Abstract
Allosteric modulation of AMPA, NR2B, mGlu2, mGlu5 and M1, targeting glutamatergic dysfunction, represents a significant area of research for the treatment of schizophrenia. Of these targets, clinical promise has been demonstrated using muscarinic activators for the treatment of Alzheimer’s disease (AD) and schizophrenia. These diseases have inspired researchers to determine the effects of modulating cholinergic transmission in the forebrain, which is primarily regulated by one of five subtypes of muscarinic acetylcholine receptor (mAChR), a subfamily of G-protein-coupled receptors (GPCRs). Of these five subtypes, M1 is highly expressed in brain regions responsible for learning, cognition and memory. Xanomeline, an orthosteric muscarinic agonist with modest selectivity, was one of the first compounds that displayed improvements in behavioral disturbances in AD patients and efficacy in schizophrenics. Since these initial clinical results, many scientists, including those in our laboratories, have strived to elucidate the role of M1 with compounds that display improved selectivity for this receptor by targeting allosteric modes of receptor activation. A survey of selected compounds in this area will be presented.
Introduction
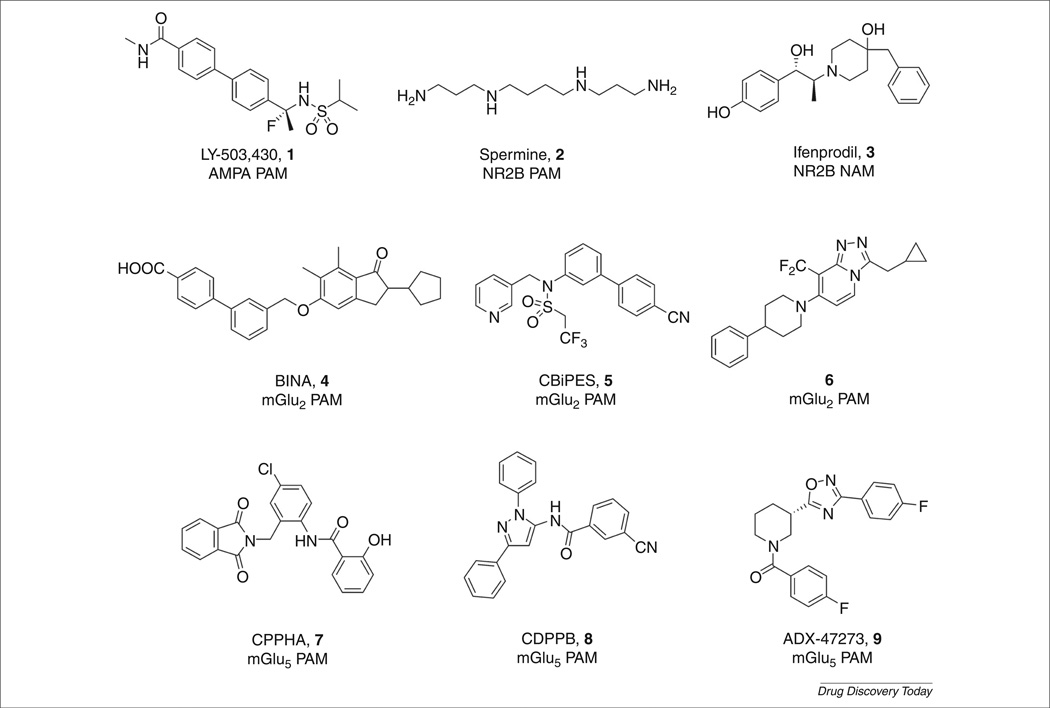
Schizophrenia is a devastating psychiatric illness that afflicts approximately 1% of the population and presents with three classical symptom clusters: positive symptoms, negative symptoms and cognitive impairments [1,2]. Cognitive (including deficits in attention, memory and executive function) and negative (social withdrawal, anhedonia and apathy) deficits that precede the first psychotic episode (delusions, hallucinations and thought disorders) are not effectively treated by current antipsychotic drugs, and account for the lifelong disability and poor outcomes associated with schizophrenia [2–5]. A host of data suggests that dysfunction in glutamatergic synaptic transmission in frontal cortical networks underlies the complex symptom clusters of schizophrenia, as opposed to altered neuro-transmitter levels [4–7]. These findings have led researchers to evaluate multiple pre- and post-synaptic mechanisms affecting glutamatergic synaptic transmission, which has elucidated a number of discrete molecular targets for therapeutic intervention. For many of these targets (AMPA, NR2B, mGlu2 and mGlu5), the development of orthosteric ligands has proven extremely difficult, from a chemical, pharmacological or safety perspective; however, targeting allosteric sites of these targets has emerged as a promising alternative [8–12]. Indeed, many recent manuscripts and reviews have detailed the virtues of allosteric modulation [10–15],and many valuable allosteric modulator tool compounds have been developed (Fig. 1, compounds 1–9) to enable key preclinical proof-of-concept studies. Because these have been extensively reviewed recently, we will focus this review on a large body of new data on the M1 muscarinic acetylcholine receptor (mAChR) and its link to the cholinergic and N-methyl-d-aspartate (NMDA) hypofunction hypotheses of schizophrenia, as well as its link to Alzheimer’s disease (AD).
FIGURE 1.
Representative allosteric modulators of AMPA, NR2B, mGlu2 and mGlu5 that address dysfunction in glutamatergic neurotransmission in cortical brain regions.
A cholinergic hypothesis of schizophrenia
The mAChRs are G-protein-coupled receptors (GPCRs) for the neurotransmitter acetylcholine (ACh) and consist of five different subtypes, termed M1–M5. These subtypes are further grouped based on their coupling to signal transduction pathways [16–18]. When stimulated by ACh, M1, M3 and M5 induce release of intracellular calcium stores through the activation of phospholipase C through Gαq. M2 and M4 couple to Gαi/o to regulate adenylyl cyclase and many ion channels (Fig. 2) [19]. Numerous preclinical and clinical studies with nonselective mAChR agonists suggest that activation of mAChRs improves cognitive function in patients suffering from various central nervous system (CNS) disorders, and these studies, along with genetic studies, indicate that M1 is the mAChR subtype mediating the procognitive effects [20,21]. Furthermore, agents that enhance cholinergic transmission, including acetylcholinesterase (AChE) inhibitors, have established efficacy in improving cognitive function in patients suffering from AD and other memory disorders [22,23]. Over 50 years ago, nonselective muscarinic antagonists, such as scopolamine, were shown to induce many of the symptoms associated with schizophrenia in healthy humans and exacerbate existing symptoms in schizophrenia patients. During this time, muscarinic agonists were shown to be moderately effective as neuroleptic agents, which gave rise to a cholinergic hypothesis of schizophrenia, decades before the now prevalent dopamine hyperfunction hypothesis. A large body of clinical, preclinical, postmortem, genetic and brain-imaging studies provides strong support for involvement of the cholinergic system in the pathophysiology of schizophrenia. Receptor protein and mRNA levels of M1 have been shown to be decreased in frontal cortex of schizophrenic patients, which led to the characterization of a subpopulation of schizophrenic patients referred to as muscarinic receptor deficient schizophrenics (MRDS) [24]. In addition, circulating antibodies against M1 have been found in the serum of schizophrenics, suggesting a link between the immune system and M1 in schizophrenics. Interestingly, it is unclear if the antipsychotic efficacy of mAChR activation is caused by direct muscarinic effects alone, or through modulatory effects on the dopaminergic system and other neurotransmitter systems.
FIGURE 2.
Schematic illustration of the muscarinic acetylcholine receptor subtypes M1–M5. The seven transmembrane domains of the family A G-protein-coupled receptors (GPCRs) are highlighted in blue. The orthosteric binding site is indicated in red. A putative allosteric site is illustrated in green and not indicative of a singular or unique site. The downstream effectors indicate M1 signaling related to the (a) amyloidogenic precursor protein (APP) and subsequent secretase activity to generate soluble amyloidogenic precursor protein alpha (sAPPα), soluble amyloidogenic precursor protein beta (sAPPβ) and Aβ. (b) NMDAR through the CAKβ and SFK pathway. Abbreviations: AC, adenylyl cyclase; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; ERK1/2, extracellular signal-regulated kinase; IP3, inositol triphosphate; PKC, protein kinase C; PLC, phospholipase C, CAKβ, cell adhesion kinase β; SFK, Src family kinases.
M1 activation and the NMDA receptor hypofunction hypothesis of schizophrenia
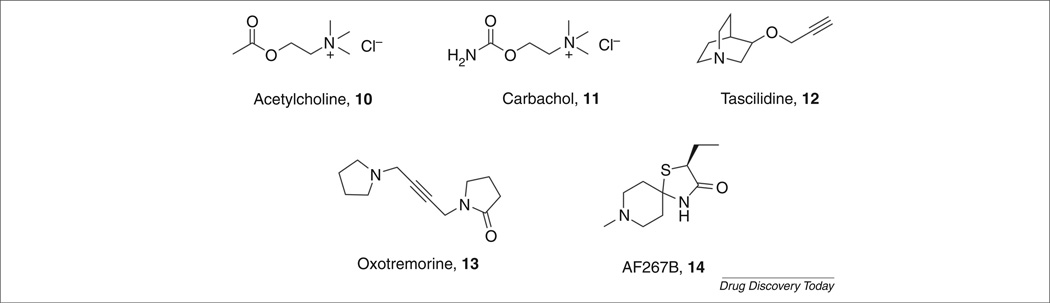
NMDA receptors have an important role in the regulation of circuits that are crucial for normal cognitive and executive functions and that are disrupted in schizophrenia and other psychotic disorders [25]. Competitive and noncompetitive antagonists of the NMDA receptor, such as ketamine and phencyclidine (PCP), can induce a psychotic state that closely resembles that seen in schizophrenic patients [26–28]. Furthermore, co-agonists at the NMDA receptor, such as glycine and d-cycloserine, produce improvements in the symptoms of schizophrenic patients and the glycine transporter 1 (GlyT1) inhibitor RG-1678 recently provided robust efficacy against negative symptoms in schizophrenia patients [29]. Thus, a large number of clinical and animal studies have led to the hypothesis that potentiation of NMDA receptor neurotransmission might help to normalize the imbalances in neural circuitry associated with schizophrenia and provide antipsychotic action and improvements for the negative and cognitive symptoms [30,31]. One of the most prominent effects of M1 activation in the hippocampus and other forebrain regions is the potentiation of NMDA receptor currents [31]. Many studies have also confirmed that M1 is colocalized with the NR1 subunit of the NMDA receptor in CA1 pyramidal cells in the hippocampus and other cortical regions. Therefore, it is proposed that M1-induced potentiation of NMDA receptor function could play an important part in the therapeutic efficacy of mAChR activation in psychotic disorders [31]. Additional strong support comes from N-desmethylclozapine (NDMC) [32], which is an M1 allosteric agonist that potentiates NMDA receptor currents in CA1 pyramidal cells in the hippocampus, and further supports the view that selective activation of M1 by allosteric agonism or potentiation compliments the ‘dopamine hyperfunction hypothesis’ and the ‘NMDA receptor hypofunction hypothesis’ of schizophrenia [32,33]. In addition, atypical antipsychotics and muscarinic agonists are efficacious in behavioral models where deficits have been induced by dopamine agonists and NMDA receptor antagonists [34–36]. The development of novel therapeutic agents for schizophrenia that induce selective M1 activation offers new hope to patients; to address the cognitive, negative and positive symptom clusters while complementing existing treatment strategies. Although muscarinic receptors are expressed throughout the body, M1 has attracted much attention owing to its expression levels in the brain, in particular its localization in the cortex, striatum and hippocampus, implicating this receptor in the regulation of signals that deal with cognition, movement and memory. For this reason, drug discovery efforts have tried to fulfill the need for a truly selective cohort of muscarinic modulators (Fig. 3, examples of muscarinic agonists 10– 14). However, the high sequence homology of the orthosteric site among this family of receptors has proven a difficult hurdle to overcome. As mentioned previously, targeting allosteric sites has rejuvenated the field, providing highly subtype-selective tools and a novel approach to modulate M1 with the promise to address multiple symptom clusters of schizophrenia.
