Abstract
Plant respiratory burst oxidase homolog (rboh) genes appear to play crucial roles in plant development, defense reactions and hormone signaling. In this study, a total of seven rboh genes from grape were identified and characterized. Genomic structure and predicted protein sequence analysis indicated that the sequences of plant rboh genes are highly conserved. Synteny analysis demonstrated that several Vvrboh genes were found in corresponding syntenic blocks of Arabidopsis, suggesting that these genes arose before the divergence of the respective lineages. The expression pattern of Vvrboh genes in different tissues was assessed by qRT-PCR and two were constitutively expressed in all tissues tested. The expression profiles were similarly analyzed following exposure to various stresses and hormone treatments. It was shown that the expression levels of VvrbohA, VvrbohB and VvrbohC1 were significantly increased by salt and drought treatments. VvrbohB, VvrbohC2, and VvrbohD exhibited a dramatic up-regulation after powdery mildew (Uncinula necator (Schw.) Burr.) inoculation, while VvrbohH was down-regulated. Finally, salicylic acid treatment strongly stimulated the expression of VvrbohD and VvrbohH, while abscisic acid treatment induced the expression of VvrbohB and VvrbohH. These results demonstrate that the expression patterns of grape rboh genes exhibit diverse and complex stress-response expression signatures.
Keywords: reactive oxygen species, synteny analysis, phylogenetic analysis, gene expression
1. Introduction
Reactive oxygen species (ROS) play multiple signaling roles in a wide range of organisms, including bacteria and mammals, and are also known to control various cellular mechanisms in plants. Indeed, there is growing evidence that ROS are key to fundamental plant metabolic processes, such as cellular growth [1,2], the hypersensitive response (HR) and abiotic stress responses [3–5]. Furthermore, ROS are integrated into many different signaling systems in plants, such as those mediated by protein kinases, calcium and hormones [6].
The major source of ROS in plants is the NADPH oxidase-catalyzed conversion of the superoxide anion (O2·−) to other ROS, such as perhydroxyl radicals, hydroxyl radicals and hydrogen peroxide [7]. The respiratory burst oxidase homolog (rboh) gene family encodes the key enzymatic subunit of the plant NADPH oxidase, the first example of which to be identified was the rice rbohA gene, which is a homologue of the mammalian gene gp91phox [8]. Following this initial discovery, rboh genes have been identified from other plant species, including Arabidopsis thaliana [9], tomato [10,11], tobacco [12–15], potato [16,17], maize [18], watermelon [19], barley [20,21], Medicago truncatula [22] and Lepidium sativum [23]. The proteins predicted to be encoded by the mammalian gp91phox gene and homologous plant rboh genes share conserved structural and functional domains, but the plant sequences differ in that they have an extended N-terminal region. This extension contains two putative calcium-binding domains (EF-hands), which may account for their direct regulation by Ca2+[3].
The rboh gene family has been most extensively characterized in Arabidopsis, where members play crucial roles in plant health and metabolism. Sagi and Fluhr [24] identified 10 rboh homologs in the Arabidopsis genome and it has been shown by microarray analysis that AtrbohH and AtrbohJ are specifically expressed in pollen, AtrbohA-G and AtrbohI are specifically expressed in roots, while AtrbohD and AtrbohF are expressed in all plant tissues (https://www.genevestigator.com) [25]. AtrbohD and AtrbohF participate in guard cell ABA signal transduction [26] and are also required for the accumulation of reactive oxygen intermediates during plant defense responses [27]. Transient RNA interference-mediated gene silencing of barley HvrbohA indicated a potential role in influencing penetration by the powdery mildew fungus Blumeria graminis f. sp. Hordei [20]. Moreover, the tobacco NtrbohD gene is responsible for ROS production in cryptogein-elicited tobacco cells [15], and NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans [14]. In addition, the Arabidopsis NADPH oxidase rbohD mediates rapid, long-distance, cell-to-cell signaling, which can be triggered by diverse stimuli, including wounding, heat, cold, high-intensity light and salinity stresses [28]. Together, these results indicate an important role for rboh genes in plant stress responses; however, there is also evidence that they also function in the regulation of plant growth and development. The Arabidopsis rhd2 mutant, which lacks a functional AtrbohC gene is defective in root hair growth, and it has been suggested that the corresponding protein affects ROS-mediated plant cell growth through the activation of Ca2+ channels [2]. In addition, cress plants in which LesarbohB expression was suppressed showed a strong seedling root phenotype that resembles those associated with defective auxin-related genes, thus indicating that LesarbohB plays a role in root development via auxin signaling [23].
To our knowledge, a functional analysis of grapevine (Vitis vinifera L.) Vvrboh genes has not yet been reported. Grapevine is one of the most important perennial fruit crops worldwide and the release of the grape genome data now allows a comprehensive genome-wide identification and analysis of Vvrboh genes. We also report an analysis of exon-intron structure, phylogenetic relationships and synteny with the Arabidopsis rboh gene family. Finally, the expression profiles of Vvrboh genes in different tissues, and in grape leaves responding to different exogenous hormones as well as abiotic and biotic stresses are presented; data that we propose will provide a solid foundation for future functional analyses.
2. Results
2.1. Identification of Vvrboh Genes in the Grape Genome
A total of seven genes from the grape genome were predicated to encode Rboh proteins (Table 1), and according to their localization in the grape genome and the widely recognized nomenclature [22] were named VvrbohA, VvrbohB, VvrbohC1, VvrbohC2, VvrbohD, VvrbohE and VvrbohH. All seven genes were mapped to a specific chromosome (1, 2, 6, 11, 14 and 19). The smallest predicted gene length was that of VvrbohH (3707 bp) and the largest was that of VvrbohC2 (15,930 bp), while their predicted open reading frames were similar in length. Computational prediction of protein localization indicated that VvRbohA, VvRbohC1 and VvRbohD are localized in the plasma membrane while the other Rbohs were predicted to reside in the chloroplast thylakoid membrane.
Table 1.
