Abstract
OBJECTIVE.
The reported frequency of aborted MRI-guided breast biopsies ranges from 8% to 17%, usually secondary to nonvisualization at attempted biopsy. Our study examines the frequency of MRI-guided breast biopsies aborted because of lesion nonvisualization and the subsequent risk of malignancy.
MATERIALS AND METHODS.
We identified 350 patients and 445 lesions scheduled for MRI-guided biopsy between January 1, 2007, and December 31, 2009. Medical records and imaging studies were reviewed to ascertain patient demographics, lesion and imaging characteristics, and subsequent pathology results. Chi-square statistics were calculated for patient level analyses.
RESULTS.
MRI-guided biopsies were aborted in 13% (56/445) of lesions and 15% (53/350; 95% CI, 11.6–19.3%) of patients because of nonvisualization of the biopsy target at the time of attempted biopsy. Of these 53 patients, 50 patients had follow-up data available. Malignancy was subsequently diagnosed in five of those 50 patients (10%; 95% CI, 3.3–21.8%) patients, three with invasive ductal carcinomas and two with ductal carcinoma in situ. The mean time to malignant diagnosis from the date of aborted biopsy was 2.6 months (range, 1.1–6.9 months).
CONCLUSION.
Informed consent for MRI-guided breast biopsies should include discussion of biopsy cancellation because of nonvisualization of the target lesion. The low yet significant risk of malignancy in patients subsequent to an aborted MRI-guided breast biopsy warrants short-term follow-up MRI after a canceled biopsy.
Keywords: breast cancer, breast MRI, MRI-guided biopsy, MRI-guided intervention
MRI-guided vacuum-assisted breast biopsy is often required to further evaluate suspicious lesions, because only 38–75% of MRI-detected masses and 11–46% of MRI-detected nonmasslike enhancing lesions are identified on “second-look” diagnostic mammography or sonography [1-3]. The MRI-guided biopsy has become a routine and safe method of breast intervention with the potential to spare the patient a surgical biopsy in some cases [4]. However, the reported frequency of canceled MRI-guided biopsies ranges from 8% to 17% [4-10]. Potential reasons for an aborted MRI-guided biopsy are numerous, with lesion nonvisualization at the time of biopsy being the most common reason for biopsy cancellation, likely related to background parenchymal enhancement, breast compression tightness, and timing of the MRI-guided biopsy relative to the patient’s menstrual cycle. The current literature suggests that, after an aborted MRI-guided biopsy, 0–10% of lesions will subsequently be diagnosed as a malignancy, such as ductal carcinoma in situ, invasive ductal carcinoma, or invasive lobular carcinoma [4-10]. The objective of this study was to calculate the frequency of an ipsilateral malignancy diagnosis in the same quadrant of the breast following a canceled MRI-guided breast biopsy secondary to nonvisualization of the lesion on the day of biopsy.
Materials and Methods
This HIPAA-compliant study was approved by the institutional review board. We retrospectively reviewed our institution’s consecutive MRI-guided breast interventions (biopsy or clip placement) between January 1, 2007, and December 31, 2009, to determine the rate of cancellation of an MRI-guided intervention. Each of the 63 canceled MRI-guided biopsies during the study period was retrospectively reviewed by a single author to determine the cause for cancellation (e.g., nonvisualization, contrast material extravasation, technical malfunction of equipment, or biopsy target positioned outside of compression grid). The 56 MRI-guided biopsies canceled because of nonvisualization of the biopsy target on the day of biopsy were the focus of this study. Seven biopsies canceled for other reasons were excluded from this study. Of the seven excluded lesions, two were not imaged within the biopsy compression grid at the time of initial biopsy attempt, one was identified in retrospect within the biopsy compression grid and the MRI-guided biopsy was promptly rescheduled, and four were not biopsied because of IV access issues, contrast material extravasation, and patient preference to discontinue biopsy procedure. For a subset of lesions not amenable to MRI-guided biopsy because of lesion location, MRI-guided clip placement may be attempted, and subsequent mammographic needle localization is performed at our institution. During the study period, no MRI-guided clip placement was canceled. No MRI-guided needle localizations were included in this study. Therefore, all procedures included in this study were attempted MRI-guided biopsies canceled because of lesion nonvisualization.
