Abstract
Calbindin-D9k (CaBP-9k) binds calcium with high affinity and regulates the distribution of free calcium in the cytoplasm. The expression of CaBP-9k is detected primarily in intestine that is vitamin D target tissue, and accumulates in the enterocytes of the duodenal villi. These enterocytes are the clearest example of vitamin D responsive cells, and the presence of CaBP-9k within them accentuates calcium absorption mediated by active transcellular calcium transport. It has been well established that the expression of CaBP-9k is mediated with vitamin D response element on its promoter and it regulates the amount of intracellular calcium in order to prevent cell death from reaching the toxicity of free calcium. There is now little doubt that glucocorticoid also decreases CaBP-9k expression in duodenal epithelial cells. In addition, it was reported that the level of CaBP-9k gene in enterocytes is increased in pregnancy when the plasma estradiol concentration is generally associated with a concomitant increase. Although calcium homeostasis was not disturbed in mice lacking the CaBP-9k gene, we found that CaBP-9k has a buffering role of free calcium in the cytosolic environment beyond that of calcium transfer. To expand our knowledge of the biological functions of CaBP-9k, our research has focused on defining the biological significance of intracellular CaBP-9k. Our findings suggest that the CaBP-9k gene is involved in compensatory induction of other calcium transporter genes in duodenal epithelial cells. This article summarizes the findings from recent studies on the expression and the functions of CaBP-9k in the small intestine.
Keywords: calbindin, transcellular pathway, vitamin D receptor, duodenum, calcium absorption, knockout mice
1. Introduction
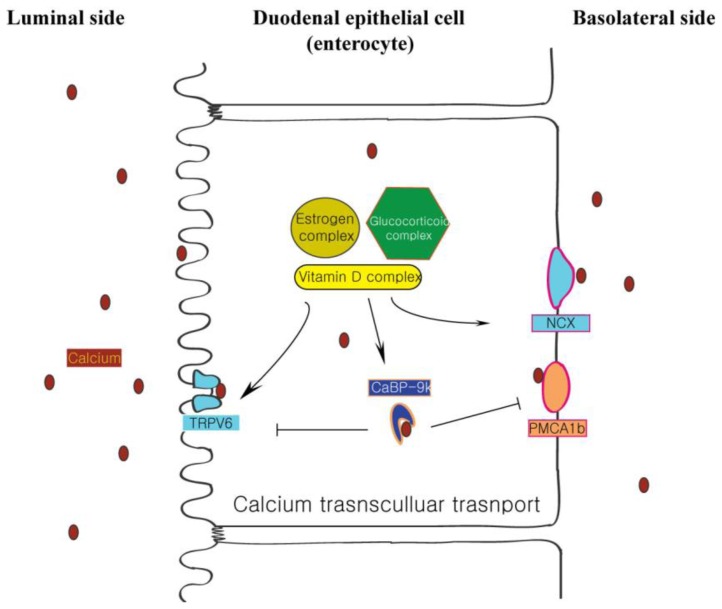
Calcium homeostasis refers to the regulation of the concentration of calcium ions in the body, and impairment of this mechanism contributes to their underlying pathologies, such as hypercalcemia or hypocalcemia. When calcium is absorbed in the intestine along either transcellular or paracellular routes, the transcellular transport of calcium ion occurred mainly in the upper intestine including the duodenum and upper jejunum. As a component of active transcelluar trasnport process, CaBP-9k (S100G in human) was originally described as a vitamin D-dependent calcium-binding protein in the intestine [1], and it binds intracellular calcium with high affinity in the cytoplasm [2]. In addition, there is evidence that CaBP-9k regulates the level of intracellular free calcium in order to prevent this mineral from reaching toxic levels [3]. Traditionally, it was thought that intracellular binding proteins transfer cytosolic calcium from the apical membrane to the basolateral membrane via trans-cellular transport of calcium [4], while calcium entry is facilitated by apical transient receptor potential vanilloid 6 (TRPV6), and calcium export is promoted by plasma membrane Ca2+-ATPase 1b (PMCA1b) as shown in Figure 1. PMCA1b facilitates the excretion of calcium ions using adenosine triphosphate (ATP) hydrolysis [5]. Like PMCA1b, NCX1 exchanges outer sodium ions for inner calcium ions [6,7]. In order to understand the influence of CaBP-9k related to other calcium related proteins, we focused our attention on the expression and function of CaBP-9k within duodenal epithelial cells.
Figure 1.
