Abstract
We characterized by antibiotic susceptibility, plasmid analysis, incompatibility grouping, and pulsed-field gel electrophoresis (PFGE) of XbaI- and SpeI-digested DNA 102 Salmonella enterica serovar Typhi (serovar Typhi) isolated from recent outbreaks of typhoid in three different parts of Kenya. Only 13.7% were fully susceptible, whereas another 82.4% were resistant to each of the five commonly available drugs: ampicillin, chloramphenicol, and tetracycline (MICs of >256 μg/ml); streptomycin (MIC, >1,024 μg/ml); and cotrimoxazole (MIC of >32 μg/ml). Resistance to these antibiotics was encoded on a 110-kb self-transferable plasmid of IncHI1 incompatibility group. The MICs of nalidixic acid (MIC, 8 to 16 μg/ml) and ciprofloxacin (MIC of 0.25 to 0.38 μg/ml) for 41.7% of the 102 serovar Typhi isolates were 5- and 10-fold higher, respectively, than for sensitive strains. Amplification by PCR and sequencing of the genes coding for gyrase (gyrA and gyrB) and topoisomerase IV (parE and parC) within the quinolone resistance-determining region revealed that the increase in the MICs of the quinolones had not resulted from any significant mutation. Analysis of genomic DNA from both antimicrobial agent-sensitive and multidrug-resistant serovar Typhi by PFGE identified two distinct subtypes that were in circulation in the three different parts of Kenya. As the prevalence of multidrug-resistant serovar Typhi increases, newer, more expensive, and less readily available antimicrobial agents will be required for the treatment of typhoid in Kenya.
Typhoid fever, caused by Salmonella enterica serovar Typhi is endemic in most parts of Central America (7, 20), Southeast Asia (12, 15, 16), and the Indian subcontinent (23, 25), and recently increasing numbers of cases have been reported in Africa (13, 14). Globally, it is estimated that typhoid causes over 16 million cases of illness each year, resulting in over 600,000 deaths (31). Typhoid fever manifests as a prolonged illness, and it is a sometimes fatal infection in both adults and children if it results in inflammatory destruction of intestines and other organs, including the bone marrow.
For more than 40 years since its discovery, chloramphenicol was the drug of choice for the treatment of typhoid. However, the emergence in the late 1980s of multidrug-resistant (MDR) serovar Typhi (isolates resistant to ampicillin, chloramphenicol, and cotrimoxazole) in outbreaks reported in the Indian subcontinent (16, 23, 25), Arabian Gulf, the Philippines (24), and South Africa (5) has led to the use of the fluoroquinolones as alternative drugs (4). Among the first reports of clinical treatment failure due to serovar Typhi resistant to nalidixic acid and showing then an increased ciprofloxacin MIC (0.125 μg/ml) was in 1991 in a patient who had recently returned to the United Kingdom from India (29). Thereafter, several cases of MDR serovar Typhi also resistant to nalidixic acid and the fluoroquinolones have been reported in Bangladesh (2), India (18) Thailand, Vietnam (21), and Tajikistan (17), raising concerns about further spread to other regions where typhoid fever is endemic. In addition, molecular characterization of the serovar Typhi outbreak strains revealed that resistance to commonly used antimicrobial agents, including chloramphenicol, ampicillin, and trimethoprim, was encoded by plasmids of the HI incompatibility group (14, 16, 24).
In most strains of serovar Typhi, resistance to the quinolones has been attributed to point mutations in the genes encoding DNA gyrase (gyrA and gyrB) or DNA topoimerase IV (parC and parE) enzymes, which are located within the quinolone resistance-determining region (QRDR) of the chromosomes of bacteria (9, 11, 22, 23). Recently, MDR serovar Typhi showing resistance to nearly all of the commonly available “first line” antimicrobial agents used for the treatment of these and other infections have been isolated in Kenya (13). Using plasmid and chromosomal DNA typing, the aim of the present study was to characterize MDR serovar Typhi strains isolated from the blood of patients in three different parts of Kenya that reported outbreaks during a period of 2 years and then compare these with isolates from previous outbreaks of typhoid fever.
MATERIALS AND METHODS
Patients.
These were adult admissions to the hospital who came from areas reporting outbreaks of typhoid fever during the period January 2001 to December 2002. Outbreaks were reported at various times during the period from three parts of Kenya: (i) Nairobi Province, with cases of typhoid fever being identified at the main referral hospital, Kenyatta National Hospital, and the Aga Khan Hospital; (ii) Embu District, located ca. 120 km north of Nairobi; and (iii) Thika, located ca. 50 km north of Nairobi. Cases from Embu District and Thika District were treated at the respective Provincial General Hospitals.