RBOH genes in grape.
| Gene ID | Gene Locus ID | Accession No. | Putative function | Chromosome | Start | End | Predicted gene length (kb) | Predicted ORF length (bp) |
|---|---|---|---|---|---|---|---|---|
| VvrbohA | GSVIVT01019429001 | XP_002277529.1 | plasma membrane | chr2 | 621477 | 631791 | 10.310 | 2769 |
| VvrbohB | GSVIVT01031128001 | XP_002283888.1 | chloroplast thylakoid membrane | chr14 | 1892425 | 1897786 | 5.362 | 2622 |
| VvrbohC1 | GSVIVT01014350001 | XP_002282296.2 | plasma membrane | chr19 | 2924506 | 2929619 | 5.114 | 2523 |
| VvrbohC2 | GSVIVT01001122001 | XP_002268604.1 | chloroplast thylakoid membrane | chr1 | 22798594 | 22814521 | 15.930 | 2484 |
| VvrbohD | GSVIVT01001123001 | XP_002268641.1 | plasma membrane | chr1 | 22815076 | 22819209 | 4.134 | 2721 |
| VvrbohE | GSVIVT01015025001 | XP_002277540.1 | chloroplast thylakoid membrane | chr11 | 542212 | 547987 | 5.776 | 2754 |
| VvrbohH | GSVIVT01025074001 | XP_002281695.1 | chloroplast thylakoid membrane | chr6 | 4762503 | 4766209 | 3.707 | 2559 |
2.2. Sequence Analysis and Domain Organization in Grape Rboh Homologs
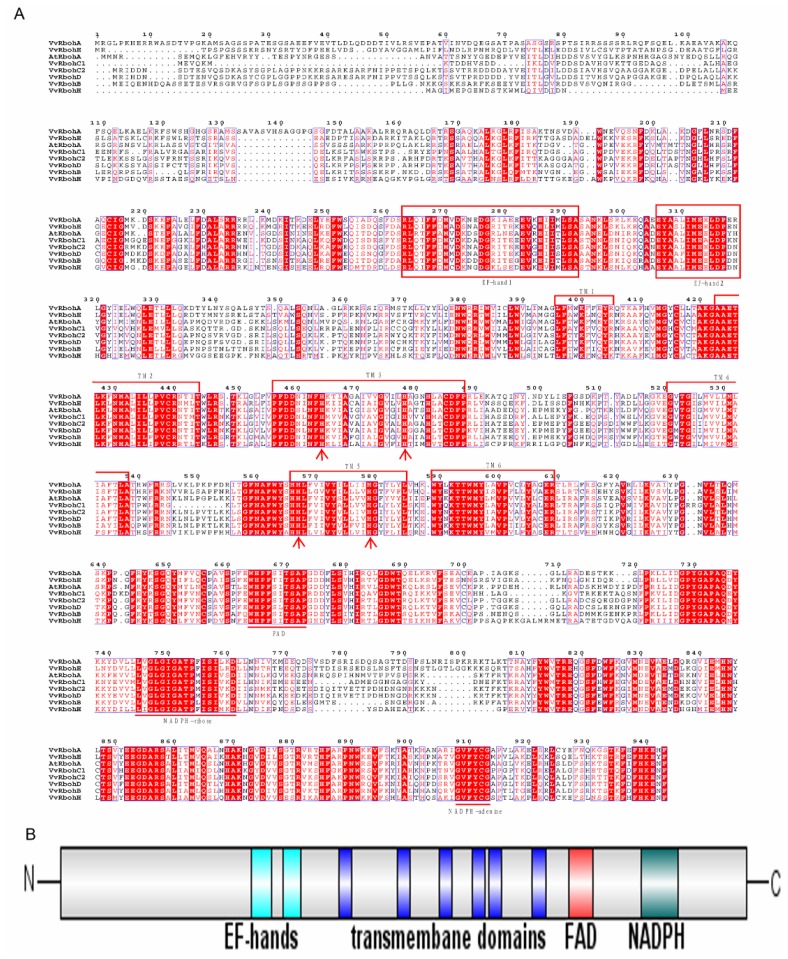
The amino acid sequences of the grapevine Rboh homologs were aligned with Arabidopsis AtRbohA to identify conserved domains, including those for binding of FAD, NADPH-ribose and NADPH-adenine in the C-terminal region (Figure 1A,B) [29,30]. The N-terminal regions of the seven predicted VvRboh proteins each contain two putative Ca2+-binding EF-hands, which are known to play a key role in the regulation of Rbohs [7,31,32] and were predicted to include six transmembrane domains (TM1–6) that correspond to those identified in plant Rbohs from Arabidopsis, rice, maize, barley, potato and tobacco, as well as the mammalian gp91phox. TM3 and TM5 also contain pairs of histidine residues that have been reported to be important for heme binding in the human gp91phox protein during electron transfer across the cell membrane [33].
Figure 1.
Protein alignment and domain structure of AtRbohA and the seven predicted grape respiratory burst oxidase homolog (Rboh) proteins. (A) The red shading indicates residues that are identical and the lighter shading represents positions with a lower level of conservation. EF-hand domains are indicated by the boxes. Conserved binding sites for flavin adenine dinucleotide (FAD), NADPH-ribose and NADPH-adenine are indicated with a straight line below the alignment. Histidine residues involved in heme binding are indicated by arrows. Putative transmembrane domains (TMs) are indicated by brackets above the alignment; and (B) Schematic representation of the grape Rboh proteins with their respective functional domains, showing that grape Rboh proteins are similar to Arabidopsis AtRbohA.
2.3. Phylogenetic Analysis of Vvrboh Genes
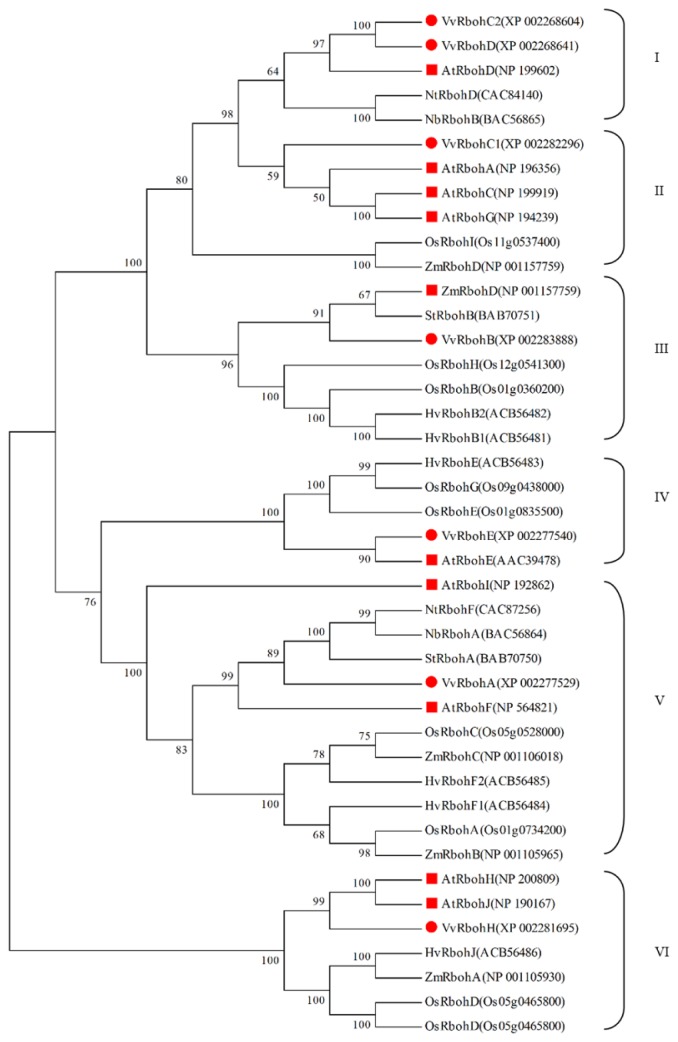
In order to infer the evolutionary relationships among plant Rbohs, the predicted VvRboh amino acid sequences were compared with each other and with divergent VvRboh from Arabidopsis, N. tabacum, Z. mays, O. sativa, S. tuberosum, N. benthamiana and H. vulgare [21]. Six groups of orthologs were identified (Figure 2), which is in agreement with previous evolutionary analyses of plant Rbohs [34]. The VvRboh sequences were distributed amongst all groups (Figure 2): VvRbohA, VvRbohB, VvRbohC1, VvRbohE and VvRbohH belong to group V, III, II, IV and VI, respectively, while VvRbohC2 and VvRbohD belong to group I.
