Abstract
Background
Atrial fibrillation is a growing public health problem without adequate therapies. Angiotensin II (Ang II) and reactive oxygen species (ROS) are validated risk factors for atrial fibrillation (AF) in patients, but the molecular pathway(s) connecting ROS and AF is unknown. The Ca2+/calmodulin-dependent protein kinase II (CaMKII) has recently emerged as a ROS activated proarrhythmic signal, so we hypothesized that oxidized CaMKIIδ(ox-CaMKII) could contribute to AF.
Methods and Results
We found ox-CaMKII was increased in atria from AF patients compared to patients in sinus rhythm and from mice infused with Ang II compared with saline. Ang II treated mice had increased susceptibility to AF compared to saline treated WT mice, establishing Ang II as a risk factor for AF in mice. Knock in mice lacking critical oxidation sites in CaMKIIδ (MM-VV) and mice with myocardial-restricted transgenic over-expression of methionine sulfoxide reductase A (MsrA TG), an enzyme that reduces ox-CaMKII, were resistant to AF induction after Ang II infusion.
Conclusions
Our studies suggest that CaMKII is a molecular signal that couples increased ROS with AF and that therapeutic strategies to decrease ox-CaMKII may prevent or reduce AF.
Keywords: atrial fibrillation, arrhythmia mechanisms, calcium/calmodulin-dependent protein kinase II, angiotensin II, reactive oxygen species
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia. AF produces lifestyle-limiting symptoms and increases the risk of stroke and death,1 but current therapies have limited efficacy. The renin-angiotensin-system is upregulated in cardiovascular disease and elevated Angiotensin II (Ang II) favors AF.2,3 Ang II activates NADPH oxidase, leading to increased ROS and fibrillating atria are marked by increased reactive oxygen species (ROS).4,5 We recently identified the multifunctional Ca2+ and calmodulin-dependent protein kinase II (CaMKII) as a ROS sensor6 and proarrhythmic signal.7 Oxidation of critical methionines (281/282) in the CaMKII regulatory domain lock CaMKII into a constitutively active, Ca2+ and calmodulin-independent conformation that is associated with cardiovascular disease.8 Based on this information, we asked if oxidized CaMKII (ox-CaMKII) could be a biomarker and proarrhythmic signal for connecting increased atrial ROS to AF. We found that ox-CaMKII was increased in atrial tissue from patients with AF compared to patients in sinus rhythm, and in atrial tissue from Ang II-infused, compared to saline-infused, mice. We used a validated mouse model of AF induction by rapid right atrial pacing9,10 and found that mice with prior Ang II infusion were at significantly higher risk of AF compared to vehicle-infused mice. We tested AF induction in Ang II and vehicle-infused mice with genetically engineered resistance to CaMKII oxidation by knock-in replacement of methionines 281/282 with valines in CaMKIIδ (MM-VV), the isoform associated with cardiovascular disease11–14 or by myocardial-targeted antioxidant therapy by transgenic over-expression of methionine sulfoxide reductase A (MsrA), an enzyme that reduces ox-CaMKII.15,16 Collectively, our results support a view that Ang II promotes AF induction by increasing ROS, ox-CaMKII, CaMKII activity, sarcoplasmic reticulum Ca2+ leak and delayed afterdepolarizations (DADs). Our findings provide novel insights into a ROS and Ang II-dependent mechanism of AF by linking oxidative stress to dysfunctional intracellular Ca2+ signaling via ox-CaMKII and identify a potential new approach for treating AF by targeted antioxidant therapy.
Methods
Human samples and immunodetection of ox-CaMKII
The human samples were provided by the Georg-August-University Goettingen and the University of Heidelberg after approval by the local ethics committee of the Georg-August-University Göttingen and the Medical Faculty Mannheim, University of Heidelberg (#2011-216N-MA). Each patient gave written informed consent. The investigation conforms to the principles outlined in the Declaration of Helsinki. Right atrial appendage tissue samples were obtained from patients undergoing thoracotomy with sinus rhythm or with AF (Table 1) as published previously.17 For immunostaining experiments a total of 9 samples were studied including 5 patients with sinus rhythm and 4 patients with AF (Table 1A). For immunoblotting a total of 51 samples were studied including 25 patients with SR and 26 patients with AF (Table 1B). The patient charts were reviewed by the authors to obtain relevant clinical information. See Supplemental Material for detailed methods.
Table 1.
Summary of patient characteristics. A. Patient characteristics for immunofluorescence studies in Figure 1A and B. B. Patient characteristics for immunoblotting experiments in Figure 1C–F. Values are mean ± SEM or N (%).
| Variable | A | B | ||
|---|---|---|---|---|
| Sinus Rhythm (N=5) |
Atrial Fibrillation (N=4) |
Sinus Rhythm (N=25) |
Atrial Fibrillation (N=26) |
|
| Age (years) | 67±6 | 72±3 | 70±2 | 74±1 |
| Males | 4 (80.0) | 3 (75.0) | 11 (44.0) | 15 (57.7) |
| Rhythm | ||||
| Paroxysmal AF | 0 (0.0) | 5 (19.2) | ||
| Persistent/Permanent AF | 4 (100.0) | 15 (57.7) | ||
| Unclassified AF | 0 (0.0) | 6 (23.1) | ||
| Surgery | ||||
| CABG | 19 (76.0) | 8 (30.8) | ||
| Valve surgery | 2 (8.0) | 9 (34.6) | ||
| CABG + Valve surgery | 4 (16.0) | 8 (30.8) | ||
| Unknown | 5 (100.0) | 4 (100.0) | 1 (3.8) | |
| Diseases | ||||
| Coronary artery disease | 3 (60.0) | 1 (25.0) | 22 (88.0) | 19 (73.1) |
| Hypertension | 4 (80.0) | 4 (100.0) | 17 (68.0) | 15 (57.7) |
| Valve disease | 1 (20.0) | 3 (75.0) | ||
| Diabetes | 1 (20.0) | 0 (0.0) | 6 (24.0) | 5 (19.2) |
| Stroke | 5 (20.0) | 4 (15.4) | ||
| Hyperlipidemia | 5 (100.0) | 2 (50.0) | ||
| Unknown | 1 (3.8) | |||
| Drug treatment | ||||
| ACEI/ARBs | 2 (40.0) | 2 (50.0) | 19 (76.0) | 18 (69.2) |
| Beta blocker | 3 (60.0) | 4 (100.0) | 17 (68.0) | 20 (76.9) |
| Ca channel blocker | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Amiodarone | 0 (0.0) | 1 (3.8) | ||
| Peripheral Calcium channel blocker | 4 (16.0) | 9 (34.6) | ||
| Diuretics | 8 (32.0) | 20 (76.9) | ||
| Statins | 4 (80.0) | 3 (75.0) | 18 (72.0) | 10 (38.5) |
| Unknown | 1 (3.8) | |||
| Echocardiography | ||||
| LA diameter (mm) | 42.3±3 | 51.7±1* | ||
| EF >45% | 13 (52.0) | 10 (38.5) | ||
| EF 35–45% | 7 (28.0) | 5 (19.2) | ||
| EF <35% | 0 (0.0) | 2 (7.7) | ||
| EF (average %) | 57.6±5.7 | 47.5±7.5 | 50±2 | 48±2 |
p<0.05, Student’s t-test, sinus rhythm versus atrial fibrillation (AF) from the same panel.
