Abstract
Biological agents are widely used for various immune-mediated diseases, with remarkable effectiveness in the treatment of rheumatoid arthritis (RA), psoriasis, psoriatic arthritis, ankylosing spondylitis and Crohn's disease. However, attention needs to be drawn to the adverse effects of these therapies and the risk of reactivating underlying granulomatous infectious diseases such as tuberculosis, leprosy, syphilis, leishmaniasis, among others. The objective of this paper is to describe a case of leprosy in a patient with RA using anti-TNF alfa, demonstrating the need for systematic investigation of skin lesions suggestive of leprosy in patients who require rheumatoid arthritis therapeutic treatment, especially in endemic regions like Brazil.
Keywords: Rheumatoid arthritis, Exposure to biological agents, Leprosy, Tumor necrosis factor-alpha
Abstract
Os agentes biológicos são amplamente utilizados em diversas doenças imuno-mediadas, com marcante eficácia no tratamento da Artrite Reumatóide (AR), Psoríase, Artrite Psoriática, Espondilite Anquilosante e Doença de Crohn. No entanto, deve-se atentar quanto aos efeitos adversos de tais terapêuticas, como o risco de reativar doenças infecciosas granulomatosas latentes, como a tuberculose, hanseníase, sífilis, leishmaniose, entre outras. O objetivo deste artigo é descrever um caso de hanseníase em paciente portador de AR em uso de terapia anti-TNF alfa, mostrando, assim, a necessidade de investigação sistematizada de lesões cutâneas sugestivas de hanseníase em pacientes com indicação de terapia anti-TNF alfa, especialmente, em regiões endêmicas como o Brasil.
INTRODUCTION
The Tumor Necrosis Factor (TNF) is a proinflammatory cytokine that has a key function in various autoimmune diseases, such as rheumatoid arthritis (RA), psoriasis, psoriatic arthritis, ankylosing spondylitis and Crohn's disease. It plays an important role in human immune response to infections.1,2 As regards infectious diseases, it encourages the release of other inflammatory cytokines, particularly interleukins IL-1, IL-6 and IL-8, and stimulates the output of protease, thus participating in the formation and maintenance of granulomas, a component of intracellular pathogen-defence.2-4
Biological disease modifying drugs (DMDs) are indicated for patients who experience persistence of the disease, though treatment involves at least two schemes with synthetic DMDs, of which at least one is the combination of DMDs. The following biological DMDs have been approved by the National Health Surveillance Agency (ANVISA) for use in Brazil: anti-TNF α, B-lymphocyte depletor, T-lymphocyte costimulation blocker and interleukin-6 (IL-6) receptor blocker.1,5
At present, three anti-TNF a agents are used to treat certain autoimmune diseases: infliximab, etanercept and adalimumab.2,5 Infliximab is a chimerical IgG monoclonal antibody, made up of 75% human protein and 25% mouse protein, the portion of which contains the binding site for TNF. This biological agent contributes through the death of cells expressing TNF on the surface via an Ac cytotoxic mechanism and dependent complement, disrupting granulomas, which can cause reactivation of latent granulomatous infections like tuberculosis and leprosy.3-6
Mycobacterium leprae causes a chronic infectious disease that presents clinically as a spectrum of symptoms associated with immune response. There are two polar clinical presentations: the tuberculoid form, represented by well-organized granulomas with few microbacteria; and the lepromatous form, characterized by less-organized lesions and a higher number of bacilli. There are also intermediary clinical forms, the dimorphous forms, with immunological instability and clinical characteristics from both polar forms. The clinical presentation is polymorphic, varying from skin alterations with areas of diminished sensitiveness and hypopigmentation, to more serious neural lesions or involvement of other organs, including bones and joints.4,7,8
CASE REPORT
Male patient, aged 49, white, mason, from the municipality of Auriflama, State of São Paulo, Brazil. The patient underwent follow-up in the rheumatology department at the Base Hospital of São José do Rio Preto, diagnosed with rheumatoid arthritis 15 years ago, refractory to conventional treatments. Therapy was initiated using infliximab and, after four doses of this anti-TNF a biological agent, with a total dose of 1600mg, during the physical exam, erythematous plaques were discovered in the dorsal region and the left forearm, with respective diameters of 8 and 5cm; and erythematous violaceous plaques in the left thigh, with diameters of 4 x 6cm, and diminished sensitivity in these lesions (Figures 1 and 2). The patient experienced increases in the number and diameter of the erythematous violaceous plaques in the midsection and limbs. The patient presented positive family epidemiology for leprosy.
FIGURE 1.
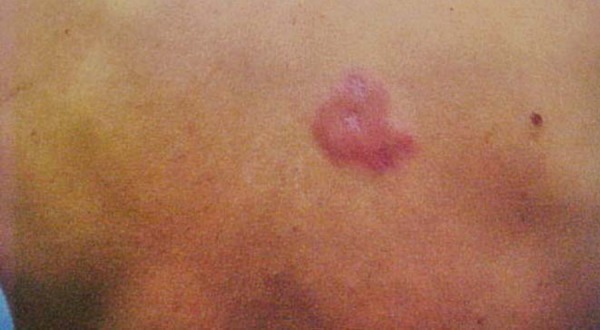
Erythematous lesion in the dorsal region
FIGURE 2.