Figure 2.
Phylogenetic analysis of grape and other plant Rboh proteins. The phylogenetic tree was constructed with Rboh domain protein sequences from V. vinifera (VvRboh), N. tabacum (NtRboh), Z. mays (ZmRboh), O. sativa (OsRboh), A. thaliana (AtRboh), S. tuberosum (StRboh), N. benthamiana (NbRboh) and H. vulgare (HvRboh). They were classified to six groups: I, II, III, IV, V, VI. VvRboh proteins are indicated with red circles and AtRboh proteins with red boxes. All accession numbers or locus IDs of the rboh genes are listed in the phylogenetic tree.
2.4. Gene Structure Analysis of Vvrboh Genes
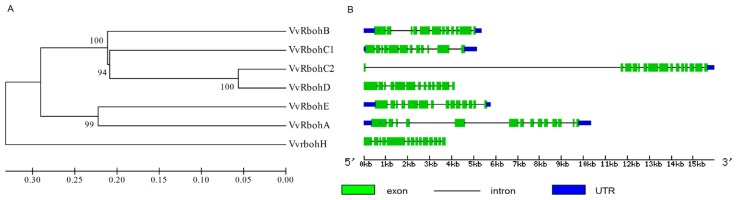
An unrooted phylogenetic tree was constructed using only the VvRboh protein sequences identified in this study (Figure 3A). Exon-intron structures were identified based on the coding sequences and the corresponding genome sequences (Figure 3B) and the similarity of the grapevine genes to those previously described in Arabidopsis, barley and rice was further reflected in the intron/exon structures. Within their coding regions HvrbohB1, HvrbohB2 and VvrbohC2 contain 12 exons, compared to the 13 exons of HvrbohE, HvrbohF2, VvrbohC1 and VvrbohE, the 14 exons of AtrbohF, OsrbohA, HvrbohF1, HvrbohJ, VvrbohA and VvrbohH and the 15 exons of VvrbohB and VvrbohD. The increase in exon number seen in several of the grapevine genes appears to be a result of insertions of introns into the exonic regions, rather than from acquisition of additional exons. The order and approximate size of exons among the Vvrboh genes is relatively conserved, while intron size is more variable. Spacing between the first and second, as well as between the eleventh and twelfth exon is particularly variable, as seen in the first exon of VvrbohB, VvrbohC1 and VvrbohD, which is different from those in the other genes and might reflect a division of the first exon into two or three exons during evolution.
Figure 3.
Phylogenetic analysis (A) and exon-intron structures (B) of Vvrboh genes. Numbers above or below branches in the tree indicate bootstrap values. Exons, introns and untranslated regions (UTR) are indicated by green boxes, black horizontal lines and blue boxes, respectively.
2.5. Evolutionary Relationships between Grape and Arabidopsis rboh Genes
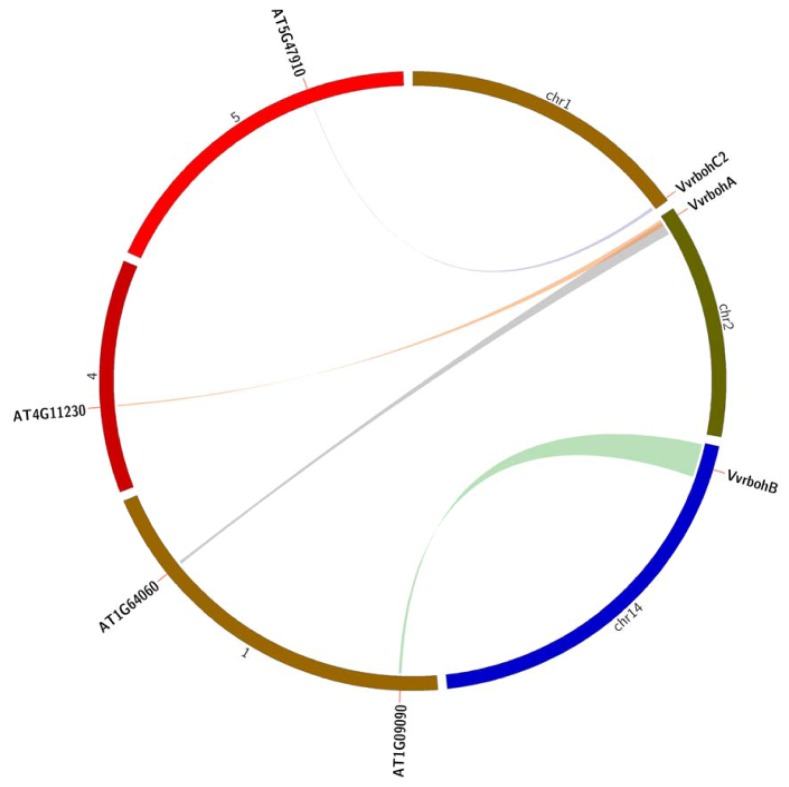
A. thaliana is one of the most important experimental model plant species and the functions of most Arabidopsis rboh genes have been well characterized. Accordingly, a comparative syntenic map between grape and Arabidopsis genomes was created in order to further study the origin, evolutionary history and putative functions of the grape homologs. Syntenic groups containing orthologs of three Vvrboh genes and four rboh genes from Arabidopsis were identified (Figure 4). According to this analysis, four paired Vvrboh-Atrboh genes (VvrbohA-AT4G11230, VvrbohA-AT1G64060, VvrbohB-AT1G09090 and VvrbohC2-AT5G47910) were located in genomic regions with synteny between the grape and Arabidopsis genomes (Table S1), indicating that these genes may be derived from a common ancestor. Based on this type of comparative genomic analysis, it is possible to deduce potential function of genes to guide future functional studies.
Figure 4.
