Abstract
Sequence analysis of Pneumocystis jiroveci internal transcribed spacer (ITS) regions has become an important epidemiological tool. The objectives of the present study were to investigate sequence variations in the ITS1-5.8S ribosomal DNA (rDNA)-ITS2 regions; determine the P. jiroveci genotypes present in Cape Town, South Africa; and resolve the lineage evolution of the types by use of the coalescent theory. ITS regions were amplified from samples collected from 19 patients. PCR products were cloned, and four to five clones were sequenced from each specimen. Statistical parsimony was applied for coalescence-based network genotype analysis. The most prevalent type was Eg (14 of 19 patients, 33 of 83 clones), followed by Gg (4 of 19 patients, 7 of 83 clones), Eu (3 of 19 patients, 5 of 83 clones), and Gh (2 of 19 patients, 2 of 83 clones). Four new combinations (Eo, Je, Ge, and No), 11 new ITS1 sequences, and 13 new ITS2 sequences were identified. A new ITS2 type was detected in three patients and was designated type u. Coinfection appeared to be common, with 15 of 19 patients harboring more than one type and with up to six types per specimen. The resultant parsimony network identified Eg as the most probable ancestral haplotype and supported the occurrence of recombinational events within the population studied. Although the 5.8S rDNA region revealed only 13 clones containing one to two nucleotide polymorphisms, it may assist in defining types. Coalescent theory proposed that Eg is an ancestral type from which microevolutionary subtypes radiate.
Pneumocystis pneumonia (PCP) is a major contributor to morbidity and mortality in immunocompromised individuals (16, 18). Many molecular epidemiological techniques are not applicable for the typing of Pneumocystis, as it cannot readily be propagated in vitro. Regions that have been investigated for use in the design of a typing method include the mitochondrial large-subunit rRNA, mitochondrial small-subunit rRNA, the arom locus, and internal transcribed spacer (ITS) regions (21). The sequence diversity of the Pneumocystis jiroveci ITS1 and ITS2 regions prompted these regions to be the basis on which typing of P. jiroveci could be conducted (10). Ribosomal DNA (rDNA) of P. jiroveci is present as a single copy in the cell and is transcribed as a single transcript, with 18S rRNA, 5.8S rRNA, and 26S rRNA occurring in tandem (4). The rRNA genes are separated by the ITS1 region between 18S rRNA and 5.8S rRNA and the ITS2 region between 5.8S rRNA and 26S rRNA (3, 6). Globally, the most frequently encountered genotypes are Eg and Ne (9, 20, 21). Latouche et al. (8) proposed that as a specific type was seen to persist during the same episode of PCP, genotype switching did not occur. However, in a study conducted with 19 patients during the same episode of PCP, genotype changes were observed in 53% of the patients (5) and coinfection with more than one genotype has been reported in a high proportion of PCP episodes (10, 12, 14, 20, 21, 22). The present understanding is that P. jiroveci infection is not clonal and that repeated de novo acquisition of ITS types is likely to occur (8, 20).
Conventional molecular phylogenetic analysis of tree building is based on homologous characters between species that are assumed to be reproductively isolated, with ascendance based on linear, dichotomous speciation events (17). These assumptions do not hold when intraspecific nucleotide evolution is analyzed at the population level. The focus is shifted to a recent evolutionary timescale, which implies that ancestral states may still exist, multiple apomorphies may be present, sexual reproduction may take place, and recombination may be involved (2, 17). Coalescent theory addresses these issues, as it models the genealogical processes of selectively neutral genes from a population by looking backward in time, whereby all lineages will eventually coalesce into a single lineage termed the most recent common ancestor of the sample (17).
The aim of the present study was to investigate which P. jiroveci ITS1-5.8S rDNA-ITS2 genotypes are present in Cape Town, South Africa, and determine the lineage evolution of the types by use of the coalescence theory.
(This work was presented in part at the 8th International Workshop on Opportunistic Protists 2003.)
MATERIALS AND METHODS
Clinical specimens (n = 20) were obtained from 19 patients with PCP attending the Tygerberg Hospital. Included were three specimens from twin babies, both of whom were human immunodeficiency virus positive. They presented with PCP simultaneously and were admitted together to the pediatric intensive care unit. Two specimens were analyzed from twin 1: a tracheal aspirate and a lung biopsy specimen (which was obtained 6 days after retrieval of the tracheal aspirate). A lung biopsy specimen was obtained from the second twin concurrently with the lung biopsy specimen from twin 1.
