Abstract
We aimed to reveal the true status of epidermal growth factor receptor (EGFR) mutations in Chinese patients with non-small cell lung cancer (NSCLC) after lung resections. EGFR mutations of surgically resected fresh tumor samples from 697 Chinese NSCLC patients were analyzed by Amplification Refractory Mutation System (ARMS). Correlations between EGFR mutation hotspots and clinical features were also explored. Of the 697 NSCLC patients, 235 (33.7%) patients had tyrosine kinase inhibitor (TKIs) sensitive EGFR mutations in 41 (14.5%) of the 282 squamous carcinomas, 155 (52.9%) of the 293 adenocarcinomas, 34 (39.5%) of the 86 adenosquamous carcinomas, one (9.1%) of the 11 large-cell carcinomas, 2 (11.1%) of the 18 sarcomatoid carcinomas, and 2 (28.6%) of the 7 mucoepidermoid carcinomas. TKIs sensitive EGFR mutations were more frequently found in female patients (p < 0.001), non-smokers (p = 0.047) and adenocarcinomas (p < 0.001). The rates of exon 19 deletion mutation (19-del), exon 21 L858R point mutation (L858R), exon 21 L861Q point mutation (L861Q), exon 18 G719X point mutations (G719X, including G719C, G719S, G719A) were 43.4%, 48.1%, 1.7% and 6.8%, respectively. Exon 20 T790M point mutation (T790M) was detected in 3 squamous carcinomas and 3 adenocarcinomas and exon 20 insertion mutation (20-ins) was detected in 2 patients with adenocarcinoma. Our results show the rates of EGFR mutations are higher in all types of NSCLC in Chinese patients. 19-del and L858R are two of the more frequent mutations. EGFR mutation detection should be performed as a routine postoperative examination in Chinese NSCLC patients.
Keywords: EGFR mutations, NSCLC, targeted therapy, ARMS, surgery, fresh tumor specimens
1. Introduction
Lung cancer remains the leading cause of cancer morbidity and mortality in males, comprising 17% of the total new cancer cases and 23% of the total cancer deaths. On the other hand, lung cancer is the fourth most commonly diagnosed cancer and the second leading cause of cancer death. In developing countries, the mortality burden for lung cancer accounts for 11% of the total female cancer deaths, as high as the burden for cervical cancer [1]. Surgery is the predominant approach for treatment of lung cancer, in combination with other approaches depending on the disease status. In recent years, targeted drug treatment has become a highlight for lung cancer, especially with the use of epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs).
Targeting EGFR is a promising strategy for the treatment of non-small cell lung cancer (NSCLC). Previous studies have demonstrated various EGFR mutations in NSCLC, including adenocarcinomas and nonadenocarcinomas [2–13]. Unlike drug resistant mutations (Exon 20 T790M point mutation (T790M) and exon 20 insertion mutation (20-ins)), NSCLC harboring EGFRTKI sensitive mutations such as exon 19 deletion mutation (19-del) and exon 21 L858R point mutation (L858R) respond to EGFR-TKIs [14]. Other drug sensitive mutations consist of exon 21 L861Q point mutation (L861Q) and exon 18 G719X point mutations (G719X, including G719C, G719S, G719A). Large-scale studies have also demonstrated that TKIs could apparently improve the therapeutic outcome of patients with EGFR-mutant NSCLC [12,15,16]. Thus, screening for EGFR mutations in NSCLC is significant in the decision-making on the treatment of NSCLC.
Surgically resected tumor specimens are the optimal DNA source for EGFR mutation detection. Complete and sufficient DNA can be extracted from surgically resected fresh samples, while specimens obtained from transbronchial lung biopsy or percutaneus aspiration lung biopsy could not demonstrate the whole tumor genomics because of the existence of intratumor genetic heterogeneity [17]. Surgical specimens can show relatively complete tumor genomics, thus avoiding or reducing false-negative results of EGFR mutation detections.
The amplification refractory mutation system (ARMS) is a method for point mutation in DNA based on allele specific polymerase chain reaction [18]. It is quick, relatively easy and more sensitive than DNA direct sequencing, and EGFR mutation detection kits according to the principle of ARMS can detect 29 common EGFR mutations [19–21]. As some mutations may be present in a minor population of tumor cells, and normal cells can be mixed in tumor tissue, this highly sensitive assay can be an appropriate method for EGFR mutation analysis.