FIGURE 3.
Orthosteric agonists of the M1 mAChR.
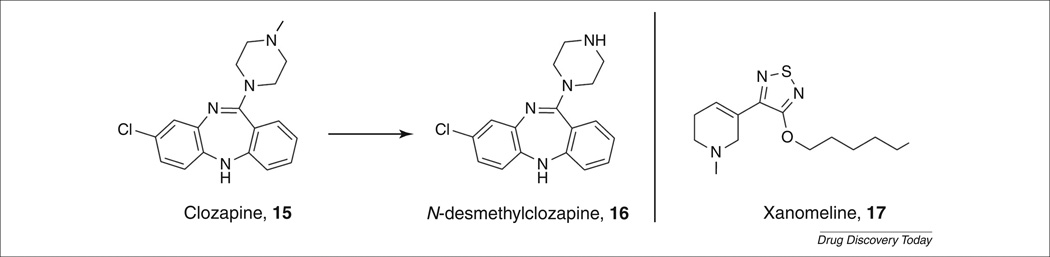
Clozapine and NDMC
Support for the cholinergic hypothesis of schizophrenia can be found in the clinical evidence generated from the atypical antipsychotic agent clozapine. Clozapine (Fig. 4, 15)is very effective as an antipsychotic treatment but, as a result of its side effects, is typically a drug of last resort for schizophrenics that do not respond to other treatments (see: http://www.nimh.nih.gov/health/publications/mental-health-medications/what-medications-are-used-to-treat-schizophrenia.shtml what-medicat). The efficacy of clozapine is partially attributed to its major metabolite, NDMC (Fig. 4, 16), which is an M1 allosteric agonist (EC50 = 115 nm), as well as the classical D2 antagonism of the parent [32,33]. It is probable that this combination of activity sets clozapine apart as a clinically effective treatment for schizophrenics through modulation of glutamatergic and muscarinic neurotransmission.
FIGURE 4.
M1 agonists for the treatment of schizophrenia.
Xanomeline
In 1997, Bodick et al. reported on the results of a large-scale clinical trial for the effect of xanomeline (Fig. 4, 17) on cognitive impairments and behavioral disturbances in AD patients [37]. In this study, the purported M1/M4-preferring muscarinic agonist xanomeline improved cognitive performance and also had robust therapeutic effects on psychotic symptoms and behavioral disturbances associated with AD, such as delusions, vocal outbursts and hallucinations. These improvements in psychotic symptoms prompted scientists at Lilly to evaluate xanomeline in a small clinical trial for its effects on schizophrenia [38,39].
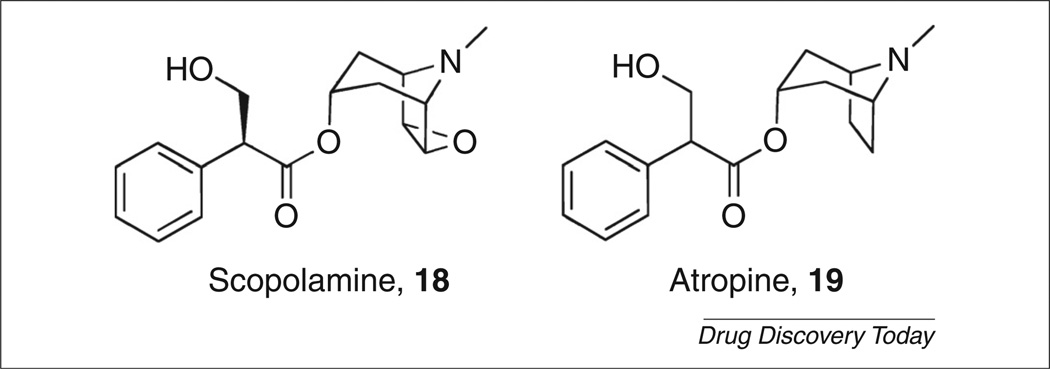
Typical and atypical antipsychotics work to increase dopamine release in the prefrontal cortex through induction of c-Fos expression. Xanomeline showed similar effects to normal antipsychotic agents olanzapine and clozapine by inducing c-Fos expression in the same brain regions and increased dopamine levels as well [40]. The effects of xanomeline further supported a novel mechanism for the treatment of psychosis and, indeed, the effect of xanomeline could be blocked by a muscarinic antagonist, scopolamine (Fig. 5, 18) [41]. In 2008, the Phase II clinical trial results for schizophrenia patients receiving xanomeline showed significant improvements in the Brief Psychiatric Rating Scale (BPRS) and the Positive and Negative Syndrome Scale (PANSS) over the placebo group scores [38]. There were robust cognitive improvements as well, with the same patients showing marked improvements in short-term memory function and vocal learning measurements.
FIGURE 5.
Nonselective muscarinic antagonists.
Despite the encouraging results, xanomeline is still marred by dose-limiting side effects. In animal models and Phase II and III clinical trials the subjects experienced moderate to severe gastrointestinal (GI) distress, salivation and sweating [42,43], which are attributed to the off-target activity of xanomeline at other mAChRs, specifically M2 and M3. It is not surprising that many researchers have endeavored to provide the scientific community with an array of selective muscarinic tools to probe the palliative and potentially disease-modifying effects of M1 activation.
M1 and the potential for disease-modifying efficacy in AD
AD is one of the most prevalent neurodegenerative disorders affecting over 26 million people worldwide (data recorded in 2006). It is a disease that predominantly affects the elderly (individuals over 65) resulting in cognitive dysfunction and severe memory loss [44]. Possessing a confounding etiology, AD is characterized by the formation of two types of brain structures: neurofibrillary tangles (from hyperphosphorylated τ proteins) and amyloid plaques (aggregated amyloid-β (Aβ) peptide) [45,46]. The hallmark of the progression of AD is the formation of amyloid plaques through Aβ accumulation, which has led to the investigation of many potential therapies that inhibit the formation of Aβ peptides (Fig. 2; and see: http://www.alz.org).
It has been postulated that increasing M1 receptor activity could not only provide symptomatic relief but also have disease-modifying outcomes in AD patients by influencing the processing of amyloid precursor protein (APP) [47–49]. APP is known to undergo proteolytic cleavage in two competing pathways: amyloidogenic and nonamyloidogenic [50]. In the amyloidogenic pathway, sequential cleavage of APP by β-secretase and γ-secretase releases the Aβ peptide, the core of amyloid plaques and source of neurotoxicity. In the nonamyloidogenic pathway, APP is cleaved by α-secretase, preventing Aβ peptide generation and forming soluble amyloid precursor protein alpha (sAPPα) [50]. Evidence suggests that activation of M1 can shunt APP processing through the nonamyloidogenic pathway, producing sAPPα, and deterring the formation of Aβ peptides and, ultimately, slowing the progression of AD [51]. Efforts to elucidate the enzymes responsible for formation of Aβ have revealed β-site APP-cleaving enzyme 1 (BACE1) to be the β-secretase responsible. Studies have conclusively shown that M1 interacts with BACE1 to regulate its proteosomal degradation and activation of M1 lowers Aβ levels in vitro. M1 activation was also shown to increase sAPPα formation in vitro thereby preventing the formation of Aβ via MAPK-and PKC-dependent pathways [48]. In addition, M1 activation decreases τ phosphorylation; therefore, M1 activation affects the major pathological hallmarks of AD [52]. These results were corroborated in vivo with the M1-positive allosteric modulator (PAM) BQCA [51,53–55]. Transgenic Tg2576 mice, which overexpress a familial AD mutant form of the APP, are impaired on compound discrimination reversal learning [56,57]. Treatment of Tg2576 mice with BQCA reverses impairment in compound discrimination and compound discrimination reversal models [51].
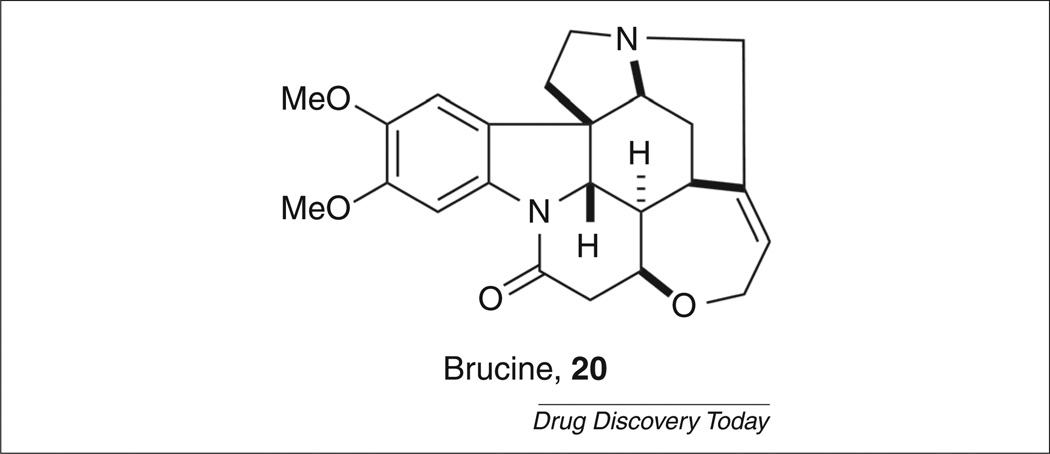
Brucine
Given the potential of M1 as a therapeutic target for the treatment of a variety of disease states, substantial effort has been dedicated to the elucidation of selective allosteric agonists of the M1 receptor. In 1998, Lazareno et al. reported that the natural product brucine (Fig. 6, 20) was a PAM selective for M1 over the other muscarinic subtypes [58]. Although brucine only weakly potentiated ACh at micromolar concentrations (<twofold increase in potency), it did establish that allosteric activation of the mAChRs was a valid strategy for obtaining subtype selectivity.