CaBP-9k of transcellular calcium transport in duodenal enterocyte. In the transportation, calcium binds to membrane bound TRPV6, which influxes from the extracellular environment. The calcium translocates into the cytoplasm and binds to calcium binding protein CaBP-9k. The calcium-bound CaBP-9k may also move to the basolateral side and transfer its bound calcium to PMCA1b or NCX channel protein, leading to uptake into serum.
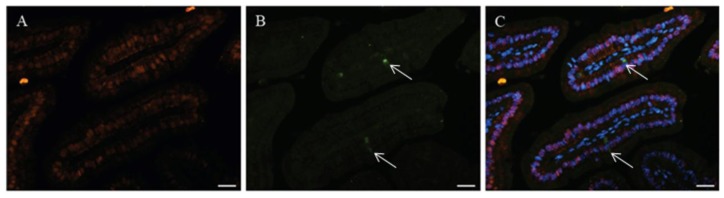
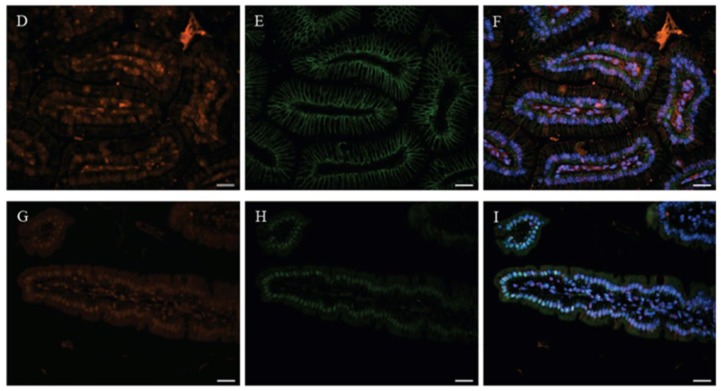
The duodenum is the first part of the small intestine in which digested nutrients are absorbed by the epithelial layer. Although the jejunum and ileum are also other sites of dietary calcium absorption via paracellular absorption [8,9], the presence of calcium-related proteins in duodenal epithelial cells also promotes uptake of calcium across the epithelium and its transport into the blood stream via transcellular absorption. We have previously shown that in the porcine intestine CaBP-9k is highly expressed in the duodenum. CaBP-9k expression decreases gradually aborally to undetectable in the distal ileum [10]. In the intestinal epithelium, three differentiated types of cells along the villus are organized with enterocytes, goblet cells, and enteroendocrine cells [11]. As intestinal absorptive cells, enterocytes are terminally differentiated cells comprising the majority of the intestinal epithelium. To further define which epithelial cell types within the duodenum express CaBP-9k, we examined in our unpublished study the duodenum of mice by double-label immuno-histochemical studies with anti-mouse CaBP-9k antibody and antibody directed against chromogranin A (enteroendocrine cell marker) or E-cadhedrin (epithelial cell maker). As previously reported for enterocyte [12], immune-reactive CaBP-9k presents in the majority of the intestinal epithelium as shown in Figure 2, and this suggest that mouse CaBP-9k in the duodenum might be confined to enterocytes, the major population of duodenal epithelial cells, because other cell types in the epithelium are present in 2%–3% population of epithelial cells in the villus.
Figure 2.
Immuno-histochemical analyses in duodenal villi of mice. A to F, immunoreactive CaBP-9k appears in the cytoplasm of many populations of epithelial cells within the duodenal villi (A and D, red) at 100× magnification by immunofluorescence detection. To identify the co-localization between CaBP-9k and other proteins, the specific antibodies for chromogranin A (B, green) and E-cadhedrin (E, green) were co-incubated with anti-mouse CaBP-9k antibodies using corresponding Alexa-Fluor conjugated secondary antibodies. DAPI (blue) was used for nuclei staining. Based on DAPI signals, co-localization of CaBP-9k and each protein is evident by superimposition (C and F) of the green and red signals, respectively. HNF-4α (nuclear protein marker, G, Red) used in an attempt to identify the GR (H, green) expression in duodenal enterocytes, both images were merged and observed as blue (I). Scale bar = 20 μm.