DNA extraction and amplification.
Mucoid specimens of 1 to 5 ml were treated with an equal volume of 0.1 M 1,4-dithiothreitol (Roche Molecular Biochemicals, Mannheim, Germany), vortexed, and incubated at 37°C for 30 min until they were liquefied. The suspension was centrifuged at 3,000 × g for 15 min, and the pellet was stored in phosphate-buffered saline (Sigma-Aldrich, St. Louis, Mo.) at −80°C. DNA extractions were performed with a High Pure Template Preparation kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the instructions of the manufacturer. Nested PCR of the ITS region (550 bp) was conducted by using the PCR conditions and primers described previously (10), but with Taq DNA polymerase in the PCR buffer (10 mM Tris-HCl [pH 9], 50 mM KCl, 0.1% Triton X-100 [Promega Corporation, Madison, Wis.]). Seven specimens were evaluated with a proofreading DNA polymerase, Pwo (Roche Molecular Biochemicals), with 3′ to 5′ exonuclease activity. Amplification was performed according to the recommendations of the manufacturer by using an Applied Biosystems GeneAmp PCR System 9700 (PE Biosystems, Foster City, Calif.). The PCR products were separated by electrophoresis in 1.4% UltraPure (GibcoBRL) agarose gel for 45 min at 80 V with Tris-acetate EDTA buffer. Ethidium bromide-stained DNA products were visualized under UV light and sized by using a 100-bp DNA molecular size marker (XIV; Roche Molecular Biochemicals).
Cloning and sequencing.
The second-round PCR products were purified with the Wizard SV Gel and PCR Clean-Up system (Promega Corporation) and cloned into plasmid pGEM-T (Promega Corporation) according to the instructions of the manufacturer. Insert (806 bp) amplification was performed with vector-specific M13 primers. ExoSap-IT (U.S. Biochemical Corporation, Cleveland, Ohio)-digested PCR products were sequenced with the ABI Prism BigDye Terminator Ready Reaction (version 3.1) Cycle Sequencing kit (Applied Biosystems, Foster City, Calif.) according to the instructions of the manufacturer. Sequencing capillary electrophoresis was performed on an ABI Prism 3100 Genetic Analyzer (Applied Biosystems) by the Central Analytical Facility, University of Stellenbosch. The sequences obtained were compared to those of ITS regions previously published by Lee et al. (9) (GenBank accession numbers AF013806 to AF013834), Nimri et al. (14) (GenBank accession numbers AF374238 to AF374265), Nevez et al. (13) (GenBank accession number AF498265), and Totet et al. (19) (GenBank accession numbers AY135711 to AY135712). Type nomenclature was assigned as described by Lee et al. (9).
Network construction.
ITS1 and ITS2 sequences were concatenated and aligned by use of the Clustal W program, which is incorporated in the BioEdit software package (version 5.06; Tom Hall, Department of Microbiology, North Carolina State University [http://www.mbio.ncsu.edu/BioEdit/page2.html]), and were subsequently optimized by visual inspection. Statistical parsimony was applied by using the computer program TCS (version 1.13; M. Clement, J. Derington, and D. Posada, Brigham Young University [http://inbio.byu.edu/Faculty/kac/crandall_lab/]) (1). Gaps were treated as missing characters, but subsequent to coalescence network analysis, they were included to reveal subdivisions within the haplotypes obtained. Unresolved loops were optimized by applying coalescence theory to obtain the most parsimonious network (17).
Nucleotide sequence accession numbers.
The nucleotide sequences of the new ITS1, ITS2, and 5.8S rDNA alleles have been deposited in GenBank under accession numbers AY328043 to AY328053, AY328054 to AY328066, and AY328067 to AY328078 and AY330724, respectively.
RESULTS
ITS1 and ITS2 genotypes.