During the study period, MRI-guided biopsies were performed by seven breast radiologists on a 1.5-T magnet with the patient prone, using a 4-channel coil (GE Breast Array Coil, GE Health-care) and an immobilization and biopsy system (Breast Immobilization and Biopsy Device MRBI 160, NORAS MRI Products). During the study period, the MRI-guided biopsy imaging protocol included a multiplanar localizing sequence and 3-mm slice thickness sagittal fat-suppressed T1-weighted sequences performed before and after injection of 20 mL of gadopentetate dimeglumine (Magnevist, Bayer HealthCare Pharmaceuticals). MRI-guided biopsies were performed using a 9-gauge vacuum-assisted device (Suros ATEC, Hologic) [4]. Before canceling an MRI-guided biopsy, the breast radiologist confirmed that the location of the biopsy target was positioned within the biopsy grid and confirmed the presence of enhancement within the breast parenchyma. At the discretion of the breast radiologist performing the procedure, subtraction images, 3-mm slice thickness axial fat-suppressed T1-weighted images, or additional 3-mm slice thickness sagittal fat-suppressed T1-weighted images following release of the compression grid were acquired before cancellation. The standard recommendation after a canceled biopsy in our department was for a short-interval follow-up breast MRI, with the duration of follow-up determined by the breast radiologist performing the attempted biopsy.
For each patient with a canceled MRI-guided biopsy secondary to lesion nonvisualization, the electronic medical record and imaging studies were reviewed to ascertain patient demographics, lesion and imaging characteristics, and subsequent diagnosis of breast malignancy. The study design included a follow-up interval of at least 2 years to determine whether a subsequent malignancy developed in the same quadrant as the aborted MRI-guided biopsy. If the lesion targeted for MRI-guided biopsy and the subsequent malignant diagnosis were in the same quadrant of the breast, we classified these cancers as MRI detected. MRI lesion characteristics, breast parenchymal volume, and background parenchymal enhancement were retrospectively reviewed for each lesion with a canceled MRI-guided biopsy by one author, a breast imaging fellowship-trained radiologist with 3 years of experience in breast MRI. Breast parenchymal volume and background parenchymal enhancement were evaluated as described elsewhere [7].
Abstracted data were entered into a computerized spreadsheet (Excel 2003, Microsoft). For patient level analyses, statistical significance was tested using chi-square statistics and 95% CIs, with a p value of less than 0.05 considered statistically significant. To formulate a pooled estimate of the risk of malignancy from the data in our study, as well as previously published data, a Fisher exact test for homogeneity was performed to compare each of the individual datasets with the pooled estimate. A p value of less than 0.05 was considered statistically significant for heterogeneity between studies.
Results
From January 1, 2007, through December 31, 2009, 445 MRI-guided biopsies were performed in 350 women. Fifty-six scheduled biopsies were aborted (13% of lesions) in 53 women (15%; 95% CI, 11.6–19.3%) because of nonvisualization of the biopsy target. Women with canceled MRI-guided biopsies were more frequently premenopausal with no personal history of a prior or current breast cancer (Table 1). Nonvisualized targets were nonmasslike enhancement in 55.4% of cases, masses in 30.3%, and foci in 14.3%. Eighty-nine percent (50/56) of lesions with a canceled biopsy had undergone a second-look diagnostic mammogram or diagnostic ultrasound with no correlative findings identified before the attempted MRI-guided biopsy. Before canceling the procedure, subtraction images, 3-mm slice thickness axial fat-suppressed T1-weighted images, or additional 3-mm slice thickness sagittal fat-suppressed T1-weighted images following release of the compression grid were acquired at the discretion of the breast radiologist in 30 of 56 biopsy attempts (54%). There was no difference in the frequency of additional image acquisition in women with and without a subsequent diagnosis of malignancy.
TABLE 1.