Mouse Models and Experimental Methods
All mice used in the study were available to us in C57Bl6 background. All experiments were performed in male mice 8–12 weeks of age. In total we studied 262 mice. Numbers for each experimental group are provided in the figures or figure legends. See Supplemental Material for detailed methods.
Statistics
Data are presented as mean ± SEM. P values were assessed with a Student’s t-test (2-tailed), ANOVA or two-way ANOVA, as appropriate, for continuous data. The effect of Ang II compared to saline on ox-CaMKII, CaMKII, and ox-CaMKII/CaMKII ratio was tested within each mouse genotype (strain) and compared among the four genotypes using the two-way analysis of variance (ANOVA). The factors that were tested in the ANOVA model were genotype (WT, MM-VV, p47−/− and MsrA TG), treatment (Ang II versus saline), and genotype treatment interaction effect. A significant genotype treatment interaction (*) indicated that the effect of Ang II (versus saline) differed significantly among the strains. Post hoc comparisons after ANOVA were performed using the Bonferroni test. Discrete variables were analyzed by Fisher’s exact test. Statistical analyses were made with GraphPadPrism or SAS version 3.9, and the null hypothesis was rejected for p ≤ 0.05. See Supplemental Material for detailed methods.
Results
Oxidized CaMKII is increased in AF
Patients with AF have increased atrial CaMKII activity18,19 and high circulating levels of serum markers for oxidative stress.4,5 We first obtained right atrial tissue from patients undergoing cardiac surgery (Table 1) and measured ox-CaMKII using a validated antiserum against oxidized Met 281/282 in the CaMKII regulatory domains.6 These pilot immunofluorescence studies on atrial tissue samples made available upon consent by patients with AF or normal sinus rhythm (Table 1A) showed significantly (p<0.05) higher (~2.5 fold) ox-CaMKII levels in patients with AF (Figure 1A and B). Based on these initial findings, we measured ox-CaMKII in atrial tissue from a larger cohort of patients (Table 1B; for complete gels see supplementary Figure 1) in sinus rhythm (N = 25) or AF (N = 26) using Western blots, and confirmed that AF patients have significantly elevated expression of ox-CaMKII, while there was no difference in total CaMKII (Figure 1C–F). The patient characteristics in the two groups (Table 1) were similar in terms of age, presence of hypertension, diabetes and left ventricular ejection fraction, recognized risk factors for AF.20 The subgroup of AF patients that were not treated with angiotensin converting enzyme inhibitor (ACE-i) or angiotensin receptor blockers (ARB) showed the highest levels of ox-CaMKII and total CaMKII (Supplementary Figure 1A and B). Taken together, these findings showed a positive association between AF and increased expression of atrial ox-CaMKII and a loss of this association in AF patients treated with ACE-i or ARBs.
Figure 1.
ox-CaMKII is increased in atria from patients with Atrial Fibrillation (AF). A. Representative immunofluorescence images using antiserum against ox-CaMKII in fixed sections of right atrial tissue from patients with sinus rhythm (SR) or AF. B. Image quantification showing significantly higher ox-CaMKII in patients with AF compared to SR (*p<0.05, Student’s t-test). C. Representative immunoblots with ox-CaMKII antiserum in right atrial tissue homogenates from patients in SR or AF. D. Quantification of immunoblots showing significantly higher ox-CaMKII expression in patients with AF compared to SR (*p<0.05, Student’s t-test). The % value indicates the mean ox-CaMKII/GAPDH ratio as normalized to the mean ox-CaMKII/GAPDH ratio in the SR group. E. Representative immunoblots with total CaMKII antiserum in right atrial tissue homogenates from patients in SR or AF. F. Quantification of immunoblots showing similar total CaMKII expression in patients with AF and SR (p=0.3, Student’s t-test). The % value indicates the mean CaMKII/GAPDH ratio as normalized to the mean CaMKII/GAPDH ratio in the SR group. The numerals shown in the bars indicate the sample size in each group, here and in subsequent figures.
Ang II treatment enhances AF susceptibility
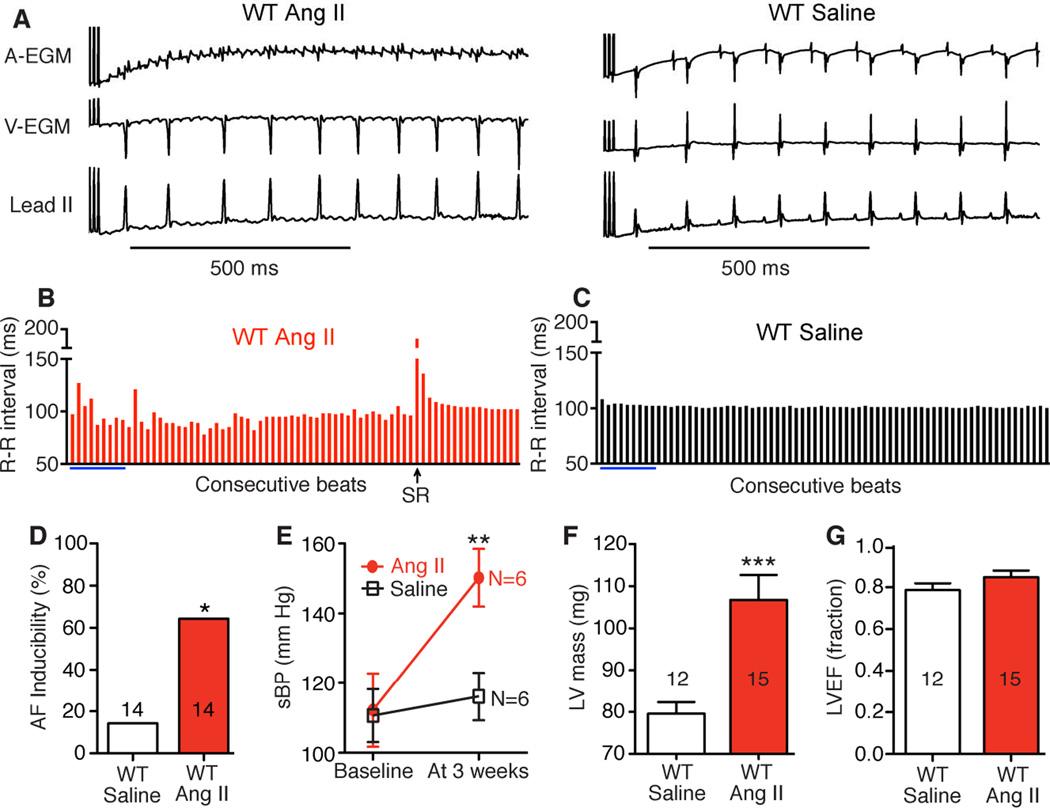
To test the hypothesis that ox-CaMKII contributes to AF we developed a mouse model of AF by infusing wild type (WT) mice with Ang II (2000 ng/kg/min) or an equal volume of normal saline via osmotic mini-pumps for three weeks. We previously established that this dose of Ang II caused a significant increase in atrial ox-CaMKII7 and resulted in serum Ang II levels similar to those measured in heart failure patients.21 In order to test if Ang II treatment can promote AF we performed burst pacing in the right atrium of anesthetized mice, using an established method (Figure 2A–C).10 Mice treated with Ang II showed significantly higher AF induction rates compared to saline treated mice (64% [9/14] versus 18% [2/14], p=0.018 Fisher’s exact test) (Figure 2D). Ang II is known to contribute to hypertension, left ventricular hypertrophy and heart failure, all established clinical risk factors for AF.20 Therefore, we measured blood pressure (BP) by tail-cuff and assessed left ventricular size and systolic function by echocardiography. As expected, Ang II treatment significantly increased systolic BP (Figure 2E; p<0.01) and left ventricular mass (Figure 2F; p<0.001). Ang II treated mice maintained a normal left ventricular ejection fraction, similar to saline-infused control mice (Figure 2G). These data showed that Ang II infusion increased the susceptibility of mice to AF induction by rapid right atrial pacing and established a framework for us to test the hypothesized role of ox-CaMKII in promoting AF.