FIGURE 6.
Brucine, an M1-selective positive allosteric modulator.
AC-42
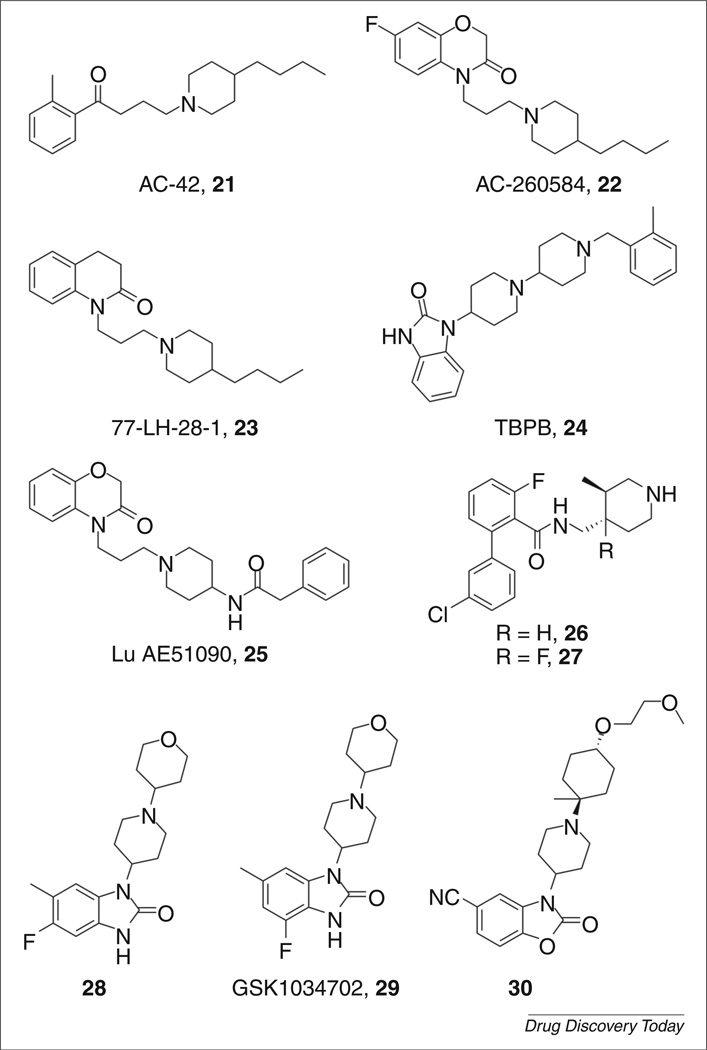
In a seminal advancement, Spalding et al. disclosed AC-42 (Fig. 7, 21) as the first allosteric agonist selective for M1 [59]. AC-42 was shown to stimulate calcium mobilization and inositol monophosphate (IP) accumulation in recombinant hM1 cell lines. It possessed an excellent selectivity profile (>400-fold) for M1 over the M2–M5 receptors. Using a series of chimeric receptors, AC-42 was shown to bind to an allosteric site at transmembrane (TM) domains one and seven, which is distinct from the orthosteric binding site (TM domains three, five and six). However, when it was evaluated in native tissues, AC-42 failed to elicit a response in single-unit cell firing of the CA1 region in the rat hippocampus. Additionally, AC-42 binds to D2 dopamine and 5HT2B biogenic amine receptors, making it a less than ideal tool for studying the role of M1 in diseases where other biogenic amine receptors might also play a part (i.e. schizophrenia and the dopamine D2 receptor) [60]. After AC-42 verified that mAChR subtype selectivity could be achieved with allosteric agonists, a number of second-generation agonists were reported in the literature including AC-260584 (22) [61], 77-LH-28-1 (23) [62] and TBPB (24) [63,64] (Fig. 7).
FIGURE 7.
Selective allosteric agonists of the M1 mAChR.
77-LH-28-1
GSK subsequently reported 77-LH-28-1 (23), a close structural analog of AC-42, as an allosteric agonist of M1 with an improved pharmacological profile [62]. In a calcium mobilization assay, 77-LH-28-1 was found to have EC50=8nM at M1. Although approximately an order of magnitude more potent than AC-42 at M1, subtype selectivity was somewhat eroded (M2 EC50 = 760nm, M3 EC50 = 159nm and M5 EC50 = 206nm). Similar to AC-42, 77-LH-28-1 also displayed activation of the human dopamine D2 and 5HT2B receptors. Pharmacokinetic studies showed that 77-LH-28-1 was rapidly metabolized (Tmax = 15 min); however, 23 did penetrate the CNS with a B:P of ~4. In a key advance over AC-42, 77-LH-28-1 was able to show efficacy in native rat tissues rather than transfected cell lines. It also stimulated single-unit firing and initiated network oscillations in rat hippocampal CA1 cells, as did the orthosteric agonist carbachol (CCh, 11; Fig. 3).
TBPB
In 2008, Jones et al. reported on 1-(1′-(2-methylbenzyl)-[1,4′-bipi-peridin]-4-yl)-1H–benzo[d]imidazol-2-(3H)-one (TBPB, 24; Fig. 7) as one of the first novel allosteric agonists of the M1 mAChR [63,64]. TBPB emerged as an unoptimized HTS hit with EC50 = 289 nm and displayed an effect at 82% of a maximal CCh response. Through an M1 receptor Y381A mutation, it was shown that TBPB behaved as an allosteric agonist at the M1 receptor [Y381A mutation robustly right-shifted a CCh response when compared to wild type (WT) rM1; TBPB EC50 = 220 nm in WT rM1; EC50 = 97 nm in Y381A rM1] [63]. TBPB was analyzed for its ability to shift APP processing to the nonamyloidogenic pathway and, indeed, was shown to increase the production of sAPPα. Treatment of PC12 cells with 1 µm TBPB increased sAPPα release by 58% compared to vehicle. Analysis of conditioned media from these cells for Aβ40 levels indicated a 61% decrease compared to vehicle control. Both of these effects could be blocked by atropine (Fig. 5, 19), a nonselective muscarinic antagonist. These results are consistent with the hypothesis that selective activation of M1 can regulate APP processing and increase sAPPα formation.
Unfortunately, more results from in vitro experiments precluded the advance of TBPB as a lead compound. TBPB showed appreciable levels of D2 antagonism (IC50 = 2.6 µm), which compromised its utility as a novel antipsychotic. Also, TBPB robustly antagonized an ACh EC80 response at M2–M5 [65]. These results demonstrated that TBPB possesses a two-site binding profile (two-site binding: a ligand that binds to a high-affinity allosteric site at low concentrations and binds to a low-affinity orthosteric site at higher concentrations). Although this antagonist activity was only seen at higher concentrations than required for agonist activity at M1, these data revealed that TBPB was not an M1-selective ligand. SAR studies were undertaken to determine if TBPB could be further optimized to remove the D2 and M2–M5 antagonism in this series. After several hundred compounds were synthesized and tested, the original screening hit could not be improved. All efforts led to a decrease in M1 efficacy or increases or decreases in M2–M5 agonism or antagonism, D2 antagonism or completely inactive compounds [49,66,67]. This ultimately led to the discontinuation of TBPB as a lead candidate for M1 activation.
Lu AE51090
Following the disclosure of TBPB, Lundbeck reported a related allosteric agonist: Lu AE51090 [68] (Fig. 7, 25). Beginning from an HTS campaign that identified two hits possessing EC50s of 13 and 130 nm at hM1, parallel synthesis was used to probe the SAR around the eastern and western amide regions, with Lu AE51090 emerging as the lead compound. Lu AE51090 provided high selectivity for hM1; no activity was observed for hM2–hM5. Screening against a panel of 69 GPCRs identified the adrenergic α1A receptor as the only major off-target liability. Its DMPK properties were evaluated, and Lu AE51090 was found to possess a good free fraction (fu) in rat and human plasma and to have moderate CNS exposure with a B:P ratio of 0.20 in rat. Compound 25 suffered from high clearance and low oral bioavailability; however, it exhibited efficacy in a dose-dependent manner in an in vivo model of working memory (delayed alternation Y-maze in mice).
GlaxoSmithKline muscarinic agonists
GlaxoSmithKline has disclosed two separate series of M1-selective allosteric agonists arising from screening of their corporate libraries [69–71]. Rescreening compounds originally designed for their M3 antagonist program against M1 yielded an initial hit that displayed M1 selectivity, albeit with EC50 = 250 nm. Iterative library synthesis yielded compounds 26 and 27 (Fig. 7), where potencies were improved to EC50 = 0.8 nm and 10 nm, respectively. These compounds were found to be pan-antagonists of M2–M5 (predominant interactions: 26 M3 IC50 = 40 nm; 27 M2 IC50 = 2.5µm). However, 26 exhibited clean ancillary pharmacology in a CEREP panel. Compounds 26 and 27 were found to have good oral bioavailability in rat, possessed a t1/2 of 2.3 hours and 3.0 hours, respectively, and penetrated the CNS with B:P ratios of 0.9 and 0.3, respectively.
A second series was reported arising from a virtual screen of their corporate compound library against the pharmacophore of AC-42. Initial optimization efforts led to the discovery of compound 28, which, although bearing significant structural similarity to TBPB,was found to exhibit divergent muscarinic activity. Compound 28 was found to be a weak agonist at M2–M5 receptors, in addition to κ-opiod receptor binding in the CEREP selectivity panel [72]. Compound 28 was evaluated in native tissues and found to increase the firing rate of hippocampal CA1 cells. Additionally, the compound displayed in vivo efficacy in a dose-dependent reversal of scopolamine-induced amnesia in a rat model. However, compound 28 was found to have limited exposure to the CNS, indicating the possible role of efflux transporters. GSK1034702 (Fig. 7, 29), which emerged from this series, is currently in clinical trials as a positron emission tomography (PET) tracer and has been shown to improve episodic memory in humans in the nicotine abstinence model of cognitive dysfunction [73,74]. Subsequent lead optimization endeavors in this series identified compounds exemplified by 30 [71] (Fig. 7). These compounds maintained selectivity for M1, although weak panagonism of M2–M5 was observed. Compound 30 was also found to be effective in a novel object recognition (NOR) model of cognition in rats.
