Abstract
The persistence and variability of 188 Haemophilus influenzae isolates in respiratory tract of 30 cystic fibrosis (CF) patients over the course of 7 years was studied. Antibiotic susceptibility testing, DNA fingerprinting, and analysis of outer membrane protein profiles were performed on all isolates. A total of 115 distinct pulsed-field gel electrophoresis profiles were identified. Ninety percent of patients were cocolonized with two or more clones over the studied period. A third of the patients were cross-colonized with one or two H. influenzae strains; 11% of the clones persisted for 3 or more months. Biotype, outer membrane protein profiles, and resistance profiles showed variation along the studied period, even in persisting clones. Four isolates (2.1%) recovered from 3 patients were type f capsulate, with three of them belonging to the same clone. β-Lactamase production was detected in 23.9% of isolates while 7% of the β-lactamase-negative isolates presented diminished susceptibility to ampicillin (β-lactamase-negative ampicillin resistance phenotype). Remarkably, 21.3% of the H. influenzae isolates presented decreased susceptibility to ciprofloxacin, which was mainly observed in persisting clones. Of the H. influenzae isolates from CF patients, 18 (14.5%) were found to be hypermutable in comparison with 1 (1.4%) from non-CF patients (P < 0.0001). Ten patients (33.3%) were colonized by hypermutable strains over the study period. A multiresistance phenotype and long-term clonal persistence were significantly associated in some cases for up to 7 years. These results suggest that H. influenzae bronchial colonization in CF patients is a dynamic process, but better-adapted clones can persist for long periods of time.
Cystic fibrosis (CF) is an autosomal recessive disorder resulting from mutations in a gene on the long arm of chromosome 7 (11). The gene product is the CF transmembrane conductance regulator, which regulates and facilitates transport of electrolytes across the epithelial cell membrane and other membranes (10).
Haemophilus influenzae is regularly involved in chronic lung infections and acute exacerbations of CF patients (26). To better study the persistence of this bacterium, several typing methods have been applied: serotyping, biotyping, outer membrane protein (OMP) electrophoresis, and genotyping methods, such as random PCR (1, 17, 18, 24, 35).
On the other hand, long-term antibiotic therapy is widely used for the treatment of lung infection in CF patients. The risk of developing antibiotic resistance in these patients is clearly higher than in other patients, and that is also applicable to H. influenzae (17). The first ciprofloxacin-resistant H. influenzae strains in Spain were recovered from CF patients and studied by our groups (4, 9).
The goals of the present study were to carry out a prospective follow-up of CF patients over time to (i) determine whether these patients maintained the same H. influenzae strains or were subsequently colonized by different strains, (ii) study the antimicrobial susceptibility profiles in comparison with non-CF patients, and (iii) discern whether antimicrobial resistance and persistence were associated. Since other studies showed high proportions of hypermutable strains in CF patients carrying Pseudomonas aeruginosa (22) and Staphylococcus aureus (25), we decided to study whether a similar trend may occur in the case of H. influenzae strains.
MATERIALS AND METHODS
Patients.
During the period 1994 to 2001, 30 CF patients (20 males and 10 females) with a history of H. influenzae infections were identified at three different hospitals in Spain (Ramón y Cajal, Madrid; Lozano Blesa, Zaragoza; and Virgen de las Nieves, Granada). The dynamics of H. influenzae colonization in all of these patients were studied for a mean period of 3.4 years (range, 1 to 6.7 years).
Bacterial isolation.
Bacterial isolates were recovered from respiratory secretions, most of them expectorated sputum specimens, during scheduled assessments or pulmonary exacerbations. Samples were homogenized with N-acetyl cysteine and processed by a modified quantitative technique (38). Columbia 5% blood, MacConkey, mannitol-salt, and a selective Burkholderia cepacia agar medium were incubated in air for 24 h at 37°C and then transferred for an additional period of 24 h at 25°C in an incubator. In addition, bacitracin-chocolate agar was plated and incubated on 5% CO2 for 48 h at 37°C. A culture was considered positive for H. influenzae when growth of this organism was observed, irrespective of the bacterial count.