Patient Demographics and Lesion Characteristics in Canceled MRI-Guided Biopsies
| Patient or Lesion Characteristic | Value |
|---|---|
|
| |
| Patient demographics (n = 53) | |
| Menopausal status | |
| Premenopausal | 35 (66.0) |
| Postmenopausal | 17 (32.1) |
| Not available | 1 (1.9) |
| Prior breast cancer | |
| Yes | 15 (28.3) |
| No | 37 (69.8) |
| Not available | 1 (1.9) |
| Current breast cancer | |
| Yes | 17 (32.1) |
| No | 36 (67.9) |
| Indication for MRI | |
| Screening | 21 (39.6) |
| Diagnostic | 32 (60.4) |
| Prior MRI available for comparison | |
| Yes | 18 (34.0) |
| No | 35 (66.0) |
| Breast parenchymal volume | |
| Fatty | 1 (1.9) |
| Scattered fibroglandular | 27 (50.9) |
| Heterogeneously dense | 21 (39.6) |
| Extremely dense | 4 (7.5) |
| Background parenchymal enhancement | |
| Minimal | 10 (18.9) |
| Mild | 26 (49.1) |
| Moderate | 14 (26.4) |
| Marked | 3 (5.7) |
| Lesion characteristics | |
| Lesion type (n = 56) | |
| Focus | 8 (14.3) |
| Nonmasslike enhancement | 31 (55.4) |
| Mass | 17 (30.3) |
| Mass size (mm), mean (range) | 7.9 (5–13) |
| Nonmasslike enhancement and focus size (mm), mean (range) | 12.6 (3–50) |
| Mass lesion kinetics (n = 17) | |
| Persistent | 8 (47.1) |
| Plateau | 5 (29.4) |
| Washout | 3 (17.6) |
| Did not register or not available | 1 (5.9) |
| Nonmasslike enhancement and focus kinetics (n = 39) | |
| Persistent | 29 (74.3) |
| Plateau | 3 (7.7) |
| Washout | 4 (10.3) |
| Did not register or not available | 3 (7.7) |
Note—Except where noted otherwise, data are number (%) of patients or lesions. Fifty-three patients had 56 nonvisualized lesions.
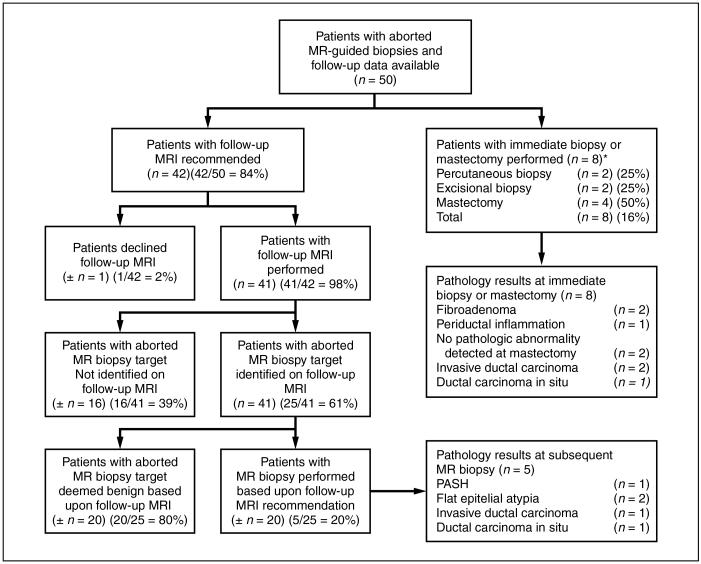
Of 53 patients with a canceled MRI-guided biopsy, 50 (94%) had follow-up data available for review, with 42 of those 50 patients (84%) recommended for a short-term follow-up MRI (Fig. 1). Short-term follow-up breast MRI was performed in 41 of the 42 patients in whom it was recommended. The time interval to the first short-term follow-up MRI ranged from 29 days to 382 days (median, 154 days). The breast radiologist recommended the first short-term follow-up breast MRI at 1 month in four patients, 3 months in 16 patients, 6 months in 18 patients, and 12 months in three patients. In 16 of these 41 patients (39%), the lesion targeted for MRI-guided biopsy was not identified on subsequent short-interval follow-up imaging. In these 16 patients, the lesions targeted for MRI-guided biopsy most likely represented physiologic enhancement and were deemed benign. In the remaining 25 of 41 patients (61%) who returned for follow-up MRI of 28 lesions, the aborted MRI-guided biopsy lesion was reidentified on the first follow-up MRI, including six lesions (21%) that had decreased in size, three lesions (11%) that had increased in size, and 19 lesions (68%) that showed no interval change in size (Fig. 2). Of these 25 patients in whom the target lesion was again identified on follow-up MRI, 20 patients (80%) had the target lesion interpreted as benign (BI-RADS category 2) based on subsequent follow-up breast MRI, requiring an average of 1.6 follow-up MRI examinations before a BI-RADS category 2 interpretation (range, 1–5 examinations). With 2–4 years of available follow-up data, none of these 20 patients had a subsequent diagnosis of malignancy in the same breast as the lesion targeted for MRI-guided biopsy.
