [DOI] [PubMed] [Google Scholar]
- 5.Garland F.C., Garland C.F., Gorham E.D., Young J.F. Geographic variation in breast cancer mortality in the United States: A hypothesis involving exposure to solar radiation. Prev. Med. 1990;19:614–622. doi: 10.1016/0091-7435(90)90058-R. [DOI] [PubMed] [Google Scholar]
- 6.Hanchette C.L., Schwartz G.G. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–2869. doi: 10.1002/1097-0142(19921215)70:12<2861::AID-CNCR2820701224>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Van Dam R.M., Huang Z., Giovannucci E., Rimm E.B., Hunter D.J., Colditz G.A., Stampfer M.J., Willett W.C. Diet and basal cell carcinoma of the skin in a prospective cohort of men. Am. J. Clin. Nutr. 2000;71:135–141. doi: 10.1093/ajcn/71.1.135. [DOI] [PubMed] [Google Scholar]
- 8.Hunter D.J., Colditz G.A., Stampfer M.J., Rosner B., Willett W.C., Speizer F.E. Diet and risk of basal cell carcinoma of the skin in a prospective cohort of women. Ann. Epidemiol. 1992;2:231–239. doi: 10.1016/1047-2797(92)90055-U. [DOI] [PubMed] [Google Scholar]
- 9.Weinstock M.A., Stampfer M.J., Lew R.A., Willett W.C., Sober A.J. Case-control study of melanoma and dietary vitamin D: Implications for advocacy of sun protection and sunscreen use. J. Invest. Dermatol. 1992;98:809–811. doi: 10.1111/1523-1747.ep12499962. [DOI] [PubMed] [Google Scholar]
- 10.Asgari M.M., Tang J., Warton M.E., Chren M.M., Quesenberry C.P., Jr., Bikle D., Horst R.L., Orentreich N., Vogelman J.H., Friedman G.D. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. J. Invest. Dermatol. 2010;130:1438–1443. doi: 10.1038/jid.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas R.M., McMichael A.J., Armstrong B.K., Smith W.T. Estimating the global disease burden due to ultraviolet radiation exposure. Int. J. Epidemiol. 2008;37:654–667. doi: 10.1093/ije/dyn017. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong B.K., Kricker A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B. 2001;63:8–18. doi: 10.1016/S1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 13.English D.R., Armstrong B.K., Kricker A., Winter M.G., Heenan P.J., Randell P.L. Case-control study of sun exposure and squamous cell carcinoma of the skin. Int. J. Cancer. 1998;77:347–353. doi: 10.1002/(sici)1097-0215(19980729)77:3<347::aid-ijc7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Rosso S., Tang J., Warton M.E., Chren M.M., Quesenberry C.P., Jr., Bikle D., Horst R.L., Orentreich N., Vogelman J.H., Friedman G.D. The multicentre south European study “Helios”. II: Different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br. J. Cancer. 1996;73:1447–1454. doi: 10.1038/bjc.1996.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zinser G.M., Sundberg J.P., Welsh J. Vitamin D3 receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis. 2002;23:2103–2109. doi: 10.1093/carcin/23.12.2103. [DOI] [PubMed] [Google Scholar]
- 16.Indra A.K., Castaneda E., Antal M.C., Jiang M., Messaddeq N., Meng X.J., Loehr C.V., Gariglio P., Kato S., Wahli W., et al. Malignant transformation of DMBA/TPA-induced papillomas and nevi in the skin of mice selectively lacking retinoid-X-receptor alpha in epidermal keratinocytes. J. Invest. Dermatol. 2007;127:1250–1260. doi: 10.1038/sj.jid.5700672. [DOI] [PubMed] [Google Scholar]