Bacterial identification and biotyping.
The isolates were identified as H. influenzae on the basis of the following biological characteristics: gram-negative small rods requiring X and V factors for growth (Mast Diagnostics, Bootle, Merseyside, United Kingdom). All of the strains were serotyped by a coagglutination test (Phadebact Haemophilus test; Boule Diagnostics AB, Huddinge, Sweden) including both specific anti-type b antibodies and a pool of specific anti-type a, c, d, e, and f antibodies. Discrimination among these serotypes was done with specific a to f antisera obtained in our laboratory and eventually confirmed by the PCR technique (7). To evaluate whether the initial H. influenzae population isolated from patients could be composed of a mixture of different strains, we selected 9 individual colonies from 11 samples and carried out a complete bacterial identification for each one of them.
The presence of hypermutable strains was studied in 124 H. influenzae isolates comprising the 111 different pulsed-field gel electrophoresis (PFGE) patterns (4 more were excluded, as the MICs of rifampin for these strains were ≥4.0 μg/ml) and all of the 13 OMP variants obtained from the sputum of 30 CF patients. As a control group for the susceptibility and hypermutability studies, a collection of 188 epidemiologically unrelated H. influenzae isolates and 71 isolates from non-CF patients matched by time of isolation, geographical source, and anatomical origin were also studied. Biotyping was performed according to the method described by Kilian (12).
OMP profiles.
An overnight culture of H. influenzae was collected in 10 ml of 10 mM HEPES buffer (pH 7.5), and the suspension was subjected to sonication. Cellular debris was removed by centrifugation at 26,450 × g for 3 min (3,000 × g for 10 min). The supernatant was then removed and centrifuged at 26,450 × g for 90 min at 4°C. The pellet containing the cell membrane material was resuspended in HEPES buffer and sodium lauryl sarcosylate (2% in HEPES buffer) for 10 min. Each preparation was then centrifuged at 26,450 × g for 60 min at 4°C. The resulting pellet was diluted in distilled water to a concentration of 1,400 μg of protein per ml. Solutions were then stored at −70°C until all samples were processed. Twenty-five microliters of each membrane suspension was added to 50 μl of sample buffer containing 50% distilled water, 12.6% 0.5 M Tris-HCl (pH 6.8), 10.5% glycerol, 21% sodium dodecyl sulfate (10% solution), and 5% bromophenol blue (0.1% solution). The final solution was boiled for 5 min. Samples were loaded onto sodium dodecyl sulfate-polyacrylamide (4% stacking gel and 10% separating gel), prepared, and run according to the method of Laemmli (13).
PFGE.
Total DNA was prepared, and PFGE was performed as described previously (5, 14). The restriction endonucleases SmaI and Bsp120 I (ApaI) (MBI Fermentas, Vilnius, Lithuania) were used at the manufacturer's suggested temperature. Restriction fragments were separated by PFGE on a 1% agarose gel in 0.5× TBE buffer (45 mM Tris, 45 mM boric acid, 1.0 mM EDTA [pH 8.0]) with the Bio-Rad CHEF Mapper apparatus (Hercules, Calif). The initial pulse time of 5 s was increased linearly to 50 s over 23.3 h at 6 V/cm at 13°C. λ ladders were applied as molecular size markers (size, 48.5 to 1,000 kb, PFGE marker I; Boehringer-Mannheim, Mannheim, Germany). Gels were then stained with ethidium bromide and photographed under UV. Fragment patterns were compared visually and interpreted with software (MVSP Shareware). Similarity analysis and dendrograms were done with this database system on the basis of molecular mass patterns (29, 30).
Antimicrobial susceptibility testing.