Figure 2.
Ang II treatment increases AF inducibility in WT mice. A. Representative atrial (AEGM) and ventricular (V-EGM) intracardiac electrograms and lead II surface ECG immediately after burst pacing show AF or SR in WT mice treated with Ang II or saline for 3 weeks. B. Contrasting R-R interval variability in AF and SR (C). Blue bars indicate calculated values from lead II ECGs shown in panel A. D. Higher AF inducibility in the Ang II treatment group (*p<0.05, Fisher’s exact test). E. Increase in systolic blood pressure (sBP) in WT mice after 3 weeks of Ang II treatment (**p<0.01, Student’s t-test). The numerals shown in the graph indicate the number of mice in each group. F. Significantly higher echocardiographically estimated left ventricular (LV) mass in Ang II treated mice compared to saline controls (***p<0.001, Student’s t-test). G. Similar LV ejection fraction (LVEF) in Ang II and saline treated mice.
ox-CaMKII is critical for AF
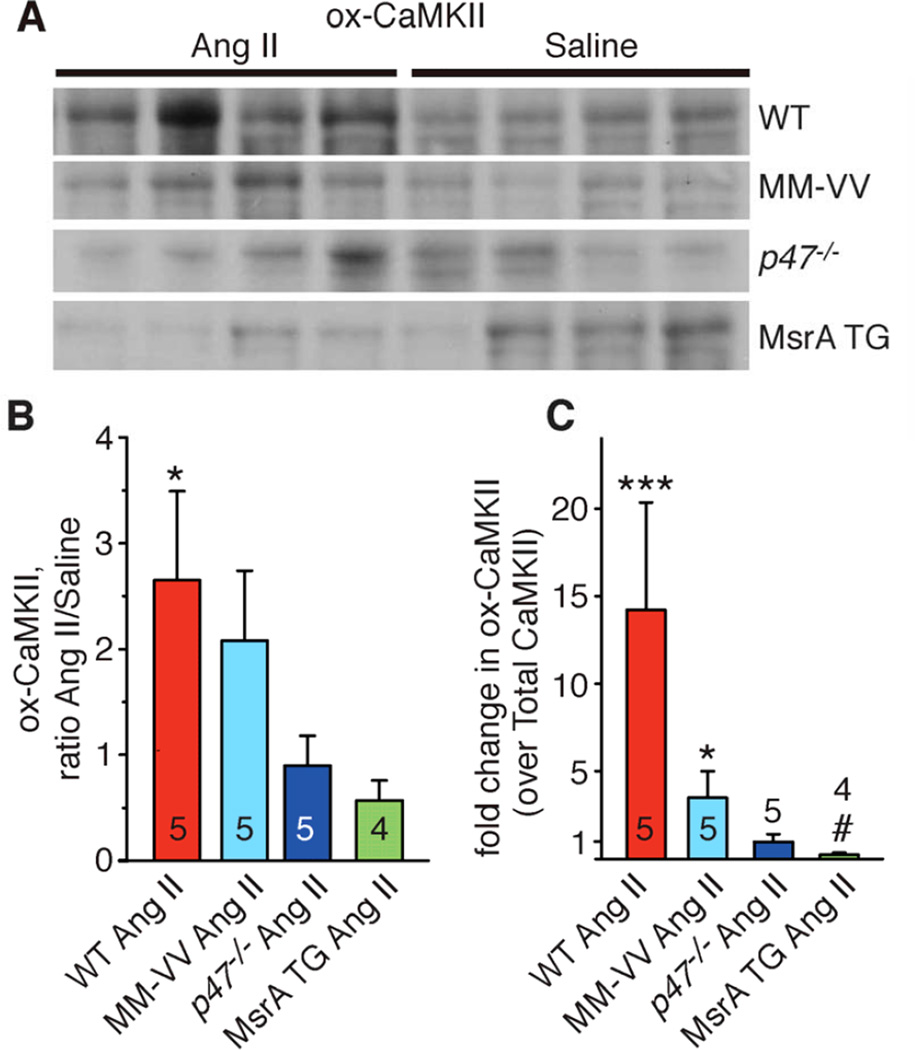
In order to test if ox-CaMKII was required for AF induction in our model we used oxidation resistant knock in MM-VV mice (Supplementary Figure 2).22 CaMKII with the MM-VV mutation is resistant to oxidative activation but retains normal Ca2+ and calmodulin dependent activation and is capable of transitioning into a Ca2+ and calmodulin independent enzyme after threonine 287 autophosphorylation.6 The MM-VV mice were significantly resistant to AF induction after Ang II infusion, compared to WT controls (Figure 3A), suggesting that ox-CaMKII is required for increased AF susceptibility in Ang II infused mice. WT mice treated with Ang II showed significantly higher (~2.7 fold; 95% confidential interval, CI: 1.4, 5.1) levels of atrial ox-CaMKII compared to saline treated mice. As expected, Ang II infusion increased ox-CaMKII less in MM-VV (~2.1 fold; 95% CI: 1.1, 4.0) than in control WT mice (Figure 4A and B). When indexed to total CaMKII levels (Supplementary Figure 3A and B) this increase in ox-CaMKII was much higher (~14.2 fold; 95% CI: 5.9, 34.5) in Ang II treated WT mice (Figure 4C). The residual increase in ox-CaMKII in the MM-VV mice likely results from expression of CaMKIIδ, a myocardial CaMKII isoform not affected by the MM-VV mutation.23 However, despite the greater increase in ox-CaMKII in WT compared to MM-VV mice, Ang II-related ROS production was increased in both WT and MM-VV mice to a similar degree (Supplementary Figure 4). Interestingly, Ang II treated WT mice showed a significant decrease in total CaMKII levels (Supplementary Figure 3A and B) suggesting feedback inhibition of total CaMKII expression.