Synteny analysis of rboh genes from grape and Arabidopsis. The positions of related Vvrboh genes and Atrboh genes are depicted in the grape chromosomes (chr1, 2 and 14) and Arabidopsis chromosomes (1, 4 and 5), respectively. Colored lines connecting two chromosomal regions indicate syntenic regions between grape and Arabidopsis chromosomes.
2.6. Expression Patterns of Vvrboh Genes in Different Tissues and Organs
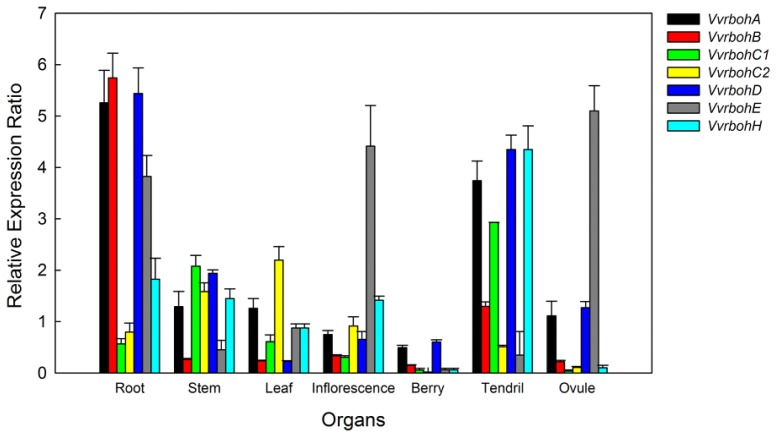
qRT-PCR was performed using RNA isolated from young leaves, roots, stems, inflorescences, berries, tendrils and ovules, revealing differential expression patterns for the seven Vvrboh genes (Figure 5). VvrbohA, VvrbohB, VvrbohD and VvrbohH were more highly expressed in roots and tendrils and VvrbohB, VvrbohC1, VvrbohC2 and VvrbohH had much lower expression in the ovules and berries. VvrbohE transcripts were more abundant in roots, inflorescences and ovules compared to other tissues/organs tested.
Figure 5.
The expression profiles of seven Vvrboh genes in various tissues. qRT-PCR analysis was conducted to visualize Vvrboh gene expression. Amplification of actin1 was used as an internal control. Error bars represent standard error (SE; n = 3).
2.7. Expression Profiles of Vvrboh Genes Following Various Stress Hormone Treatments
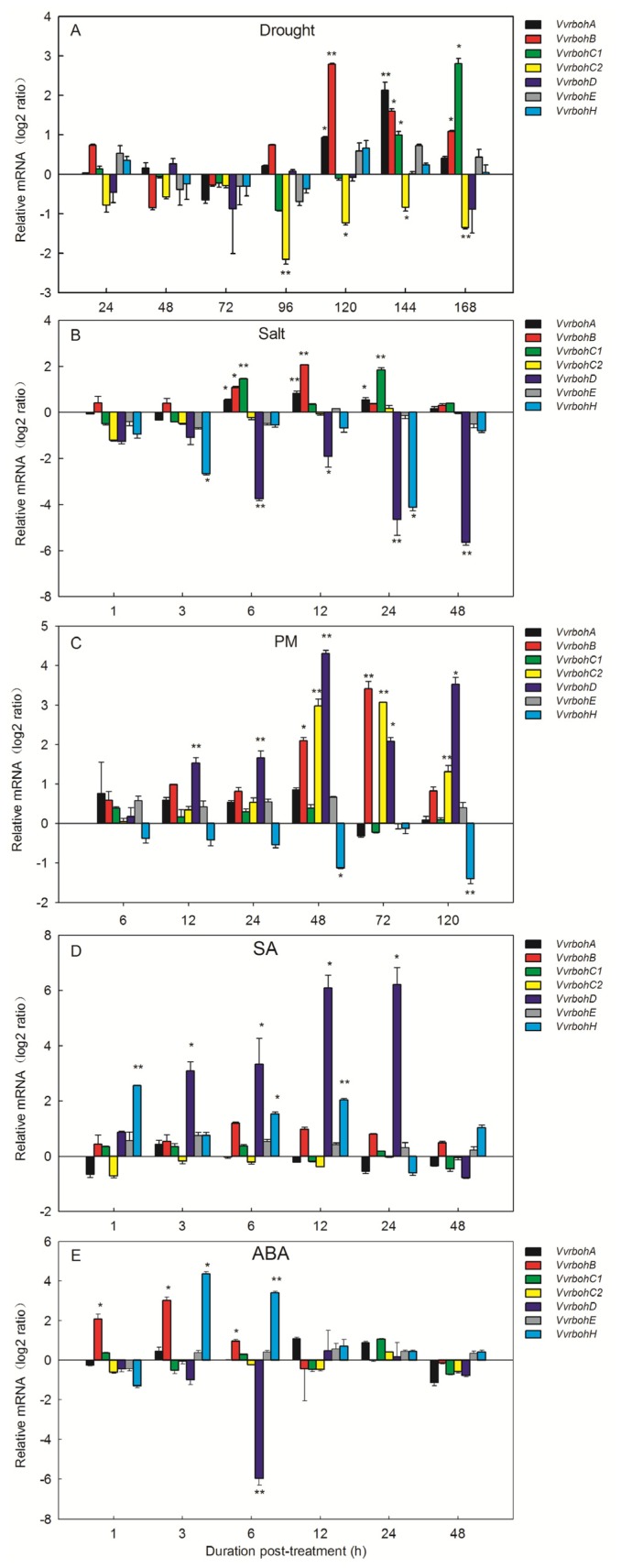
In order to determine whether the Vvrboh genes responded to stress conditions, we examined their expression patterns in response to a series of stress and exogenous hormone treatments. To test abiotic stress effects, drought and salinity treatments were performed. As shown in Figure 6A,B, the expression levels of VvrbohA, VvrbohB and VvrbohC1 were significantly increased by salt and drought treatments, while VvrbohC2 was down-regulated under drought treatment. Only the transcripts of VvrbohD and VvrbohH were down-regulated by the salt treatment, while VvrbohE expression was not altered by either treatment.
Figure 6.
Effect of drought (A); salt (B); powdery mildew (PM) (C); salicylic acid (SA) (D) and abscisic acid (E) on the expression of Vvrboh gene expression in leaves was investigated by qRT-PCR. The grape actin1 gene was used as the reference gene. Error bars represent SE (n = 3). Asterisks indicate levels of significance of differential expression (t-test, *p ≤ 0.05, **p ≤ 0.01).