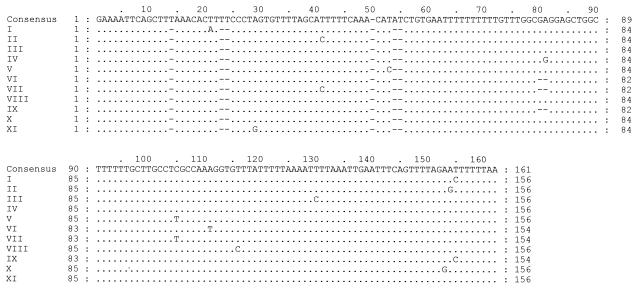
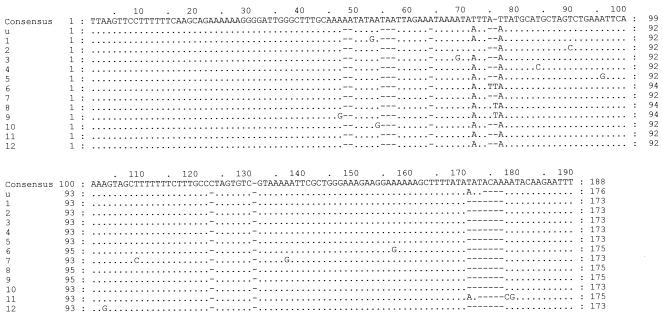
A total of 83 clones were sequenced from the 20 clinical specimens (19 patients), and ITS types were assigned. Eleven new ITS1 sequences (Fig. 1, Roman numerals) and 13 new ITS2 sequences (Fig. 2, Arabic numerals) were found. A new ITS2 type was identified in three patients and was designated type u (Table 1; Fig. 2). Four new combinations of previously reported ITS1 and ITS2 sequences were demonstrated: Eo, Je, Ge, and No. A single ITS type was detected in three specimens, five specimens contained two types, eight specimens contained three types, two specimens contained four types, one specimen contained five types, and one specimen contained six types. On sequencing 14 clones from a tracheal aspirate and a biopsy specimen, 9 types were demonstrated from one patient (Table 1, specimens 2 and 3, respectively). The most frequent type detected in the sampled population was Eg (14 of 19 patients, 33 of 83 clones), followed by Gg (4 of 19 patients, 7 of 83 clones), Eu (3 of 19 patients, 5 of 83 clones), and Gh (2 of 19 patients, 2 of 83 clones) (Table 1).
FIG. 1.
Alignment of new ITS1 sequences. GenBank accession numbers are AY328043 to AY328053 for sequences I to XI, respectively.
FIG. 2.
Alignment of new ITS2 sequences. The GenBank accession number for type u is AY328054; the GenBank accession numbers for sequences 1 to 12 are AY328055 to AY328066, respectively.
TABLE 1.
ITS types demonstrated from 20 specimensa
| Specimen | ITS type(s) obtained from each specimen |
|---|---|
| 1 | Eg, Eu |
| 2b,c | Je, Ne, Ig, Eo, Eg, IIg |
| 3b,c | Eg, IIIg, IV1, Ng |
| 4c | Eg, Ge, No |
| 5 | Gb, Eb, Eg |
| 6 | E2, Eg |
| 7 | V3, Eg, E4 |
| 8 | Gg, Gh, VIg |
| 9 | E5, Eg |
| 10 | VII6, E7, Gh, G6 |
| 11 | Eg |
| 12 | E8, Eg, E9, G8 |
| 13 | Eg, Gg |
| 14 | Eg |
| 15 | VIII10, Gg, IXg |
| 16 | Eg, Eu, Xg |
| 17 | Ea |
| 18 | Eu |
| 19 | Eg, XI11, E12 |
| 20 | Eg, Gg |
A total of 83 clones were obtained. A novel ITS2 type was demonstrated from three patients and was designated type u. New ITS1 and ITS2 sequences that were only detected once are indicated by Roman and Arabic numerals, respectively.
Specimens 2 and 3 (a tracheal aspirate and a biopsy specimen, respectively) were from the same patient.
Specimens from twins (twin 1, specimens 2 and 3; twin 2, specimen 4).
PCR with DNA polymerase Pwo demonstrated a reduced specificity compared to that obtained with Taq DNA polymerase reactions. Although Pwo produced amplicons of the expected size, upon cloning and sequencing of 35 clones, most PCR products were shown to be human DNA and/or the homologous ITS regions of Candida albicans. However, successful amplification of four new sequences and the new type (Eu, Xg, E12, and XI11 [Table 1 and Fig. 1 and 2]) was confirmed with Pwo.