Bacterial isolation.
Blood for culture was obtained from suspected cases before antibiotic treatment was commenced and cultured in brain heart infusion broth (Oxoid, Ltd., Basingstoke, United Kingdom) with p-aminobenzoic acid (Sigma-Aldrich, Dorset, United Kingdom). The blood cultures were incubated at 37°C and subcultured when turbid onto sheep blood and MacConkey agar (Oxoid) plates. Bacterial isolates were evaluated by biochemical tests with API 20E strips (bioMerieux, Basingstoke, United Kingdom), and the isolates were then serotyped by using agglutinating antisera (Murex Diagnostics, Dartford, United Kingdom). Serovar Typhi isolates were stored at −70°C on protect beads (Technical Service Consultants, Ltd., Heywood, United Kingdom) until analyzed.
Antibiotic susceptibility testing.
Serovar Typhi isolates were tested for susceptibility to antimicrobials by a controlled disk diffusion technique on IsoSensitest agar (Oxoid) plates containing 5% lysed horse blood. The antibiotic disks (all from Oxoid) contained ampicillin (10 μg), tetracycline (30 μg), cotrimoxazole (1:25 μg), chloramphenicol (30 μg), streptomycin (30 μg), gentamicin (10 μg), co-amoxyclav (20:10 μg), ciprofloxacin (5 μg), cefuroxime (30 μg), ceftriaxone (30 μg), and nalidixic acid (10 μg). MICs of these antibiotics were determined by using the E-test strips (AB Biodisk, Solna, Sweden) according to the manufacturer's instructions. Escherichia coli ATCC 25922 (with known MICs) was used as a control for potency of antibiotics. Disk susceptibility tests and MICs were interpreted according to the guidelines provided by the National Committee for Clinical Laboratory Standards (NCCLS) (19).
Mating experiments.
Mating experiments were performed as described previously (13) with Escherichia coli K-12 (F−, nalidixic acid resistant) as recipient. Briefly, both donor isolates (serovar Typhi resistant to one or more antimicrobial agents, including ampicillin or chloramphenicol) and recipient E. coli K-12 were grown in nutrient broth on a shaker at 37°C for 18 h. Both donor and recipient cultures were diluted 1:10 in freshly prepared and prewarmed (37°C) nutrient broth (Oxoid). Donor and recipient broth cultures were then mixed in equal proportions in 4-ml volumes and incubated without shaking at 37°C for 18 h. Transconjugants were selected on IsoSensitest agar containing either ampicillin and nalidixic acid or chloramphenicol and nalidixic acid (32 μg/ml each). In order to determine transferability of antibiotic resistance, E. coli K-12 transconjugants were retested by disk diffusion for antibiotic susceptibility to the range of antimicrobial agents previously tested on the serovar Typhi donor isolates. To obtain information on the transferable resistance-encoding plasmids, plasmid DNA was extracted from both donor and transconjugant strains by using a Plasmid Mini-Prep kit (Qiagen, Ltd., West Sussex, United Kingdom) according to the manufacturer's instructions. Plasmid molecular sizes were determined by coelectrophoresis with plasmids of known molecular sizes from E. coli strains V517 (35.8, 4.8, 3.7, 2.6, 2.0, 1.8, and 1.4 MDa) and 39R861 (98, 42, 24, and 4.6 MDa) on 1% horizontal agarose gels.
Incompatibility grouping of plasmids by PCR.
Plasmids isolated from both the donor serovar Typhi, and their transconjugants were subjected to PCR to determine whether they belonged to the IncHI1 incompatibility group. The RepHI1A replicon, present in IncHI plasmids, was amplified by using PCR with the primers 5′-GGTCCAACCCATTGCTTTAC-3′ and 5′-CACGGAAAGAAATCACAAC-3′ as previously reported (8, 14) on a Techgene MiniCycler (Techne, Inc., Princeton, N.J.). Reaction conditions consisted of 50 ng of plasmid DNA and 100 nM concentrations of each primer in a buffer composed of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, a 200 μM concentration of deoxynucleoside triphosphate mixture, and 1 U of Taq polymerase in a final volume of 100 μl. Amplification conditions were 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, with a final extension step of 72°C for 10 min. PCR products were resolved by electrophoresis on 1.2% gels at 120 V run for 1 h. Amplicons of 365 bp were considered positive for the RepHI1A replicon, as previously reported (14, 25).
PCR of the gyrA, gyrB, parC, and parE genes in the QRDR of serovar Typhi.