The frequency of EGFR mutations in NSCLC is known to be associated with many factors, including race, gender, smoking status, and tumor histology. About 8%–15% of European patients with NSCLC harbor EGFR mutations. In Asian patients, the frequency is up to 31% and the mutations are associated with Asian ethnicity, female gender, never-smoking or light-smoking, and adenocarcinoma [7,12,13]. As most previous large-scale studies on EGFR mutations primarily focused on adenocarcinoma, few studies have evaluated the frequency of EGFR mutations in non-adenocarcinoma NSCLC, such as squamous-cell carcinoma, adenosquamous carcinoma, and large-cell carcinoma. In this study, we used ARMS to demonstrate the status of EGFR mutations in Chinese patients with NSCLC after lung resection and clarify correlations between EGFR mutations and clinical features.
2. Results
2.1. Patient Characteristics
Patient characteristics are summarized in Table 1. The 697 enrolled patients consisted of 476 males (68.3%) and 221 females (31.7%) with a median age of 55.3 years (range, 38–76 years), and 366 smokers (52.5%) vs. 331 non-smokers (47.5%). Postoperative pathological evaluation revealed 282 squamous-cell carcinomas (40.5%), 293 adenocarcinomas (42.0%), 86 adenosquamous carcinomas (12.3%), 11 large-cell carcinomas (1.6%), 18 sarcomatoid carcinomas (2.6%), and 7 mucoepidermoid carcinomas (1.0%). All the pathological diagnoses were based on the criteria of the WHO/IASLC Histological Classification of Lung and Pleural Tumors. According to the seventh edition of the TNM Classification of malignant tumors, cases in stage Ia, Ib, IIa, IIb, IIIa, IIIb, IV were 105, 110, 153, 159, 155, 12, respectively.
Table 1.
Patient characteristics.
| Characteristics | N | Mutation *,% | p-value |
|---|---|---|---|
| Age | 0.066 | ||
| <60 years | 378 | 116, 30.7% | |
| ≥60 years | 319 | 119, 37.3% | |
| Gender | <0.001 | ||
| Male | 476 | 108, 22.7% | |
| Female | 221 | 127, 57.5% | |
| Smoking status | 0.047 | ||
| Never | 331 | 124, 37.5% | |
| Ever | 366 | 111, 30.3% | |
| Histology | <0.001 | ||
| Squamous-cell carcinoma | 282 | 41, 14.5% | |
| Adenocarcinoma ** | 293 | 155, 52.9% | |
| Adenosquamous carcinoma | 86 | 34, 39.5% | |
| Large-cell carcinoma | 11 | 1, 9.1% | |
| Sarcomatoid carcinoma | 18 | 2, 11.1% | |
| Mucoepidermoid carcinoma | 7 | 2, 28.6% | |
| Stage | 0.898 | ||
| Ia | 105 | 34, 32.4% | |
| Ib | 110 | 40, 36.4% | |
| IIa | 153 | 49, 32.0% | |
| IIb | 159 | 56, 35.2% | |
| IIIa | 155 | 53, 34.2% | |
| IIIb | 12 | 2, 16.7% | |
| IV | 3 | 1, 33.3% |
Tyrosine Kinase Inhibitors (TKIs) sensitive EGFR mutations (19-del, L858R, L861Q, G719X);
Partition of Chi-square showed higher mutation rate in adenocarcinomas (α′ = 0.003).