2. Vitamin D-Dependent CaBP-9k Expression in the Small Intestine
It is generally accepted that vitamin D3 plays a key role in calcium uptake, and there is evidence that vitamin D3 is activated by 25-hydroxylation and 1α-hydroxylation in the liver and kidney, respectively. Metabolized calcitriol (1,25-dihydroxyvitamin D3) enters enterocytes by passive diffusion and interacts with the nuclear vitamin D receptor (VDR), which heterodimerizes with the retinoid X receptor [13]. Knockout mice lacking VDR or 1α-hydroxylase have previously been used for evaluation of the effects of vitamin D on expression of the calcium transport gene and transcellular calcium transport [14,15]. Because calcitriol is essential for intestinal calcium absorption [16], this calciotropic hormone facilitates maintenance of calcium homeostasis via the VDR by acting on the vitamin D response element (VDRE) in target genes, such as CaBP-9k [17] and TRPV6 [18]. More importantly, VDR is expressed in epithelial cells involved in calcium absorption. Using knockout mouse models, deficiencies in plasma calcitriol or intracellular VDR expression were found to show an association with the development of rickets and hypocalcemia [14,19]. In 1α-hydroxylase knockout mice, reduced plasma calcium concentrations are restored to normal levels by exogenous calcitriol, suggesting that this hormone induces calcium uptake mediated by expression of calcium transporters [20]. Therefore, it was concluded that expression of CaBP-9k mRNA and protein is induced by calcitriol in the intestine due to the presence of a VDRE in the CaBP-9k gene promoter [21]. Expression of the CaBP-9k mRNA in the intestine is substantially reduced in mice lacking 1α-hydroxylase, a metabolic enzyme for the production of calcitriol [14]. Regardless of the presence of calcitriol, intestinal CaBP-9k mRNA and protein was reduced in VDR-null mice [22], and this observation suggests that CaBP-9k transcription is mediated by binding of ligand-bound VDR to the VDRE located within the promoter regions. Although vitamin-D-mediated regulation is involved in reproductive tissues [19,23], we confirmed that CaBP-9k mRNA does not respond to vitamin-deficient diet or active vitamin D despite the presence of VDR in the uterus, as described previously [24].
To further determine whether the calcium-binding properties of CaBP-9k in gastrointestinal tissues are responsible for the enhanced VDR expression, we explored the link between CaBP-9k and VDR expression in human intestine. Following the increase of CaBP-9k mRNA and decrease of blood calcium revealed in an age-dependent manner, our study shows that the link between age and VDR mRNA expression in the human duodenum does not appear to correlate [25]. While an earlier study reported that the levels of active vitamin D (calcitriol) are reduced in an age-dependent manner [26], we failed to address the link between CaBP-9k levels and calcium absorption because calcium absorption declines even when the level of CaBP-9k mRNA remains high in the duodenal epithelium [25]. These results imply that CaBP-9k may not be a critical factor for duodenal calcium absorption, and the latter was confirmed by our previous study using a CaBP-9k knockout mouse model [27]. Although the calcium absorption does not correspond with CaBP-9k expression in the duodenum, it is still considered that CaBP-9k has a role as a transport protein because it increases the total amount of intracellular calcium [9].
3. Other Physiological Factors that Affect CaBP-9k Expression
Glucocorticoids have been reported to inhibit calcium absorption by reducing active transport and brush border uptake [28,29]. Although renal CaBP-9k levels were significantly decreased in adrenalectomized mice from which endogenous cortisol had been removed by a surgical method, we did not observe any difference in expression of intestinal CaBP-9k mRNA or protein, compared with sham-operated animals. It is widely accepted that glucocorticoids reduce intestinal calcium absorption by inhibition of active calcium transcellular transport and brush border vehicle uptake [29,30]. In addition, cortisol reduced the production of intestinal calcium binding protein in chicks [31]. Previous studies have reported that the glucocorticoid receptor (GR) is highly expressed in epithelial cells of the duodenum, and glucocorticoid appears to control differentiation and mineral absorption of the epithelial cell [32,33]. We observed that accumulation of CaBP-9k occurs within the cytoplasm of the same duodenal epithelium where we detected GR expression in the nuclei as shown in Figure 2. After treatment with dexamethasone, used as a GR agonist, the level of CaBP-9k expression was substantially decreased in duodenal enterocyte [34]. We also demonstrated that the reduced expression of duodenal CaBP-9k via dexamethasone administration is reversed by treatment with a GR antagonist [34], and our data all suggest that the ligand-bound GR decreased CaBP-9k expression in duodenal epithelial cells. In addition to the intestine, we also observed that the reduction of renal CaBP-9k expression in adrenalectomized mice was restored by administration of dexamethasone [34]. Renal CaBP-9k proteins expressed in the distal convoluted tubule have been suspected of active calcium reabsorption [34], like a function of CaBP-28K which has been implicated in active calcium absorption in the kidney [35].