- 17.Ellison T.I., Smith M.K., Gilliam A.C., MacDonald P.N. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J. Invest. Dermatol. 2008;128:2508–2517. doi: 10.1038/jid.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teichert A.E., Elalieh H., Elias P.M., Welsh J., Bikle D.D. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J. Invest. Dermatol. 2011;131:2289–2297. doi: 10.1038/jid.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bikle D.D. Vitamin D metabolism and function in the skin. Mol. Cell Endocrinol. 2011;347:80–89. doi: 10.1016/j.mce.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan E.F., Gat U., McNiff J.M., Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat. Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- 21.Hahn H., Wicking C., Zaphiropoulos P.G., Gailani M.R., Shanley S., Chidambaram A., Vorechovsky I., Holmberg E., Unden A.B., Gillies S., et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/S0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 22.Daya-Grosjean L., Sarasin A. The role of UV induced lesions in skin carcinogenesis: An overview of oncogene and tumor suppressor gene modifications in xeroderma pigmentosum skin tumors. Mutat. Res. 2005;571:43–56. doi: 10.1016/j.mrfmmm.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y.J., Bikle D.D. LncRNA profiling reveals a new mechanism for VDR protection against skin cancer formation. J. Steroid Biochem. Mol. Biol. 2013 doi: 10.1016/j.jsbmb.2013.11.018. in press. [DOI] [PubMed] [Google Scholar]
- 24.Bikle D.D., Oda Y., Teichert A. The vitamin D receptor: A tumor suppressor in skin. Discovery Med. 2011;11:7–17. [PMC free article] [PubMed] [Google Scholar]
- 25.Bikle D.D., Elalieh H., Chang S., Xie Z., Sundberg J.P. Development and progression of alopecia in the vitamin D receptor null mouse. J. Cell Physiol. 2006;207:340–353. doi: 10.1002/jcp.20578. [DOI] [PubMed] [Google Scholar]
- 26.Oda Y., Ishikawa M.H., Hawker N.P., Yun Q.C., Bikle D.D. Vitamin D receptor and coactivators SRC 2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J. Invest. Dermatol. 2009;129:1367–1378. doi: 10.1038/jid.2008.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oda Y., Ishikawa M.H., Hawker N.P., Yun Q.C., Bikle D.D. Differential role of two VDR coactivators, DRIP205 and SRC-3, in keratinocyte proliferation and differentiation. J. Steroid Biochem. Mol. Biol. 2007;103:776–780. doi: 10.1016/j.jsbmb.2006.12.069. [DOI] [PubMed] [Google Scholar]
- 28.He T.C., Sparks A.B., Rago C., Hermeking H., Zawel L., da Costa L.T., Morin P.J., Vogelstein B., Kinzler K.W. Identification of c-Myc as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 29.Xie Z., Bikle D.D. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J. Biol. Chem. 2007;282:8695–8703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- 30.Bikle D.D. The vitamin D receptor: A tumor suppressor in skin. Discov. Med. 2011;11:7–17. [PMC free article] [PubMed] [Google Scholar]
- 31.Bienz M. beta-Catenin: A pivot between cell adhesion and Wnt signaling. Curr. Biol. 2005;15:R64–R67. doi: 10.1016/j.cub.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 32.Tu C., Chang W., Xie Z., Bikle D.D. Inactivation of the calcium sensing receptor inhibits E-cadherin-mediated cell-cell adhesion and calcium-induced differentiation in human epidermal keratinocytes. J. Biol.Chem. 2008;283:3519–3528. doi: 10.1074/jbc.M708318200. [DOI] [PubMed] [Google Scholar]
- 33.Bikle D.D. University of California San Francisco; San Francisco, CA, USA: 2013. Unpublished work. [Google Scholar]
- 34.Jiang Y.J., Teichert A.E., Fong F., Oda Y., Bikle D.D. 1,25(OH)2-Dihydroxyvitamin D3/VDR protects the skin from UVB-induced tumor formation by interacting with the B-catenin pathway. J. Steroid Biochem. Mol. Biol. 2013;136:229–232. doi: 10.1016/j.jsbmb.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huelsken J., Vogel R., Erdmann B., Cotsarelis G., Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/S0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 36.Niemann C., Owens D.M., Hulsken J., Birchmeier W., Watt F.M. Repression of B-catenin signaling in mouse epidermis results in transdifferentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development. 2002;129:95–109. doi: 10.1242/dev.129.1.95. [DOI] [PubMed] [Google Scholar]