Total DNA was prepared by boiling serovar Typhi for 10 min, followed by centrifugation at 13,000 rpm for 2 min to obtain the supernatant. PCR was performed on a Techgene MiniCycler (Techne) with the four primer pairs shown in Table 1. Reaction conditions consisted of 50 ng of plasmid DNA and 100 nM concentrations of each primer in a buffer composed of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, a 200 μM deoxynucleoside triphosphate mixture, and 1 U of Taq polymerase in a final volume of 100 μl. Amplification conditions consisted of 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, with a final extension step of 72°C for 10 min. To determine whether a mutation had occurred at the gyrase A site, 2 μl of purified PCR product was digested with 5 U of HinfI at 37°C for 2 h. PCR products were resolved by electrophoresis on 1.2% gels at 120 V run for 1 h.
TABLE 1.
Primers used for PCR amplification and sequencing of genes coding for the quinolone resistance
| Primer | Primer sequence | Reference |
|---|---|---|
| GYRA1 | 5′-ATGAGCGACCTTGCGAGAGAAATTAC ACCG-3′ | 3 |
| GYRA2 | 5′-CTTCTGTAGTCGTAACTTCCCGACTAC CTT-3′ | |
| GYRB1 | 5′-AAGCGCGATGGCAAAGAAG-3′ | 11 |
| GYRB2 | 5′-AACGGTCTGCTCATCAGAAAGG-3′ | |
| PARC1 | 5′-ATGAGCGATATGGCAGAGCG-3′ | 9 |
| PARC2 | 5′-TGACCGAGTTCGCTTAACAG-3′ | |
| PARE1 | 5′-GACCGAGCTGTTCCTTGTGG-3′ | 9 |
| PARE2 | 5′-GCGTAACTGCATCGGGTTCA-3′ |
DNA sequencing.
DNA sequencing was carried out by using automated sequencing (Lark Technologies, Inc., Essex, United Kingdom). DNA sequences were analyzed by using a commercial software (Lasergene; DNAStar, Inc., Madison, Wis.). BLAST (1) was used to compare the nucleotide sequences from the four PCRs with the published genome sequence of serovar Typhi strain CT18 (complete chromosome accession number AL513382).
PFGE of macrorestricted chromosomal DNA.
Chromosomal DNA from serovar Typhi isolates was prepared in agarose plugs as described previously (13). DNA in agarose plugs was digested by using 25 U each of XbaI and SpeI (Roche Diagnostics GmbH, Mannheim, Germany). PFGE of agarose plug inserts was then performed on a CHEF-DR III system (Bio-Rad Laboratories, Hercules, Calif.) on a horizontal 1% agarose gel for 20 h at 120 V, with a pulse time of 5 to 40 s at 14°C. A lambda DNA digest consisting of a ladder (∼22 fragments) of increasing size from 50 to ∼1,000 kb was included as a DNA size standard. The gel was stained with ethidium bromide and photographed on a UV transilluminator (UVP, Inc., San Gabriel, Calif.). The restriction endonuclease digest patterns were compared, and their similarities were scored by the method of Tenover et al. (26) and by using the Dice similarity coefficient formula: 2 h/(a + b), where h is the number of matching bands and a + b is the total number of matching and nonmatching bands.
RESULTS
Bacterial isolates.
During the period of study from January 2000 to December 2002 a total of 102 serovar Typhi isolates were obtained: 85 isolates from patients in Nairobi Province, 14 isolates from patients from Embu District, and 3 isolates from patients treated at Thika District Hospital (Table 2). In addition, serovar Typhi isolates from recent (i.e., 1999 and 2000) outbreaks in Pakistan (Rawalpindi) (six MDR serovar Typhi isolates) and South Africa (Natal, 1992 outbreak of typhoid) (three MDR serovar Typhi and two sensitive isolates) were included for epidemiological comparison.
TABLE 2.
Phenotype and genotype distribution of serovar Typhi isolates from sporadic outbreaks in three different parts of Kenya
| Source of serovar Typhi | No. of isolates | No. (%) of MDRa isolates | No. (%) with increased MICsb | No. (%) of isolates with XbaI and SpeI PFGE pattern:
|
|||
|---|---|---|---|---|---|---|---|
| 1
|
2
|
||||||
| MDR | Sensitive | MDR | Sensitive | ||||
| KNH | 85 | 72 (84.7) | 38 (44.7) | 34 (40) | 10 (11.8) | 38 (44.7) | 3 (3.5) |
| Embu | 14 | 13 (92.8) | 7 (50) | 9 (64.3) | 0 | 4 (28.6) | 1 (17.1) |
| Nakuru | 3 | 3 (100) | 3 (100) | 0 | 0 | 3 (100) | 0 |
That is, serovar Typhi strains resistant to three or more antimicrobial agents.
The nalidixic acid and ciprofloxacin MICs were 8 to 16 μg/ml and 0.25 to 0.38 μg/ml, respectively.