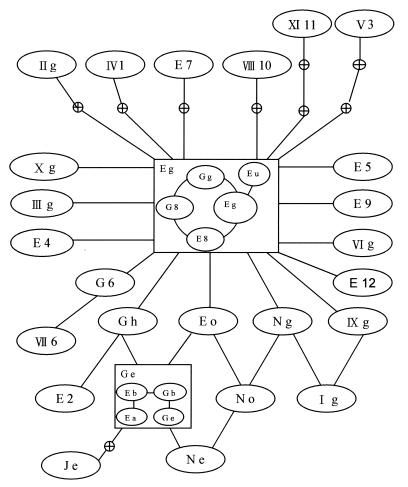
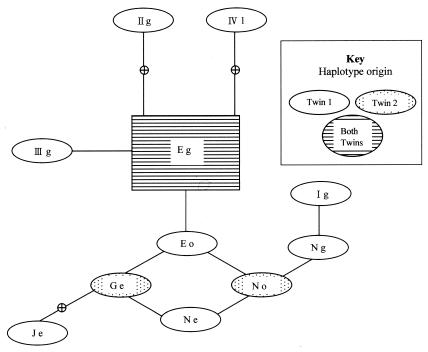
The 95% parsimony distance matrix and the resultant statistical parsimony network are shown in Table 2 and Fig. 3, respectively. Coalescence suggests that the major ancestral haplotype is Eg, which radiates microevolutionarily minor haplotypes. Unresolved loops are indicated between types Eo, Ge, Ne, and No, as well as between types Eo, No, and Ng and types Ng, Ig, and IXE. It appears that several haplotypes can be considered missing from the sampling performed. Of major interest were the specimens received from baby twins presenting with PCP at the same time (Table 1). The tracheal aspirate of twin 1 revealed ITS types Je, Ne, Eg, Eo, Ig, and IIg; and the biopsy specimen of twin 1 revealed types Eg, Ne, IIIg, and IV1. In total, twin 1 presented with nine types obtained from 14 clones, with only type (type Eg) common to both specimens. Three types (types Eg, Ge, and No) were identified from six clones from the biopsy specimen from twin 2. The only type common to both twins was Eg (Fig. 4).
TABLE 2.
Real distance matrix analysis with the 33 different ITS1 and ITS2 sequences obtaineda
| Haplotype | Haplotype no. | Real distance
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | ||
| Eg | 1 | 4 | 3 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 3 | 1 | 1 | 1 | 1 | 3 | 2 | 1 | 1 | 2 | 1 | 1 | 3 | 1 | |
| Je | 2 | 4 | 3 | 6 | 3 | 6 | 2 | 4 | 5 | 6 | 5 | 4 | 7 | 5 | 3 | 5 | 5 | 6 | 6 | 4 | 5 | 6 | 5 | 5 | 6 | 5 | |
| Ne | 3 | 3 | 3 | 3 | 2 | 5 | 1 | 1 | 4 | 5 | 2 | 3 | 6 | 4 | 2 | 4 | 4 | 5 | 5 | 3 | 4 | 5 | 4 | 4 | 5 | 4 | |
| Ig | 4 | 2 | 6 | 3 | 3 | 4 | 4 | 2 | 3 | 4 | 1 | 4 | 5 | 3 | 3 | 3 | 3 | 5 | 4 | 3 | 3 | 4 | 1 | 3 | 5 | 3 | |
| Eo | 5 | 1 | 3 | 2 | 3 | 3 | 1 | 1 | 2 | 3 | 2 | 3 | 4 | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 2 | 3 | 2 | 2 | 3 | 2 | |
| IIg | 6 | 2 | 6 | 5 | 4 | 3 | 4 | 4 | 3 | 4 | 3 | 4 | 5 | 3 | 3 | 3 | 3 | 3 | 4 | 3 | 3 | 4 | 3 | 3 | 5 | 3 | |
| Ge | 7 | 2 | 2 | 1 | 4 | 1 | 4 | 2 | 3 | 3 | 3 | 2 | 5 | 3 | 1 | 3 | 3 | 4 | 4 | 2 | 3 | 4 | 3 | 3 | 4 | 3 | |
| No | 8 | 2 | 4 | 1 | 2 | 1 | 4 | 2 | 3 | 4 | 1 | 4 | 5 | 3 | 3 | 3 | 3 | 4 | 4 | 2 | 3 | 4 | 3 | 3 | 4 | 3 | |