We have observed that CaBP-9k expression is induced in the uterus and pituitary glands by endogenous/exogenous estrogens [36–38]. Although the expression and regulation of CaBP-9k in these tissues have been well described, the regulation of CaBP-9k expression remains obscure in the duodenum. At least, the promoter region of CaBP-9k gene contains a putative estrogen receptor-binding site located in the first intron [39]. In an earlier study, the regulation of CaBP-9k expression resulted in response in the rat uterus by estrogen, but not in the intestine [40]. Nevertheless, after menopause estrogen deficiency is associated with increased renal calcium loss [41]. Interestingly, there is considerable evidence that estrogen has a physiological role in regulation of intestinal calcium absorption. According to one report, estrogen might promote calcium absorption by increasing the expression of apical channels in duodenal epithelial cells [12]. In our recent study, the expression of duodenal CaBP-9k mRNA was disrupted by exogenous estrogen or an estrogenic compound during late pregnancy, when endogenous estrogen production shows a significant increase [42]. This effect of exogenous compound may disrupt the activity of the estrogen receptor, which controls an estrogen-regulated CaBP-9k expression.
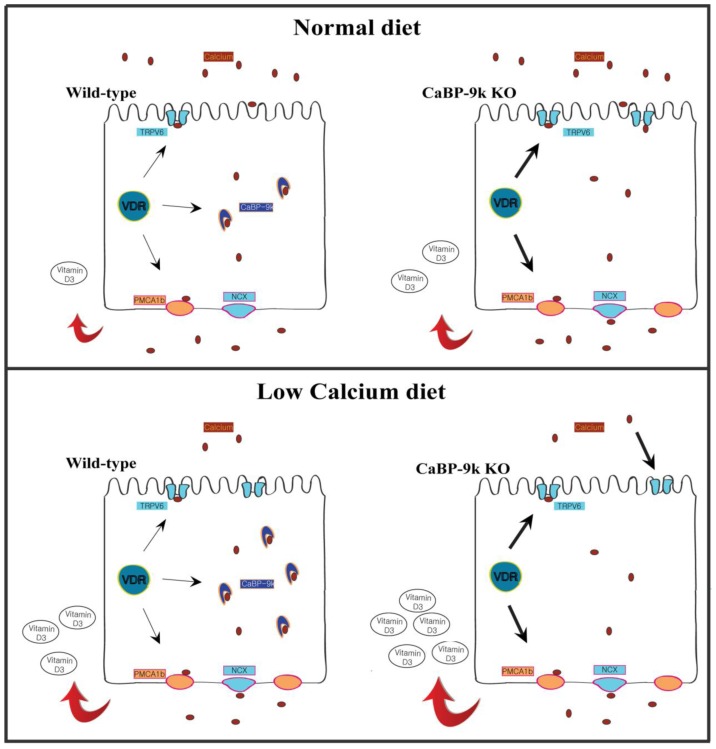
Since the intestinal calcium transporting system was activated in mice fed a low-calcium diet, dietary calcium is also considered to be another regulator of TRPV6 or CaBP-9k gene expression [43]. For example, TRPV6 or CaBP-9k mRNA expression of wild-type mice was higher in a low-calcium diet compared to a normal-calcium diet as shown in Figure 3. In addition, the TRPV6 expression in CaBP-9k-null mice was also higher than that of wild-type mice when the mice were fed a normal diet. CaBP-9k was also reported to regulate calcium influx across the TRPV channel by buffering intracellular calcium [44]. When the animals were fed a low calcium diet, the serum calcitriol level was significantly increased in CaBP-9k null mice compared with the wild-type mice [27]. Intuitively, one might suspect that the absence of CaBP-9k gene would accelerate the production of calcitriol in the kidney, because this hormone is generally considered to be the main regulator of TRPV6 expression. It was also reported that the repression of PMCA1b expression was shown by calcium deficiency, but NCX1 expression was stimulated in the duodenum by low calcium diet [27]. Furthermore, duodenal PMCA1b mRNA in VDR-null mice was reduced by a calcium deficiency diet [12]. These reports raise the possibility that dietary calcium can regulate the expression of other membrane-bound calcium channels beyond that of intracellular calcium binding protein as shown in Figure 3.
Figure 3.
The role of CaBP-9k in the extracellular and intracellular environment of enterocytes. In calcium absorption, the abolishment of intracellular CaBP-9k stimulates compensative increase of vitamin D, and the increased vitamin D induces the expression of apical or basolateral membrane bound channels. In addition, a low calcium diet may also stimulate the expression of membrane bound channels in CaBP-9k knockout mice.
