Abstract
An international collaborative study was performed in order to propose quality control limits for voriconazole disk diffusion tests on Mueller-Hinton agar with 2% glucose and 0.5 μg of methylene blue per ml. The supplement may be added to the agar before autoclaving, or Mueller-Hinton agar plates may be flooded with a glucose-methylene blue solution. Replicate tests on both types of agar plates with 1-μg voriconazole disks generated data to propose zone size limits for tests of Candida parapsilosis ATCC 22019 (28 to 37 mm), Candida albicans ATCC 90028 (31 to 42 mm), and Candida krusei ATCC 6258 (16 to 25 mm). Candida tropicalis ATCC 750 was not useful for this purpose.
Voriconazole is a new extended-spectrum triazole that has recently been approved for primary treatment of invasive aspergillosis (4-6, 8). Although not yet approved by the Food and Drug Administration for the treatment of candidiasis, the excellent in vitro activity of voriconazole against Candida spp. is well documented (3, 10). In Europe, voriconazole is approved for the treatment of serious invasive infections with fluconazole-resistant Candida spp. (including Candida krusei) (9). In an effort to provide a practical means of testing voriconazole against Candida spp., an agar disk diffusion test based on the method described by Barry et al. (2) has been developed using Mueller-Hinton agar (MHA) supplemented with 2% glucose and 0.5 μg of methylene blue per ml (MH-GMB) and a 1-μg voriconazole disk (11). The addition of glucose and methylene blue serves to enhance the growth of organisms tested and to provide sharp, clear zones surrounding the voriconazole disk (1, 2, 7, 11). A good correlation between voriconazole disk zone diameters and MICs obtained with the NCCLS M27-A2 broth microdilution method has been demonstrated (11). Given this level of standardization (described in NCCLS document M44-P [7]), it is now necessary to establish quality control (QC) limits for the voriconazole disk test for Candida.
Plates with MH-GMB may be prepared in advance and stored until needed, but the shelf life of such plates has not yet been determined. An alternative approach that may be better suited for the clinical laboratory, where susceptibility testing of Candida is performed only sporadically and where medium preparation facilities are limited, employs preprepared MHA plates that are supplemented by flooding the surface with GMB solution (1). Both prepared MH-GMB plates and MHA plates flooded with GMB solution are recommended in NCCLS document M44-P (7) and were used in this study.
Flooding procedure.
The flooding procedure is that described by Barry et al. (1). Briefly, the GMB solution was prepared by adding 200 μl of a stock methylene blue solution (5 mg/ml) to 100 ml of a 40% glucose solution. The GMB solution was dispensed into screw-cap tubes (3.5 ml for 150-mm-diameter plates or 1.5 ml for 100-mm-diameter plates) and then sterilized by autoclaving. The tubes with GMB solution were then stored at 5 to 8°C until used in the study. The day before testing, GMB-containing tubes were allowed to warm to room temperature, and at the same time MHA plates were dried in a 35°C incubator until all surface moisture evaporated (1 to 2 h). The dried agar surfaces were then flooded with the GMB solution, and that solution was allowed to absorb overnight at room temperature.
QC study.
An international collaborative study was performed at the eight institutions represented by the authors. Three lots of MHA (Acumedia lot 0101-126, Difco lot 10015002, and Becton Dickinson lot 1031005) were prepared as MH-GMB agar plates or poured for flooding with a GMB solution when needed. Two lots of 1-μg voriconazole disks (Becton Dickinson lot 0309724 and Remel lot 244626) were tested on each prepared or flooded agar plate. The four control strains were Candida albicans ATCC 90028, C. krusei ATCC 6258, Candida parapsilosis ATCC 22019, and Candida tropicalis ATCC 750. The GMB solutions used for flooding and the agar plates were prepared at, and distributed from, a central source, as were the disks and control strains.
Disk diffusion tests were performed by preparing an inoculum suspension in saline that was then adjusted to match the turbidity of a McFarland standard of 0.5. The surface of each 150-mm-diameter agar plate was inoculated with a sterile cotton swab that was moistened with the inoculum suspension. One voriconazole disk from each lot was applied to the surface of each inoculated plate. The plates were then inverted and incubated at 35°C for 20 to 24 h. Calipers were used to measure the diameter of each zone of incubation from the point where there was a sharp decline in the density of growth.
On each of 10 separate days, each participant tested the four control strains on both types (prepared and flooded) of MH-GMB agar plates. For each control strain, every participant was to record 60 zone diameters on prepared MH-GMB agar plates and 60 zone diameters on flooded MHA plates (three lots of MHA and two lots of voriconazole disks tested on 10 separate test days). Because of logistical problems, our target of 480 zone measurements for each strain on prepared and flooded agar plates was not achieved, but 454 to 479 zones were recorded for each strain.