- 37.Gat U., DasGupta R., Degenstein L., Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/S0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 38.Palmer H.G., Anjos-Afonso F., Carmeliet G., Takeda H., Watt F.M. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS One. 2008;3:e1483. doi: 10.1371/journal.pone.0001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aszterbaum M., Rothman A., Johnson R.L., Fisher M., Xie J., Bonifas J.M., Zhang X., Scott M.P., Epstein E.H., Jr. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J. Invest. Dermatol. 1998;110:885–888. doi: 10.1046/j.1523-1747.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- 40.Barnfield P.C., Zhang X., Thanabalasingham V., Yoshida M., Hui C.C. Negative regulation of Gli1 and Gli2 activator function by Suppressor of fused through multiple mechanisms. Differentiation. 2005;73:397–405. doi: 10.1111/j.1432-0436.2005.00042.x. [DOI] [PubMed] [Google Scholar]
- 41.Svard J., Heby-Henricson K., Persson-Lek M., Rozell B., Lauth M., Bergstrom A., Ericson J., Toftgard R., Teglund S. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev. Cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Regl G., Kasper M., Schnidar H., Eichberger T., Neill G.W., Ikram M.S., Quinn A.G., Philpott M.P., Frischauf A.M., Aberger F. The zinc-finger transcription factor GLI2 antagonizes contact inhibition and differentiation of human epidermal cells. Oncogene. 2004;23:1263–1274. doi: 10.1038/sj.onc.1207240. [DOI] [PubMed] [Google Scholar]
- 43.Regl G., Kasper M., Schnidar H., Eichberger T., Neill G.W., Philpott M.P., Esterbauer H., Hauser-Kronberger C., Frischauf A.M., Aberger F. Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res. 2004;64:7724–7731. doi: 10.1158/0008-5472.CAN-04-1085. [DOI] [PubMed] [Google Scholar]
- 44.Regl G., Neill G.W., Eichberger T., Kasper M., Ikram M.S., Koller J., Hintner H., Quinn A.G., Frischauf A.M., Aberger F. Human GLI2 and GLI1 are part of a positive feedback mechanism in Basal Cell Carcinoma. Oncogene. 2002;21:5529–5539. doi: 10.1038/sj.onc.1205748. [DOI] [PubMed] [Google Scholar]
- 45.Grachtchouk M., Mo R., Yu S., Zhang X., Sasaki H., Hui C.C., Dlugosz A.A. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat. Genet. 2000;24:216–217. doi: 10.1038/73417. [DOI] [PubMed] [Google Scholar]
- 46.Nilsson M., Unden A.B., Krause D., Malmqwist U., Raza K., Zaphiropoulos P.G., Toftgard R. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc. Natl. Acad. Sci. USA. 2000;97:3438–3443. doi: 10.1073/pnas.97.7.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bijlsma M.F., Spek C.A., Zivkovic D., van de Water S., Rezaee F., Peppelenbosch M.P. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4:e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang J.Y., Xiao T.Z., Oda Y., Chang K.S., Shpall E., Wu A., So P.L., Hebert J., Bikle D., Epstein E.H., Jr. Vitamin D3 inhibits hedgehog signaling and proliferation in murine Basal cell carcinomas. Cancer Prev. Res. 2011;4:744–751. doi: 10.1158/1940-6207.CAPR-10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Youssef K.K., Lapouge G., Bouvrée K., Rorive S., Brohéem S., Appelstein O., Larsimont J.-C., Sukumaran V., van de Sande B., Pucci D., et al. Adult interfollicular tumour-initiating cells are reprogrammed into an embryonic hair follicle progenitor-like fate during basal cell carcinoma initiation. Nat. Cell Biol. 2012;14:1282–1294. doi: 10.1038/ncb2628. [DOI] [PubMed] [Google Scholar]