4. The Influence of Intracellular CaBP-9k in Expression of Other Calcium-Associated Proteins
To further define the role of CaBP-9k in calcium transport, we generated CaBP-9k-null mice and evaluated the phenotype of these animals. While calcium homeostasis is disrupted in VDR-null or 1α-hydroxylase-null mice [20,45], CaBP-9k-null mice were phenotypically normal for the birth and survivality for one year [27]. Since abolishment of CaBP-9k gene did not affect calcium absorption or blood calcium levels in the mice, we searched for other factors that might be compensated by CaBP-9k expression. In an earlier study, it was observed that the role of CaBP-9k is to stimulate the rate of extrusion of calcium via increase of the calcium pump [46]. During the pre-weaning period, we found that the absence of CaBP-9k enhances the compensatory expression of membrane-bound calcium channels, such as TRPV6 and PMCA1b [27]. It has also been demonstrated that calcitriol may be involved in the expression of duodenal CaBP-9k expression, but not involved in that of PMCA1b expression [47]. Since VDR expression of CaBP-9k-null mice was similar to that of wild-type mice, the compensatory induction of TRPV6 expression in CaBP-9k null mice may be related to the level of active vitamin D in the blood (Figure 3). We have explored this expectation in a series of experiments that lead us to conclude that the absence of CaBP-9k within specific cell types, such as renal epithelial cells and duodenal epithelial cells, accentuates the vitamin D environment in the body. We demonstrate also that TRPV6 mRNA in CaBP-9k null mice is halted when the animals are fed a vitamin D3-deficient diet [48]. In addition, the expression of TRPV6 and PMCA1b revealed a compensatory increase in CaBP-9k null mice as shown in Figure 3, and their inductions are reduced by exogenous glucocorticoid, which regulates duodenal VDR transcription in CaBP-9k null mice [49]. While abolishment of CaBP-9k gene did not reveal significant affection for calcium absorption or blood calcium levels, the CaBP-9k could control the blood calcium level via compensatory expression of TRPV6 and PMCA1b. It was reported that intestinal calcium absorption in TRPV6-null mice is limited, and these animals develop calcium deficiency despite activity by calcitriol [50]. Conversely, expression of CaBP-9k mRNA in the intestine of TRPV6-null mice is significantly altered compared to the wild-type counterparts [51]. These findings provide evidence that the absence of intracellular calcium-binding proteins might induce compensatory expression of other genes for maintenance of calcium absorption.
5. Conclusions
Since identification of CaBP-9k as a vitamin D-dependent calcium-binding protein within the intestine, its role has been examined in various physiological and experimental environments. Until recently, CaBP-9k was thought to facilitate calcium transfer through the cytosol or aid in regulation of free calcium levels by mediation of calcium absorption. However, our recent studies demonstrated that CaBP-9k is not essential for active intestinal calcium absorption. The CaBP-9k-null mouse model fails to explain how the absence of CaBP-9k in duodenal epithelial cells directly restricts calcium uptake. Nevertheless, our results from the CaBP-9k-null mouse support the concept that CaBP-9k may play a role in calcium absorption of the cytosolic environment during active calcium transport, which could act as a potential modulator of other membrane-bound calcium channels. Since the genetic inactivation of CaBP-9k gene does not disrupt the calcium homeostasis, this therefore raises the obvious question of whether the generation of a double or triple knockout mouse model could contribute to impair calcium homeostasis.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2013-010514).
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- 1.Wasserman R.H., Taylor A.N. Vitamin D3-induced calcium-binding protein in chick intestinal mucosa. Science. 1966;152:791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]
- 2.Ingersoll R.J., Wasserman R.H. Vitamin D3-induced calcium-binding protein. Binding characteristics, conformational effects, and other properties. J. Biol. Chem. 1971;246:2808–2814. [PubMed] [Google Scholar]