VU0357017 and VU0364572
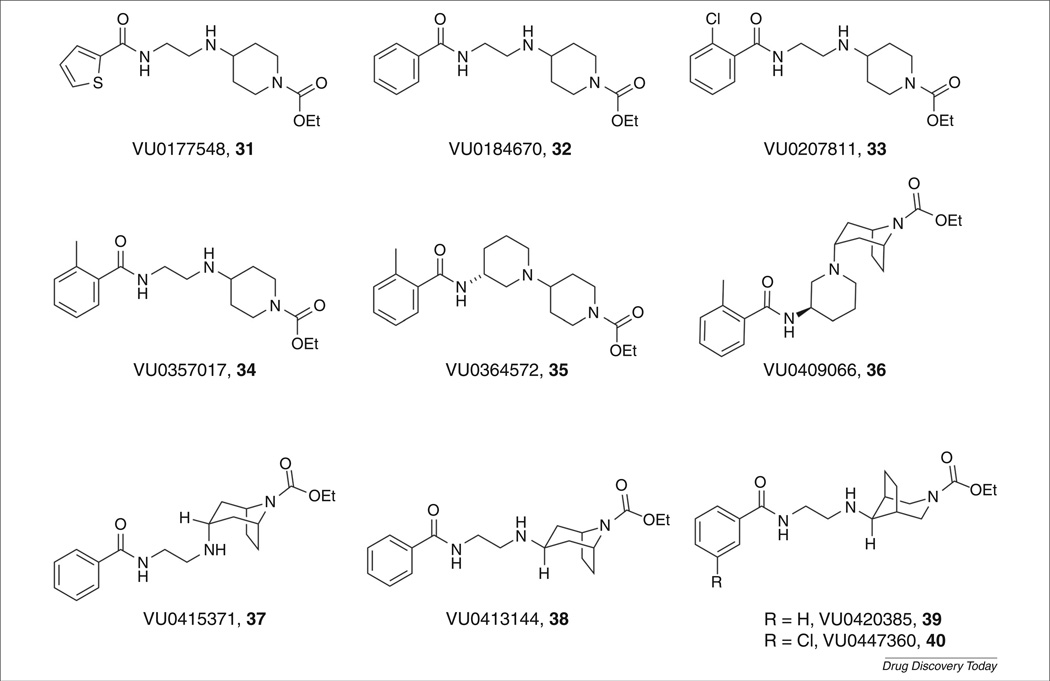
In an effort to expand the diversity of novel M1 activators, our laboratories initiated work on compounds such as VU0177548 (31) and VU0184670 (32) [10,65,75,76] (Fig. 8). Beginning from a HTS of the 65,000 member MLPCN library, two hits: VU0177548 (31) and VU0207811 (33), were identified as allosteric agonists selective for M1 over M2–M5. Employing an iterative parallel synthesis screening approach, optimization of the HTS hits was met with steep SAR. Changes to the western amide, introduction of fluorine onto the piperidine ring, amine or amide alkylation, alternative chain lengths and introduction of basic heterocycles on the western amide abolished M1 activity. However, subtle changes to the aryl amide were tolerated, and 32 and VU0357017 (34) were identified as lead compounds with M1 agonist activity (EC50 = 152 nm and 198 nm, respectively, in a high-expressing rat M1 CHO cell line) [65]. Compounds 32 and 34 maintained selectivity at M2–M5 (EC50 > 30 µm). A putative allosteric binding site, located on extracellular loop three, was identified via site-directed mutagenesis studies. Schild analysis through treatment with 19 and competition binding experiments with [3H]-N-methylscopolamine led to 34 being initially identified as an allosteric agonist. In addition to 34 displaying selectivity for M1 over the other muscarinic subtypes, it also exhibited selectivity in a panel of 68 GPCRs (exhibited binding selectivity <50% radioligand displacement at 10 µm), including biogenic amine receptors. However, it did show weak functional D2 antagonism (IC50 = 4.5µm). Finally, 34 possessed a desirable drug metabolism and pharmacokinetic (DMPK) profile; it was CNS penetrant (B:P = 4:1), low to moderately cleared (Clobs = 13.8 mL/min/kg; t1/2 = 1.1 hours), orally bioavailable and, as a result of its high aqueous solubility, it could be dosed in saline.
FIGURE 8.
M1-selective allosteric agonists from the Vanderbilt Center for Neuroscience Drug Discovery.
Further optimization efforts explored introducing cyclic constraints into the ethyl linker of 34. Again, steep SAR was encountered; however, replacement of the ethyl linker with a three-amino piperidine resulted in a novel class of M1 agonists, with the lead structure VU0364572 (35) [77] (Fig. 8). Evaluation of each enantiomer showed that the R-enantiomer was active (EC50 = 110 nm in high-expressing cell lines), whereas the S-enantiomer was inactive, illustrating the first example of enantioselective activation of M1. Compound 35 maintained selectivity for M2–M5 in a functional calcium mobilization assay, and was selective when screened against a panel of 68 GPCRs (<30% displacement at 10 µm). Furthermore, the functional D2 antagonism displayed by 34 was ablated in 35. Compound 35 was also found to have good free fraction, no cytochrome P450 (CYP450) inhibition, low to moderate clearance (Clobs = 14.5 mL/min/kg; t1/2 = 45 min), oral bioavailability and to be CNS penetrant (B:P = 1.5). Whereas calcium assays were originally conducted in high-expressing cell lines, tetracycline-inducible rat M1 and human M1 cell lines were developed with expression levels similar to native tissues to determine if selectivity was still present. In these cell lines, the EC50s of 34 and 35 were attenuated (EC50 = 16 µm and EC50 = 2.3 µm, respectively) [78]. This change in activity as a function of differing receptor reserve raised the possibility that 34 and 35 could act as full agonists or weak partial agonists.
Compounds 34 and 35 were tested for their ability to affect APP processing. A 2 µm concentration of 34 showed the same increase in sAPPα as a 10 µm CCh concentration in a stably expressed tetracycline repressor protein hM1 (TREx293-hM1) cell line. Compound 35 showed a robust increase in the formation of sAPPα, in this case a threefold increase (normalized to a 10 µm CCh response, in TREx293-hM1 cell line) [78]. Once again, these findings support the hypothesis that activation of M1 can regulate APP processing and increase sAPPα formation, and could therefore have a disease-modifying role in the treatment of AD.
Tropane modifications to VU0357017
Our continued efforts to introduce cyclic constraints to improve upon 32 led to the development of a potent series of tropane derivatives. Replacement of the eastern piperidine ring of 35 with a tropane scaffold resulted in VU0409066, a potent M1 agonist (Fig. 8, 36; hM1 EC50 = 59 nm) [79]. Similarly, replacement of the eastern piperidine of 32 with a tropane scaffold afforded the endo-(VU0415371, 37) and exo- (VU0413144, 38) isomers, which possessed an EC50 of 110 nm and 970 nm, respectively. Flat SAR was again encountered in the exo-tropane series, with many different amide modifications showing little to no improvement in potency relative to 38. Moving the bicyclic bridge of the tropane scaffold afforded compounds VU0420385 (39) and VU0447360 (40), representing the most potent agonists in this system (EC50 = 92 nm and 47 nm, respectively). This novel series of M1 agonists was also tested for ability to enhance the release of sAPPα processing. In all cases, these compounds (36–40 at 2 µm) stimulated the release of sAPPα to the same extent as a 10 µm dose of CCh (TREx293-hM1 cell line). These experiments continue to support the belief that activation of M1 is potentially a disease-modifying treatment of AD.
Although much effort was spent to determine tractable SAR, the tropane replacements in this series possessed some blemishes. Compound 36 was analyzed for its PK properties and was found to have high clearance (Clobs = 189 mL/min/kg; Vss = 11.8 l/kg, t1/2 = 46 min) and good oral bioavailability (%F = 70). Unfortunately, 36 had a poor selectivity profile, lacking in muscarinic subtype selectivity (hM2 EC50 = 1.8 µm; hM5 EC50 = 3.7 µm; weak agonist at hM3 and hM4) [79]. Other structural modifications did not improve their muscarinic selectivity profile, exemplified by 37. This compound displayed antagonist activity at the other muscarinic subtypes (hM2–hM5 weak to mid micromolar antagonists; Table 1) reminiscent of the ancillary pharmacology associated with TBPB. This lack of selectivity in this series raised concerns that compounds with higher potency would engender poorer selectivity across the other subtypes. Initially, changes to the bridgehead location to deliver alternative tropanes 39 and 40 were promising. Both compounds displayed improved hM1 potencies; however, the off-target activity was mixed. Compound 39 was a weak antagonist at hM3 and hM4 but showed partial agonist activity at hM5 (hM5 EC50 = 2.8 µm). Similarly, 40 was a weak antagonist at hM4 but showed partial agonist activity at hM5 (hM5 EC50 = 4.2 µm).
TABLE 1.