- 50.Yang S.H., Andl T., Grachtchouk V., Wang A., Liu J., Syu L.J., Ferris J., Wang T.S., Glick A.B., Millar S.E., et al. Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/beta3-catenin signaling. Nat. Genet. 2008;40:1130–1135. doi: 10.1038/ng.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider F.T., Schanzer A., Czupalla C.J., Thom S., Engels K., Schmidt M.H., Plate K.H., Liebner S. Sonic hedgehog acts as a negative regulator of {beta}-catenin signaling in the adult tongue epithelium. Am. J. Pathol. 2010;177:404–414. doi: 10.2353/ajpath.2010.091079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freeman S.E., Hacham H., Gange R.W., Maytum D.J., Sutherland J.C., Sutherland B.M. Wavelength dependence of pyrimidine dimer formation in DNA of human skin irradiated in situ with ultraviolet light. Proc. Natl. Acad. Sci. USA. 1989;86:5605–5609. doi: 10.1073/pnas.86.14.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Besaratinia A., Synold T.W., Chen H.H., Chang C., Xi B., Riggs A.D., Pfeifer G.P. DNA lesions induced by UV A1 and B radiation in human cells: Comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc. Natl. Acad. Sci. USA. 2005;102:10058–10063. doi: 10.1073/pnas.0502311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hussein M.R. Ultraviolet radiation and skin cancer: Molecular mechanisms. J. Cutan. Pathol. 2005;32:191–205. doi: 10.1111/j.0303-6987.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 55.Ziegler A., Leffell D.J., Kunala S., Sharma H.W., Gailani M., Simon J.A., Halperin A.J., Baden H.P., Shapiro P.E., Bale A.E. Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc. Natl. Acad. Sci. USA. 1993;90:4216–4220. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziegler A., Jonason A.S., Leffell D.J., Simon J.A., Sharma H.W., Kimmelman J., Remington L., Jacks T., Brash D.E. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 57.Brash D.E., Rudolph J.A., Simon J.A., Lin A., McKenna G.J., Baden H.P., Halperin A.J., Ponten J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA. 1991;88:10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bito T., Ueda M., Ahmed N.U., Nagano T., Ichihashi M. Cyclin D and retinoblastoma gene product expression in actinic keratosis and cutaneous squamous cell carcinoma in relation to p53 expression. J. Cutan. Pathol. 1995;22:427–434. doi: 10.1111/j.1600-0560.1995.tb00758.x. [DOI] [PubMed] [Google Scholar]
- 59.Chen R.H., Maher V.M., McCormick J.J. Effect of excision repair by diploid human fibroblasts on the kinds and locations of mutations induced by (+/−)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene in the coding region of the HPRT gene. Proc. Natl. Acad. Sci. USA. 1990;87:8680–8684. doi: 10.1073/pnas.87.21.8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wood R.D. DNA damage recognition during nucleotide excision repair in mammalian cells. Biochimie. 1999;81:39–44. doi: 10.1016/S0300-9084(99)80036-4. [DOI] [PubMed] [Google Scholar]
- 61.Mellon I., Bohr V.A., Smith C.A., Hanawalt P.C. Preferential DNA repair of an active gene in human cells. Proc. Natl. Acad. Sci. USA. 1986;83:8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanawalt P.C. Transcription-coupled repair and human disease. Science. 1994;266:1957–1958. doi: 10.1126/science.7801121. [DOI] [PubMed] [Google Scholar]
- 63.Wood R.D., Mitchell M., Sgouros J., Lindahl T. Human DNA repair genes. Science. 2001;291:1284–1289. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- 64.Demetriou S.K., Ona-Vu K., Teichert A.E., Cleaver J.E., Bikle D.D., Oh D.H. Vitamin D receptor mediates DNA repair and is UV inducible in intact epidermis but not in cultured keratinocytes. J. Invest. Dermatol. 2012;132:2097–2100. doi: 10.1038/jid.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dixon K.M., Deo S.S., Wong G., Slater M., Norman A.W., Bishop J.E., Posner G.H., Ishizuka S., Halliday G.M., Reeve V.E., et al. Skin cancer prevention: A possible role of 1,25dihydroxyvitamin D3 and its analogs. J. Steroid Biochem. Mol. Biol. 2005;97:137–143. doi: 10.1016/j.jsbmb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 66.Gupta R., Dixon K.M., Deo S.S., Holliday C.J., Slater M., Halliday G.M., Reeve V.E., Mason R.S. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J. Invest. Dermatol. 2007;127:707–715. doi: 10.1038/sj.jid.5700597. [DOI] [PubMed] [Google Scholar]