Antibiotic susceptibility testing.
Of the 102 serovar Typhi isolates studied, only 14 (13.7%) isolates were fully susceptible to all 11 antibiotics tested. Four strains showed different susceptibilities: one strain each from Nairobi hospitals were resistant to chloramphenicol and tetracycline, respectively; one strain was resistant to ampicillin, chloramphenicol, and tetracycline, and one strain was resistant to ampicillin, streptomycin, chloramphenicol, and tetracycline. The fourth isolate from Embu was also resistant to ampicillin, streptomycin, chloramphenicol, and tetracycline. The rest of the isolates 84 (82.4%) were all uniformly resistant to ampicillin, chloramphenicol, and tetracycline (MICs of >256 μg/ml), streptomycin (MIC of >1,024 μg/ml), and cotrimoxazole (MIC of >32 μg/ml), which are among the most readily available antibiotics in Kenya. Nearly half (48 [47.1%]) of all the isolates showed increased nalidixic acid and ciprofloxacin MICs (8 to 16 μg/ml and 0.25 to 0.38 μg/ml, respectively) compared to fully sensitive isolates (nalidixic acid and ciprofloxacin MICs of 2 to 4 μg/ml and 0.02 to 0.1 μg/ml, respectively), a 5- to 10-fold increase in the MIC level. However, increased nalidixic acid and ciprofloxacin MICs remained within the sensitive range (MICs of ≤1 and ≤16 μg/ml, respectively) according to NCCLS criteria (19). There was no correlation between increased nalidixic acid and ciprofloxacin MICs and the MDR phenotype. For example, for a total of 20 (19.6%) isolates, the nalidixic acid (MIC of 2 to 4 μg/ml) and ciprofloxacin (MIC of 0.02 to 0.09 μg/ml) MICs were low, even though these isolates were multiply resistant to all five commonly available antimicrobial agents. There was also no correlation between the source of serovar Typhi (by region) and susceptibility or resistance to the antimicrobial agents tested.
Plasmid incompatibility and in vitro conjugation tests.
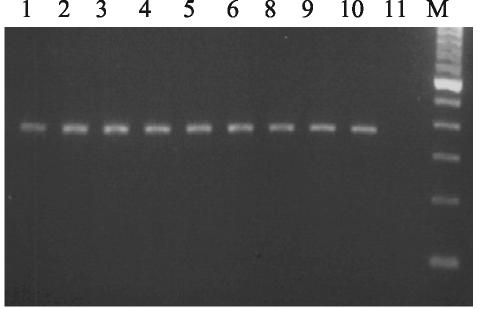
All 88 MDR serovar Typhi contained a single 110-kb plasmid, but 6 isolates contained one to two additional plasmids of 4 to 10 kb. No plasmids were isolated from fully sensitive isolates. On the basis of in vitro conjugation tests, the MDR serovar Typhi all transferred their full resistance phenotype to five antibiotics (ampicillin, chloramphenicol, tetracycline, streptomycin, and cotrimoxazole) to E. coli K-12 on the 110-kb plasmids. Plasmid preparations from MDR serovar Typhi and total DNA from five fully sensitive isolates (negative controls) were used as a template, along with incompatibility group HI1A-specific primers, in order to amplify a 365-bp region specific for the RepHI1A replicon. Only the MDR serovar Typhi isolates were positive for the 365-bp PCR product (Fig. 1). HindIII digestion of the 365-bp product resulted in two fragments (165 and 220 bp, respectively) for all of the MDR serovar Typhi isolates.
FIG. 1.
Incompatibility grouping for IncHI1 plasmid by PCR. The product is 365 bp. Lanes: M, 100-bp ladder; 1 to 10, MDR serovar Typhi; 11, antibiotic-sensitive serovar Typhi.
Analysis of QRDR of serovar Typhi.