Fig. 1.
Patient outcomes after canceled MRI-guided biopsy. *Includes two patients who underwent follow-up preoperative MRI after aborted MRI guided biopsy to assess response to neoadjuvant chemotherapy. **During 2–4 year follow-up interval, no subsequent malignancies were identified in same quadrant of ipsilateral breast in any of these patients. PASH = pseudoangiomatous stromal hyperplasia.
Fig. 2.
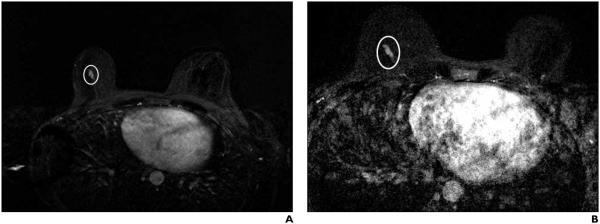
45-year-old woman who underwent bilateral breast screening MRI.
A, On bilateral dynamic contrast-enhanced breast MRI with subtraction, focal area of nonmasslike enhancement (oval) in lower central right breast was recommended for MRI-guided biopsy. MRI-guided biopsy was canceled. Patient underwent serial follow-up breast MRI.
B, Bilateral dynamic contrast-enhanced breast MRI with subtraction performed 2 years and 1 month after canceled MRI-guided biopsy found no interval change in morphologic features or size of focal area of nonmasslike enhancement (oval) in lower central right breast, after accounting for difference in FOV. Finding was considered benign and no additional follow-up breast MRI was recommended at that time.
Of the 25 patients with the MRI-guided biopsy target reidentified on short-term follow-up MRI, five patients had a recommendation for a repeat attempt at MRI-guided biopsy because of interval growth or suspicious morphologic or kinetic features (Fig. 1). An average of 1.4 follow-up breast MRI examinations was performed before repeat biopsy recommendation. All five patients underwent a successful repeat attempt at MRI-guided biopsy. Two patients had flat epithelial atypia with apocrine metaplasia, one patient was diagnosed with pseudoangiomatous stromal hyperplasia, and two patients were diagnosed with a malignancy. In each of these two patients diagnosed with a malignancy on repeat MRI-guided biopsy, the lesion showed no interval change in size on the first short-term follow-up breast MRI (one performed at 6 weeks and one performed at 3 months). In one of the malignancies, repeat MRI-guided biopsy was recommended because of a change in lesion morphologic features on the first follow-up MRI (performed at 6 weeks following the canceled MRI-guided biopsy). In the second patient subsequently diagnosed with a malignancy, a second short-term follow-up breast MRI 6 months after the canceled MRI-guided biopsy showed increased prominence of the originally targeted lesion, prompting repeat biopsy recommendation.
Of the 50 patients with aborted MRI-guided biopsies and follow-up data available for review, eight patients underwent an excisional biopsy (n = 2), core biopsy (n = 2), or mastectomy (n = 4) rather than short-term follow-up imaging (Fig. 1). Within the mastectomy specimens, two of four women were diagnosed with a malignancy in the same quadrant as the canceled MRI-guided biopsy lesion, with pathologic measurement of disease similar to that of the MRI measurement of the lesion targeted for MRI-guided biopsy (Fig. 3). Two of eight patients underwent a needle localized excisional biopsy of a mammographic lesion thought to correspond to the targeted lesion on MRI, with excisional pathology showing fibroadenoma in one patient and invasive ductal carcinoma in the second patient. Of note, the patient subsequently diagnosed with the invasive ductal carcinoma had the breast MRI performed for a site of mammographic architectural distortion without sonographic correlate, to determine whether a percutaneous diagnosis of malignancy could be made. Two patients underwent a repeat attempt at MRI-guided biopsy without a short-term follow-up MRI, with successful identification of the target lesion and benign pathology results on both repeat MRI-guided biopsies.