2.2. EGFR Mutation Status
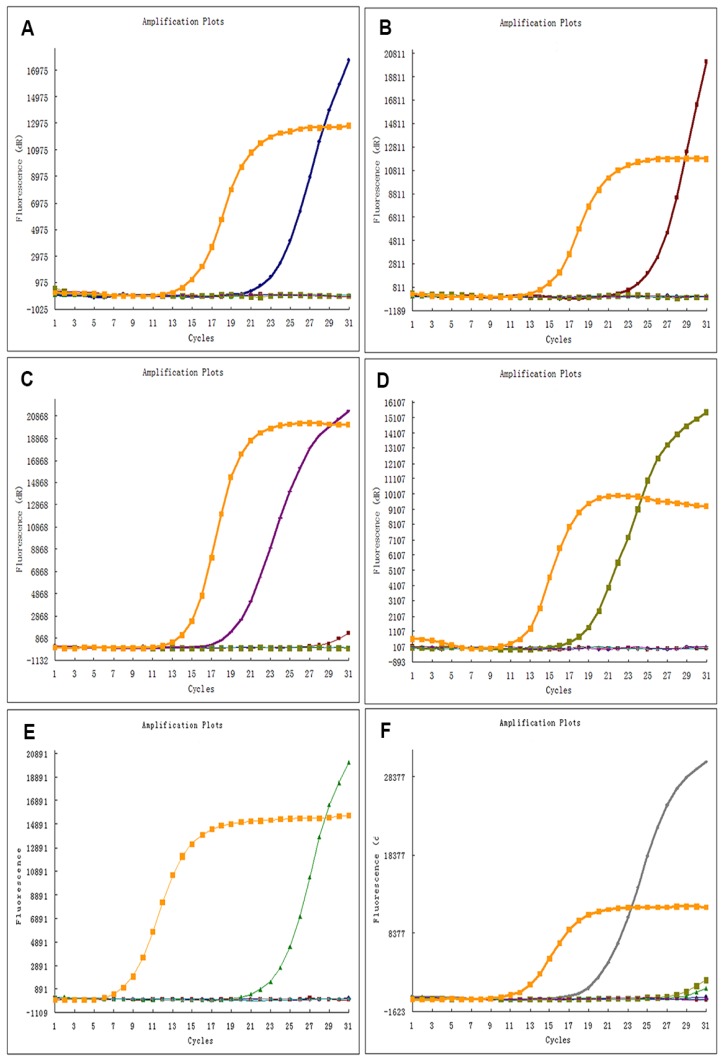
Typical results for ADx-ARMS are demonstrated in Figure 1. Of the 697 Chinese patients with NSCLC, 235 (33.7%) patients had TKIs sensitive EGFR mutations, comprising 41 (14.5%) of the 282 squamous-cell carcinomas, 155 (52.9%) of the 293 adenocarcinomas, 34 (39.5%) of the 86 adenosquamous carcinomas, 1 (9.1%) of the 11 large-cell carcinomas, 2 (11.1%) of the 18 sarcomatoid carcinomas, and 2 (28.5%) of the 7 mucoepidermoid carcinomas (Table 1). As shown in Tables 2–4, exon 19 deletion and exon 21 L858R point mutation were two of the most common subtypes of EGFR mutations, accounting for 43.4% (n = 102/235) and 48.1% (n = 113/235) respectively. L861Q was detected in 4 (1.7%) and G719X was detected in 16 (6.8%) of the 235 EGFR mutant patients. In addition, EGFR T790M mutation was identified in 6 patients and EGFR exon 20 insertion mutation in 2 patients. The data of the 8 patients are shown in Table 5. No coexisting mutations were found in our study.
Figure 1.
Representative results for ADx-ARMS of EGFR mutation testing. (A–F) demonstrate the typical results of exon 19 deletion mutation, exon 21 L858R point mutation, exon 21 L861R point mutation, exon 18 G719X point mutation, exon 20 T790M point mutation, exon 20 insertion mutation, respectively. The orange lines are the internal control signal (HEX), the blue lines, red lines, purple lines, dark green lines, light green lines and the gray lines are mutant signal (FAM), representing 19-del, L858R, G719X, T790M and 20-ins, respectively.
Table 2.
Subtypes of TKIs sensitive EGFR mutations according to gender.
| Mutation spot | Gender | p-value | |
|---|---|---|---|
|
| |||
| Male | Female | ||
| 19-Del | 48 | 54 | 0.864 |
| L858R | 51 | 62 | |
| L861Q | 1 | 3 | |
| G719X | 8 | 8 | |
19-Del: exon 19 deletion mutation; L858R: exon 21 L858R point mutation; L861Q: exon 21 L861R point mutation; G719X: exon 18 G719X point mutation.
Table 3.
Subtypes of TKIs sensitive EGFR mutations according to the smoking status.
| Mutation spot | Smoking Status | p-value | |
|---|---|---|---|
|
| |||
| Ever | Never | ||
| 19-Del | 22 | 80 | 0.202 |
| L858R | 34 | 79 | |
| L861Q | 1 | 3 | |
| G719X | 7 | 9 | |
19-Del: exon 19 deletion mutation; L858R: exon 21 L858R point mutation; L861Q: exon 21 L861R point mutation; G719X: exon 18 G719X point mutation.
Table 4.