| IIIg | 9 | 1 | 5 | 4 | 3 | 2 | 3 | 3 | 3 | 3 | 2 | 3 | 4 | 2 | 2 | 2 | 2 | 4 | 3 | 2 | 2 | 3 | 2 | 2 | 4 | 2 | |
| IV1 | 10 | 2 | 6 | 5 | 4 | 3 | 4 | 3 | 4 | 3 | 3 | 4 | 5 | 3 | 2 | 2 | 3 | 4 | 4 | 2 | 3 | 4 | 2 | 3 | 5 | 3 | |
| Ng | 11 | 1 | 5 | 2 | 1 | 2 | 3 | 3 | 1 | 2 | 3 | 3 | 4 | 2 | 2 | 2 | 2 | 4 | 3 | 2 | 2 | 3 | 2 | 2 | 4 | 2 | |
| E2 | 12 | 2 | 4 | 3 | 4 | 3 | 4 | 2 | 4 | 3 | 4 | 3 | 5 | 3 | 1 | 3 | 3 | 5 | 4 | 3 | 3 | 4 | 3 | 3 | 5 | 3 | |
| V3 | 13 | 3 | 7 | 6 | 5 | 4 | 5 | 5 | 5 | 4 | 5 | 4 | 5 | 4 | 4 | 4 | 4 | 4 | 5 | 4 | 4 | 5 | 4 | 4 | 6 | 4 | |
| E4 | 14 | 1 | 5 | 4 | 3 | 2 | 3 | 3 | 3 | 2 | 3 | 2 | 3 | 4 | 2 | 2 | 2 | 4 | 3 | 2 | 2 | 3 | 2 | 2 | 4 | 2 | |
| Gh | 15 | 1 | 3 | 2 | 3 | 2 | 3 | 1 | 3 | 2 | 2 | 2 | 1 | 4 | 2 | 2 | 2 | 4 | 3 | 2 | 2 | 3 | 2 | 2 | 4 | 2 | |
| VIg | 16 | 1 | 5 | 4 | 3 | 2 | 3 | 3 | 3 | 2 | 2 | 2 | 3 | 4 | 2 | 2 | 2 | 4 | 3 | 2 | 2 | 3 | 2 | 2 | 4 | 2 | |
| E5 | 17 | 1 | 5 | 4 | 3 | 2 | 3 | 3 | 3 | 2 | 3 | 2 | 3 | 4 | 2 | 2 | 2 | 4 | 3 | 2 | 2 | 3 | 2 | 2 | 4 | 2 | |
| VII6 | 18 | 3 | 6 | 5 | 5 | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 5 | 4 | 4 | 4 | 4 | 4 | 5 | 2 | 5 | 5 | 4 | 4 | 5 | 4 | |
| E7 | 19 | 2 | 6 | 5 | 4 | 3 | 4 | 4 | 4 | 3 | 4 | 3 | 4 | 5 | 3 | 3 | 3 | 3 | 5 | 3 | 3 | 4 | 3 | 3 | 5 | 3 | |
| G6 | 20 | 1 | 4 | 3 | 3 | 1 | 3 | 2 | 2 | 2 | 2 | 2 | 3 | 4 | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 2 | 2 | 3 | 2 | |
| E9 | 21 | 1 | 5 | 4 | 3 | 2 | 3 | 3 | 3 | 2 | 3 | 2 | 3 | 4 | 2 | 2 | 2 | 2 | 5 | 3 | 3 | 3 | 2 | 2 | 4 | 2 | |
| VIII10 | 22 | 2 | 6 | 5 | 4 | 3 | 4 | 4 | 4 | 3 | 4 | 3 | 4 | 5 | 3 | 3 | 3 | 3 | 5 | 4 | 3 | 3 | 3 | 3 | 5 | 3 | |
| IXg | 23 | 1 | 5 | 4 | 1 | 2 | 3 | 3 | 3 | 2 | 2 | 2 | 3 | 4 | 2 | 2 | 2 | 2 | 4 | 3 | 2 | 2 | 3 | 2 | 4 | 2 | |
| Xg | 24 | 1 | 5 | 4 | 3 | 2 | 3 | 3 | 3 | 2 | 3 | 2 | 3 | 4 | 2 | 2 | 2 | 2 | 4 | 3 | 2 | 2 | 3 | 2 | 4 | 2 | |
| XI11 | 25 | 3 | 6 | 5 | 5 | 3 | 5 | 4 | 4 | 4 | 5 | 4 | 5 | 6 | 4 | 4 | 4 | 4 | 5 | 5 | 3 | 4 | 5 | 4 | 4 | 4 | |
| E12 | 26 | 1 | 5 | 4 | 3 | 2 | 3 | 3 | 3 | 2 | 3 | 2 | 3 | 4 | 2 | 2 | 2 | 2 | 4 | 3 | 2 | 2 | 3 | 2 | 2 | 4 | |
The distances were determined with the TCS program (version 1.13; Clement et al., Brigham Young University). ITS1 and ITS2 sequences were concatenated. Gaps were treated as missing data. The parsimony limit was 95%.