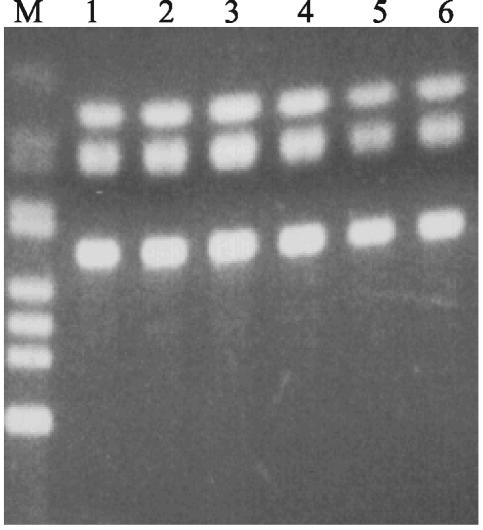
We examined a total of 15 serovar Typhi with raised MICs of nalidixic acid and ciprofloxacin (MICs of 8 to 16 μg/ml and 0.25 to 0.38 μg/ml, respectively) and a negative control strain (MICs of 4 μg/ml and 0.08 μg/ml, respectively) for mutations in the gyrA, gyrB, parC, and parE genes within the QRDR. Amplification of the gyrA gene by using PCR and restriction analysis with HinfI of the 620-bp product revealed three bands (Fig. 2) at the HinfI sites corresponding to Ser-83 and Asp-87 of gyrA, a finding which indicated that there were no alterations of the HinfI restriction sites. Nucleotide sequence analysis also showed that no point mutation had occurred in the QRDR in the gyrA, gyrB, parC, and parE genes.
FIG. 2.
HinfI digests of a 620-bp product from amplification of gyrA gene by PCR, resulting in three fragments. This experiment shows that there was no point mutation to abolish HinfI sites within gyrA region. Lanes: M, 123-bp ladder; 1 to 6, HinfI restriction digests of PCR product containing fully sensitive serovar Typhi (lanes 1 to 3), serovar Typhi resistant to ampicillin and cotrimoxazole (lane 4), and MDR serovar Typhi resistant to ampicillin, cotrimoxazole, tetracycline, streptomycin and with increased nalidixic acid and ciprofloxacin MICs (lanes 5 and 6).
PFGE.
In the analysis of fragments produced by XbaI and SpeI digestion of genomic DNA, fragments of <100 kb were excluded because of the possibility of including plasmid DNA from the MDR serovar Typhi. Analysis by PFGE of restriction fragments from the two enzymes, XbaI and SpeI, produced two distinct but similar patterns with either enzyme (Fig. 3, left panel). Restriction fragment pattern I (14 fragments each with XbaI and SpeI) consisted of 75.5% of isolates, whereas pattern II (14 fragments with XbaI and 12 fragments with SpeI) consisted of 24.5% of isolates. There were >8 band differences in migration on PFGE gels between the two restriction fragment patterns. The Dice coefficient value, excluding plasmid DNA, for both XbaI and SpeI digest fragment patterns was 42.9%. There was, however, no correlation between the antimicrobial susceptibility phenotype of serovar Typhi and the PFGE restriction fragment pattern (Table 2).
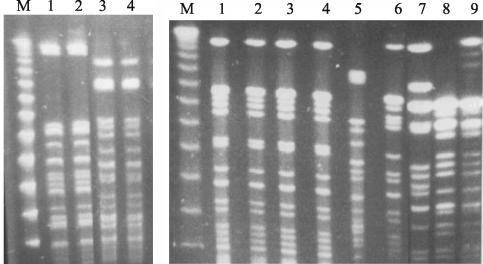
FIG. 3.
(Left panel) Gel picture showing two different digest patterns of MDR serovar Typhi from Kenya: PFGE pattern 1 (lanes 1 and 2) and PFGE pattern 2 (lanes 3 and 4) with SpeI restriction endonuclease. Lane M, 50-kb lambda molecular size ladder. (Right panel) Gel picture showing XbaI digest pattern I of MDR serovar Typhi from Kenya (lanes 1 to 3), a similar PFGE pattern of MDR serovar Typhi from South Africa (lane 4) but different from the digest patterns of MDR serovar Typhi from Hong Kong (lane 5), Pakistan (lane 6), and the fully sensitive serovar Typhi from 1988 to 1990 outbreaks in Kenya (lanes 7 to 9). Lane M, 50-kb lambda molecular size ladder.
With both XbaI and SpeI, the PFGE pattern I was identical to the pattern for the earlier (i.e., 1999) serovar Typhi outbreak strain (13) but differed significantly from the banding patterns of sensitive isolates from the 1988 to 1990 outbreaks (Fig. 3, right panel). In addition, the Kenyan serovar Typhi strains of pattern I were identical in their PFGE restriction fragment pattern to a MDR strain (ampicillin, chloramphenicol, tetracycline [MICs of >256 μg/ml] and streptomycin [MIC of >1,024 μg/ml]) from South Africa, for which the nalidixic acid and ciprofloxacin MICs were 6 and 0.09 μg/ml, respectively. However, these serovar Typhi strains of PFGE pattern I were different from the sensitive serovar Typhi isolates from South Africa and the MDR strains from Pakistan and Hong Kong (Fig. 3, right panel).