- 67.Sequeira V.B., Rybchyn M.S., Tongkao-On W., Gordon-Thomson C., Malloy P.J., Nemere I., Norman A.W., Reeve V.E., Halliday G.M., Feldman D., et al. The role of the vitamin D receptor and ERp57 in photoprotection by 1alpha,25-dihydroxyvitamin D3. Mol. Endocrinol. 2012;26:574–582. doi: 10.1210/me.2011-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moll P.R., Sander V., Frischauf A.M., Richter K. Expression profiling of vitamin D treated primary human keratinocytes. J. Cell Biochem. 2007;100:574–592. doi: 10.1002/jcb.21061. [DOI] [PubMed] [Google Scholar]
- 69.Mercer T.R., Dinger M.E., Mattick J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 70.Mattick J.S. Long noncoding RNAs in cell and developmental biology. Semin. Cell Dev. Biol. 2011;22 doi: 10.1016/j.semcdb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 71.Spitale R.C., Crisalli P., Flynn R.A., Torre E.A., Kool E.T., Chang H.Y. RNA SHAPE analysis in living cells. Nat. Chem. Biol. 2013;9:18–20. doi: 10.1038/nchembio.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibb E.A., Vucic E.A., Enfield K.S.S., Stewart G.L., Lonergan K.M., Kennett J.Y., Becker-Santos D.D., MacAulay C.E., Lam S., Brown C.J., et al. Human cancer long non-coding RNA transcriptomes. PLoS One. 2011;6:e25915. doi: 10.1371/journal.pone.0025915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gutschner T., Diederichs S. The hallmarks of cancer: A long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X., Wu Z., Fu X., Han W. Long Noncoding RNAs: Insights from biological features and functions to diseases. Med. Res. Rev. 2013;33:517–553. doi: 10.1002/med.21254. [DOI] [PubMed] [Google Scholar]
- 75.Barsyte-Lovejoy D., Lau S.K., Boutros P.C., Khosravi F., Jurisica I., Andrulis I.L., Tsao M.S., Penn L.Z. The c-Myc oncogene directly induces the H19 noncoding RNA by allele-specific binding to potentiate tumorigenesis. Cancer Res. 2006;66:5330–5337. doi: 10.1158/0008-5472.CAN-06-0037. [DOI] [PubMed] [Google Scholar]
- 76.Matouk I.J., DeGroot N., Mezan S., Ayesh S., Abu-lail R., Hochberg A., Galun E. The H19 non-coding RNA is essential for human tumor growth. PLoS One. 2007;2:e845. doi: 10.1371/journal.pone.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berteaux N., Lottin S., Monté D., Pinte S., Quatannens B., Coll J., Hondermarck H., Curgy J.-J., Dugimont T., Adriaenssens E. H19 mRNA-like noncoding RNA promotes breast cancer cell proliferation through positive control by E2F1. J. Biol. Chem. 2005;280:29625–29636. doi: 10.1074/jbc.M504033200. [DOI] [PubMed] [Google Scholar]
- 78.Wang K.C., Yang Y.W., Liu B., Sanyal A., Corces-Zimmerman R., Chen Y., Lajoie B.R., Protacio A., Flynn R.A., Gupta R.A., et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pandey R.R., Mondal T., Mohammad F., Enroth S., Redrup L., Komorowski J., Nagano T., Mancini-Dinardo D., Kanduri C. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell. 2008;32:232–246. doi: 10.1016/j.molcel.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 80.Huarte M., Guttman M., Feldser D., Garber M., Koziol M.J., Kenzelmann-Broz D., Khalil A.M., Zuk O., Amit I., Rabani M., et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moumen A., Magill C., Dry K.L., Jackson S.P. ATM-dependent phosphorylation of heterogeneous nuclear ribonucleoprotein K promotes p53 transcriptional activation in response to DNA damage. Cell Cycle. 2013;12:698–704. doi: 10.4161/cc.23592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Redrup L., Branco M.R., Perdeaux E.R., Krueger C., Lewis A., Santos F., Nagano T., Cobb B.S., Fraser P., Reik W. The long noncoding RNA Kcnq1ot1 organises a lineage-specific nuclear domain for epigenetic gene silencing. Development. 2009;136:525–530. doi: 10.1242/dev.031328. [DOI] [PMC free article] [PubMed] [Google Scholar]