FIG. 3.
Resultant ITS haplotype network derived from Table 2 by coalescent theory (15). Lines connecting haplotypes are equivalent to one mutational difference, with empty nodes representing haplotypes not found in the population. Haplotype subdivisions incorporating insertion-deletion (indel) information (one indel is depicted by a connecting line) are shown in boxed haplotypes Eg and Ge.
FIG. 4.
ITS haplotype network focusing on samples from twins who presented concurrently with PCP. Lines connecting haplotypes are equivalent to one mutational difference, with empty nodes representing haplotypes not found in the population.
5.8S rRNA genotypes.
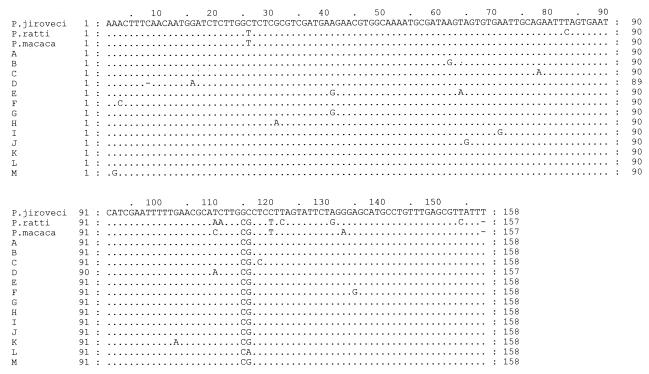
Thirteen different 5.8S rDNA sequences were detected among the 83 clones analyzed (Fig. 5). Eighty-two clones contained a CG at positions 115 and 116. Of the 20 clinical specimens examined, 13 contained a single 5.8S rDNA type, 3 contained two types, 3 contained three types, and 1 contained four different types. Among the specimens from the twins, the tracheal aspirate from twin 1 possessed four different 5.8S rRNA types and the biopsy specimen possessed two types, with only one type common to both the biopsy specimen and the tracheal aspirate. Twin 2 harbored two 5.8S rDNA types. One type, associated with ITS types Eg and Ge, was also present in both the tracheal aspirate and the biopsy specimen from twin 1; but the second type exhibited a genotype that was demonstrated only in twin 2 (linked to ITS type No) (Fig. 4). No linkage could be demonstrated between the ITS and 5.8S rDNA types obtained.
FIG. 5.
Alignment of new 5.8S rDNA sequences. The GenBank accession numbers are as follows: A to M, AY328067 to AY328078 and AY330724, respectively; P. ratti, L27658; P. macaca, AF288848; P. jiroveci (P. carinii f. sp. hominis), AF013954.
DISCUSSION
The most prevalent ITS1 and ITS2 types demonstrated were Eg (14 of 19 patients), as in studies from other continents (19, 20), and Gg (4 of 19 patients), Eu (3 of 19 patients), and Gh (2 of 19 patients). The ITS type combinations Eo, Je, Ge, and No were unique to the South African samples; and in addition, 12 new ITS1 sequences and 13 new ITS2 sequences were demonstrated. A new ITS2 type that was detected in three different temporally separated patients was designated type u (GenBank accession number AY328054). Coinfection in the patient population appeared to be common, with 15 of 19 patients (79%) harboring more than one genotype. Two specimens from one patient, taken only 6 days apart, revealed the presence of nine different genotypes. In a study conducted by Lee et al. (9), it was suggested that samples containing six or more genotypes had resulted from cross-contamination during processing. However, in the present study cross-amplicon contamination could be excluded, as types Je, Ne, Ig, Eo, and IIg found in one specimen were not detected in any other specimens analyzed. Other studies that have sequenced up to five clones from each specimen have also reported the presence of three to five types from a single specimen (5, 10, 13, 14).