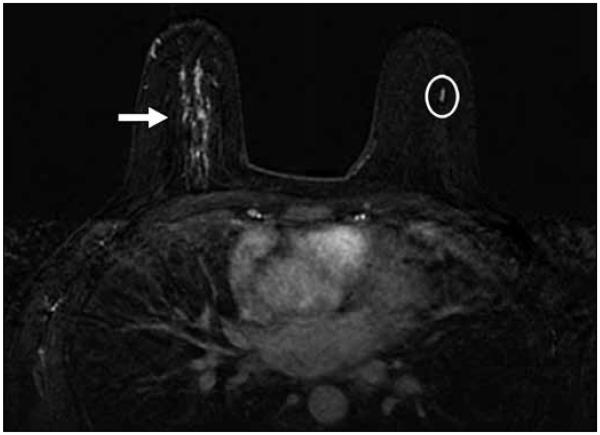
Fig. 3.
40-year-old woman with current diagnosis of right breast invasive ductal carcinoma underwent bilateral breast MRI. Linear nonmasslike enhancement (oval) in left central breast seen on bilateral dynamic contrast-enhanced breast MRI with subtraction was recommended for MRI-guided biopsy. MRI-guided biopsy was canceled. Patient elected contralateral prophylactic mastectomy given her young age and current history of breast cancer in right breast (arrow). Ductal carcinoma in situ was diagnosed on her left mastectomy specimen in same quadrant as aborted MRI biopsy lesion with pathologic measurement of disease similar to MRI measurement of lesion targeted for MRI-guided biopsy.
In summary, five of 50 patients (10%; 95% CI, 3.3–21.8%) were subsequently shown to have a malignancy following an aborted MRI-guided biopsy. Of the five malignant lesions, three were invasive ductal carcinomas and two were ductal carcinoma in situ. On MRI, the five malignant lesions included three masses, two nonmasslike enhancement, two lesions with progressive enhancement kinetics, one lesion with plateau, and two with wash-out kinetics. The mean time to malignant diagnosis from the date of aborted biopsy was 2.6 months (range, 1.1–6.9 months).
Discussion
Our study shows that cancellation of an MRI-guided biopsy secondary to lesion nonvisualization occurs in 13% of targeted lesions, similar to the 8–13% reported in the literature (Table 2). The published rates of a subsequent diagnosis of malignancy range from 0 to 10%. Because all published studies are retrospective single-site studies with sample sizes of fewer than 100 aborted biopsies, we pooled data across studies as part of our review of the literature. Pooled data, including our study, suggest that the risk of a diagnosis of subsequent malignancy for a lesion after a canceled MRI-guided biopsy is approximately 5% (95% CI, 2.2–8.7%). Although a formal meta-analysis was not performed, a Fisher exact test for homogeneity suggests that no statistically significant heterogeneity exists within the individual studies (p = 0.22). The variation in the published outcomes of canceled biopsies is likely related to the relatively small sample sizes in single-institution retrospective studies. Although our data are also from a single institution, our study is the second largest study published to date that has reviewed the risk of malignancy in patients with MRI-guided biopsies canceled because of nonvisualization. We think that our study lends additional support to the growing body of literature surrounding the small, but not insignificant, risk of malignancy following MRI-guided biopsy cancellation.
TABLE 2.
Frequency of Subsequent Malignancy After Canceled MRI-Guided Biopsy
| Study | No. of Lesions Recommended for MRI-Guided Biopsy |
No. (%) of Lesions With MRI-Guided Biopsy Canceled Because of Nonvisualization |
No. of Women With Canceled Biopsies and Available Follow-Up Data |
No. (%) of Women With Canceled Biopsies and Subsequent Malignancy in Ipsilateral Quadrant |
|---|---|---|---|---|
|
| ||||
| Brennan et al. [7] | 911 | 74 (8) | 61 | 1 (2) |
| Han et al. [5] | 172 | 22 (13) | 16 | 0 (0) |
| Hefler et al. [6] | 291 | 37 (13) | 29 | 3 (10) |
| Liberman et al. [4] | 112 | 14 (13) | 13 | 0 (0) |
| Perlet et al. [8]a | 410 | 49 (12) | Not recorded | Not recorded |
| Viehweg et al. [9] | 83 | 9 (11) | 9 | 0 (0) |
| Johnson et al. [10] | 117 | 15 (13) | 14 | 0 (0) |
| This study | 445 | 56 (13) | 50 | 5 (10) |
| Overall | 2541 | 276 (11) | 192 | 9 (5) |
Aborted biopsies occurred in 72 lesions, including 52 because of nonvisualization. Three of 52 aborted biopsies were attributed to incorrect original interpretation secondary to motion artifact, resulting in 49 lesions with aborted biopsies because of nonvisualization. Follow-up data were not provided in this study.