Powdery mildew was used to infect grapevine to test for responses to biotic stress. VvrbohB, VvrbohC2, and VvrbohD exhibited a dramatic up-regulation after powdery mildew inoculation, while VvrbohH was down-regulated (Figure 6C). The expression of the remaining Vvrboh genes did not change appreciably after inoculation with powdery mildew. Responses to exogenous hormone treatments were also evaluated by spraying grape leaves with either SA or ABA. After SA treatment (Figure 6D), VvrbohA, VvrbohB, VvrbohC1, VvrbohC2 and VvrbohE showed a constitutive expression pattern, while VvrbohD and VvrbohH were significantly induced. As shown in Figure 6E, VvrbohD showed obvious decreases in expression 6 h after ABA treatment while VvrbohB and VvrbohH showed an increase in expression at 1 to 6 h post-treatment. All the remaining Vvrboh genes showed no appreciable changes in transcript levels following ABA treatment, and the expression level of VvrbohE did not change appreciably under any tested condition.
3. Discussion
3.1. Identification and Sequence Analysis of Vvrboh Genes
In this study we identified seven grape rboh genes (Table 1), of which VvrbohA, VvrbohC1 and VvrbohD were predicted to encode proteins that are located in the plasma membrane, suggesting that their functions are similar to those of other plant homologs. In contrast to Arabidopsis and rice, where Rbohs are predicted to localize to the plasma membrane, VvRbohB, VvRbohC2, VvRbohE and VvRbohH were predicted to be located in the thylakoid membrane of the chloroplast, indicating other functions. We are interested in the potential connection between cellular localization and functionalities of the Vvrboh protein and further experimental analyses are being carried out to try to analyze it. A comparison of the predicted VvRboh protein sequences with other plant homologs revealed several well-conserved functional domains (Figure 1). To date, all identified plant Rbohs have conserved binding sites for FAD, NADPH-ribose and NADPH-adenine, six TM domains with pairs of histidine residues in TM3 and 5, and two EF-hand, domains that are absent from the mammalian phagocyte pg91phox protein [3]. EF-hands can bind Ca2+, which could account for the direct regulation of plant Rbohs by Ca2+[35]. Thus, the regulation of the plant proteins might be different from NADPH oxidases in mammalian phagocytes.
3.2. The Evolution of Rboh Proteins in Grape and Arabidopsis and Functional Prediction of Vvrboh Genes
Genomic comparison is a convenient and often effective way to transfer knowledge of genome structure and function gained from a well-studied taxon to a species where less information is available [36]. Thus, the predicted function of the Vvrboh genes might be suggested by a comparison with their respective orthologs in the model plant Arabidopsis, whose rboh genes have previously been characterized. A synteny analysis comparing the grape and Arabidopsis genomes showed that four paired Vvrboh-Atrboh genes located to syntenic genomic regions (Figure 4, Table S1) and the synteny analysis further indicated that these genes were derived from a common ancestor. The four orthologs in the syntenic map were also clustered together in the phylogenetic tree and may exhibit similar functions. AT1G09090, AT5G47910, AT1G64060 and AT4G11230, correspond to AtrbohB and AtrbohD, AtrbohF, AtrbohI, respectively. Publicly available microarray data show that the expression of AT4G11230 (AtrbohI) can be induced by anoxia, cycloheximide and norflurazone, and is only expressed in the root elongation zone [24]. AtrbohB is primarily expressed in germinating seeds and knocking out the expression of this gene disrupts seed germination [37]. Finally, AtRbohD and AtRbohF function in pathogen responses and stomatal closure [8,27]. It is possible that the grape homologs of these four Arabidopsis proteins could be involved in similar functions; however, further experimental analyses are necessary to confirm this.
3.3. Spatial Expression Patterns of Vvrboh Genes in Various Grape Tissues
There are several similarities in the spatial expression models of Vvrboh genes and those from other plant species. The AtRbohE, HvRbohE, and VvRbohE proteins are closely related and this is also reflected by their presence within the same phylogenetic group (IV) (Figure 2). AtrbohE is expressed preferentially in roots and seed tissues [24], and the barley gene HvrbohE is strongly expressed in roots, head and coleoptile tissues [21], while the closely related VvrbohE is highly expressed in roots, ovules and inflorescences. Members of groups I, III and V also appear to have similar expression patterns to each other. Torres, Onouchi, Hamada, Machida, Hammond-Kosack and Jones [9] as well as Sagi and Fluhr [24] reported that AtrbohF was expressed in all tested tissues/organs, and HvrbohF1 an HvrbohF2 were also constitutively expressed in all the tissues/organs examined [21]. This is similar to the expression patterns observed for VvrbohA. HvrbohB1 and HvrbohB2 are expressed in all tissues [21], as is VvrbohB, VvrbohD and AtrbohD. It therefore seems that members of individual rboh groups have similar expression signatures, again suggesting that there may be conserved functionality amongst members of the same groups.
3.4. Vvrboh Genes Respond to a Range of Biological Stresses
It has been reported that plant Rbohs mediate a wide range of responses to stimuli such as abiotic stress and development cues [3]. Several groups have reported that rboh genes are transcriptionally up-regulated by pathogens or fungal elicitors [11,15,16,38]. For example, AtrbohD and AtrbohF are required for accumulation of ROS during plant defense responses and studies of Arabidopsis mutants lacking functional AtrbohD and AtrbohF showed that AtrbohD is responsible for nearly all the ROS produced in response to avirulent bacterial or oomycete pathogens [27]. NtrbohD is responsible for ROS production after treatment of tobacco cells with the fungal elicitor cryptogein [15] and experiments using virus-induced gene silencing (VIGS) indicated that NbrbohA and NbrbohB are required for ROS accumulation and for resistance to Phytophthora [14]. StrbohA and StrbohB were induced by hyphal wall components from P. infestans, arachidonic acid and SA in potato tubers [16] and it has also been shown that transient RNAi-mediated gene silencing of HvrbohA led to an increase of basal penetration resistance during the penetration process of the powdery mildew fungus B. graminis f. sp. hordei [20].
ROS that is generated by plant Rboh proteins has also been implicated in regulating abiotic stress responses. For example AtrbohD and AtrbohF, which are expressed in guard cells, are transcriptionally induced by ABA treatment [4], and AtrbohD and AtrbohA have been shown to be involved in salt stress responses [24]. Gene expression studies in rice showed that OsNox8 (OsrbohI) expression was significantly stimulated by NaCl stress, while OsNox1 (OsrbohB) and OsNox9 (OsrbohH) were strongly up-regulated by drought stress [35]. In addition, salt stress reduced the levels of OsNox1 transcripts, but had no effect on OsNox9 expression. We found by qRT-PCR analysis that six Vvrboh genes showed differential expression in response to at least one abiotic stress (Figure 6A,B), indicating their putative important roles in protecting grape from abiotic stresses. It is well known that ABA plays a crucial role in plant responses to abiotic stress, such as drought, salinity, cold, and hypoxia, and we found that the expression level of VvrbohD was strongly decreased following ABA treatment and salt stress. In contrast, VvrbohB was strongly up-regulated by exogenous ABA, drought and salt treatments and we propose that ROS produced by Vvrboh genes contributes to the response to drought via the ABA signaling pathways. In support of this idea, Pei, Murata, Benning, Thomine, Klüsener, Allen, Grill and Schroeder [4] demonstrated that ABA-induced H2O2 production and the H2O2-activated Ca2+ channels are important mechanisms for ABA-induced stomatal closing. Here VvrbohA and VvrbohC1 were significantly stimulated by both drought and salt stress, but not to ABA treatment. This may be because the expression of these Vvrboh genes is regulated by ABA-independent signaling pathways when subjected to drought and salt stresses. Indeed, stress-responsive genes have previously been proposed to be regulated by both ABA-dependent and ABA-independent signaling pathways Shinozaki and Yamaguchi-Shinozaki [39].