DISCUSSION
Although typhoid outbreaks caused by MDR serovar Typhi in most endemic parts Asia and the Indian subcontinent (4, 6, 11, 16) have been well characterized, data from areas in Africa where there have been outbreaks are scarce. We characterized by antimicrobial susceptibility testing and by plasmid and genomic DNA typing a total of 102 serovar Typhi isolates from recent outbreaks in three parts of Kenya.
Since the first report of MDR serovar Typhi outbreaks, which occurred in Kenya in 1997 to 1999 (13) when the prevalence of the MDR phenotype was 50 to 65%, continuous surveillance has shown that the prevalence of MDR serovar Typhi has been rising steadily and that, at present, 70 to 78% of all serovar Typhi isolates from blood cultures from the main referral hospital in Nairobi are MDR. This figure is much higher than the 52% prevalence for MDR serovar Typhi reported in outbreaks of typhoid in Ghana (14) but is close to the high prevalence of MDR serovar Typhi noted in South African outbreaks (5). Based on the increases in the prevalence of the MDR phenotype, it appears that MDR serovar Typhi strains have been spreading to other parts of Kenya and are gradually replacing the fully sensitive strain type, probably due to their survival advantage over sensitive strains.
The initial screening for mutations within the gyrase gene by using HinfI restriction sites produced three bands (137, 43, and 15 bp) in both the low- and the higher-antimicrobial-agent-MIC serovar Typhi isolates, showing that no significant changes had occurred within the HinfI sites, as would normally occur with nalidixic acid-resistant isolates. In addition, sequencing of the PCR products did not reveal any significant point mutations in the gyrA, gyrB, parC, and parE genes within the QRDR. This could be because our isolates, although showing raised quinolones MICs compared to fully sensitive isolates, are still within sensitive category on the basis of NCCLS criteria. Full resistance to the quinolones has normally been traced to sequential point mutations within the QRDR of the chromosome of serovar Typhi, with each mutation encoding higher levels of resistance. The increased quinolone MICs is not surprising since, within the last 5 years, the quinolones, especially norfloxacin and ciprofloxacin, have become the mainstay treatment choices for typhoid fever after resistance developed to previously commonly available antibiotics, including chloramphenicol and cotrimoxazole. The high prevalence of MDR serovar Typhi strains for which the nalidixic acid and ciprofloxacin MICs are high compared to those for sensitive strains means that soon resistance may emerge that renders these drugs ineffective, as has happened in Southeast Asia (4, 17, 18, 28), where even moderate rises in MICs has led to clinical treatment failure. The major concern in Kenya has been the unregulated over-the-counter sale of the quinolones and indeed other antibiotics mainly for self-treatment of suspected infection in humans and, to a lesser extent, for use in animals without prescription, which will inevitably lead to the emergence and rapid dissemination of resistance. In addition, there will be a need to ensure that cheaper generic drugs available in the market for the treatment of typhoid are of good quality and are used rationally. As in MDR serovar Typhi outbreaks reported from many parts of the world (16, 17, 30), multidrug resistance in Kenyan serovar Typhi (which is resistant to five commonly available antimicrobial agents) was encoded on large self-transferable IncHI1 plasmids of ∼110 kb. It is likely that resistance was acquired through the horizontal transfer of plasmids to previously sensitive strains irrespective of susceptibility to the quinolones commonly in use in Kenya: nalidixic acid, norfloxacin, and ciprofloxacin.
PFGE of XbaI- and SpeI-digested chromosomal DNA from serovar Typhi isolates, including both MDR and fully sensitive strains, produced two distinct patterns, showing that two strain types were in circulation in the affected parts of the country and hence likely to represent outbreak-related strains. In addition, 75.5% of serovar Typhi (PFGE pattern I) from the recent outbreaks had PFGE patterns similar to those of isolates from a previous 1997-to-1999 typhoid outbreak in Nairobi (13), and this strain type seems to be predominant in the three study areas examined here. Most outbreaks in Asia have been due to single or closely related serovar Typhi strains, as characterized by PFGE (10, 29). In contrast, outbreaks of typhoid in Pakistan have previously been caused by clonally unrelated strains (16), whereas MDR serovar Typhi isolates from Thailand (27) were found to be distinct and independently coexisted with sensitive strains. Our observations seem to be in contrast to these findings since the MDR and sensitive serovar Typhi strains shared similar PFGE patterns and were not specific to any one regional outbreak. In addition, the two PFGE patterns of serovar Typhi from Kenya were unrelated to patterns observed in the isolates we analyzed from recent outbreaks in Pakistan and Hong Kong. However, the most predominant PFGE pattern I was indistinguishable from that of an MDR serovar Typhi resistant to four antimicrobial agents (ampicillin, chloramphenicol, tetracycline, and streptomycin), but for which the quinolone MICs were low, that we obtained from an outbreak in 1992 in Natal, South Africa. It is likely that outbreaks from these two regions were caused by related strains.