As numerous new ITS1 and ITS2 sequences were found, 7 of 20 samples were reamplified by using the proofreading DNA polymerase Pwo. Pwo provided valuable confirmation of the sequences of types Eu, Xg, E12, and XI11. PCR with Pwo and the specified cycling conditions, however, proved to be unsuitable for the amplification of ITS regions of P. jiroveci from clinical specimens.
ITS analysis of Pneumocystis macacae by classical phylogenetic approaches, as performed by Hsueh et al. (7), does not appear to provide adequate intraspecies resolution. If Pneumocystis ITS regions undergo recombination or if there is a high rate of homoplasy, classic analysis would, in effect, provide no resolution due to a saturation effect. On application of coalescence-based statistical parsimony analysis to ITS regions from P. jiroveci, the parsimony network clearly shows linkage loops between haplotypes, indicative of homoplasies or recombination. Morphological investigations with Pneumocystis conducted in 1984 (11) certainly support recombination, in that synaptonemal complexes, indicative of meiosis in the early precyst stage, were reported.
On the basis of sequence information and haplotype frequency data, genotype Eg was identified as an outgroup and the most probable major ancestral haplotype within the population group. In support of the coalescence approach adopted, genotyping conducted worldwide has shown that the most frequently encountered ITS type is Eg (8, 9, 13, 14, 19, 20, 21). As certain genotypes, most notably type Eg, are overrepresented, dissemination of specific ITS types appears to be the major mode of propagation.
Linkage of 5.8S rDNA types with ITS types was not evident, indicating that different parental strains may harbor very similar ITS types or that recombination may occur. Although the relevance of 5.8S rDNA sequence polymorphisms as an adjunct to analysis of ITS regions necessitates further investigations, they may assist in distinguishing strain types within populations with similar ITS genotypes. When the number of different ITS1 and ITS2 genotype combinations reported here is considered, recombinational events could well contribute to the degree of heterogeneity observed worldwide.
Acknowledgments
This work was supported by a South African Medical Research Council grant and a bursary awarded to F. J. L. Robberts.
We thank J. Goodway for computer assistance and diagram formatting.
REFERENCES
- 1.Clement, M., D. Posada, and K. A. Crandall. 2000. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 9:1657-1659. [DOI] [PubMed] [Google Scholar]
- 2.Crandall, K. A., and A. R. Templeton. 1993. Empirical tests of some predictions from coalescent theory with applications to intraspecific phylogeny reconstruction. Genetics 134:959-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edman, J. C., J. A. Kovacs, H. Masur, D. V. Santi, H. J. Elwood, and M. L. Sogin. 1989. Ribosomal RNA genes of Pneumocystis carinii. J. Protozool. 36:18S-20S. [DOI] [PubMed] [Google Scholar]
- 4.Giunoli, D., S. L. Stringer, and J. R. Stringer. 1994. Extraordinarily low number of ribosomal RNA genes in P. carinii. J. Eukaryot. Microbiol. 41:88S. [PubMed] [Google Scholar]
- 5.Helweg-Larsen, J., C.-H. Lee, S. Jin, J. Y. Hsueh, T. L. Benfield, J. Hansen. J. D. Lundgren, and B. Lundgren. 2001. Clinical correlation of variations in the internal transcribed spacer regions of rRNA genes in Pneumocystis carinii f. sp. hominis. AIDS 15:451-459. [DOI] [PubMed] [Google Scholar]
- 6.Hillis, D. M., and M. T. Dixon. 1991. Ribosomal DNA: molecular evolution and phylogenetic inference. Q. Rev. Biol. 66:411-453. [DOI] [PubMed] [Google Scholar]