In the current study, qRT-PCR analysis showed that VvrbohB, VvrbohC2 and VvrbohD expression was up-regulated after powdery mildew inoculation (Figure 6C). This corresponds well with the predicted functions of VvrbohC2 based on the syntenic analysis, which showed that VvrbohC2-AT5G47910 (AtrbohD) represent an ortholog pair (Figure 4). Taking together, these results indicate that rboh genes in group I (Figure 2), VvrbohC2, VvrbohD, NbrbohB, NtrbohD and AtrbohD, are involved in pathogen resistance. However, although the three Vvrboh genes (VvrbohB, -C2 and -D) may participate in resistance against powdery mildew, more work is needed to confirm their functions. Another interesting finding was that VvrbohH showed down-regulated expression after powdery mildew inoculation, which is in contrast with studies of other rboh genes, such as Arabidopsis AtrbohF, a mutation in which results increased resistance to a weakly virulent strain of the oomycete Peronospora parasitica [27]. This supports the idea that different Vvrboh genes exhibit divergent responses to pathogens and maybe even respond differently to distinct pathogens.
SA is one of the most widely studied plant stress-signaling molecules and its role in plant resistance to pathogens and other stress factors is well documented [40,41]. SA and ROS have been proposed to be involved in a positive feedback loop that amplifies signals leading to defense responses and cell death, and so ROS-dependent cell death and the accumulation of SA are intimately associated [42]. The expression level of VvrbohD strongly increased from 3 to 24 h after SA treatment (Figure 6D), as did that of VvrbohH, indicating a role for both genes in the SA signaling pathway.
4. Experimental Section
4.1. Identification and Annotation of Grape Respiratory Burst Oxidase Homolog (Vvrboh) Genes
Grape rboh genes (Vvrboh) were identified in the Grape Genome Database (12X) (http://www.genoscope.cns.fr) using the Hidden Markov Model (HMM) profile of the EF-hand binding domain (pfam00036), NAD binding domain (pfam08030) and FAD-binding domain (pfam08022) obtained from Pfam (http://pfam.sanger.ac.uk/). Subsequently, protein, gene and virtual cDNA sequences were all retrieved from the Grape Genome Database (12X) (http://www.cns.fr/externe/GenomeBrowser/Vitis).
4.2. Amino Acid Sequence Alignment and Phylogenetic Analysis
The predicted VvRboh protein sequences were aligned with homologous sequences in the public databases using the ClustalX [43], and the alignments were edited using ESPrit 2.2-ENDscript 1.0 (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi; [44]). Putative transmembrane domains (TM), EF-hands (EF) and conserved binding sites for flavin adenine dinucleotide (FAD), NADPH-ribose and NADPH-adenine were predicated using the SMART program (http://smart.embl-heidelberg.de) and TMpred (http://ch.embnet.org/software/TMPRED_form.html; [45]). A schematic representation of the VvRboh protein functional domains was made using the DOG 1.0 software (http://dog.biocuckoo.org; [46]) and phylogenetic trees were constructed using the MEGA 5.0 software (Arizona State University, Tempe, AZ, USA) with the neighbor-joining (NJ) method and the 1000 bootstrap test replicates [47].
4.3. Exon/Intron Structure Analysis of Vvrboh Genes
The exon/intron structures of the Vvrboh genes were determined based on alignments of their coding sequences with corresponding genomic sequences using the est2genome program [48]. A diagram of exon/intron structures was obtained using the online Gene Structure Display Server (GSDS: http://gsds.cbi.pku.edu.ch; [49]), which indicates both exon position and gene length.
4.4. Synteny Analysis
Synteny blocks within the grape genome and between the grape and Arabidopsis genomes were downloaded from the Plant Genome Duplication Database (http://chibba.agtec.uga.edu/duplication) and those containing Vvrboh gene sequences were identified. Visualization of blocks was performed with Circos as described by Krzywinski M et al. [50].
4.5. Targeting Signal Prediction
Targeting signals of the predicted VvRboh proteins was performed with the aid of PSORT (http://psort.hgc.jp/form.html) [51].
4.6. Plant Materials
Grape tissues/organs, including young roots, stems, leaves, and tendrils, as well as inflorescences at the time of flower opening, berries and ovules at 33 days after flowering, were harvested from eight year-old “Kyoho” (V. labrusca × V. vinifera) grapevines grown in the field. Two year-old “Kyoho” juvenile plants were used for high salt, drought stress, exogenous hormone treatments and powdery mildew (Uncinula necator (Schw.) Burr.) inoculation. Grapevines were grown in the grape germplasm resource orchard of Northwest A&F University, Yangling, China (34°20′ N, 108°24′ E).
4.7. Abiotic and Biotic Stress Treatments and Hormone Applications
For abiotic stress assays, two year-old “Kyoho” grape juvenile plants grown in pots were irrigated with 2 L 250 mM NaCl [52]. After treatment for 1, 3, 6, 12, 24 and 48 h, respectively, fully unfolded young leaves were sampled. For drought treatment, watering was withheld for up to 7 days from potted “Kyoho” plants grown in the field in June, until the leaves showed wilting [53]. Briefly, the fully expanded young leaves of the plants were harvested at 24, 48, 72, 96, 120, 144 and 168 h post treatment. For salt and drought treatments, plants watered every three days and grown under the same conditions were used as a control.
Hormone treatments were conducted by spraying young leaves with 100 μM salicylic acid (SA) [54] or 200 μM abscisic acid (ABA) [55] followed by sampling at 1, 3, 6, 12, 24 and 48 h post-treatment. Leaves sprayed with sterile distilled water at the same time points were used as a negative control. Pathogen treatment was carried out by inoculating young leaves of “Kyoho” with powdery mildew as previously described with some modification [56]. Prior to inoculation, leaves were sprayed with sterile water, and then harvested at 6, 12, 24, 48, 72 and 120 h post-inoculation. Control plants were sprayed with sterile water at the same time points and not inoculated. Each treatment included three biological replicates with leaves from three plants being pooled and snap-frozen in liquid nitrogen and stored at −80 °C until further use.