Subtypes of TKIs sensitive EGFR mutation according to histology.
| Mutation spot | Pathology | ||||
|---|---|---|---|---|---|
|
| |||||
| Squamous-cell carcinoma | Adeno-carcinoma | Adeno-squamous carcinoma | Others * | p-value | |
| 19-Del | 17 | 66 | 17 | 2 | 0.671 |
| L858R | 19 | 76 | 16 | 2 | |
| L861Q | 0 | 4 | 0 | 0 | |
| G719X | 5 | 9 | 1 | 1 | |
19-Del: exon 19 deletion mutation; L858R: exon 21 L858R point mutation; L861Q: exon 21 L861R point mutation; G719X: exon 18 G719X point mutation;
Others includes large-cell carcinoma, sarcomatoid carcinoma and mucoepideroid carcinoma.
Table 5.
Listing of features of patients whose tumors harbored EGFR TKI resistant mutations.
| Subtype | Age (years) | Gender | Smoking status | Tumor Histology | Stage |
|---|---|---|---|---|---|
| T790M | 53 | Male | Ever | Squamous-cell carcinoma | IIIa |
| T790M | 67 | Male | Ever | Adenocarcinoma | IIIb |
| T790M | 65 | Male | Never | Squamous-cell carcinoma | Ib |
| T790M | 50 | Female | Never | Squamous-cell carcinoma | IIa |
| T790M | 68 | Female | Never | Adenocarcinoma | IIb |
| T790M | 71 | Female | Never | Adenocarcinoma | Ib |
| 20-Ins | 73 | Female | Never | Adenocarcinoma | IIIa |
| 20-Ins | 62 | Female | Never | Adenocarcinoma | IIb |
T790M: exon 20 T790M point mutation; 20-Ins: exon 20 insertion mutation.
Furthermore, a significant correlation was noticed between TKIs sensitive EGFR mutations and the clinical features of the female gender (p < 0.001), non-smoking history (p = 0.047) and adenocarcinoma subtype (p < 0.001), while patient age and tumor stage were not significantly associated with EGFR mutations (p = 0.060, p = 0.584 respectively). In addition, no significant difference was observed between the subtypes of TKIs sensitive EGFR mutations and gender (p = 0.634), smoking status (p = 0.349), and histology of tumors (p = 0.819) in a subgroup analysis where only mutation-positive patients were included (Tables 2–4).
3. Discussion
The present study was intended to improve our understanding about the EGFR mutation status in NSCLC, especially in non-adenocarcinoma.
Our results showed that the overall frequency of TKIs sensitive EGFR mutations was 33.7%, and the EGFR mutation rate in squamous-cell carcinoma (14.5%), adenocarcinoma (52.9%) and adenosquamous carcinoma (39.5%) was higher than the data from previous Asian population-based studies [2,6–9,13]. We also identified that one of the 11 large-cell carcinomas, 2 of the 18 sarcomatoid carcinomas and 2 of the 11 mucoepidermoid carcinomas harbored TKIs sensitive EGFR mutations. Meanwhile, we found that TKIs sensitive EGFR mutations were associated with the female gender (p < 0.001), non-smoking history (p = 0.045), and adenocarcinoma subtype (p < 0.001).
There are several underlying causes for the higher frequency of TKIs sensitive EGFR mutations in our study. Fresh and sufficient tumor samples after lung resection could be one of them. Owing to tumor heterogeneity and limitations of needle biopsy, the small amount of tissue from needle biopsies may not be emblematic of the complete pictures of tumors, leading to false negative results; while adequate surgical samples can provide enough DNA for EGFR mutation detection. On the other hand, as formaldehyde may cause crosslinking and degradation of DNA, surgically resected fresh samples can offer less damaged DNA than the formalin-fixed ones, thus making the results of EGFR mutation detection more accurate. Another reason for the higher rate of TKIs sensitive EGFR mutations could be the use of the more sensitive method ARMS. EGFR mutation is a somatic mutation, the detection of which requires a highly specific and sensitive method, for EGFR mutant cells are mixed with wild type cells in the tumor sample. ARMS is based on allele specific polymerase chain reaction with a sensitivity at 1% (this means that at least 1% mutant DNAs can be detected within a “normal” DNA background via ARMS), which also makes the results more accurate.