- 7.Hsueh, J. Y. C., R. P. Bohm, P. J. Didier, X. Tang, M. E. Lasbury, B. Li, S. Jin, M. S. Bartlett, J. W. Smith, and C.-C. Lee. 2001. Internal transcribed spacer regions of rRNA genes of Pneumocystis carinii from monkeys. Clin. Diagn. Lab. Immunol. 8:503-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latouche, S., J.-L. Poirot, C. Bernard, and P. Roux. 1997. Study of internal transcribed spacer and mitochondrial large-subunit genes of Pneumocystis carinii hominis isolated by repeated bronchoalveolar lavage from human immunodeficiency virus-infected patients during one or several episodes of pneumonia. J. Clin. Microbiol. 35:1687-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, C.-H, J. Helweg-Larsen, X. Tang, S. Jin, B. Li, M. S. Bartlett, J.-J. Lu, B. Lundgren, J. D. Lundgren, M. Olsson, S. B. Lucas, P. Roux, A. Cargnel, C. Atzori, O. Matos, and J. W. Smith. 1998. Update on Pneumocystis carinii f. sp. hominis typing based on nucleotide sequence variations in internal transcribed spacer regions of rRNA genes. J. Clin. Microbiol. 36:734-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu, J.-J., M. S. Bartlett, M. M. Shaw, S. F. Queener, J. W. Smith, M. Ortiz-Rivera, M. J. Liebowitz, and C.-H. Lee. 1994. Typing of Pneumocystis carinii strains that infect humans based on nucleotide sequence variations of internal transcribed spacers of rRNA genes. J. Clin. Microbiol. 32:2904-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto, Y., and Y. Yoshida. 1984. Sporogeny in Pneumocystis carinii: synaptonemal complexes and meiotic nuclear divisions observed in precysts. J. Protozool. 31:420-428. [DOI] [PubMed] [Google Scholar]
- 12.Nahimana, A., D. S. Blank, P. Francioli, J. Bille, and P. M. Hauser. 2000. Typing of Pneumocystis carinii f. sp. hominis by PCR-SSCP to indicate a high frequency of co-infection. J. Med. Microbiol. 49:753-758. [DOI] [PubMed] [Google Scholar]
- 13.Nevez, G., A. Totet, V. Jounieaux, J.-L. Schmit, E. Dei-Cas, and C. Raccurt. 2003. Pneumocystis jiroveci internal transcribed spacer types in patients colonized by the fungus and in patients with pneumocystosis from the same French geographic region. J. Clin. Microbiol. 41:181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nimri, L. F., I. N. S. Moura, L. Huang, C. del Rio, D. Rimland, J. S. Duchin, E. M. Dotson, and C. B. Beard. 2002. Genetic diversity of Pneumocystis carinii f. sp. hominis based on variations in nucleotide sequences of internal transcribed spacers of rRNA genes. J. Clin. Microbiol. 40:1146-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posada, A., and K. A. Crandall. 2001. Intraspecific gene genealogies: trees grafting into networks. Trends Ecol. Evol. 16:37-45. [DOI] [PubMed] [Google Scholar]
- 16.Rimland, D., T. R. Navin, and J. L. Lennox. 2002. Prospective study of etiologic agents of community-acquired pneumonia in patients with HIV infection. AIDS 16:85-95. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg, N. A., and M. Nordborg. 2002. Genealogical trees, coalescent theory and the analysis of genetic polymorphisms. Nature 3:380-390. [DOI] [PubMed] [Google Scholar]
- 18.Ruffini, D. D., and S. A. Madhi. 2002. The high burden of Pneumocystis carinii pneumonia in African HIV-1-infected children hospitalised for severe pneumonia. AIDS 16:105-112. [DOI] [PubMed] [Google Scholar]
- 19.Totet, A., J.-C. Pautard, C. Raccurt, P. Roux, and G. Neves. 2003. Genotypes at the internal transcribed spacers of the nuclear rRNA operon of Pneumocystis jiroveci in nonimmunosuppressed infants without severe pneumonia. J. Clin. Microbiol. 41:1173-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsolaki, A. G., R. F. Miller, A. P. Underwood, S. Banerji, and A. E. Wakefield. 1996. Genetic diversity at the internal transcribed spacer regions of the rRNA operon among isolates of Pneumocystis carinii from AIDS patients with recurrent pneumonia. J. Infect. Dis. 174:141-156. [DOI] [PubMed] [Google Scholar]
- 21.Tsolaki, A. G., P. Beckers, and A. E. Wakefield. 1998. Pre-AIDS era isolates of Pneumocystis carinii f. sp. hominis: high genotypic similarity with contemporary isolates. J. Clin. Microbiol. 36:90-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsolaki, A. G., R. F. Miller, and A. E. Wakefield. 1999. Oropharyngeal samples for genotyping and monitoring response to treatment in AIDS patients with Pneumocystis carinii pneumonia. J. Med. Microbiol. 48:897-905. [DOI] [PubMed] [Google Scholar]