Figure 3.
CaMKII oxidation is critical to Ang II mediated AF. A. MM-VV, p47−/− and MsrA TG mice were resistant to Ang II mediated AF (*p<0.05 versus Ang II treated MM-VV, p47−/− and MsrA TG mice, Fisher’s exact test). B. All mice in panel A (WT, MM-VV, p47−/− and MsrA TG) showed a pressor response to Ang II. C. Ang II treatment induced cardiac hypertrophy as assessed by heart weight normalized to body weight (all comparisons versus saline controls from each genotype after 3 weeks of treatment; *p<0.05, ** p<0.01 and ***p<0.001, Student’s t-test).
Figure 4.
ox-CaMKII in atria after Ang II or saline treatment. A. Atrial lysate immunoblots from WT, MM-VV, p47−/− and MsrA TG mice treated with Ang II or saline for 3 weeks and probed with an antiserum for ox-CaMKII. For quantification, ox-CaMKII bands were normalized to the total protein loading as assessed with Coomassie staining of the membrane. B. Increase in ox- CaMKII with Ang II treatment expressed as relative to the saline treated group. From each genotype 4 saline treated mice were used as controls. *p<0.05, for WT Ang II versus WT saline (*), in all other genotypes Ang II versus saline p>0.05; in addition, p=0.02 for WT Ang II versus MsrA TG Ang II and p=0.05 for MM-VV Ang II versus MsrA TG Ang II. C. Fold change in ox- CaMKII (over total CaMKII) in Ang II as relative to saline treated mice of the same genotype. From each genotype 4 saline treated mice were used as controls. ***p<0.001 versus WT saline, *p<0.05 versus MM-VV saline, #p<0.05 versus MsrA TG saline. WT Ang II versus p47−/− Ang II, P = 0.001, WT Ang II versus MsrA TG Ang II, P<.0001, MM-VV Ang II versus MsrA TG Ang II, P=0.001. Data were analyzed using two-way ANOVA (for treatment and genotype) with Bonferroni post-hoc comparisons.
Atrial lysates from MM-VV mice showed significantly less Ca2+ and calmodulin-independent activity after Ang II treatment, but retained WT level CaMKII activity increases in response to isoproterenol (Supplementary Figure 2A). At 8 weeks MM-VV mice had body weight (Supplementary Figure 2B) and BP (Figure 3B) that were similar to WT mice, suggesting CaMKIIδ methionine 281/282 oxidation did not affect basal BP or developmentally appropriate growth. CaMKII is known to regulate the chronotropic response to stress and mice with CaMKII inhibition have a smaller increase in heart rate with isoproterenol treatment compared to controls.24 Isolated Langendorff-perfused hearts from WT and MM-VV mice had similar resting heart rates (Supplementary Figure 2C) and comparable heart rate increases after isoproterenol treatment (Supplementary Figure 2D), suggesting that CaMKII dependent physiological heart rate increases do not require CaMKIIδ methionine oxidation. L-type Ca2+ currents were similar in MM-VV and WT mice, and L-type Ca2+ current facilitation, a CaMKII-dependent phenotype, was also preserved in MM-VV mice.25,26 KN-93, a small molecule CaMKII inhibitor,27 significantly reduced facilitation in WT and MM-VV mice (Supplementary Figure 5). MM-VV mice and WT controls showed similar increases in systolic BP (Figure 3B) and heart weight (Figure 3C) or left ventricular mass estimated by echocardiography after Ang II infusion (Supplementary Figure 6), suggesting that ox-CaMKIIδ is dispensable for hypertensive and myocardial hypertrophic actions of Ang II. Taken together, these findings indicate loss of methionines 281/282 in CaMKIIδ selectively reduce the pro-arrhythmic actions of Ang II in a pacing-induced model of AF.
NADPH oxidase and MsrA regulate ox-CaMKII and AF susceptibility
Ang II increases intracellular ROS in myocardium by activating NADPH oxidase and p47−/− mice28, lacking functional NADPH oxidase, are resistant to Ang II dependent increases in ROS and ox-CaMKII.6 Atrial lysates from Ang II treated p47−/− mice did not show an increase in ox-CaMKII (Figure 4), and the p47−/− mice were also resistant to Ang II-mediated increases in AF (Figure 3A), but showed similar increases in BP (Figure 3B), overall heart weight (Figure 3C) and estimated left ventricular mass (Supplementary Figure 6) after Ang II treatment compared to WT controls. ox-CaMKII is reduced by MsrA15 and transgenic mice with myocardial-delimited MsrA overexpression (MsrA TG) have increased atrial MsrA protein (Supplementary Figure 3C) and are resistant to ROS induced myocardial injury.16 We found that Ang II treated MsrA TG mice showed decreased AF induction compared to Ang II-treated WT mice (Figure 3A) and had similar atrial ox-CaMKII expression compared to saline treated controls (Figure 4). Ang II induced increases in ROS production seen in WT atria were absent in atria from MsrA TG mice (Supplementary Figure 4), suggesting that MsrA sensitive targets represent an important component of Ang II mediated atrial oxidation. The protection from AF in MsrA TG mice appeared to be independent of pressor effects of Ang II, because MsrA TG and WT mice showed similar increases in BP (Figure 3B). Taken together, these findings suggest that NADPH oxidase dependent ROS and elevated ox-CaMKII are critical for the proarrhythmic actions of Ang II in pacing-induced AF and that targeted antioxidant therapy, by MsrA over-expression, can reduce or prevent AF in Ang II-infused mice.