In a subgroup analysis where the subtypes of EGFR mutations were studied, we found that exon 19 deletion and exon 21 L858R point mutation were the two dominant subtypes of TKIs sensitive EGFR mutations in Chinese patients with NSCLC, comprising 43.4% and 48.1% of all the TKIs sensitive EGFR mutations respectively. It is interesting that the frequency was not consistent with previous Asian population-based studies [13,22,23], which demonstrated that exon 19 in-frame deletion was more frequent. It could be explained by unselected tumor subtypes and stages in our study, ethnic variations between Chinese and other populations, or sampling errors.
Two major TKIs resistant EGFR mutations (exon 20 insertion and T790M point mutation) were found in Chinese patients with NSCLC prior to any treatment. Exon 20 insertion mutations are associated with primary TKIs resistance. It can promote the activation of EGFR kinase domain, leading to carcinogenesis. It can also affect ATP and the affinity of EGFRs to gefitinib or erlotinib, causing the resistance against gefitinib or its sister drug. Patients harboring exon 20 insertion mutations should receive irreversible inhibitors rather than gefitinib or erlotinib. T790M point mutation in exon 20 is responsible for approximately 50% patients with acquired resistance against TKIs [24]. This mutation does not reduce the affinity of gefitinib or erlotinib to the receptors but it enhances the affinity to ATP and thereby causes resistance. However, de novo T790M mutations were found in Chinese patients with NSCLC before administration of TKIs in our study, indicating that T790M could also lead to primary resistance against TKIs; this might confirm that a low frequency T790M mutation may have been present in the primary cancer, but under the selective pressure imposed by targeted therapies it may expand and lead to TKIs resistance [25]. As reported by Tetsuya Mitsudomi et al. ([14]; ASCO 2012, abstract 7521), compared with patients harboring T790M wild type, the T790M mutant counterparts could enjoy longer progress free survival (PFS) and overall survival (OS), no matter what type of treatments they received (TKIs or chemotherapy). Thus, the identification of these resistant EGFR mutations is as important as the identification of TKIs sensitive ones for the treatment patterns of NSCLC.
In addition, our study failed to find a significant association between the subtypes of EGFR mutations and gender, smoking status, and tumor histology, which was different from the study of Tanaka et al. [13], who reported that there was a gender difference in EGFR mutation subtypes. Tumor stages (unselected vs. advanced stage), mutation detection methods (ARMS vs. PNA-LNA PCR clamp), specimen types (all fresh cases vs. fresh cases/archival tissue), racial differences (Chinese vs. Japanese) or sampling errors are likely to be the underlying causes of the discordance between our study and Tanaka’s. More studies are required to elucidate such discord.
Even though targeting therapy is a brand new strategy for the treatment of non-small cell lung cancer, surgical resection is still regarded as the predominant method of controlling the tumor. Apart from the early stage cases, patients at late stages, provided the risks of the procedure are low, could also undergo surgeries for more sufficient tumor samples for pathologic and molecular diagnoses rather than needle biopsies and benefit the most from treatments. The explanations for this are mentioned above. In addition to that, drug resistance and heterogeneity of the EGFR mutations that results from tumor heterogeneity, contribute to the rationale for resections when the disease recurs or metastasizes, for significantly more adequate tissue could make the reevaluations of the pathologic and molecular status of the diseases more accurate.
Using ARMS to detect EGFR mutations in NSCLC, led to three limitations in our study. First, the primers in the ADx EGFR Mutations Detection Kit we used are designed for the 29 already known EGFR mutations, in some rare instances polymorphisms may be present that would not be recognized by this assay. Second, although ARMS is sensitive, routinely being able to detect at least 1% mutant in a normal DNA background, when the DNA concentration is below that level, the results would be false-negative; also, if the samples are contaminated, the results would be less accurate. Third, compared with DNA sequencing, ARMS is not readily available and less economic, although it is superior to sequencing in both sensitivity and robustness on a large and diverse set of clinical tumor samples.
4. Experimental Section
4.1. Patients and Specimen Sampling
Fresh tumor specimens were obtained from 697 consecutive Chinese patients with NSCLC who underwent surgical resection at the Department of Thoracic Surgery, Tangdu Hospital (Xi’an, Shaanxi, China) during the period from January 2012 to August 2013, including 312 patients who underwent video assisted thoracoscopic lobectomy and 385 patients who underwent open thoracotomy. All the patients suffered from NSCLC for the first time, and none of them received any treatment (including TKIs) before operation. Clinical data of each patient were retrieved from their medical records. Prior to the study, written informed consent was obtained from all patients for the use of their tumor samples for molecular and pathologic analysis. The study protocol was approved by the ethics committee of Tangdu Hospital and performed according to principles of good clinical practice.