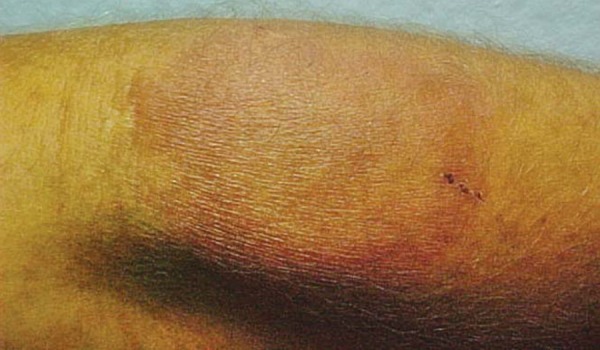
Erythematous violaceous plaque in left thigh
The biopsy of the cutaneous lesions on the back and left thigh revealed borderline leprosy, with a tendency toward the lepromatous pole, with whole, resistant alcohol-acid resistant bacilli (particularly in nerves), a lymph smear (AARB) and positive Mitsuda test, with a diameter of 11mm (Figure 3). The clinical classification, following the result of the Mitsuda test, was reactional tuberculoid leprosy and the patient developed a clinical, downgrading reverse reaction, similar anatomopathologically to dimorphous leprosy. The anti-TNF therapy was interrupted and a specific treatment for leprosy was started with multibacillary polychemotherapy (PCT/MB/WHO), presenting significant improvement in the clinical leprosy.
FIGURE 3.
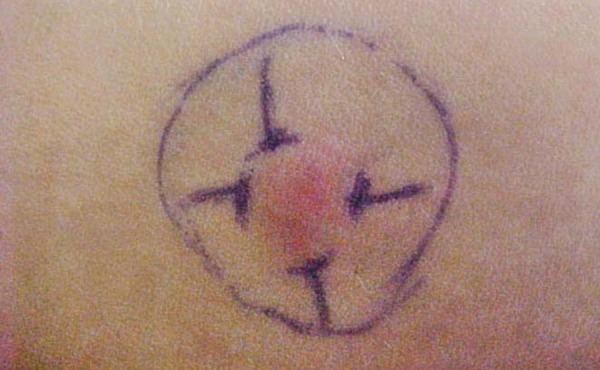
Mitsuda test: 11mm
DISCUSSION
Leprosy is a granulomatous, chronic, infectious disease of the skin and peripheral nerves caused by Mycobacterium leprae, and is transmitted through contact with contagious carriers on the part of susceptible individuals. In Brazil, the disease is endemic.4,7
Infliximab, an anti-TNF biological agent, is indicated for patients with rheumatoid arthritis, in order to reduce signs and symptoms, prevent structural joint damage and promote improvement of physical capacities in individuals with the active form of the disease, which does not respond to conventional therapeutic options.5
Since TNF-α is a modulator of cell immune response, there is a risk of opportunist infections through suppression of endogenous TNF-a, an important cytokine for the formation of granulomas. Thus, the risk of latent tuberculosis and other infections needs to be evaluated, along with that of leprosy, before beginning treatment.2,3,6,8
The patient in question underwent four applications of this biological agent, with a total dose of 1600mg. He was diagnosed with leprosy and, taking into account the symptoms, anatomopathology and immunology (Mitsuda 11mm), the specific diagnosis was downgrading hansenic reverse reaction tuberculoid leprosy (positive AARB in the biopsy with dimorphous characteristics), which occurred due to immunosuppression with infliximab. In the literature, two cases have been described of patients who developed leprosy after taking anti-TNF-a drugs; two with infliximab and two with adalimumab.4,6,8
Recently, the introduction of biological agents as an alternative to conventional treatments for autoimmune diseases has brought about positive therapeutic responses, with improvements in quality of life and the psychological wellbeing of patients in treatment. However, the development of opportunist infections or reactivation of latent granulamatous infections, such as leprosy and tuberculosis, highlight the need for careful indication of treatment.
This study demonstrates the possibility of leprosy developing in patients using infliximab, and recommends systematized investigation into this pathology through epidemiological research, neurocutaneous exams and, if necessary, lymph smear testing following the protocol for the indication of biological agents, especially in endemic countries.
Footnotes
* Study carried out at the Outpatient Service of the Dermatology Department of the Base Hospital at the State Medical School of São José do Rio Preto, São Paulo.
Financial support: None.
Conflict of interests: None.
REFERENCES
- 1.Consenso 2012 da Sociedade Brasileira de Reumatologia para o tratamento da artrite reumatoide. Rev Bras Reumatol. 2012;52:152–174. [Google Scholar]
- 2.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38:1261–1265. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 3.Winthrop KL. Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat Clin Pract Rheumatol. 2006;2:602–610. doi: 10.1038/ncprheum0336. [DOI] [PubMed] [Google Scholar]
- 4.Freitas DS, Machado N, Andrigueti FV, Reis ET, Neto, Pinheiro MM. Hanseníase virchowiana associada ao uso de inibidor do fator de necrose tumoral alfa. Rev Bras Reumatol. 2010;50:333–339. [PubMed] [Google Scholar]
- 5.Faleiro LR, Araújo LHR, Varavallo MA. A Terapia Anti-TNF-α na Artrite Reumatóide. Semina Cienc Biol Saude. 2011;32:77–94. [Google Scholar]
- 6.Oberstein EM, Kromo O, Tozman EC. Type I reaction of Hansen's disease with exposure to Adalimumabe: A case report. Arthritis Rheum. 2008;59:1040–1043. doi: 10.1002/art.23815. [DOI] [PubMed] [Google Scholar]
- 7.Munk ME, Anding P, Schettini AP, Cunha MG, Kaufmann SH. Soluble tumor necrosis factor alpha receptors in Sera from Leprosy patients. Infect Immun. 1999;67:423–425. doi: 10.1128/iai.67.1.423-425.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scollard DM, Joyce MP, Gillis TP. Development of Leprosy and type 1 reactions after treatment with Infliximab: A report of 2 cases. Clin Infect Dis. 2006;43:e19–e22. doi: 10.1086/505222. [DOI] [PubMed] [Google Scholar]