- 3.Schwaller B. Cytosolic Ca2+ buffers. Cold Spring Harb. Perspect. Biol. 2010;2:a004051. doi: 10.1101/cshperspect.a004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoenderop J.G., Nilius B., Bindels R.J. Calcium absorption across epithelia. Physiol. Rev. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- 5.Jensen T.P., Buckby L.E., Empson R.M. Expression of plasma membrane Ca2+ ATPase family members and associated synaptic proteins in acute and cultured organotypic hippocampal slices from rat. Brain Res. Dev. Brain Res. 2004;152:129–136. doi: 10.1016/j.devbrainres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 6.DiPolo R., Beauge L. Sodium/calcium exchanger: Influence of metabolic regulation on ion carrier interactions. Physiol. Rev. 2006;86:155–203. doi: 10.1152/physrev.00018.2005. [DOI] [PubMed] [Google Scholar]
- 7.Yu S.P., Choi D.W. Na+–Ca2+ exchange currents in cortical neurons: Concomitant forward and reverse operation and effect of glutamate. Eur. J. Neurosci. 1997;9:1273–1281. doi: 10.1111/j.1460-9568.1997.tb01482.x. [DOI] [PubMed] [Google Scholar]
- 8.Bronner F., Pansu D. Nutritional aspects of calcium absorption. J. Nutr. 1999;129:9–12. doi: 10.1093/jn/129.1.9. [DOI] [PubMed] [Google Scholar]
- 9.Bronner F., Slepchenko B., Wood R.J., Pansu D. The role of passive transport in calcium absorption. J. Nutr. 2003;133:1426–1427. doi: 10.1093/jn/133.5.1426. [DOI] [PubMed] [Google Scholar]
- 10.Jeung E.B., Krisinger J., Dann J.L., Leung P.C. Cloning of the porcine Calbindin-D9k complementary deoxyribonucleic acid by anchored polymerase chain reaction technique. Biol. Reprod. 1992;47:503–508. doi: 10.1095/biolreprod47.4.503. [DOI] [PubMed] [Google Scholar]
- 11.Crosnier C., Stamataki D., Lewis J. Organizing cell renewal in the intestine: Stem cells, signals and combinatorial control. Nat. Rev. Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 12.Van Cromphaut S.J., Rummens K., Stockmans I., van Herck E., Dijcks F.A., Ederveen A.G., Carmeliet P., Verhaeghe J., Bouillon R., Carmeliet G. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J. Bone Miner. Res. 2003;18:1725–1736. doi: 10.1359/jbmr.2003.18.10.1725. [DOI] [PubMed] [Google Scholar]
- 13.Kimmel-Jehan C., Jehan F., DeLuca H.F. Salt concentration determines 1,25-dihydroxyvitamin D3 dependency of vitamin D receptor-retinoid X receptor—Vitamin D-responsive element complex formation. Arch. Biochem. Biophys. 1997;341:75–80. doi: 10.1006/abbi.1997.9952. [DOI] [PubMed] [Google Scholar]
- 14.Dardenne O., Prud’homme J., Arabian A., Glorieux F.H., St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 2001;142:3135–3141. doi: 10.1210/endo.142.7.8281. [DOI] [PubMed] [Google Scholar]
- 15.Van Cromphaut S.J., Dewerchin M., Hoenderop J.G., Stockmans I., van Herck E., Kato S., Bindels R.J., Collen D., Carmeliet P., Bouillon R., et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: Functional and molecular aspects. Proc. Natl. Acad. Sci. USA. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wasserman R.H., Fullmer C.S. Vitamin D and intestinal calcium transport: Facts, speculations and hypotheses. J. Nutr. 1995;125:1971S–1979S. doi: 10.1093/jn/125.suppl_7.1971S. [DOI] [PubMed] [Google Scholar]
- 17.Colnot S., Ovejero C., Romagnolo B., Porteu A., Lacourte P., Thomasset M., Perret C. Transgenic analysis of the response of the rat calbindin-D9k gene to vitamin D. Endocrinology. 2000;141:2301–2308. doi: 10.1210/endo.141.7.7557. [DOI] [PubMed] [Google Scholar]
- 18.Balesaria S., Sangha S., Walters J.R. Human duodenum responses to vitamin D metabolites of TRPV6 and other genes involved in calcium absorption. Am. J. Physiol. Gastrointest Liver Physiol. 2009;297:1193–1197. doi: 10.1152/ajpgi.00237.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwiecinksi G.G., Petrie G.I., DeLuca H.F. 1,25-Dihydroxyvitamin D3 restores fertility of vitamin D-deficient female rats. Am. J. Physiol. 1989;256:483–487. doi: 10.1152/ajpendo.1989.256.4.E483. [DOI] [PubMed] [Google Scholar]
- 20.Hoenderop J.G., Dardenne O., van Abel M., van der Kemp A.W., van Os C.H., St-Arnaud R., Bindels R.J. Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3-1alpha-hydroxylase knockout mice. FASEB J. 2002;16:1398–1406. doi: 10.1096/fj.02-0225com. [DOI] [PubMed] [Google Scholar]