4.8. Quantitative RT-PCR Analysis
Total RNA from grapevine was extracted using the E.Z.N.A.® Plant RNA Kit (Omega Bio-tek, Norcross, GA, USA, R6827-01), then 500 ng of total RNA was used for first-strand cDNA synthesis using PrimeScript™ RTase (TaKaRa Biotechnology, Dalian, China). Products from this reaction were diluted six times and stored at −40 °C. Quantitative RT-PCR was conducted in triplicate using SYBR green (TaKaRa Biotechnology, Dalian, China) and an IQ5 real time PCR machine (Bio-Rad, Hercules, CA, USA). The conditions for the reactions were 95 °C for 30 s, 40 cycles of 95 °C for 5 s and 60 °C for 30 s, followed by a melt-curve analysis of 95 °C for 15 s and a constant increase from 60 to 95 °C. Grapevine Actin1 (GenBank accession number AY680701) was amplified as an internal control. Primers used for qRT-PCR are listed in Table S2. Besides the technical replicates, three independent biological replicates were also analyzed. Relative expression levels were analyzed with the IQ5 software (Bio-Rad, Hercules, CA, USA) using the Normalized Expression method and a student t-test performed using the SPSS software (SPSS 17.0®, Chicago, IL, USA).
5. Conclusions
In the present study, seven grape Vvrboh genes were identified and partially characterized, thereby contributing to the growing knowledge of plant homologs of the human phagocyte gp91phox gene and its relatives. Syntenic and phylogenetic analysis helped to refine the resolution of the relationship between Rboh family members in various plant species and suggested possible functional roles for the grape Rbohs. The expression patterns of Vvrboh genes varied under different treatments, indicating diverse functions in plant stress responses. Future work will focus on functional analysis of the corresponding proteins.
Supplementary Information
Acknowledgments
The authors would like to thank Yi Zheng for providing help with bioinformatic analysis. This work was supported by the National Natural Science Foundation of China (31272136); the 948 Project from the Ministry of Agriculture of China (2012-S12); as well as the Program for Innovative Research Team of Grape Germplasm Resources and Breeding (2013KCT-25).
Abbreviations
- ABA
abscisic acid
- At
Arabidopsis thaliana
- HR
hypersensitive response
- ORF
open reading frame
- qRT-PCR
quantitative reverse transcription PCR
- SA
salicylic acid
- Vv
Vitis vinifera
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mittler R., Vanderauwera S., Gollery M., van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Foreman J., Demidchik V., Bothwell J.H., Mylona P., Miedema H., Torres M.A., Linstead P., Costa S., Brownlee C., Jones J.D. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 3.Torres M.A., Dangl J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Pei Z.-M., Murata Y., Benning G., Thomine S., Klüsener B., Allen G.J., Grill E., Schroeder J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 5.Apel K., Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 6.Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V.B., Vandepoele K., Gollery M., Shulaev V., van Breusegem F. ROS signaling: The new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Sagi M., Fluhr R. Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol. 2001;126:1281–1290. doi: 10.1104/pp.126.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groom Q.J., Torres M.A., Fordham-Skelton A.P., Hammond-Kosack K.E., Robinson N.J., Jones J.D. RbohA, a rice homologue of the mammalian gp91phox respiratory burst oxidase gene. Plant J. 1996;10:515–522. doi: 10.1046/j.1365-313x.1996.10030515.x. [DOI] [PubMed] [Google Scholar]
- 9.Torres M.A., Onouchi H., Hamada S., Machida C., Hammond-Kosack K.E., Jones J.D. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox) Plant J. 1998;14:365–370. doi: 10.1046/j.1365-313x.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- 10.Sagi M., Davydov O., Orazova S., Yesbergenova Z., Ophir R., Stratmann J.W., Fluhr R. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell. 2004;16:616–628. doi: 10.1105/tpc.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orozco-Cárdenas M.L., Narváez-Vásquez J., Ryan C.A. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell. 2001;13:179–191. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H., Fang Q., Zhang Z., Wang Y., Zheng X. The role of respiratory burst oxidase homologues in elicitor-induced stomatal closure and hypersensitive response in Nicotiana benthamiana. J. Exp. Bot. 2009;60:3109–3122. doi: 10.1093/jxb/erp146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asai S., Ohta K., Yoshioka H. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell. 2008;20:1390–1406. doi: 10.1105/tpc.107.055855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshioka H., Numata N., Nakajima K., Katou S., Kawakita K., Rowland O., Jones J.D., Doke N. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell. 2003;15:706–718. doi: 10.1105/tpc.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon-Plas F., Elmayan T., Blein J.P. The plasma membrane oxidase NtrbohD is responsible for AOS production in elicited tobacco cells. Plant J. 2002;31:137–147. doi: 10.1046/j.1365-313x.2002.01342.x. [DOI] [PubMed] [Google Scholar]
- 16.Yoshioka H., Sugie K., Park H.-J., Maeda H., Tsuda N., Kawakita K., Doke N. Induction of plant gp91phox homolog by fungal cell wall, arachidonic acid, and salicylic acid in potato. Mol. Plant Microbe Inter. 2001;14:725–736. doi: 10.1094/MPMI.2001.14.6.725. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M., Ohura I., Kawakita K., Yokota N., Fujiwara M., Shimamoto K., Doke N., Yoshioka H. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell. 2007;19:1065–1080. doi: 10.1105/tpc.106.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin F., Ding H., Wang J., Zhang H., Zhang A., Zhang Y., Tan M., Dong W., Jiang M. Positive feedback regulation of maize NADPH oxidase by mitogen-activated protein kinase cascade in abscisic acid signalling. J. Exp. Bot. 2009;60:3221–3238. doi: 10.1093/jxb/erp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Si Y., Dane F., Rashotte A., Kang K., Singh N.K. Cloning and expression analysis of the Ccrboh gene encoding respiratory burst oxidase in Citrullus colocynthis and grafting onto Citrullus lanatus (watermelon) J. Exp. Bot. 2010;61:1635–1642. doi: 10.1093/jxb/erq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trujillo M., Altschmied L., Schweizer P., Kogel K.-H., Hückelhoven R. Respiratory burst oxidase homologue A of barley contributes to penetration by the powdery mildew fungus Blumeria graminis f. sp. hordei. J. Exp. Bot. 2006;57:3781–3791. doi: 10.1093/jxb/erl191. [DOI] [PubMed] [Google Scholar]
- 21.Lightfoot D.J., Boettcher A., Little A., Shirley N., Able A.J. Identification and characterisation of barley (Hordeum vulgare) respiratory burst oxidase homologue family members. Funct. Plant Biol. 2008;35:347–359. doi: 10.1071/FP08109. [DOI] [PubMed] [Google Scholar]