After surgery, each sample was divided into two parts for pathological re-evaluation and EGFR mutation analysis. Pathologic assessment of all samples was performed by two experienced pathologists. All tumor samples were routinely assessed by sectioning, hematoxylin-eosin staining, and visualization under a microscope. Pathological diagnosis was made according to the criteria of the WHO/IASLC Histological Classification of Lung and Pleural Tumors [26]. Patients diagnosed with squamous-cell carcinoma, adenocarcinoma, adenosquamous carcinoma, large-cell carcinoma, sarcomatoid carcinoma, or mucoepidermoid carcinoma were enrolled for further analysis.
4.2. EGFR Mutation Analysis Using ARMS
Genomic DNA was extracted from fresh tumor specimens using TIANamp Genomic DNA Kit (Tiangen Biotech (Beijing) Co., Ltd, Beijing, China) according to the manufacturer’s instructions. After that, EGFR mutation analysis was performed via ARMS, according to the protocol of the ADx EGFR Mutations Detection Kit (Amoy Diagnostics, Xiamen, China) using the principle of Amplified Refractory Mutation System (ARMS) and covering 29 EGFR mutation hotspots from exon 18 to 21. The assay was carried out according to the manufacturer’s protocol with the MX3005P (Stratagene, La Jolla, CA, USA) real-time PCR system. A positive or negative result could be reached if it met the criterion that was defined by the manufacturer’s instructions.
4.3. Statistical Analysis
The relationship between EGFR mutations and clinical features was analyzed using the Chi-square and Fisher’s Exact test when appropriate (in statistical analysis of R × C contingency table, if more than 20% of cells have expected count less than 5, the Fisher’s exact test is appropriate). Statistical analysis was carried out via SPSS software, version 13.0 (SPSS Company, Chicago, IL, USA, 2004). Two-sided p values less than 0.05 were considered to indicate statistical significance.
5. Conclusions
In conclusion, for the relatively large sample capacity, more effective tumor samples and the use of a more sensitive method in our study, our data should better represent the current situation of TKIs sensitive EGFR mutations in Chinese patients with NSCLS, especially the mutation status of squamous-cell carcinoma, adenocarcinoma and adenosquamous carcinoma. It is recommended that EGFR mutation analysis of NSCLC including adenocarcinoma and nonadenocarcinoma should be a routine molecular test in China after lung resection.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81000938). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Kosaka T., Yatabe Y., Endoh H., Kuwano H., Takahashi T., Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: Biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 3.Lynch T.J., Bell D.W., Sordella R., Gurubhagavatula S., Okimoto R.A., Brannigan B.W., Harris P.L., Haserlat S.M., Supko J.G., Haluska F.G., et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 4.Paez J.G., Janne P.A., Lee J.C., Tracy S., Greulich H., Gabriel S., Herman P., Kaye F.J., Lindeman N., Boggon T.J., et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 5.Bell D.W., Lynch T.J., Haserlat S.M., Harris P.L., Okimoto R.A., Brannigan B.W., Sgroi D.C., Muir B., Riemenschneider M.J., Iacona R.B., et al. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: Molecular analysis of the IDEAL/INTACT gefitinib trials. J. Clin. Oncol. 2005;23:8081–8092. doi: 10.1200/JCO.2005.02.7078. [DOI] [PubMed] [Google Scholar]
- 6.Soung Y.H., Lee J.W., Kim S.Y., Seo S.H., Park W.S., Nam S.W., Song S.Y., Han J.H., Park C.K., Lee J.Y., et al. Mutational analysis of EGFR and K-RAS genes in lung adenocarcinomas. Virchows Arch. 2005;446:483–488. doi: 10.1007/s00428-005-1254-y. [DOI] [PubMed] [Google Scholar]
- 7.Tsao A.S., Tang X.M., Sabloff B., Xiao L., Shigematsu H., Roth J., Spitz M., Hong W.K., Gazdar A., Wistuba I. Clinicopathologic characteristics of the EGFR gene mutation in non-small cell lung cancer. J. Thorac. Oncol. 2006;1:231–239. doi: 10.1016/s1556-0864(15)31573-2. [DOI] [PubMed] [Google Scholar]