- 21.Darwish H.M., DeLuca H.F. Identification of a 1,25-dihydroxyvitamin D3-response element in the 5′-flanking region of the rat calbindin D9k gene. Proc. Natl. Acad. Sci. USA. 1992;89:603–607. doi: 10.1073/pnas.89.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y.C., Pirro A.E., Demay M.B. Analysis of vitamin D-dependent calcium-binding protein messenger ribonucleic acid expression in mice lacking the vitamin D receptor. Endocrinology. 1998;139:847–851. doi: 10.1210/endo.139.3.5803. [DOI] [PubMed] [Google Scholar]
- 23.Uhland A.M., Kwiecinski G.G., DeLuca H.F. Normalization of serum calcium restores fertility in vitamin D-deficient male rats. J. Nutr. 1992;122:1338–1344. doi: 10.1093/jn/122.6.1338. [DOI] [PubMed] [Google Scholar]
- 24.L’Horset F., Perret C., Brehier A., Thomasset M. 17 beta-estradiol stimulates the calbindin-D9k (CaBP9k) gene expression at the transcriptional and posttranscriptional levels in the rat uterus. Endocrinology. 1990;127:2891–2897. doi: 10.1210/endo-127-6-2891. [DOI] [PubMed] [Google Scholar]
- 25.Lee G.S., Choi K.C., Park S.M., An B.S., Cho M.C., Jeung E.B. Expression of human Calbindin-D(9k) correlated with age, vitamin D receptor and blood calcium level in the gastrointestinal tissues. Clin. BioChem. 2003;36:255–261. doi: 10.1016/s0009-9120(03)00010-9. [DOI] [PubMed] [Google Scholar]
- 26.Armbrecht H.J., Zenser T.V., Davis B.B. Effect of vitamin D metabolites on intestinal calcium absorption and calcium-binding protein in young and adult rats. Endocrinology. 1980;106:469–475. doi: 10.1210/endo-106-2-469. [DOI] [PubMed] [Google Scholar]
- 27.Lee G.S., Lee K.Y., Choi K.C., Ryu Y.H., Paik S.G., Oh G.T., Jeung E.B. Phenotype of a calbindin-D9k gene knockout is compensated for by the induction of other calcium transporter genes in a mouse model. J. Bone Miner. Res. 2007;22:1968–1978. doi: 10.1359/jbmr.070801. [DOI] [PubMed] [Google Scholar]
- 28.Kimberg D.V., Baerg R.D., Gershon E., Graudusius R.T. Effect of cortisone treatment on the active transport of calcium by the small intestine. J. Clin. Invest. 1971;50:1309–1321. doi: 10.1172/JCI106610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shultz T.D., Bollman S., Kumar R. Decreased intestinal calcium absorption in vivo and normal brush border membrane vesicle calcium uptake in cortisol-treated chickens: Evidence for dissociation of calcium absorption from brush border vesicle uptake. Proc. Natl. Acad. Sci. USA. 1982;79:3542–3546. doi: 10.1073/pnas.79.11.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee D.B. Unanticipated stimulatory action of glucocorticoids on epithelial calcium absorption. Effect of dexamethasone on rat distal colon. J. Clin. Invest. 1983;71:322–328. doi: 10.1172/JCI110772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feher J.J., Wasserman R.H. Intestinal calcium-binding protein and calcium absorption in cortisol-treated chicks: effects of vitamin D3 and 1,25-dihydroxyvitamin D3. Endocrinology. 1979;104:547–551. doi: 10.1210/endo-104-2-547. [DOI] [PubMed] [Google Scholar]
- 32.Bastl C.P., Schulman G., Cragoe E.J., Jr. Low-dose glucocorticoids stimulate electroneutral NaCl absorption in rat colon. Am. J. Physiol. 1989;257:1027–1038. doi: 10.1152/ajprenal.1989.257.6.F1027. [DOI] [PubMed] [Google Scholar]
- 33.Sheppard K.E., Li K.X., Autelitano D.J. Corticosteroid receptors and 11beta-hydroxysteroid dehydrogenase isoforms in rat intestinal epithelia. Am. J. Physiol. 1999;277:541–547. doi: 10.1152/ajpgi.1999.277.3.G541. [DOI] [PubMed] [Google Scholar]
- 34.Lee G.S., Choi K.C., Jeung E.B. Glucocorticoids differentially regulate expression of duodenal and renal calbindin-D9k through glucocorticoid receptor-mediated pathway in mouse model. Am. J. Physiol. Endocrinol. Metab. 2006;290:299–307. doi: 10.1152/ajpendo.00232.2005. [DOI] [PubMed] [Google Scholar]