Summary of muscarinic acetylcholine receptor (mAChR) ligands reported to be selective for M1
| Name | Institution year |
M1 | M2–M5 | Ancillary | DMPK | In vitro | In vivo |
|---|---|---|---|---|---|---|---|
| AC-42 (21)a | Acadia, 2002 | 320 nm | M5 EC50 = 6.93 µm | hD2Ki = 20 nm, 5HT2BKi = 450 nm |
Data not available | No effects in native tissue |
Data not available |
| AC-260584 (22)a | Acadia, 2008 | 41 nm | M2 IC50 >10 µm, M3 EC50 = 5.9 µm, M4 IC50 >10 µm, M5 EC50 = 1.0 µm |
hD2Ki = 50 nm, 5HT2BKi = 1.59 µm |
High clearance, 26% oral bioavailability |
Promotes Gαq-mediated signaling, reduced activation of arrestin signaling |
Increases ERK1/2 phosphorylation in mouse hippocampus; improvement in NOR mouse model |
| 77-LH-28-1 (23)a | GSK, 2008 | 8nM | M2 EC50 >765 nm, M3 EC50 = 159 nm, M4 EC50 >10 µm, M5 EC50 = 206 nm |
hD2Ki = 60 nm, 5HT2BKi = 950 nm |
B:P = 4, rapidly cleared, subcutaneous admin. optimal |
Stimulate CA1 cell firing and gamma frequency oscillations in rat hippocampus |
Enhances NMDA-mediated neuronal excitation in hippocampus |
| TBPB (24) | Merck, Vanderbilt, 2006 |
289 nm | M2 IC50 = 1.1 µm, M3 IC50 = 3.0 µm, M4 IC50 = 415 nm, M5 IC50 = 10 µm |
hD2 IC50 = 2.6 µm | Data not available | Promotes Gαq-mediated signaling, reduced activation of arrestin signaling, enhances sAPPα, potentiates NMDA receptor currents |
Reverses amphetamine hyperlocomotion in rat model |
| VU0357017 (34) | Vanderbilt, 2008 |
198 nm (16 µm)b |
M2–M5 >30 µm | <50% 68 GPCRs, functional D2 antagonist |
Low-moderate clearance, CNS penetrant, dosable in saline |
Stimulates Gαq-signaling, little effect on arrestin signaling; efficacious in hippocampus, little effect in other brain regions |
Effective in contextual fear conditioning model; inactive in spatial memory and AHL reversal |
| VU0364572 (35) | Vanderbilt, 2011 |
110 nm
(2.3 µm)b |
M2–M5 >30 µm | <30% 68 GPCRs, no D2 antagonism |
Low-moderate clearance, CNS penetrant, oral bioavailable |
Stimulates Gαq-signaling, littleeffect on arrestin signaling;efficacious in hippocampus, little effect in other brain regions |
Effective in contextual fear conditioning and spatial memory; inactive in AHL reversal |
| VU0409066 (36) | Vanderbilt, 2012 |
59 nm | M2 EC50 = 1.8 µm, M3 EC50 >10 µm, M4 EC50 >10 µm, M5 EC50 = 3.7 µm |
Data not available | High clearance, t1/2 = 46 min, orally bioavailable |
Enhance sAPPα | Data not available |
| VU0415371 (37) | Vanderbilt, 2012 |
110 nm | M2 IC50 >10 µm, M3 IC50 >10 µm, M4 IC50 = 3.1 µm, M5 IC50 = 4.9 µm |
Data not available | Data not available | Enhance sAPPα | Data not available |
| VU0413144 (38) | Vanderbilt, 2012 |
970 nm | M2–M5 >30 µm | Data not available | Data not available | Enhance sAPPα | Data not available |
| VU0420385 (39) | Vanderbilt, 2012 |
92 nm | M3 IC50 >10 µm, M4 IC50 >10 µm, M5 EC50 = 2.8 µm |
Data not available | Data not available | Enhance sAPPα | Data not available |
| VU0447360 (40) | Vanderbilt, 2012 |
47 nm | M4 IC50 >10 µm, M5 EC50 = 4.2 µm |
Data not available | Data not available | Enhance sAPPα | Data not available |
| NDMC (16)a | 115 nm | M2 EC50 = 295 nm, M3 EC50 = 31 nm, M4 EC50 = 1.23 µm, M5 EC50 = 50 nm |
hD2Ki = 180 nm, 5HT2CKi = 5 nm, 5HT2BKi = 4 nm |
Data not available | Data not available | Data not available | |
| Lu AE51090 (25) | Lundbeck, 2010 | 61 nm | M2Ki = 2.2 µm, M3Ki = 7.0 µm, M4Ki = 6.9 µm, M5Ki = 8.9 µm |
hα1AKi = 260 nm, hα1BKi = 910 nm, hH1Ki = 780 nm |
High clearance, low oral bioavailability, moderate B:P |
Data not available | Observable dose-dependent improvement in learning and memory in mouse Y-maze task |
| 26 | GSK, 2010 | 0.8 nm | M2 IC50 = 200 nm, M3 IC50 = 40 nm, M4 IC50 = 158 nm, M5 IC50 = 500 nm |
No inhibition in CEREP panel |
Good brain exposure (AUC = 1655 ng h/g), t1/2 = 2.3 hours, B:P = 0.9 |
Data not available | Data not available |
| 27 | GSK, 2010 | 10 nm | M2 IC50 = 2.5 µm, M3 IC50 = 5.0 µm, M4 IC50 = 3.2 µm, M5 IC50 = 6.3 µm |
Not determined | Oral bioavailability (F = 57%), brain exposure (AUC = 2221 ng h/g), t1/2 = 3.0 hours, Cl = 35 mL/min/kg |
Data not available | Data not available |
| 28 | GSK, 2010 | 8nM | M2 EC50 = 630 nm, M3 EC50 = 2.5 nm, M4 EC50 = 500 nm, M5 EC50 = 790 nm |
hERG IC50 = 12 µm, κ-OR 53% inhibition at 1µm, hD2Ki = 264 nm, 5HT2CKi = 52 nm, 5HT2BKi = 12 nm |
Cli <0.7 ml/min/kg, oral bioavailability (F = 49%), B:P = 0.6 |
Data not available | Increases cell firing of hippocampal CA1 cells; dose-dependent reversal of scopolamine model |
| 30 | GSK, 2010 | 10 nm | M2 EC50 = 2.5 µm, M3 EC50 = 4.0 µm, M4 EC50 = 790 nm, M5 EC50 = 1.0 µm |
CEREP panel sigma-receptor (75% inhibition at 10 µm) |
Cl = 23 mL/min/kg, free concentration brain = 261 nm, free concentration blood = 265 nm |
Data not available | Dose-dependent improvement of novel object recognition of temporal induced memory deficit in rat |
| Xanomelinea (17) | Eli Lilly, 1994 | 0.3 nm | M2 EC50 = 93 nm, M3 EC50 = 5 nm, M4 EC50 = 52 nm, M5 EC50 = 42 nm |
hD2Ki = 264 nm, 5HT2CKi = 52 nm, 5HT2BKi = 12 nm |
3–7% oral bioavailabilty, t1/2 = 32 min (rat) |
Enhance sAPPα | Reverses amphetamine hyperlocomotion in rat model; reverses apomorphine-induced deficit in PPI in rat model; effective in conditioned emotional response in rat model (anxiety); did not induce catalepsy in rats; advanced to Phase II and Phase III clinical trials |
Abbreviations: AHL, amphetamine-induced hyperlocomotion; CNS, central nervous system; DMPK, drug metabolism and pharmacokinetics; GPCR, G-protein-coupled receptor; NMDA, N-methyl-d-aspartate; NOR, novel object recognition; sAPPα, soluble amyloid precursor protein alpha.
Ancillary data taken from Ref. [49]; values differ somewhat from original report.
EC50 values reported are from TREx293 hM1 cell line with low receptor reserve. These data were taken from Ref. [67].
These modifications to provide potent tropane agonists brought into focus the inherent challenges in working with this class of agonists. Although high selectivity could be achieved for the M1 receptor through an allosteric interaction at lower concentrations, 34, 35 and later analogs resulted in orthosteric interactions at higher concentrations and a loss of muscarinic subtype selectivity for more-potent compounds within the same series (similar two-site binding profiles observed with TBPB, 34 and 35) [80]. Although the loss of subtype selectivity and dependence on receptor reserve can preclude their development into strong lead candidates, there remains inherent value in the study of their in vitro pharmacology and electrophysiological effects, because muscarinic receptor density is not uniform throughout the CNS.
Signal differentiation
Characterization of novel allosteric agonists has brought to light interesting differential effects in the downstream signaling pathways of orthosteric and allosteric M1 agonists. AC-260584 (Fig. 7, 22), TBPB (24) and CCh (11) were found to be functionally active in calcium efflux and extracellular regulated signal kinase (ERK1/ 2) phosphorylation assays, which are downstream responses in the Gαq signaling pathway [61,81]. A second Gαq-independent pathway acts to regulate the response of M1 by recruitment of arrestin proteins, the most important of which is thought to be β-arrestin. In addition to their role in receptor desensitization and endocytosis, arrestins have also been found to play a part in chemotaxis, stress fiber formation and protein synthesis. When cells were treated with CCh, β-arrestin was recruited to the surface of the cell within 5 min, with additional binding studies showing significant degradation (~25%) of the M1 receptor after 24 hours. Furthermore, cells pretreated with CCh showed almost no response to a subsequent CCh challenge. Allosteric modulators 22 and 24, however, showed no significant change in arrestin localization after 5 min. Upon incubating overnight, TBPB only achieved 20% CCh maximum arrestin recruitment, whereas 22 showed a delayed but robust response with 80% CCh arrestin recruitment. Both allosteric agonists showed no significant degradation of M1 and pretreatment of cells with either agonist did not desensitize a subsequent CCh challenge. Allosteric agonists exhibiting this pharmacological profile can avoid desensitization to treatment with prolonged exposure. These results serve to highlight the complexity associated with developing M1 allosteric agonists, as well as the need for additional studies to determine ligand-biased signaling in other scaffolds. Similarly, 34 and 35 failed to promote β-arrestin recruitment.
It is known that induction of calcium release, ERK1/2 phosphorylation and β-arrestin recruitment are all activated post-M1 stimulation, eliciting responses to induce a range of physiological effects [82]. Therefore, differential activation of some M1-mediated responses will have impact in determining the therapeutic potential of novel M1 agonists. The pharmacologies of 34 and 35 were further characterized in cellular assays examining ERK phosphorylation and β-arrestin recruitment. Although both compounds induced a robust response in calcium assays, only compound 35 induced robust ERK1/2 phosphorylation, and neither compound recruited β-arrestin [78]. When the responses of these two agonists were examined in an inducible cell line they were found to show reduced potency in lower expressing cell lines, behaving as weak partial agonists. On the basis of these observations, it was postulated that 34 and 35 could show differential effects in M1-containing CNS regions thought to be important for in vivo therapeutic effects. To characterize their properties further, CCh 11, 34 and 35 were tested for their ability to enhance long-term potentiation (LTP) and long-term depression (LTD) in the CA1 region of rat hippocampal slices, where M1 is the predominant subtype expressed (60% of total mAChR expression). All three compounds were found to potentiate NMDA receptor currents in hippocampal pyramidal cells and significantly enhance LTP, a response thought to be mediated exclusively by the M1 mAChR. Additionally, high concentrations of CCh (50 µm, effect not observed at 30 µm) and 35 robustly potentiated LTD, whereas 34 did not. This was one of the first results in native tissues that demonstrated that all M1 activation is not equal.