Although contamination by a burst sewer line of a river used by local people for drinking water was incriminated in the typhoid outbreak in Embu District for the spread of infection, no clear sources were associated with the outbreaks in Nairobi and Nakuru. However, overcrowding in urban centers and subsequent deterioration of sanitary conditions may be responsible for the typhoid outbreaks reported at the respective hospitals. In conclusion, we observe that outbreaks caused by MDR strains will require more expensive and not so readily available drugs for effective treatment, and this will be an added burden to the healthcare sector. Improvements in sanitation, prompt diagnosis, and the rational use of available effective drugs in treatment will be important in managing typhoid and in arresting any further escalation in outbreaks.
Acknowledgments
We thank the Director of the Kenya Medical Research Institute for permission to publish this study.
S.K. is supported by a Wellcome Trust Training Fellowship.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Asna, S. M., J. A. Haq, and M. M. Rahman. 2003. Nalidixic acid-resistant Salmonella enterica serovar Typhi with decreased susceptibility to ciprofloxacin caused treatment failure: a report from Bangladesh. Jpn. J. Infect. Dis. 56:32-33. [PubMed] [Google Scholar]
- 3.Brown, J. C., P. M. Shanahan, M. V. Jesudason, C. J. Thomson, and S. G. Amyes. 1996. Mutations responsible for reduced susceptibility to 4-quinolones in clinical isolates of multi-resistant Salmonella typhi in India. J. Antimicrob. Chemother. 37:891-900. [DOI] [PubMed] [Google Scholar]
- 4.Chinh, N. T., C. M. Parry, N. T. Ly, H. D. Ha, M. X. Thong, T. S. Diep, J. Wain, N. J. White, and J. J. Farrar. 2000. A randomized controlled comparison of azithromycin and ofloxacin for treatment of multidrug-resistant or nalidixic acid-resistant enteric fever. Antimicrob. Agents Chemother. 44:1855-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coovadia, Y. M., V. Gathiram, A. Bhamjee, K. Mlisana, N. Pillay, R. M. Garratt, T. Madlalose, and M. Short. 1992. The emergence of multi-antibiotic-resistant strains of Salmonella typhi in northern Natal-KwaZulu. S. Afr. Med. J. 81:280-281. [PubMed] [Google Scholar]
- 6.Connerton, P., J. Wain, T. T. Hien, T. Ali, C. Parry, N. T. Chinh, H. Vinh, V. A. Ho, T. S. Diep, N. P. Day, N. J. White, G. Dougan, and J. J. Farrar. 2000. Epidemic typhoid in Vietnam: molecular typing of multiple-antibiotic-resistant Salmonella enterica serotype typhi from four outbreaks. J. Clin. Microbiol. 38:895-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fica, A. E., S. Prat-Miranda, A. Fernandez-Ricci, K. D'Ottone, and F. C. Cabello. 1996. Epidemic typhoid in Chile: analysis by molecular and conventional methods of Salmonella typhi strain diversity in epidemic (1977 and 1981) and nonepidemic. 1990. years. J. Clin. Microbiol. 34:1701-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabant, P., P. Newnham, D. Taylor, and M. Couturier. 1993. Isolation and location on the R27 map of two replicons and an incompatibility determinant specific for IncHI1 plasmids. J. Bacteriol. 175:7697-7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giraud, E., A. Brisabois, J. L. Martel, and E. Chaslus-Dancla. 1999. Comparative studies of mutations in animal isolates and experimental in vitro- and in vivo-selected mutants of Salmonella spp. suggest a counterselection of highly fluoroquinolone-resistant strains in the field. Antimicrob. Agents Chemother. 43:2131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampton, M. D., L. R. Ward, B. Rowe, and E. J. Threlfall. 1998. Molecular fingerprinting of multidrug-resistant Salmonella enterica serotype Typhi. Emerg. Infect. Dis. 4:317-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirose, K., A Hashimoto, K. Tamura, Y. Kawamura, T. Ezaki, H. Sagara, and H. Watanabe. 2002. DNA sequence analysis of DNA gyrase and DNA topoisomerase IV quinolone resistance-determining regions of Salmonella enterica serovar Typhi and serovar Paratyphi A. Antimicrob. Agents Chemother. 46:3249-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling, J. M., N. W. Lo, Y. M. Ho, K. M. Kam, N. T. Hoa, L. T. Phi, and A. F. Cheng. 2000. Molecular methods for the epidemiological typing of Salmonella enterica serotype Typhi from Hong Kong and Vietnam. J. Clin. Microbiol. 38:292-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kariuki, S., C. Gilks, G. Revathi, and C. A. Hart. 2000. Genotypic analysis of multidrug-resistant Salmonella enterica Serovar Typhi, Kenya. Emerg. Infect. Dis. 6:649-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mills-Robertson, F., M. E. Addy, P. Mensah, and S. S. Crupper. 2002. Molecular characterization of antibiotic resistance in clinical Salmonella typhi isolated in Ghana. FEMS Microbiol. Lett. 215:249-253. [DOI] [PubMed] [Google Scholar]