Ang II increases Ca2+ sparks and triggered action potentials
CaMKII contributes to increased sarcoplasmic reticulum Ca2+ leak in mice with a RyR2 mutation modeled after a human arrhythmia syndrome, catecholaminergic polymorphic ventricular tachycardia,9 in a goat model of AF and in atrial myocytes isolated from patients with AF.18,29 Atrial myocytes from patients with AF show increased CaMKII activity and increased CaMKII-dependent ryanodine receptor phosphorylation at serine 2814.29 Furthermore, CaMKII inhibition with KN-93 reduced the open probability of single RyR2 channels and prevented the increased frequency of sarcoplasmic reticulum Ca2+ sparks in atrial myocardium biopsied from AF patient.18,29 Based on this knowledge, we asked if increased RyR2 Ca2+ leak also contributed to the mechanism of AF in WT Ang II infused mice and measured diastolic Ca2+ sparks, a marker of RyR2 Ca2+ leak.30 Atrial myocytes from Ang II treated WT mice showed a significant (p<0.05) increase in spontaneous Ca2+ sparks compared to atrial myocytes from saline treated control mice (Figure 5A and B). Other Ca2+ spark parameters and sarcoplasmic reticulum Ca2+ content were not different between the saline and Ang II treated WT mice (Supplementary Figure 7). In contrast to findings in WT mice, the atrial myocytes isolated from Ang II treated MM-VV mice did not show an increase in Ca2+ sparks compared to saline treated MM-VV mice (Figure 5A and B). A significantly greater proportion of atrial myocytes isolated from Ang II treated WT mice showed DADs, compared to atrial myocytes from saline treated mice (Figure 5C and D, p=0.03; Fisher's exact test). In contrast, atrial myocytes from Ang II infused MM-VV mice did not show a significant increase in DADs compared to the atrial myocytes from saline treated MM-VV mice. We interpret these data to suggest that the proarrhythmic effects of Ang II infusion depend upon an increase in ox-CaMKII, sarcoplasmic reticulum Ca2+ leak and DADs.
Figure 5.
Ang II promotes Ca2+ sparks and DADs. A. Representative examples of Ca2+ sparks in atrial myocytes from Ang II and saline treated WT and MM-VV mice. B. Summary of Ca2+ spark frequency data in atrial myocytes from Ang II treated mice compared to saline treated mice (*p<0.05 versus saline; Student’s t-test); WT saline (N=23 cells from 5 mice), WT Ang II (N=30 cells from 4 mice), MM-VV saline (N=36 cells from 4 mice) and MM-VV Ang II (N=28 cells from 4 mice). C. Examples of stimulated action potentials and a spontaneous, DAD triggered action potential. D. Higher incidence of DADs in atrial myocytes from Ang II treated WT mice (*p<0.05 versus saline, Fisher’s exact test) but not in Ang II treated MM-VV mice compared to saline controls. Numerals show cells with DADs/total cells studied for each group.
Mice with CaMKII-resistant RyR2 are protected from AF after Ang II infusion
Enhanced CaMKII-mediated phosphorylation of serine 2814 on RyR2 is associated with an increased susceptibility to acquired arrhythmias, including AF.31 Based on our findings that atrial myocytes from Ang II infused WT mice developed more Ca2+ sparks than atrial myocytes from saline-infused mice, we hypothesized that the proarrhythmic actions of ox-CaMKII require access to RyR2 serine 2814. We tested this hypothesis by treating mutant S2814A knock-in mice (lacking serine 2814)9 with Ang II or saline and performing right atrial burst pacing. The S2814A mice were highly resistant to Ang II mediated AF (Figure 6A). Similarly, AC3-I mice with transgenic myocardial expression of a CaMKII inhibitory peptide32 were also resistant to the proarrhythmic effects of Ang II infusion on pacing-induced AF (Figure 6A). S2814A, AC3-I and WT mice, all developed similar BP increases (Figure 6B) and cardiac hypertrophy (Figure 6C) in response to Ang II, indicating that these mice were not resistant to the hemodynamic effects of Ang II, but were nevertheless protected from AF.
Figure 6.
CaMKII activation and RyR2 serine 2814 are required for AF in Ang II infused mice. A. AC3-I and S2814A mice were treated with Ang II for 3 weeks and then burst paced to induce AF. AC3-I and S2814A mice were resistant to Ang II mediated AF promotion compared to WT Ang II treated mice (*p<0.05 versus all, Fisher’s Exact test, N=number of mice tested in each group). B. AC3-I and S2814A mice show similar systolic blood pressure (sBP) elevation after treatment with Ang II. Final sBP measurements were performed on three consecutive days prior to AF induction as shown in panel A. The numerals in the graph indicate the number of mice in each group. C. Ang II treatment causes similar cardiac hypertrophy in AC3-I and S2814A mice compared to saline controls (***p<0.001 versus AC3-I saline and **p=0.01 versus S2814A saline).
Discussion
AF usually develops in patients with underlying structural heart disease, such as left ventricular hypertrophy, coronary artery disease, valve disease and congestive heart failure.20 Elevated ROS is a common feature of these conditions.33 The dose of Ang II used in our model produces a fourfold increase in plasma Ang II compared to saline controls,7 similar to increases in Ang II observed in heart failure patients compared to non-hypertensive controls.21 Despite the extensive evidence of elevated ROS in structural heart disease, clinical trials with antioxidants have generally been unsatisfactory.34–36 One potential obstacle to developing effective antioxidant therapies is lack of detailed understanding of molecular pathways that are affected by ROS. The renin-angiotensin-system is one of the best understood pathways that contributes to ROS production in AF patients.37 In the current study, we created a model of AF by infusing mice with Ang II for three weeks and assembled a cohort of genetically altered mice to rigorously test a novel molecular pathway that links oxidative stress to AF (Figure 7). Our current study provides strong evidence that CaMKII is a critical ROS sensor for transducing increased ROS into enhanced AF susceptibility in mice and suggests that atrial ox-CaMKII could contribute to AF in patients.
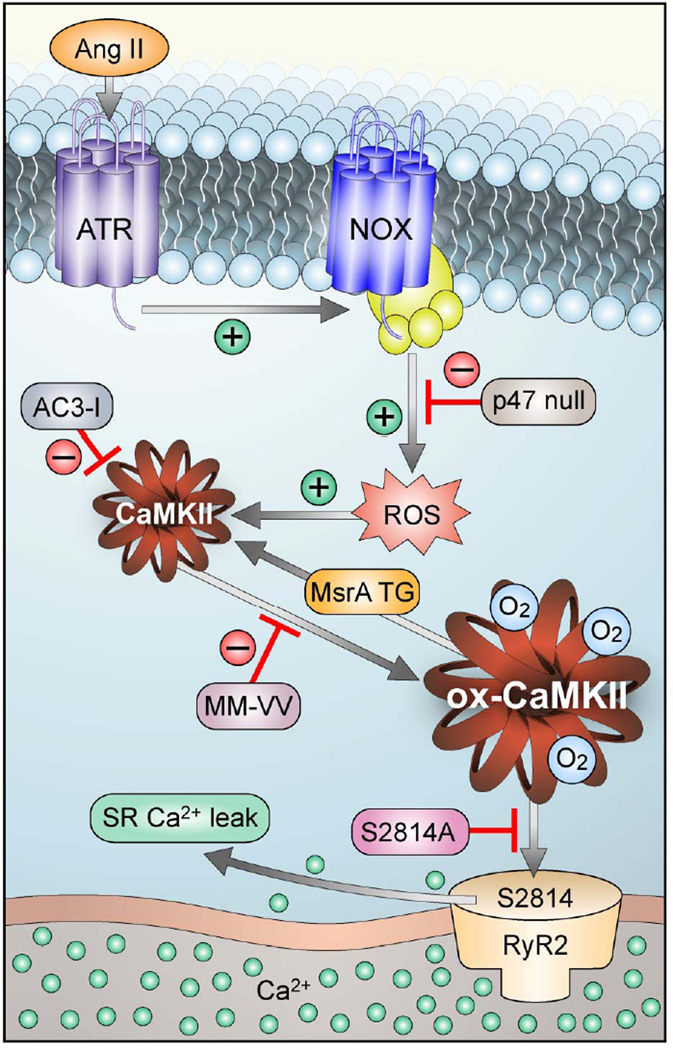
Figure 7.