- 22.Marino D., Andrio E., Danchin E.G., Oger E., Gucciardo S., Lambert A., Puppo A., Pauly N. A Medicago truncatula NADPH oxidase is involved in symbiotic nodule functioning. New Phytol. 2011;189:580–592. doi: 10.1111/j.1469-8137.2010.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller K., Linkies A., Leubner-Metzger G., Kermode A.R. Role of a respiratory burst oxidase of Lepidium sativum (cress) seedlings in root development and auxin signalling. J. Exp. Bot. 2012;63:6325–6334. doi: 10.1093/jxb/ers284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sagi M., Fluhr R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006;141:336–340. doi: 10.1104/pp.106.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmermann P., Hirsch-Hoffmann M., Hennig L., Gruissem W. Genevestigator. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 2004;136:2621–2632. doi: 10.1104/pp.104.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwak J.M., Mori I.C., Pei Z.-M., Leonhardt N., Torres M.A., Dangl J.L., Bloom R.E., Bodde S., Jones J.D., Schroeder J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres M.A., Dangl J.L., Jones J.D. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller G., Schlauch K., Tam R., Cortes D., Torres M.A., Shulaev V., Dangl J.L., Mittler R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009;2 doi: 10.1126/scisignal.2000448. [DOI] [PubMed] [Google Scholar]
- 29.Vignais P. The superoxide-generating NADPH oxidase: Structural aspects and activation mechanism. Cell Mol. Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida L.S., Saruta F., Yoshikawa K., Tatsuzawa O., Tsunawaki S. Mutation at histidine 338 of gp91phox depletes FAD and affects expression of cytochrome b558 of the human NADPH oxidase. J. Biol. Chem. 1998;273:27879–27886. doi: 10.1074/jbc.273.43.27879. [DOI] [PubMed] [Google Scholar]
- 31.Wong H.L., Pinontoan R., Hayashi K., Tabata R., Yaeno T., Hasegawa K., Kojima C., Yoshioka H., Iba K., Kawasaki T. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell. 2007;19:4022–4034. doi: 10.1105/tpc.107.055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeda S., Gapper C., Kaya H., Bell E., Kuchitsu K., Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319:1241–1244. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- 33.Finegold A.A., Shatwell K.P., Segal A.W., Klausner R.D., Dancis A. Intramembrane bis-heme motif for transmembrane electron transport conserved in a yeast iron reductase and the human NADPH oxidase. J. Biol. Chem. 1996;271:31021–31024. doi: 10.1074/jbc.271.49.31021. [DOI] [PubMed] [Google Scholar]
- 34.Wang G.-F., Li W.-Q., Li W.-Y., Wu G.-L., Zhou C.-Y., Chen K.-M. Characterization of rice NADPH oxidase genes and their expression under various environmental conditions. Int. J. Mol. Sci. 2013;14:9440–9458. doi: 10.3390/ijms14059440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller T., Damude H.G., Werner D., Doerner P., Dixon R.A., Lamb C. A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell. 1998;10:255–266. doi: 10.1105/tpc.10.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyons E., Pedersen B., Kane J., Alam M., Ming R., Tang H., Wang X., Bowers J., Paterson A., Lisch D. Finding and comparing syntenic regions among Arabidopsis and the outgroups papaya, poplar, and grape: CoGe with rosids. Plant Physiol. 2008;148:1772–1781. doi: 10.1104/pp.108.124867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller K., Carstens A.C., Linkies A., Torres M.A., Leubner-Metzger G. The NADPH oxidase AtrbohB plays a role in Arabidopsis seed after ripening. N. Phytol. 2009;184:885–897. doi: 10.1111/j.1469-8137.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 38.Desikan R., Reynolds A., Hancock J., Neill S. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem. J. 1998;330:115–120. doi: 10.1042/bj3300115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shinozaki K., Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007;58:221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- 40.Shirasu K., Nakajima H., Rajasekhar V.K., Dixon R.A., Lamb C. Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell. 1997;9:261–270. doi: 10.1105/tpc.9.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao M.V., Davis K.R. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: The role of salicylic acid. Plant J. 1999;17:603–614. doi: 10.1046/j.1365-313x.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- 42.Draper J. Salicylate, superoxide synthesis and cell suicide in plant defence. Trends Plant Sci. 1997;2:162–165. [Google Scholar]
- 43.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Higgins D.G. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 44.Hervé C., Tonon T., Collén J., Corre E., Boyen C. NADPH oxidases in Eukaryotes: Red algae provide new hints! Curr. Genet. 2006;49:190–204. doi: 10.1007/s00294-005-0044-z. [DOI] [PubMed] [Google Scholar]
- 45.Letunic I., Doerks T., Bork P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012;40:D302–D305. doi: 10.1093/nar/gkr931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren J., Wen L., Gao X., Jin C., Xue Y., Yao X. DOG 1.0: Illustrator of protein domain structures. Cell Res. 2009;19:271–273. doi: 10.1038/cr.2009.6. [DOI] [PubMed] [Google Scholar]
- 47.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rice P., Longden I., Bleasby A. EMBOSS: The European molecular biology open software suite. Trends Genet. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 49.Guo A.Y., Zhu Q.H., Chen X., Luo J.C. GSDS: A gene structure display server. Yi Chuan. 2007;29:1023–1026. [PubMed] [Google Scholar]
- 50.Krzywinski M., Schein J., Birol İ., Connors J., Gascoyne R., Horsman D., Jones S.J., Marra M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakai K., Kanehisa M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics. 1992;14:897–911. doi: 10.1016/S0888-7543(05)80111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu X., Guo R., Cheng C., Zhang H., Zhang Y., Wang X. Overexpression of ALDH2B8, an aldehyde dehydrogenase gene from grapevine, sustains Arabidopsis growth upon salt stress and protects plants against oxidative stress. Plant Cell Tiss. Org. 2013;114:187–196. [Google Scholar]
- 53.Peng S., Zhu Z., Zhao K., Shi J., Yang Y., He M., Wang Y. A novel heat shock transcription factor, VpHsf1, from Chinese wild Vitis pseudoreticulata is involved in biotic and abiotic stresses. Plant Mol. Biol. Rep. 2013;31:240–247. [Google Scholar]
- 54.Hou H., Yan Q., Wang X., Xu H. A SBP-box gene VpSBP5 from Chinese wild Vitis species responds to Erysiphe necator and defense signaling molecules. Plant Mol. Biol. Rep. 2013;31:1261–1270. [Google Scholar]
- 55.Xiao H., Nassuth A. Stress-and development-induced expression of spliced and unspliced transcripts from two highly similar dehydrin 1 genes in V. riparia and V. vinifera. Plant Cell Rep. 2006;25:968–977. doi: 10.1007/s00299-006-0151-4. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., Liu Y., He P., Chen J., Lamikanra O., Lu J. Evaluation of foliar resistance to Uncinula necator in Chinese wild Vitis species. Vitis. 1995;34:159–164. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.