- 35.Lambers T.T., Mahieu F., Oancea E., Hoofd L., de Lange F., Mensenkamp A.R., Voets T., Nilius B., Clapham D.E., Hoenderop J.G., et al. Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport. EMBO J. 2006;25:2978–2988. doi: 10.1038/sj.emboj.7601186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee G.S., Choi K.C., Kim H.J., Jeung E.B. Effect of genistein as a selective estrogen receptor beta agonist on the expression of Calbindin-D9k in the uterus of immature rats. Toxicol. Sci. 2004;82:451–457. doi: 10.1093/toxsci/kfh296. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen T.H., Lee G.S., Ji Y.K., Choi K.C., Lee C.K., Jeung E.B. A calcium binding protein, calbindin-D9k, is mainly regulated by estrogen in the pituitary gland of rats during estrous cycle. Brain Res. Mol. Brain Res. 2005;141:166–173. doi: 10.1016/j.molbrainres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Seifert M.F., Gray R.W., Bruns M.E. Elevated levels of vitamin D-dependent calcium-binding protein (calbindin-D9k) in the osteosclerotic (oc) mouse. Endocrinology. 1988;122:1067–1073. doi: 10.1210/endo-122-3-1067. [DOI] [PubMed] [Google Scholar]
- 39.Darwish H., Krisinger J., Furlow J.D., Smith C., Murdoch F.E., DeLuca H.F. An estrogen-responsive element mediates the transcriptional regulation of calbindin D9K gene in rat uterus. J. Biol. Chem. 1991;266:551–558. [PubMed] [Google Scholar]
- 40.Delorme A.C., Danan J.L., Acker M.G., Ripoche M.A., Mathieu H. In rat uterus 17 beta-estradiol stimulates a calcium-binding protein similar to the duodenal vitamin D-dependent calcium-binding protein. Endocrinology. 1983;113:1340–1347. doi: 10.1210/endo-113-4-1340. [DOI] [PubMed] [Google Scholar]
- 41.Price N.T., Smith A.J., Rogers L.J. Relationship of the flavodoxin isoforms from. Porphyra umbilicalis. Phytochemistry. 1991;30:2841–2843. doi: 10.1016/s0031-9422(00)98209-8. [DOI] [PubMed] [Google Scholar]
- 42.Kim S., An B.S., Yang H., Jeung E.B. Effects of octylphenol and bisphenol A on the expression of calcium transport genes in the mouse duodenum and kidney during pregnancy. Toxicology. 2013;303:99–106. doi: 10.1016/j.tox.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 43.Brown A.J., Krits I., Armbrecht H.J. Effect of age, vitamin D, and calcium on the regulation of rat intestinal epithelial calcium channels. Arch. BioChem. Biophys. 2005;437:51–58. doi: 10.1016/j.abb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Vennekens R., Hoenderop J.G., Prenen J., Stuiver M., Willems P.H., Droogmans G., Nilius B., Bindels R.J. Permeation and gating properties of the novel epithelial Ca2+ channel. J. Biol. Chem. 2000;275:3963–3969. doi: 10.1074/jbc.275.6.3963. [DOI] [PubMed] [Google Scholar]
- 45.Li Y.C., Pirro A.E., Amling M., Delling G., Baron R., Bronson R., Demay M.B. Targeted ablation of the vitamin D receptor: An animal model of vitamin D-dependent rickets type II with alopecia. Proc. Natl. Acad. Sci. USA. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walters J.R. Calbindin-D9k stimulates the calcium pump in rat enterocyte basolateral membranes. Am. J. Physiol. 1989;256:124–128. doi: 10.1152/ajpgi.1989.256.1.G124. [DOI] [PubMed] [Google Scholar]
- 47.Walters J.R., Howard A., Charpin M.V., Gniecko K.C., Brodin P., Thulin E., Forsen S. Stimulation of intestinal basolateral membrane calcium-pump activity by recombinant synthetic calbindin-D9k and specific mutants. BioChem. Biophys. Res. Commun. 1990;170:603–608. doi: 10.1016/0006-291x(90)92134-l. [DOI] [PubMed] [Google Scholar]
- 48.Lee G.S., Jung E.M., Choi K.C., Oh G.T., Jeung E.B. Compensatory induction of the TRPV6 channel in a calbindin-D9k knockout mouse: Its regulation by 1,25-hydroxyvitamin D3. J. Cell Biochem. 2009;108:1175–1183. doi: 10.1002/jcb.22347. [DOI] [PubMed] [Google Scholar]
- 49.Kim M.H., Lee G.S., Jung E.M., Choi K.C., Oh G.T., Jeung E.B. Dexamethasone differentially regulates renal and duodenal calcium-processing genes in calbindin-D9k and-D28k knockout mice. Exp. Physiol. 2009;94:138–151. doi: 10.1113/expphysiol.2008.044339. [DOI] [PubMed] [Google Scholar]
- 50.Bianco S.D., Peng J.B., Takanaga H., Suzuki Y., Crescenzi A., Kos C.H., Zhuang L., Freeman M.R., Gouveia C.H., Wu J., et al. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J. Bone Miner. Res. 2007;22:274–285. doi: 10.1359/jbmr.061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benn B.S., Ajibade D., Porta A., Dhawan P., Hediger M., Peng J.B., Jiang Y., Oh G.T., Jeung E.B., Lieben L., et al. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology. 2008;149:3196–3205. doi: 10.1210/en.2007-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]