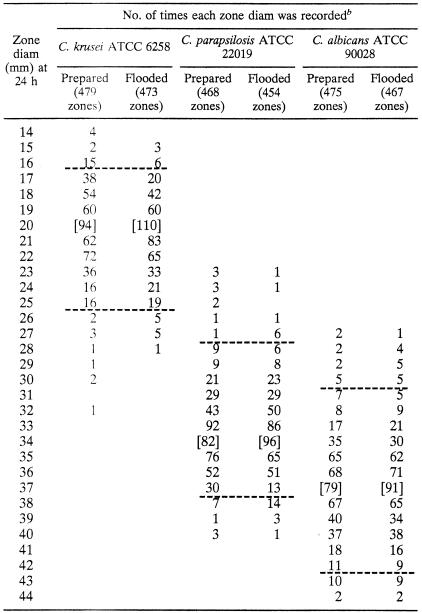
Table 1 displays the overall distributions of zone diameters recorded for three out of the four control strains when determined after 20 to 24 h of incubation. As was the case with the fluconazole disk test (1), zones were also measured after 48 h, but the 20- to 24-h results were more reproducible. C. tropicalis ATCC 750 was not useful for monitoring tests of voriconazole because the 469 zone diameters were broadly distributed over a range of 18 to 36 mm (data not shown). Useful QC limits could not be defined for this strain.
TABLE 1.
Results of replicate tests in eight laboratories using 1-μg voriconazole disks in prepared MH-GMB plates and MHA plates flooded with GMBa
MHA was supplemented by adding GMB before autoclaving (prepared agar) or by flooding MHA plates with GMB solution the day before testing (flooded agar).
Horizontal broken lines designate the QC limits for tests with each control strain, and brackets designate the median zone diameter for each data set. The QC limits were 16 to 25 mm for C. krusei ATCC 6258, 28 to 37 mm for C. parapsilosis ATCC 22019, and 31 to 42 mm for C. albicans ATCC 90028. For C. parapsilosis, of the 19 zones with diameters of <28 mm, 18 were reported by laboratory 1, and the 29 zones with diameters of >37 mm were reported by a variety of laboratories. For C. albicans, of the 26 zones with diameters of <31 mm, 22 were reported by laboratory 6, and of the 23 zones with diameters of >42 mm, 18 were reported by laboratory 3. With these QC limits, 96.8, 94.8, and 94.8% of the total number of zones examined for C. krusei, C. parapsilosis, and C. albicans, respectively, were included.
The overall spread of zone diameters for C. parapsilosis ATCC 22019 was skewed by data from one laboratory that reported unusually small zones for this strain but not for the other strains. Two other laboratories reported either unusually small zones (laboratory 6) or unusually large zones (laboratory 3) for C. albicans ATCC 90028 but not for the other strains. Control limits for each of the three strains were selected to best fit the data from all eight laboratories. Control limits could be defined to include a 10- to 12-mm range in median zone diameter for each strain and encompassing 94.8 to 96.8% of results for each strain for all labs (Table 1). The results are comparable to those reported previously for fluconazole (1).
As with fluconazole (1), the two methods of preparing MH-GMB agar plates provided essentially identical results for voriconazole with the three control strains (Table 1). The median zone sizes in each case were identical, and thus we propose one set of QC limits that can be used for each type of MH-GMB agar plate. These QC limits have been approved by the NCCLS Subcommittee on Antifungal Susceptibility Tests and are included in document M44-P (7).
Acknowledgments
This study was supported in part by unrestricted research grants from Pfizer Pharmaceuticals.
Linda Elliott and Shanna Duffy provided excellent support in the preparation of the manuscript. We appreciate the contributions of the research staff in each of the participating laboratories.
REFERENCES
- 1.Barry, A., J. Bille, S. Brown, D. Ellis, J. Meis, M. Pfaller, R. Rennie, M. Rinaldi, T. Rogers, and M. Traczewski. 2003. Quality control limits for fluconazole disk susceptibility tests on Mueller-Hinton agar with glucose and methylene blue. J. Clin. Microbiol. 41:3410-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, A. L., M. A. Pfaller, R. P. Rennie, P. C. Fuchs, and S. D. Brown. 2002. Precision and accuracy of fluconazole susceptibility testing by broth microdilution, Etest, and disk diffusion methods. Antimicrob. Agents Chemother. 46:1781-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espinel-Ingroff, A., K. Boyle, and D. J. Sheehan. 2001. In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia 150:101-115. [DOI] [PubMed] [Google Scholar]
- 4.Florea, N. R., J. L. Kuti, and R. Quintiliani. 2002. Voriconazole: a novel azole antifungal. Formulary 37:1-10. [Google Scholar]
- 5.Ghannoum, M. A., and D. M. Kuhn. 2002. Voriconazole—better chances for patients with invasive mycoses. Eur. J. Med. Res. 7:242-256. [PubMed] [Google Scholar]
- 6.Johnson, L. B., and C. A. Kauffman. 2003. Voriconazole: a new triazole antifungal agent. Clin. Infect. Dis. 36:630-637. [DOI] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. 2003. Method for antifungal disk diffusion susceptibility testing of yeasts: proposed guideline M44-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.Pearson, M. M., P. D. Rogers, J. D. Cleary, and S. W. Chapman. 2003. Voriconazole: a new triazole antifungal agent. Ann. Pharmacother. 37:420-432. [DOI] [PubMed] [Google Scholar]
- 9.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nübling, H. T. Schlamm, I. Lustar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 10.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and D. J. Diekema. 2002. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic antifungal agents against 6,970 clinical isolates of Candida spp. Antimicrob. Agents Chemother. 46:1723-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, and R. J. Hollis. 2003. Activities of fluconazole and voriconazole against 1,586 recent clinical isolates of Candida species determined by broth microdilution, disk diffusion, and Etest methods: report from the ARTEMIS Global Antifungal Susceptibility Program, 2001. J. Clin. Microbiol. 41:1440-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]