Schematic to illustrate the proposed mechanism of AF in Ang II infused mice. Ang II binding activates NADPH oxidase (NOX) to increase reactive oxygen species (ROS), leading to oxidation of methionines 281/282 in CaMKII (ox-CaMKII). Elevated ox-CaMKII phosphorylates serine 2814 on RyR2, causing enhanced diastolic Ca2+ leak that promotes AF triggering DADs. Genetically modified mice were used to test key steps of the proposed pathway.
CaMKII and increased ROS are now widely recognized to contribute to cardiac arrhythmias.8,38,39 Recent studies suggest that patients with persistent AF have elevated markers of oxidative stress in serum4 and depleted levels of atrial glutathione.40 Under increased oxidative stress CaMKII is activated by oxidation of methionines (M281/282),6 which lock it into a constitutively active conformation, suggesting a possible role for ox-CaMKII as a ROS activated proarrhythmic signal in AF.39 Our laboratory recently demonstrated that ox-CaMKII plays a major role in sinus node dysfunction,7,22 adverse post-myocardial infarct remodeling6 and cardiac rupture16. In the current study, we investigated the role of ox-CaMKII in AF. Human atria (Figure 1) and Ang II treated WT mouse atria showed significantly elevated ox-CaMKII (Figure 4). Atrial myocytes from Ang II treated WT mice had a higher frequency of spontaneous Ca2+ sparks and DADs compared to controls (Figure 5). Based on these findings we hypothesized that oxidation of methionines 281/282 on CaMKIIδ causes diastolic sarcoplasmic reticulum Ca2+ leak and DADs, cellular AF triggers. To definitively test this hypothesis we used a recently developed knock-in mouse (MM-VV) where CaMKIIδ, the myocardial CaMKII isoform implicated in myocardial disease,12,13 is resistant to oxidative activation.22 Ang II treatment did not increase Ca2+ and calmodulin independent CaMKII activity (Supplementary Figure 2A), Ca2+ sparks (Figure 5A and B), DADs (Figure 5C and D) or enhance AF susceptibility in MM-VV mice (Figure 3A). It is important to note that the MM-VV mutant form of CaMKIIδ selectively ablates the response to oxidation while retaining other aspects of CaMKII molecular physiology, such as activation by Ca2+ and calmodulin and constitutive activation by threonine 287 autophosphorylation.6 Thus, the residual AF observed in Ang II infused MM-VV mice could be a result of non-oxidation-dependent mechanisms for CaMKIIδ activation in our model. We found that atrial tissue from AF patients treated with ACE-i or ARBs did not show elevated ox-CaMKII, suggesting that Ang II stimulation oxidizes CaMKII in human atria and that ox-CaMKII independent pathways are operative in AF patients. AF in patients is more complex than AF in our Ang II infused mice. In particular, patients present with variable chronicity, tissue and structural changes. In contrast the triggers for our mice are uniform (i.e. Ang II infusion and rapid right atrial pacing) and result in a similar, modest degree of hypertrophy. We interpret the data showing that an increase in ox-CaMKII in AF patients is reduced or eliminated by clinical antagonist drugs that reduce Ang II signaling to validate our findings in mice that Ang II increases ox-CaMKII. However, we suppose that the presence of AF in patients on ACE-i or ARBs means that other pathways also result in AF. Our sample is not powered to ask if AF resistance to Ang II antagonist drugs represents later stage disease, but this is our hypothesis. Furthermore, CaMKII can be activated independently of oxidation, although oxidation appears to be the primary pathway for activating CaMKII during Ang II infusion. Thus, it is unknown if CaMKII is also important for AF progression in the group of patients treated by Ang II antagonist drugs who exhibit normal levels of ox-CaMKII.
Although we did not see higher total CaMKII in AF patients (as compared with patients in sinus rhythm), the sub-group of AF patients who were not treated with ACE-i or ARBs did show significantly elevated CaMKII levels, supporting prior studies that reported elevated CaMKII activity in AF18,19. In contrast to the situation in patients, total CaMKII expression was reduced in mice after sub-acute Ang II infusion. While the mechanism(s) for the variable response of CaMKII expression in mice and patients is unclear, the change in CaMKII expression in mice and in humans in response to manipulation of the Ang II pathway supports the idea that CaMKII is a fundamental component of Ang II signaling. The relatively small number of patient samples is not powered for analysis of AF subtypes, but human AF may transition from paroxysmal to persistent and permanent (chronic) forms.41 In contrast, our mouse model is simpler because it is triggered by a single upstream event (i.e. Ang II infusion) and elicited in a highly controlled environment by rapid atrial pacing. The resistance of MM-VV mice to AF provides new evidence that oxidative activation of CaMKIIδ is important for initiation of AF, while the finding that ox-CaMKII is elevated in atrial tissue from AF patients and particularly in AF patients naive to Ang II antagonist therapies suggests this pathway may also participate in human AF. Thus, our findings in MM-VV mice provide strong, mechanistic evidence that ox-CaMKII plays a critical role in proarrhythmic responses to Ang II. Our studies showed that mice deficient in NADPH oxidase (p47−/−) and mice expressing increased MsrA are also resistant to AF (Figure 3A), suggesting that selectively targeted antioxidant therapies could be effective in preventing or reducing AF.
CaMKII has the potential to catalyze phosphorylation of diverse targets, such as ion channels and Ca2+ homeostatic proteins, and is therefore poised to promote AF by several candidate mechanisms.42 RyR2 serine 2814 is a validated CaMKII phosphorylation target and multiple recent studies show that serine 2814 phosphorylation promotes diastolic sarcoplasmic reticulum Ca2+ release with the potential to trigger atrial and ventricular arrhythmia triggering DADs, including AF.31 Increased frequency of atrial ectopic impulses and AF are positively associated43 and this relationship may be important in our mouse model of Ang II infusion and pacinginduced AF. RyR2R176Q/+ knock-in mice have diastolic sarcoplasmic reticulum Ca2+ leak and rapid atrial pacing increases phosphorylation of RyR2 serine 2814 leading to significantly more AF than in WT mice.9 We found that S2814A mice were highly resistant to Ang II mediated AF (Figure 6A). Our studies do not rule out a potential contribution of other CaMKII substrate proteins in AF,16,44 but do support the conclusion that RyR2 serine 2814 is an important downstream CaMKII target for proarrhythmic actions of Ang II.