- 15.Mirza, S. H., N. J. Beeching, and C. A. Hart. 1996. Multidrug resistant typhoid: a global problem. J. Med. Microbiol. 44:317-319. [DOI] [PubMed] [Google Scholar]
- 16.Mirza, S., S. Kariuki, K. Z. Mamun, N. J. Beeching, and C. A. Hart. 2000. Analysis of plasmid and chromosomal DNA of multidrug-resistant Salmonella enterica serovar Typhi from Asia. J. Clin. Microbiol. 38:1449-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murdoch, D. A., N. A. Banatvala, A. Bone, B. I. Shoismatulloev, L. R. Ward, and J. E. Threlfall. 1998. Epidemic of ciprofloxacin-resistant Salmonella typhi in Tajikistan. Lancet 351:339. [DOI] [PubMed] [Google Scholar]
- 18.Nath, G., A. Tikoo, H. Manocha, A. K. Tripathi, and A. K. Gulati. 2000. Drug resistance in Salmonella typhi in North India with special reference to ciprofloxacin. J. Antimicrob. Chemother. 46:149-150. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 2000. Method for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.Olarte, J., and E. Galindo. 1973. Salmonella typhi resistant to chloramphenicol, ampicillin and other antimicrobial agents: strains isolated during an extensive typhoid fever epidemic in Mexico. Antimicrob. Agents Chemother. 4:597-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parry, C., J. Wain, N. T. Chinh, H. Vinh, and J. J. Farrar. 1998. Quinolone-resistant Salmonella typhi in Vietnam. Lancet 351:1289. [DOI] [PubMed] [Google Scholar]
- 22.Phung le, V., H. Ryo, and T. Nomura. 2002. Specific gyrA mutation at codon 83 in nalidixic acid-resistant Salmonella enterica serovar Typhi strains isolated from Vietnamese patients. Antimicrob. Agents Chemother. 46:2052-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahman, M., A. Ahmad, and S. Shoma. 2002. Decline in epidemic of multidrug resistant Salmonella typhi is not associated with increased incidence of antibiotic-susceptible strain in Bangladesh. Epidemiol. Infect. 129:29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowe, B., L. R. Ward, and E. J. Threlfall. 1997. Multidrug-resistant Salmonella typhi: a worldwide epidemic. Clin. Infect. Dis. 24(Suppl. 1):S106-S109. [DOI] [PubMed] [Google Scholar]
- 25.Shanahan, P. M., K. A. Karamat, C. J. Thomson, and S. G. Amyes. 1998. Molecular analysis of and identification of antibiotic resistance genes in clinical isolates of Salmonella typhi from India. J. Clin. Microbiol. 36:1595-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thong, K. L., Z. A. Bhutta, and T. Pang. 2000. Multidrug-resistant strains of Salmonella enterica serotype typhi are genetically homogenous and coexist with antibiotic-sensitive strains as distinct, independent clones. Int. J. Infect. Dis. 4:194-197. [DOI] [PubMed] [Google Scholar]
- 28.Threlfall, J. E., and L. R. Ward. 2001. Decreased susceptibility to ciprofloxacin in Salmonella enterica serotype Typhi, United Kingdom. Emerg. Infect. Dis. 7:448-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wain, J., N. T. Hoa, N. T. Chinh, H. Vinh, M. J. Everett, T. S. Diep, N. P. Day, T. Solomon, N. J. White, L. J. Piddock, and C. M. Parry. 1997. Quinolone-resistant Salmonella typhi in Viet Nam: molecular basis of resistance and clinical response to treatment. Clin. Infect. Dis. 25:1404-1410. [DOI] [PubMed] [Google Scholar]
- 30.Wain, J., L. T. Diem Nga, C. Kidgell, K. James, S. Fortune, T. Song Diep, T. Ali, P. Gaora, C. Parry, J. Parkhill, J. Farrar, N. J. White, and G. Dougan. 2003. Molecular analysis of IncHI1 antimicrobial resistance plasmids from Salmonella serovar Typhi strains associated with typhoid fever. Antimicrob. Agents Chemother. 47:2732-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. 1996. The world health report 1996: fighting disease, fostering development. World Health Organization, Geneva, Switzerland.