- 8.Kang S.M., Kang H.J., Shin J.H., Kim H., Shin D.H., Kim S.K., Kim J.H., Chung K.Y., Chang J. Identical epidermal growth factor receptor mutations in adenocarcinomatous and squamous cell carcinomatous components of adenosquamous carcinoma of the lung. Cancer. 2007;109:581–587. doi: 10.1002/cncr.22413. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki H., Endo K., Yukiue H., Kobayashi Y., Yano M., Fujii Y. Mutation of epidermal growth factor receptor gene in adenosquamous carcinoma of the lung. Lung Cancer. 2007;55:129–130. doi: 10.1016/j.lungcan.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y.T., Kim T.Y., Lee D.S., Park S.J., Park J.Y., Seo S.J., Choi H.S., Kang H.J., Hahn S., Kang C.H., et al. Molecular changes of epidermal growth factor receptor (EGFR) and KRAS and their impact on the clinical outcomes in surgically resected adenocarcinoma of the lung. Lung Cancer. 2008;59:111–118. doi: 10.1016/j.lungcan.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Jang T.W., Oak C.H., Chang H.K., Suo S.J., Jung M.H. EGFR and KRAS mutations in patients with adenocarcinoma of the lung. Korean J. Intern. Med. 2009;24:48–54. doi: 10.3904/kjim.2009.24.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosell R., Moran T., Queralt C., Porta R., Cardenal F., Camps C., Majem M., Lopez-Vivanco G., Isla D., Provencio M., et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T., Matsuoka M., Sutani A., Gemma A., Maemondo M., Inoue A., Okinaga S., Nagashima M., Oizumi S., Uematsu K., et al. Frequency of and variables associated with the EGFR mutation and its subtypes. Int. J. Cancer. 2010;126:651–655. doi: 10.1002/ijc.24746. [DOI] [PubMed] [Google Scholar]
- 14.Mitsudomi T., Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007;98:1817–1824. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mok T.S., Wu Y.L., Thongprasert S., Yang C.H., Chu D.T., Saijo N., Sunpaweravong P., Han B., Margono B., Ichinose Y., et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 16.Mitsudomi T., Morita S., Yatabe Y., Negoro S., Okamoto I., Tsurutani J., Seto T., Satouchi M., Tada H., Hirashima T., et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 17.Gerlinger M., Rowan A.J., Horswell S., Larkin J., Endesfelder D., Gronroos E., Martinez P., Matthews N., Stewart A., Tarpey P., et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton C.R., Graham A., Heptinstall L.E., Powell S.J., Summers C., Kalsheker N., Smith J.C., Markham A.F. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS) Nucleic Acids Res. 1989;17:2503–2516. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellison G., Donald E., McWalter G., Knight L., Fletcher L., Sherwood J., Cantarini M., Orr M., Speake G. A comparison of ARMS and DNA sequencing for mutation analysis in clinical biopsy samples. J. Exp. Clin. Cancer Res. 2010;29:132. doi: 10.1186/1756-9966-29-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mok T., Wu Y.L., Zhang L. A small step towards personalized medicine for non-small cell lung cancer. Discov. Med. 2009;8:227–231. [PubMed] [Google Scholar]
- 21.Kimura H., Fujiwara Y., Sone T., Kunitoh H., Tamura T., Kasahara K., Nishio K. High sensitivity detection of epidermal growth factor receptor mutations in the pleural effusion of non-small cell lung cancer patients. Cancer Sci. 2006;97:642–648. doi: 10.1111/j.1349-7006.2006.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokumo M., Toyooka S., Kiura K., Shigematsu H., Tomii K., Aoe M., Ichimura K., Tsuda T., Yano M., Tsukuda K., et al. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin. Cancer Res. 2005;11:1167–1173. [PubMed] [Google Scholar]
- 23.Sonobe M., Manabe T., Wada H., Tanaka F. Mutations in the epidermal growth factor receptor gene are linked to smoking-independent, lung adenocarcinoma. Br. J. Cancer. 2005;93:355–363. doi: 10.1038/sj.bjc.6602707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pao W., Miller V.A., Politi K.A., Riely G.J., Somwar R., Zakowski M.F., Kris M.G., Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner N.C., Reis-Filho J.S. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012;13:e178–e185. doi: 10.1016/S1470-2045(11)70335-7. [DOI] [PubMed] [Google Scholar]
- 26.Brambilla E., Travis W.D., Colby T.V., Corrin B., Shimosato Y. The new World Health Organization classification of lung tumours. Eur. Respir. J. 2001;18:1059–1068. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]