Clinically, there appears to be an overlap in sinus node dysfunction and AF.45–47 Almost half of patients enrolled in the Mode Selection Trial (MOST) with sinus node dysfunction had a history of AF48, but a clear mechanistic link between increased risk of AF and sinus node dysfunction is unknown. In recent studies we showed that Ang II and diabetes-induced CaMKII oxidation caused sinus node dysfunction by increased pacemaker cell death and fibrosis,7 while MM-VV mice are resistant to sinus node dysfunction evoked by hyperglycemia.22 Here we provide evidence that ox-CaMKII increases susceptibility for AF via increased diastolic sarcoplasmic reticulum Ca2+ release, showing that the proarrhythmic actions of ox-CaMKII may occur in cardiomyocytes by increasing sarcoplasmic reticulum Ca2+ leak or by enhanced cell death. Our findings suggest that the clinical association between sinus node dysfunction and AF might have a mechanistic basis because sinus node dysfunction and AF are downstream consequences of elevated ox-CaMKII.
Supplementary Material
Clinical Commentary.
Atrial fibrillation is associated with hyperactivity of renin-angiotensin II signaling, enhanced oxidant stress and increased activity of the multifunctional Ca2+ and calmodulin-dependent protein kinase II (CaMKII). Excessive CaMKII activity promotes arrhythmia initiation by enhancing Ca2+ leak from intracellular stores. We recently identified a mechanism whereby CaMKII is activated by oxidation of regulatory domain methionines in response to angiotensin II stimulation and, motivated by these findings, developed new mouse models to test the potential role of oxidation activated CaMKII in atrial fibrillation. We identified increased oxidized CaMKII in atria from patients with atrial fibrillation compared to non-fibrillating controls and determined that atrial fibrillation patients treated with ACE inhibitors or angiotensin receptor blockers (ARBs) did not have increased atrial oxidized CaMKII. These findings suggested that CaMKII is oxidized by the renin-angiotensin II pathway and is associated with atrial fibrillation in the subgroup of patients not treated with ACE inhibitors or ARBs. Angiotensin II infused mice also showed increased atrial oxidized CaMKII and high rates of atrial fibrillation after rapid right atrial pacing. In contrast, genetically engineered mice with oxidation resistant CaMKII and mice with atrial over-expression of a methionine reducing enzyme, methionine sulfoxide reductase A, were resistant to atrial fibrillation induction, intracellular Ca2+ leak and to angiotensin II infusion induced increases in oxidized CaMKII. These findings suggest CaMKII is a critical component of a proarrhythmic oxidant pathway and that CaMKII inhibition could be an effective antioxidant and antiarrhythmic therapy.
Acknowledgements
We are thankful to Dr Miriam B. Zimmerman for her help with statistical analysis of the data. We are also thankful to Kathy Zimmerman and Melissa Davis who performed mouse echocardiograms. We acknowledge Jinying Yang for her assistance in maintaining mouse colonies. We thank Chantal Allamargot and the University of Iowa Microscopy Research Facility for their assistance in microscopic imaging and Shawn Roach for graphic art. The authors thank cardiac surgeons of Heart Centers Göttingen and Heidelberg for kind provision of human atrial tissue samples.
Funding Sources: This work was funded by National Institutes of Health (NIH) Grants R01-HL 079031, R01-HL096652, and R01-HL070250, R01-HL071140 (to MEA), RR026293, the European Union (large-scale FP7 grant “European Network for Translational Research in Atrial Fibrillation, EUTRAF, to DD), the University of Iowa Research Foundation, two Fondation Leducq grants (“Transatlantic Alliance for CaMKII Signaling”, 08CVD01 to MEA, LSM, XHTW and “European-North American Atrial Fibrillation Research Alliance”, 07CVD03, to DD), and by DZHK (German Center for Cardiovascular Research to D.D.). AP’s salary was supported by an American Heart Association (AHA) post-doctoral fellowship grant 10POST3620047. AGR is supported by a Career Development Award from the Fondation Leducq. NL’s salary was supported by AHA grant 12BGIA12050207. XHTW is supported by AHA grant 13EIA14560061 and NIH grants R01-HL089598 and R01-HL091947. PDS’s salary was supported by a Kenneth M Rosen Fellowship and Max Schaldach Fellowship from the Heart Rhythm Society and University of Iowa Cardiovascular Research Center Interdisciplinary Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The manuscript and its contents are confidential, intended for journal review purposes only, and not to be further disclosed.
Conflict of Interest Disclosures: MEA is a cofounder of Allosteros Therapeutics, a biotech aiming to develop enzyme inhibitors to treat arrhythmias.