The small yet significant risk of subsequent malignancy after a canceled MRI-guided biopsy warrants routine discussion of the possibility of an aborted biopsy and subsequent short-term follow-up MRI when informed consent for the biopsy procedure is obtained. At the time of the aborted biopsy, the breast radiologist should evaluate the biopsy images in the context of background parenchymal enhancement. If the expected lesion and breast parenchyma show minimal or no enhancement, the following steps should be considered: verify adequate gadolinium-based contrast material administration and lack of extravasation, review the breast parenchyma outside the confines of the compression grid to exclude the possibility of inadvertently missing the lesion, and consider releasing the immobilizing grid, because excessive compression may prevent gadolinium-based contrast material enhancement [8, 11]. Marked parenchymal enhancement on the biopsy images may limit lesion identification, so timing the MRI-guided biopsy to the menstrual cycle, when possible, may be considered.
Our study has several limitations. First, we were unable to determine whether each patient’s canceled MRI-guided biopsy was timed to her menstrual cycle; therefore, we are not able to determine the effect of menstrual cycle timing on the MRI-guided biopsy cancellation rate in our study. A previous report suggests that MRI-guided biopsy cancellation rate may be increased with marked and moderate background parenchymal enhancement compared with minimal or mild enhancement, and background parenchymal enhancement varies with phase of the menstrual cycle [7, 12, 13]. However, in our study, only 32% of studies showed moderate or marked background parenchymal enhancement on the diagnostic breast MRI recommending an MRI-guided biopsy, suggesting that obscuration of the biopsy target due to profound background parenchymal enhancement likely accounts for the minority of canceled MRI-guided biopsies at our institution.
Another potential limitation of our study relates to the imaging parameters used for diagnostic and screening MRI compared with MRI-guided biopsies. For our diagnostic and screening MRI, we perform 2-mm slices for axial acquisitions and 3-mm slices for sagittal acquisitions on an 8-channel coil (GE Breast Array Coil, GE Healthcare). We routinely perform our breast biopsies on a 4-channel coil with 3-mm sagittal acquisitions. If the breast radiologist performing a biopsy acquires axial imaging at the time of biopsy, it is routinely performed at 3-mm slice thickness at the time of biopsy. Although it is possible that the differences in image acquisition may account for difficulty in identifying the lesion at the time of biopsy, we think that this is unlikely because the mean sizes of lesions that had an aborted biopsy were 8 mm for masses and 13 mm for nonmasslike enhancement.
An additional limitation of our study is the small sample size, which precludes determination of the optimal follow-up interval for short-term follow-up MRI after a canceled MRI-guided biopsy. Short-term follow-up imaging would ideally show resolution of the enhancement for which biopsy was attempted, consistent with physiologic enhancement. However, persistence of the lesion targeted for MRI-guided biopsy on short-term follow-up imaging is common. In our study, one of two lesions with short-term follow-up MRI that was subsequently diagnosed as a malignancy showed no change in size until the follow-up MRI performed 6 months later. Published studies also report no significant change in size of some missed malignancies with follow-up MRI performed less than 6 months after an attempted biopsy [14, 15]. Short-term follow-up breast MRI performed within 6 months after the attempted biopsy may result in earlier diagnosis of malignancy, as it did in one lesion subsequently diagnosed as a malignancy in our study, as well as one lesion subsequently diagnosed as a malignancy in a previously published report [14]. Although the optimal timing of short-term follow-up MRI is yet to be established, repeat imaging no later than 6 months after the aborted biopsy seems reasonable. At our institution, the duration of the short-interval follow-up period is left to the discretion of the radiologist who attempted the biopsy. The majority of breast radiologists at our institution recommended the first short-term follow-up MRI at 3–6 months after biopsy, taking into account the lesion size, morphologic features, and kinetic characteristics, as well as the patient’s underlying risk factors for breast cancer.