Following these studies, 34 and 35 were examined in tissues with lower M1 receptor density (low receptor reserve). In rat striatal medium spiny neurons (MSNs) M1 activation is believed to be responsible for locomotor activity through M1 agonism. Compunds 34 and 35 showed a significant, albeit weak, increase in the firing rate of MSNs; however, both compounds showed no effect in the depolarization of mouse prefrontal cortex (mPFC) pyramidal cells [78]. Extending these findings into in vivo systems, 34 and 35 were evaluated in two assays assessing hippocampal-dependent learning and a separate striatal-dependent antipsychotic assay. In the hippocampal-dependent assays, 34 and 35 showed robust responses in contextual fear conditioning, yet only 35 showed efficacy in a Morris water maze test to probe spatial learning. Both compounds showed limited effects on the striatum, which is implicated in antipsychotic affects associated with the M1 receptor. In an amphetamine-induced hyperlocomotion(AHL)assay, neither 34 nor 35 showed efficacy. These data support the hypothesis that relying on a single assay (in this case: potency in calcium mobilization) to advance chemical lead optimization might not deliver compounds with the desired in vivo CNS action. These data illustrate the importance of advancing key compounds by assaying in multiple signaling pathways and under conditions where receptor reserve is known, because these ‘agonists’ display receptor reserve-dependent and, hence, brain-region-dependent pharmacology. This phenomenon will make it difficult to achieve the necessary selectivity versus M3 in humans (high M3 receptor reserve in the GI tract) to avoid GI side effects noted for orthosteric agonists [83].
M1 PAMs as a way forward
Challenges exemplified in this review serve to highlight the difficulty in the development of truly selective M1 agonists owing to the high conservation of the orthosteric binding site among this family of receptors. In addition, the two-site binding profile possessed by many of these agonists often results in panmuscarinic activation or antagonism in low receptor reserve systems [80]. To avoid these hurdles, targeting ligands that bind exclusively to a less-conserved allosteric site, which is topologically distinct from the orthosteric site, imparts a level of subtype selectivity not observed with two-site binding agonists. Allosteric ligands possess several modes of pharmacology, including allosteric agonism, PAMs and negative allosteric modulators (NAMs) [14,84]. PAMs are characterized by eliciting an increase in the efficacy or affinity of a native orthosteric agonist, such as ACh, as a result of a change in conformation of the receptor, but possess no intrinsic pharmacology alone. PAMs have offered advantages over classical agonists by conferring greater subtype and receptor selectivity. Also, by operating in conjunction with physiological signaling conditions, they offer an exquisite level of temporal selectivity not seen with traditional muscarinic activators.
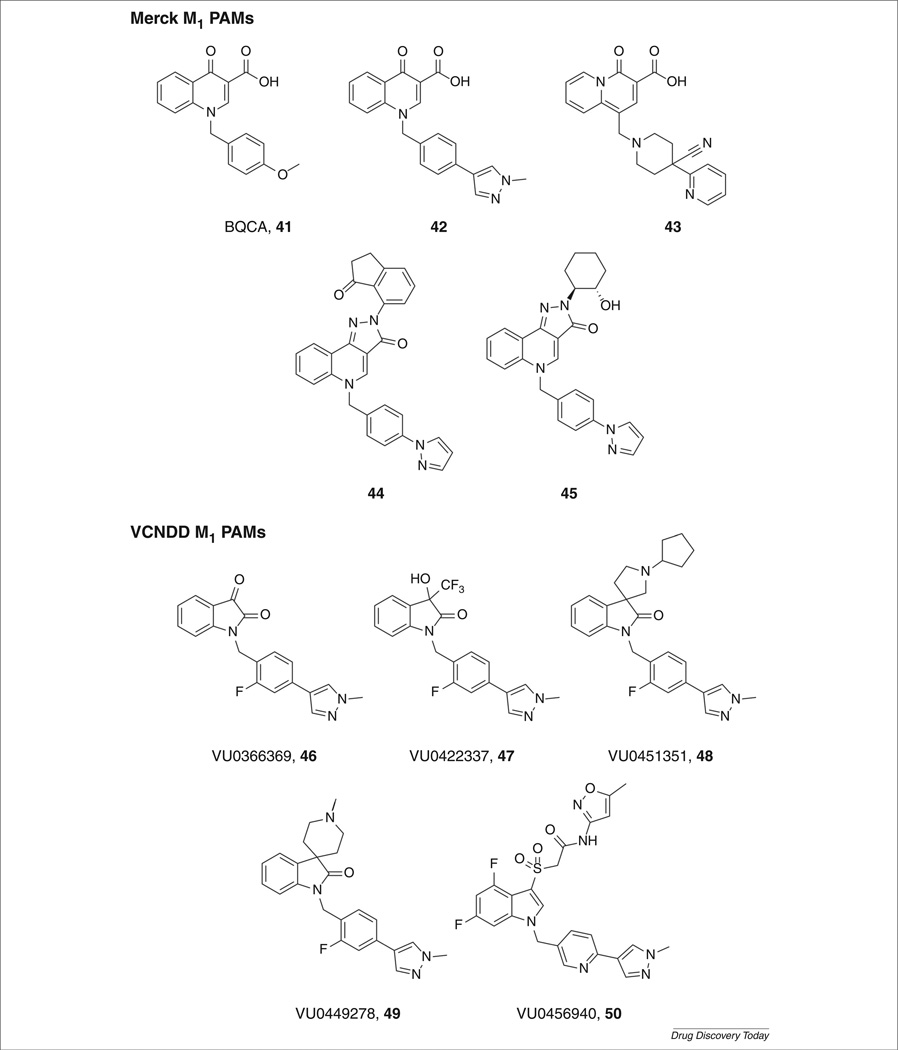
A proof-of-concept M1 PAM, benzylquinolone carboxylic acid (BQCA, 41; Fig. 9) was discovered by scientists at Merck Laboratories. BQCA is a potent and highly selective M1 PAM (hM1 EC50 = 840 nm, 129-fold leftward shift of the ACh CRC; hM2– hM5 inactive) with acceptable pharmacokinetics and CNS exposure [53–55]. It was shown to have no competitive interactions with the orthosteric binding site (determined through mutagenesis studies) and increased M1 affinity for CCh. BQCA increased spontaneous excitatory postsynaptic currents (sEPSCs) in the mPFC and induced a robust inward current. These effects were absent in brain slices from M1 knockout mice. BQCA regulated the nonamyloidogenic pathway for APP processing, increasing the release of sAPPα in the presence of a 50 nm CCh dose (displayed no activity in the absence of CCh) and restored discrimination reversal learning in a transgenic mouse model of AD. Both of these effects provide further support that M1 PAMs can potentially be disease modifying in a similar manner to M1 agonists. Moreover, BQCA was efficacious in reversing AHL [54], which led Merck to pursue this series actively through extensive chemical lead optimization delivering 42–45 [85–90].
FIGURE 9.
M1-selective positive allosteric modulators from Merck and Vanderbilt Center for Neuroscience Drug Discovery.
Researchers at our laboratories recently reported on VU0456940 (Fig. 9, 50), a potent M1 PAM with excellent selectivity (hM1 EC50 = 340 nm, 14-fold leftward shift of the ACh CRC; hM2–hM5 inactive), derived from a weak HTS hit [52,91]. Further development of this series of M1 PAM was precluded owing to problems with high clearance and moderate CYP450 inhibition, but 50 was tested in native tissues for its ability to potentiate M1. Compound 50 potentiated the excitation of a subthreshold concentration of CCh in MSNs. Also, 50 shifted APP processing and engaged the nonamyloidogenic pathway inducing the release of sAPPα in the presence of a 100 nm CCh dose (displayed no activity in the absence of CCh) [52]. A structurally distinct series arose from an M1, M3, M5 PAM, which, through chemical optimization efforts, afforded several subseries (Fig. 9, 46–49) of highly M1-selective PAMs [92–94]. The development of novel M1 PAMs related to 46– 49 are currently underway.
Concluding remarks
Following the promising clinical efficacy of the orthosteric agonist xanomeline for the treatment of psychosis and cognitive deficits in schizophrenia and AD patients, tremendous effort has been dedicated to finding suitable therapeutics to target mAChRs, with subtype selectivity at the orthosteric site representing the major hurdle in these efforts. With the disclosure of AC-42, targeting less-conserved allosteric sites has become the paradigm for engaging the mAChRs. A number of allosteric agonists have subsequently been reported with improved potency, muscarinic selectivity, attenuated ancillary pharmacology and desirable pharmacokinetic profiles. These compounds have aided in further understanding the role of the M1 receptor in cognitive functions. Numerous compounds have been shown to potentiate the NMDA receptor, CA1 firing in hippocampal cells and enhance soluble APP processing, perhaps offering a disease-modifying treatment for AD, as well as showing efficacy in multiple rodent cognition models. 13C-labeled compound 29 has entered clinical trials as a PET tracer. However, despite these advances, the development of M1 allosteric agonists possessing suitable profiles to advance through clinical trials has remained an unmet challenge. Many of the reported compounds exhibit binding to an orthosteric and an allosteric site, display poor selectivity or display a wide range of pharmacology based on receptor reserve in vitro and in vivo. A promising strategy that has emerged for addressing this challenge is the use of mAChR subtype-selective PAMs to potentiate the effect of ACh. PAMs such as these exhibit lower receptor desensitization, have less ancillary pharmacology (and are therefore more selective) and are generally not subject to issues associated with receptor reserve; however, PAMs are not a panacea because M1 PAMs must still be critically evaluated for ligand-biased signaling (β-arrestin, ERK, among others) and across safety species (rat, dog, nonhuman primate) to ensure no variation in potency or efficacy. Despite the challenges and caveats, the past five years have witnessed a revolution in muscarinic drug discovery efforts. Currently, Merck & Co. has advanced M1 PAM MK-7622 into Phase II clinical trials as an adjunct therapy to donepezil in patients with AD (http://clinicaltrials.gov/ct2/show/NCT01852110). This illustrates that we are closer than ever before to assessing the efficacy of a selective M1 activator in humans for the treatment of schizophrenia and AD.
Biographies

Bruce J. Melancon, PhD, is a research instructor for the Vanderbilt Center for Neuroscience Drug Discovery (VCNDD). Bruce received his doctorate in 2008 from the University of Notre Dame under the direction of Professor Richard E. Taylor on the development of methodology for asymmetric syntheses of 1,2-disubstituted cyclopropanes via cationic pathways. Subsequently, he received an NIH postdoctoral fellowship at Vanderbilt University Medical Center under the direction of Professor Gary A. Sulikowski to develop small molecule inhibitors of Wnt signaling. Bruce accepted a senior scientist position in the VCNDD in 2010 and was promoted to the faculty in the School of Medicine in 2012. His research focuses on the development of allosteric modulators of mAChR M1 and M4 for the treatment of schizophrenia and Alzheimer’s disease.