References
- 1.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 2.Khatib R, Joseph P, Briel M, Yusuf S, Healey J. Blockade of the renin-angiotensin-aldosterone system (RAAS) for primary prevention of non-valvular atrial fibrillation: A systematic review and meta analysis of randomized controlled trials. Int J Cardiol. 2013;165:17–24. doi: 10.1016/j.ijcard.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 4.Shimano M, Shibata R, Inden Y, Yoshida N, Uchikawa T, Tsuji Y, Murohara T. Reactive oxidative metabolites are associated with atrial conduction disturbance in patients with atrial fibrillation. Heart Rhythm. 2009;6:935–940. doi: 10.1016/j.hrthm.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Neuman RB, Bloom HL, Shukrullah I, Darrow LA, Kleinbaum D, Jones DP, Dudley SC. Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007;53:1652–1657. doi: 10.1373/clinchem.2006.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson JR, Joiner M-LA, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham A-JL, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner M-LA, Kutschke W, Yang J, Donahue JK, Weiss RM, Grumbach IM, Ogawa M, Chen P-S, Efimov I, Dobrev D, Mohler PJ, Hund TJ, Anderson ME. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011;121:3277–3288. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev. 2011;91:889–915. doi: 10.1152/physrev.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Müller FU, Schmitz W, Schotten U, Anderson ME, Valderrábano M, Dobrev D, Wehrens XHT. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verheule S, Sato T, Everett T, Engle SK, Otten D, Rubart-von der, Lohe M, Nakajima HO, Nakajima H, Field LJ, Olgin JE. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94:1458–1465. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Bers DM, Heller Brown J. The delta C isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 12.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Heller Brown J. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA, Katus HA, Bassel-Duby R, Maier LS, Olson EN. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci USA. 2009;106:2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott JA, Xie L, Li H, Li W, He JB, Sanders PN, Carter AB, Backs J, Anderson ME, Grumbach IM. The multifunctional Ca2+/Calmodulin-dependent kinase II regulates vascular smooth muscle migration through matrix metalloproteinase 9. Am J Physiol Heart Circ Physiol. 2012;302:H1953–H1964. doi: 10.1152/ajpheart.00978.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci USA. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He BJ, Joiner M-LA, Singh MV, Luczak ED, Swaminathan PD, Koval OM, Kutschke W, Allamargot C, Yang J, Guan X, Zimmerman K, Grumbach IM, Weiss RM, Spitz DR, Sigmund CD, Blankesteijn WM, Heymans S, Mohler PJ, Anderson ME. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17:1610–1618. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voigt N, Trausch A, Knaut M, Matschke K, Varró A, Van Wagoner DR, Nattel S, Ravens U, Dobrev D. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhyth Electrophysiol. 2010;3:472–480. doi: 10.1161/CIRCEP.110.954636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schöndube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 19.Tessier S, Karczewski P, Krause EG, Pansard Y, Acar C, Lang-Lazdunski M, Mercadier JJ, Hatem SN. Regulation of the transient outward K+ current by Ca2+/calmodulin-dependent protein kinases II in human atrial myocytes. Circ Res. 1999;85:810–819. doi: 10.1161/01.res.85.9.810. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 21.Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation. 1990;82:1730–1736. doi: 10.1161/01.cir.82.5.1730. [DOI] [PubMed] [Google Scholar]
- 22.Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest. 2013;123:1262–1274. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer HA, Benscoter HA, Schworer CM. Novel Ca2+/calmodulin-dependent protein kinase II gamma-subunit variants expressed in vascular smooth muscle, brain, and cardiomyocytes. J Biol Chem. 1997;272:9393–9400. doi: 10.1074/jbc.272.14.9393. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Gao Z, Chen B, Koval OM, Singh MV, Guan X, Hund TJ, Kutschke W, Sarma S, Grumbach IM, Wehrens XHT, Mohler PJ, Song L-S, Anderson ME. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci USA. 2009;106:5972–5977. doi: 10.1073/pnas.0806422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 26.Koval OM, Guan X, Wu Y, Joiner ML, Gao Z, Chen B, Grumbach IM, Luczak ED, Colbran RJ, Song LS, Hund TJ, Mohler PJ, Anderson ME. CaV1.2 -subunit coordinates CaMKII-triggered cardiomyocyte death and afterdepolarizations. Proc Natl Acad Sci USA. 2010;107:4996–5000. doi: 10.1073/pnas.0913760107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun. 1991;181:968–975. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- 28.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XHT, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 31.Wehrens XHT. CaMKII regulation of the cardiac ryanodine receptor and sarcoplasmic reticulum calcium release. Heart Rhythm. 2011;8:323–325. doi: 10.1016/j.hrthm.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R, Khoo MSC, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Thiel W, Guatimosim S, Song L-S, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 33.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hare JM, Mangal B, Brown J, Fisher C, Freudenberger R, Colucci WS, Mann DL, Liu P, Givertz MM, Schwarz RP. OPT-CHF Investigators. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J Am Coll Cardiol. 2008;51:2301–2309. doi: 10.1016/j.jacc.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 35.Marchioli R, Levantesi G, Macchia A, Marfisi RM, Nicolosi GL, Tavazzi L, Tognoni G, Valagussa F. GISSI-Prevenzione Investigators. Vitamin E increases the risk of developing heart failure after myocardial infarction: Results from the GISSI-Prevenzione trial. J Cardiovasc Med (Hagerstown) 2006;7:347–350. doi: 10.2459/01.JCM.0000223257.09062.17. [DOI] [PubMed] [Google Scholar]
- 36.Negi S, Shukrullah I, Veledar E, Bloom HL, Jones DP, Dudley SC. Statin Therapy for the Prevention of Atrial Fibrillation Trial (SToP AF trial) J Cardiovasc Electrophysiol. 2010;22:414–419. doi: 10.1111/j.1540-8167.2010.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iravanian S, Dudley SC. The renin-angiotensin-aldosterone system (RAAS) and cardiac arrhythmias. Heart Rhythm. 2008;5:S12–S17. doi: 10.1016/j.hrthm.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie L-H, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomaselli GF, Barth AS. Sudden cardio arrest: oxidative stress irritates the heart. Nat Med. 2010;16:648–649. doi: 10.1038/nm0610-648. [DOI] [PubMed] [Google Scholar]
- 40.Carnes CA, Janssen PML, Ruehr ML, Nakayama H, Nakayama T, Haase H, Bauer JA, Chung MK, Fearon IM, Gillinov AM, Hamlin RL, Van Wagoner DR. Atrial glutathione content, calcium current, and contractility. J Biol Chem. 2007;282:28063–28073. doi: 10.1074/jbc.M704893200. [DOI] [PubMed] [Google Scholar]
- 41.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 42.Dobrev D, Carlsson L, Nattel S. Novel molecular targets for atrial fibrillation therapy. Nat Rev Drug Discov. 2012;11:275–291. doi: 10.1038/nrd3682. [DOI] [PubMed] [Google Scholar]
- 43.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 44.Anderson ME. Multiple downstream proarrhythmic targets for calmodulin kinase II: moving beyond an ion channel-centric focus. Cardiovasc Res. 2007;73:657–666. doi: 10.1016/j.cardiores.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Healey JS, Martin JL, Duncan A, Connolly SJ, Ha AH, Morillo CA, Nair GM, Eikelboom J, Divakaramenon S, Dokainish H. Pacemaker-Detected Atrial Fibrillation in Patients With Pacemakers: Prevalence, Predictors, and Current Use of Oral Anticoagulation. Can J Cardiol. 2013;29:224–228. doi: 10.1016/j.cjca.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 46.Chang HY, Lin YJ, Lo LW, Chang SL, Hu YF, Li CH, Chao TF, Yin WH, Chen SA. Sinus node dysfunction in atrial fibrillation patients: the evidence of regional atrial substrate remodelling. Europace. 2013;15:205–211. doi: 10.1093/europace/eus219. [DOI] [PubMed] [Google Scholar]
- 47.Lee JMS, Kalman JM. Sinus node dysfunction and atrial fibrillation: two sides of the same coin? Europace. 2013;15:161–162. doi: 10.1093/europace/eus223. [DOI] [PubMed] [Google Scholar]
- 48.Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee R, Marinchak RA, Flaker G, Schron E, Orav EJ, Hellkamp AS, Greer S, McAnulty J, Ellenbogen K, Ehlert F, Freedman RA, Estes NAM, III, Greenspon A, Goldman L. Ventricular Pacing or Dual-Chamber Pacing for Sinus-Node Dysfunction. N Engl J Med. 2002;346:1854–1862. doi: 10.1056/NEJMoa013040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.