After a canceled MRI-guided biopsy, careful review of each patient’s breast MRI and MRI-guided biopsy images is critical. Consideration should be given to reviewing these images during an internal breast imaging conference, which may foster discussion among colleagues. Given the low yet significant risk of subsequent malignancy for a lesion after a canceled MRI-guided biopsy, we recommend a short-term follow-up MRI within 3–6 months for each patient after an aborted MRI-guided biopsy.
References
- 1.LaTrenta LR, Menell JH, Morris EA, Abramson AF, Dershaw DD, Liberman L. Breast lesions detected with MR imaging: utility and histopathologic importance of identification with US. Radiology. 2003;227:856–861. doi: 10.1148/radiol.2273012210. [DOI] [PubMed] [Google Scholar]
- 2.Meissnitzer M, Dershaw DD, Lee CH, Morris EA. Targeted ultrasound of the breast in women with abnormal MRI findings for whom biopsy has been recommended. AJR. 2009;193:1025–1029. doi: 10.2214/AJR.09.2480. [DOI] [PubMed] [Google Scholar]
- 3.Wiratkapun C, Duke D, Nordmann AS, et al. Indeterminate or suspicious breast lesions detected initially with MR imaging: value of MRI-directed breast ultrasound. Acad Radiol. 2008;15:618–625. doi: 10.1016/j.acra.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Liberman L, Bracero N, Morris E, Thornton C, Dershaw DD. MRI-guided 9-gauge vacuum-assisted breast biopsy: initial clinical experience. AJR. 2005;185:183–193. doi: 10.2214/ajr.185.1.01850183. [DOI] [PubMed] [Google Scholar]
- 5.Han BK, Schnall MD, Orel SG, Rosen M. Outcome of MRI-guided breast biopsy. AJR. 2008;191:1798–1804. doi: 10.2214/AJR.07.2827. [DOI] [PubMed] [Google Scholar]
- 6.Hefler L, Casselman J, Amaya B, et al. Follow-up of breast lesions detected by MRI not biopsied due to absent enhancement of contrast medium. Eur Radiol. 2003;13:344–346. doi: 10.1007/s00330-002-1713-7. [DOI] [PubMed] [Google Scholar]
- 7.Brennan SB, Sung JS, Dershaw DD, Liberman L, Morris EA. Cancellation of MR imaging-guided breast biopsy due to lesion nonvisualization: frequency and follow-up. Radiology. 2011;261:92–99. doi: 10.1148/radiol.11100720. [DOI] [PubMed] [Google Scholar]
- 8.Perlet C, Heinig A, Prat X, et al. Multicenter study for the evaluation of a dedicated biopsy device for MR-guided vacuum biopsy of the breast. Eur Radiol. 2002;12:1463–1470. doi: 10.1007/s00330-002-1376-4. [DOI] [PubMed] [Google Scholar]
- 9.Viehweg P, Bernerth T, Kiechle M, et al. MR-guided intervention in women with a family history of breast cancer. Eur J Radiol. 2006;57:81–89. doi: 10.1016/j.ejrad.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Johnson KS, Baker JA, Lee SS, Soo MS. Cancelation of MRI guided breast biopsies for suspicious breast lesions identified at 3.0 T MRI: reasons, rates, and outcomes. Acad Radiol. 2013;20:569–575. doi: 10.1016/j.acra.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Kuhl CK, Leutner C, Mielcarek P, Gieseke J, Schild HH. Breast compression interferes with lesion enhancement in contrast-enhanced breast MR imaging. Radiology. 1997;205(P):538. (abstract) [Google Scholar]
- 12.Delille JP, Slanetz PJ, Yeh ED, Kopans DB, Garrido L. Physiologic changes in breast magnetic resonance imaging during the menstrual cycle: perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. Breast J. 2005;11:236–241. doi: 10.1111/j.1075-122X.2005.21499.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuhl CK, Bieling HB, Gieseke J, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclicalphase dependency. Radiology. 1997;203:137–144. doi: 10.1148/radiology.203.1.9122382. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Dershaw DD, Lee CH, Kaplan J, Morris EA. MRI follow-up after concordant, histologically benign diagnosis of breast lesions sampled by MRI-guided biopsy. AJR. 2009;193:850–855. doi: 10.2214/AJR.08.2226. [DOI] [PubMed] [Google Scholar]
- 15.Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7:18–27. doi: 10.1016/j.jacr.2009.09.022. [DOI] [PubMed] [Google Scholar]