James C. (Chris) Tarr, PhD, is a medicinal chemist at the Vanderbilt Center for Neuroscience Drug Discovery. Chris completed his doctoral studies in 2010 at the University of North Carolina, Chapel Hill, under the direction of Professor Jeffrey S. Johnson on the development of methodology to employ acyl silanes as latent acyl anion equivalents. In 2010, Chris accepted a postdoctoral position with Professor Craig W. Lindsley at Vanderbilt University where he worked on the development of allosteric agonists and PAMs of the M1 mAChR. In 2011, Chris accepted a senior staff scientist position at the VCNDD. His research focuses on the development of allosteric modulators of mAChR M1, M4 and mGluR5.

Craig W. Lindsley, PhD, is Director of Medicinal Chemistry for the Vanderbilt Center for Neuroscience Drug Discovery and holds the William K. Warren, Jr. Chair in Medicine. He received his doctorate in 1996 from the University of California, Santa Barbara, and pursued postdoctoral studies at Harvard University. In 2001, he moved to Merck & Co. and developed a streamlined approach for lead optimization, resulting in delivery of six preclinical candidates. He also provided preclinical proof-of-concept for the first isoenzyme selective, allosteric AKT kinase inhibitors, the first mGluR5 and M1 PAMs. In 2006, he accepted associate professor appointments in Pharmacology and Chemistry at Vanderbilt University. He serves as Editor-in-Chief of ACS Chemical Neuroscience. Now full professor, he serves as Principal Investigator of the Vanderbilt Specialized Chemistry Center for Accelerated Probe Development.
Footnotes
Disclosure statement
The authors declare no competing financial interest. Dr Lindsley has received, or is receiving, funding from Johnson & Johnson and AstraZeneca for the development of allosteric modulators for the treatment of schizophrenia, as well as support form NIH/NIMH for the development of allosteric M1 ligands.
References
- 1.Mueser K, McGurk S. Schizophrenia. Lancet. 2004;363:2063–2072. doi: 10.1016/S0140-6736(04)16458-1. [DOI] [PubMed] [Google Scholar]
- 2.Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu. Rev. Clin. Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19:1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 4.Green M. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Keefe RS, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch. Gen. Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 6.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am. J. Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 7.Ross C, et al. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Menniti FS, et al. Allosteric modulation for the treatment of schizophrenia: targeting glutamatergic networks. Curr. Top. Med. Chem. 2013;13:26–54. doi: 10.2174/1568026611313010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindsley CW, et al. Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr. Top. Med. Chem. 2006;8:771–784. doi: 10.2174/156802606777057599. [DOI] [PubMed] [Google Scholar]
- 10.Field JR, et al. Targeting glutamate synapses in schizophrenia. Trends Mol. Med. 2011;17:689–698. doi: 10.1016/j.molmed.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantrowitz JT, Javitt DC. N-Methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res. Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin R, et al. Mechanism of positive allosteric modulators acting on AMPA receptors. J. Neurosci. 2005;25:9027–9036. doi: 10.1523/JNEUROSCI.2567-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conn PJ, et al. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conn PJ, et al. Allosteric modulation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melancon BJ, et al. Allosteric modulation of 7 transmembrane spanning receptors: theory, practice and opportunities for CNS drug discovery. J. Med. Chem. 2012;55:1445–1464. doi: 10.1021/jm201139r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AI. Immunological localization of m1–m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- 17.Abrams P, et al. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br. J. Pharmacol. 2006;148:565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volpicelli LA, Levey AI. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog. Brain Res. 2004;145:59–66. doi: 10.1016/S0079-6123(03)45003-6. [DOI] [PubMed] [Google Scholar]
- 19.Salter MW, et al. Src kinases: a hub for NMDA receptor regulation. Nat. Rev. Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 20.Langmead CJ, et al. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 2008;117:232–243. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophr. Res. 2004;72:21–28. doi: 10.1016/j.schres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Green MF, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr. Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 23.Green MF, et al. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr. Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Salh-Uddin H, et al. Altered M1 muscarinic acetylcholine receptor (CHRM1)-Gα(q/11) coupling in a schizophrenia endotype. Neuropsychopharmacology. 2009;34:2156–2166. doi: 10.1038/npp.2009.41. [DOI] [PubMed] [Google Scholar]
- 25.Olney JW, et al. NMDA receptor hypofunction model of schizophrenia. J. Psychiat. Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 26.Malhotra AK, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 27.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 28.Duncan GE, et al. Comparison of brain metabolic activity patterns induced by ketamine, MK-801 and amphetamine in rats: support for NMDA receptor involvement in responses to subanesthetic dose of ketamine. Brain Res. 1999;843:171–183. doi: 10.1016/s0006-8993(99)01776-x. [DOI] [PubMed] [Google Scholar]
- 29.Chue P. Glycine reuptake inhibition as a new therapeutic approach in schizophrenia: focus on the glycine transporter 1 (GlyT1) Curr. Pharm. Des. 2013;19:1311–1320. doi: 10.2174/138161213804805766. [DOI] [PubMed] [Google Scholar]
- 30.Lipina T, et al. Modulators of the glycine site on NMDA receptors, d-serine and ALX 5407, display similar beneficial effects to clozapine in mouse models of schizophrenia. Psychopharmacology. 2005;179:54–67. doi: 10.1007/s00213-005-2210-x. [DOI] [PubMed] [Google Scholar]
- 31.Marino MJ, et al. Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-d-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. PNAS. 1998;95:11465–11470. doi: 10.1073/pnas.95.19.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sur C, et al. N-Desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-d-aspartate receptor activity. PNAS. 2003;100:13674–13679. doi: 10.1073/pnas.1835612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller AD, Blaha CD. Midbrain muscarinic receptor mechanisms underlying regulation of mesoaccumbens and nigrostriatal dopaminergic transmission in the rat. Eur. J. Neurosci. 2005;21:1837–1846. doi: 10.1111/j.1460-9568.2005.04017.x. [DOI] [PubMed] [Google Scholar]
- 34.Jones CK, et al. Pharmacologic interactions between the muscarinic cholinergic and dopaminigeric systems in the modulation of prepulse inhibition in rats. J. Pharmacol. Exp. Ther. 2005;312:1055–1063. doi: 10.1124/jpet.104.075887. [DOI] [PubMed] [Google Scholar]
- 35.Mirza NR, et al. Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists. CNS Drug Rev. 2003;9:159–186. doi: 10.1111/j.1527-3458.2003.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barak S, Weiner I. The M1/M4 preferring agonist xanomeline reverses amphetamine-, MK-801- and scopolamine-induced abnormalities of latent inhibition: putative efficacy against positive, negative and cognitive symptoms in schizophrenia. Int. J. Neuropsychopharmacol. 2011;14:1233–1246. doi: 10.1017/S1461145710001549. [DOI] [PubMed] [Google Scholar]
- 37.Bodick NC, et al. Effects of xanomeline, a selective muscarinic receptor agonist,on cognitive function and behavioral symptoms in Alzheimer disease. Arch. Neurol. 1997;54:465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- 38.Shekhar A, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry. 2008;165:1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- 39.Lieberman J, et al. Cholinergic agonists as novel treatments for schizophrenia: the promise of rational drug development for psychiatry. Am. J. Psychiatry. 2008;165:931–936. doi: 10.1176/appi.ajp.2008.08050769. [DOI] [PubMed] [Google Scholar]
- 40.Perry KW, et al. The muscarinic agonist xanomeline increases monoamine release and immediate early gene expression in the rat prefrontal cortex. Biol. Psychiatry. 2001;49:716–725. doi: 10.1016/s0006-3223(00)01017-9. [DOI] [PubMed] [Google Scholar]
- 41.Shannon HE, et al. Xanomeline, an M1/M4 preferring muscarinic cholinergic receptor agonist, produces antipsychotic-like activity in rats and mice. Schizophr. Res. 2000;42:249–259. doi: 10.1016/s0920-9964(99)00138-3. [DOI] [PubMed] [Google Scholar]
- 42.Wienrich M, et al. Talsaclidine (WAL 2014 FU), a muscarinic M1 receptor agonist for the treatment of Alzheimer’s disease. Drug Dev. Res. 2002;56:321–334. [Google Scholar]
- 43.Clader JW, Wang Y. Muscarinic receptor agonists and antagonists in the treatment of Alzheimer’s disease. Curr. Pharm. Des. 2005;11:3353–3361. doi: 10.2174/138161205774370762. [DOI] [PubMed] [Google Scholar]
- 44.Fillit H, et al. Health care utilization and costs of Alzheimer’s disease: the role of comorbid conditions, disease stage, and pharmacotherapy. Fam. Med. 2002;34:528–535. [PubMed] [Google Scholar]
- 45.Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch. Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 46.Welsh KA, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 47.Davis AA, et al. Deletion of M1 muscarinic acetylcholine receptors increases amyloid pathology in vitro and in vivo . J. Neurosci. 2010;30:4190–4196. doi: 10.1523/JNEUROSCI.6393-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang S, et al. M1 muscarinic acetylcholine receptor interacts with BACE1 and regulates its proteosomal degradation. Neurosci. Lett. 2012;515:125–130. doi: 10.1016/j.neulet.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 49.Bridges TM, et al. Synthesis and SAR of analogues of the M1 allosteric agonist TBPB. Part I. Exploration of alternative benzyl and privileged structure moieties. Bioorg. Med. Chem. Lett. 2008;18:5439–5442. doi: 10.1016/j.bmcl.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nunan J, Small DH. Regulation of APP cleavage by α-, β- and γ-secretases. FEBS Lett. 2000;483:6–10. doi: 10.1016/s0014-5793(00)02076-7. [DOI] [PubMed] [Google Scholar]
- 51.Shirey JK, et al. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J. Neurosci. 2009;29:14271–14286. doi: 10.1523/JNEUROSCI.3930-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarr JC, et al. Targeting selective activation of M1 for the treatment of Alzheimer’s disease: further chemical optimization and pharmacological characterization of the M1 positive allosteric modulator ML169. ACS Chem. Neurosci. 2012;3:884–895. doi: 10.1021/cn300068s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindsley C, et al. Merck & Co., Inc. Benzyl-substituted quinolone M1 receptor positive allosteric modulators. WO Patent. 2008 2,008,002,621. [Google Scholar]
- 54.Ma L, et al. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. PNAS. 2009;106:15950–15955. doi: 10.1073/pnas.0900903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang FV, et al. Parallel synthesis of N-birayl quinolone carboxylic acids as selective M1 positive allosteric modulators. Bioorg. Med. Chem. Lett. 2010;20:531–536. doi: 10.1016/j.bmcl.2009.11.100. [DOI] [PubMed] [Google Scholar]
- 56.Zhou JM, et al. Early discrimination reversal learning impariment and preserved spatial learning in a longitudinal study of Tg2576 APPsw mice. Neurobiol. Aging. 2007;28:1248–1257. doi: 10.1016/j.neurobiolaging.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 57.Zhou JM, et al. An increase in Abeta42 in the prefrontal cortex is associated with a reversal-learning impairment in Alzheimer’s disease model Tg2576 APPsw mice. Curr. Alzheimer Res. 2008;5:385–391. doi: 10.2174/156720508785132280. [DOI] [PubMed] [Google Scholar]
- 58.Lazareno S, et al. Subtype-selective positive cooperative interactions between brucine analogues and acetylcholine at muscarinic receptors: radioligand binding studies. Mol. Pharmacol. 1998;53:573–589. doi: 10.1124/mol.53.3.573. [DOI] [PubMed] [Google Scholar]
- 59.Spalding TA, et al. Discovery of an ectopic activation site on the M1 muscarinic receptor. Mol. Pharmacol. 2002;61:1297–1302. doi: 10.1124/mol.61.6.1297. [DOI] [PubMed] [Google Scholar]
- 60.Heinrich JN, et al. Pharmacological comparison of muscarinic ligands: historical versus more recent muscarinic M1-preferring receptor agonists. Eur. J. Pharmacol. 2009;605:53–56. doi: 10.1016/j.ejphar.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 61.Bradley SR, et al. AC-260584, an orally bioavailable M1 muscarinic receptor allosteric agonist, improves cognitive performance in an animal model. Neuropharmacology. 2010;58:365–373. doi: 10.1016/j.neuropharm.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Langmead C, et al. Characterization of a CNS penetrant, selective M1 muscarinic receptor agonist, 77-LH-28-1. Br. J. Pharmacol. 2008;154:1104–1115. doi: 10.1038/bjp.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones CK, et al. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J. Neurosci. 2008;28:10422–10433. doi: 10.1523/JNEUROSCI.1850-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones C, et al. TBPB is a highly selective M1 allosteric muscarinic receptor agonist in vitro and produces robust antipsychotic-like effects in vivo . Neuropsychopharmacology. 2006;31:S116–S117. [Google Scholar]
- 65.Lebois EP, et al. Discovery and characterization of novel subtype-selective allosteric agonists for the investigation of M1 receptor function in the central nervous system. ACS Chem. Neurosci. 2010;1:104–121. doi: 10.1021/cn900003h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller NR, et al. Synthesis and SAR of analogs of the M1 allosteric agonist TBPB. Part II. Amides, sulfonamides and ureas—the effect of capping the distal basic piperidine nitrogen. Bioorg. Med. Chem. Lett. 2008;18:5443–5447. doi: 10.1016/j.bmcl.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheffler DJ, et al. Further exploration of M1 allosteric agonists: subtle structural changes abolish M1 allosteric agonism and result in pan-mAChR orthosteric antagonism. Bioorg. Med. Chem. Lett. 2013;23:223–227. doi: 10.1016/j.bmcl.2012.10.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sams AG, et al. Discovery of N-{1-[3-(3-oxo-2,3-dihydrobenzo [1,4] oxazin-4-yl) propyl] piperidin-4-yl}-2-phenylacetamide (Lu AE51090): an allosteric muscarinic M1 receptor agonist with unprecedented selectivity and procognitive potential. J. Med. Chem. 2010;53:6386–6397. doi: 10.1021/jm100697g. [DOI] [PubMed] [Google Scholar]
- 69.Budzik B, et al. 2′ biaryl amides as novel and subtype selective M1 agonists. Part I. Identification, synthesis, and initial SAR. Bioorg. Med. Chem. Lett. 2010;20:3540–3544. doi: 10.1016/j.bmcl.2010.04.128. [DOI] [PubMed] [Google Scholar]
- 70.Budzik B, et al. 2′ biaryl amides as novel and subtype selective M1 agonists. Part II. Further optimization and profiling. Bioorg. Med. Chem. Lett. 2010;20:3545–3549. doi: 10.1016/j.bmcl.2010.04.127. [DOI] [PubMed] [Google Scholar]
- 71.Johnson DJ, et al. The discovery of a series of N-substituted 3-(4-piperidinyl)-1,3-benzoxazolinones and oxindoles as highly brain penetrant, selective muscarinic M1 agonists. Bioorg. Med. Chem. Lett. 2010;20:5434–5438. doi: 10.1016/j.bmcl.2010.07.097. [DOI] [PubMed] [Google Scholar]
- 72.Budzik B, et al. Novel N-substituted benzimidazolones as potent, selective, CNS-penetrant, and orally active M1 mAChR agonists. ACS Med. Chem. Lett. 2010;1:244–248. doi: 10.1021/ml100105x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huiban M, et al. Fully automated synthesis of the M1 receptor agonist 11C GSK1034702 for clinical use on an Eckert & Ziegler Modular Lab system. Appl. Radiat. Isotopes. 2011;69:1390–1394. doi: 10.1016/j.apradiso.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 74.Nathan PJ, et al. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int. J. Neuropsychopharmacol. 2013;16:721–731. doi: 10.1017/S1461145712000752. [DOI] [PubMed] [Google Scholar]
- 75.Digby GJ, et al. Allosteric activators of muscarinic receptors as novel approaches for treatment of CNS disorders. Mol. BioSyst. 2010;6:1345–1354. doi: 10.1039/c002938f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dencker D, et al. Muscarinic acetylcholine receptor subtypes as potential drug targets for the treatment of schizophrenia, drug abuse, and Parkinson’s disease. ACS Chem. Neurosci. 2011;3:80–89. doi: 10.1021/cn200110q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lebois EP, et al. Development of a highly selective, orally bioavailable and CNS penetrant M1 agonist derived from the MLPCN probe ML071. Bioorg. Med. Chem. Lett. 2011;21:6451–6455. doi: 10.1016/j.bmcl.2011.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Digby GJ, et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J. Neurosci. 2012;32:8532–8544. doi: 10.1523/JNEUROSCI.0337-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Melancon BJ, et al. Continued optimization of the MLPCN probe ML071 into highly potent agonists of the hM1 muscarinic acetylcholine receptor. Bioorg. Med. Chem. Lett. 2012;22:3467–3472. doi: 10.1016/j.bmcl.2012.03.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Digby GJ, et al. Chemical modification of the M1 agonist VU0364572 reveals molecular switches in pharmacology and a bitopic binding mode. ACS Chem. Neurosci. 2012;3:1025–1036. doi: 10.1021/cn300103e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davis AA, et al. Differential effects of allosteric M1 muscarinic acetylcholine receptor agonists on receptor activation, arrestin 3 recruitment, and receptor downregulation. ACS Chem. Neurosci. 2010;1:542–551. doi: 10.1021/cn100011e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomas RL, et al. Contrasting effects of allosteric and orthosteric agonists on m1 muscarinic acetylcholine receptor internalization and down-regulation. J. Pharmacol. Exp. Ther. 2009;331:1086–1095. doi: 10.1124/jpet.109.160242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin S, et al. Expression of muscarinic receptor subtypes in rat gastric smooth muscle. Dig. Dis. Sci. 1997;42:907–914. doi: 10.1023/a:1018808329603. [DOI] [PubMed] [Google Scholar]
- 84.Bridges TM, Lindsley CW. G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem. Biol. 2008;3:530–541. doi: 10.1021/cb800116f. [DOI] [PubMed] [Google Scholar]
- 85.Kuduk SD, et al. Novel M1 allosteric ligands: a patent review. Expert Opin. Ther. Pat. 2012;22:1385–1398. doi: 10.1517/13543776.2012.731395. [DOI] [PubMed] [Google Scholar]
- 86.Kuduk SD, et al. Quinolizidinone carboxylic acids as CNS pentrant, selective M1 allosteric modulators. ACS Med. Chem. Lett. 2010;1:263–267. doi: 10.1021/ml100095k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuduk SD, et al. Heterocyclic fused pyridone carboxylic acid M1 positive allosteric modulators. Bioorg. Med. Chem. Lett. 2010;20:1710–1715. doi: 10.1016/j.bmcl.2010.02.096. [DOI] [PubMed] [Google Scholar]
- 88.Kuduk SD, et al. Pyridine containing M1 positive allosteric modulators with reduced plasma protein binding. Bioorg. Med. Chem. Lett. 2010;20:2533–2537. doi: 10.1016/j.bmcl.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 89.Kuduk SD, et al. Identification of amide amides as carboxylic acid surrogates for quinolizidinone based M1 positive allosteric modulators. ACS Med. Chem. Lett. 2012;3:1070–1074. doi: 10.1021/ml300280g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Uslaner JM, et al. The muscarinic M1 receptor positive allosteric modulator PQCA improves cognitive performance in rat, cynomolgus macaque and rhesus macaque. Psychopharmacology. 2013;225:21–30. doi: 10.1007/s00213-012-2788-8. [DOI] [PubMed] [Google Scholar]
- 91.Reid PR, et al. Discovery and optimization of a novel, selective and brain penetrant M1 positive allosteric modulator (PAM): the development of ML169, an MLPCN Probe. Bioorg. Med. Chem. Lett. 2011;21:2697–2701. doi: 10.1016/j.bmcl.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bridges TM, et al. Chemical optimization of an M1, M3, M5 positive allosteric modulator (PAM) lead. Part II. Development of a highly selective M1 PAM. Bioorg. Med. Chem. Lett. 2010;20:1972–1975. doi: 10.1016/j.bmcl.2010.01.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Poslusney MS, et al. Spirocyclic replacements for the isatin moiety in the highly selective muscarinic M1 PAM ML137: the continued optimization of an MLPCN probe. Bioorg. Med. Chem. Lett. 2013;23:1860–1864. doi: 10.1016/j.bmcl.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Melancon BJ, et al. Isatin replacements applied to the highly selective, muscarinic M1 PAM ML137: the continued optimization of an MLPCN probe molecule. Bioorg. Med. Chem. Lett. 2013;23:412–416. doi: 10.1016/j.bmcl.2012.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]