For antibiotic susceptibility screening purposes, the disk diffusion susceptibility test for H. influenzae was performed on standard Haemophilus test medium (6) with the following antibiotics: ampicillin, amoxicillin-clavulanic acid, cefaclor, cefixime, cefuroxime, cefotaxime, sulfamethoxazole-trimethoprim, chloramphenicol, tetracycline, kanamycin, rifampin, ciprofloxacin, and nalidixic acid. The isolates were classified as susceptible, intermediate, or resistant according to the criteria of the NCCLS (20). MICs of the following antibiotics were determined by the Epsilon-test (E-test) method: ampicillin, amoxicillin-clavulanic acid, cefotaxime, sulfamethoxazole-trimethoprim, chloramphenicol, ciprofloxacin, and nalidixic acid. In the case of conflicting susceptibility data results, disk diffusion tests and MICs were replicated. The control strains were H. influenzae ATCC 49247 and ATCC 51907. β-Lactamase activity was studied by the chromogenic cephalosporin test with nitrocefin as the substrate (21).
Determination of mutation frequencies.
To determine mutation frequencies, one bacterial colony was resuspended in 20 ml of brain heart infusion broth and grown at 37°C with 5% CO2 overnight. Bacterial cells were then collected at 3,000 rpm for 5 min and resuspended in 1 ml of brain heart infusion broth. A 100-μl sample from this suspension, as well as samples from successive dilutions, was plated onto Haemophilus test medium with and without rifampin (10 μg/ml). After 48 h of incubation, the number of colonies was counted and the mutation frequencies were determined as relative proportions to the total counts of viable organisms plated. For every strain yielding >30 colonies on antibiotic-containing medium, the experiment was repeated in triplicate, and results were indicated as mean values. A strain was considered to be hypermutable when the mutation frequency was higher than 10−7, i.e., at least 10 times higher than the average mutation frequency found for H. influenzae strains in non-CF patients (22, 25).
Analysis of results.
Management of data and statistical calculations were carried out by using the Whonet Programme (WHO/CSR/DRS/99.1; World Health Organization). Mean mutation frequencies were compared by the Mann-Whitney test; categorical variables were compared by Fisher's exact test. P values were considered significant at a value of 0.05.
RESULTS
Frequency and density of H. influenzae colonization.
Seven hundred ninety-four sputum specimens from 30 CF patients were analyzed (median, 26.5 samples per patient; range, 4 to 82 samples per patient). The median follow-up was 40.8 months (range, 12 to 80 months). In total, 188 H. influenzae strains were obtained (mean number of isolates per patient, 6.3; range, 1 to 27 isolates per patient) from the 30 patients studied (Table 1). In the group of 11 clinical patients (see Materials and Methods) in which nine independent H. influenzae colonies were studied, only one clonal type was identified per patient, suggesting that cocolonization is a rare event in our series. The quantitative study in the sputa showed an average of 108 CFU/ml (range, 103 to 109 CFU/ml). The cross-colonizing clones persisting for more than 3 months did not reached higher cell densities in the sputa than the total average (7.7 × 107 and 9.1 × 107 CFU/ml, respectively).
TABLE 1.
Results of typing of 188 H. influenzae strains isolated from 30 patients with CF at three different hospitals in Spaina
| Patient no. | Sexb | Patient age at isolate collection (yr/mo) | Sampling period (mo) | No. of H. influenzae strainsc | PFGE pattern(s)d | Strain distribution according to PFGE pattern(s) | Persistence of PFGE pattern (mo) | OMP variant(s) | Biotype(s) (no. of strains) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 37/10 | 80 | 15 | 1A, 1B, 2 | 10, 4, 1 | 80, 15, 0 | 1Aa, 1Ab, 1Ac | VI (6)-VIII (2)-VII (2) VI, VI |
| 2 | F | 18/11 | 39 | 3 | 3, 4 | 2, 1 | 39, 0 | VII, V | |
| 3 | F | 12/4 | 41 | 18 | 5, 6, 7, 8 | 14, 2, 1, 1 | 26, 10, 0, 0 | 5a(5), 5b, 5c(4), 6a | V, III, II, III |
| 4 | M | 4/3 | 58 | 7 | 9, 10, 11, 12, 13 | 1, 2, 1, 1, 2 | 0, 3, 0, 0, 2 | II, I, I, II, I | |
| 5 | M | 15/9 | 60 | 3 | 14, 15 | 2, 1 | 1, 0 | II, III | |
| 6 | F | 3/10 | 61 | 15 | 16, NT, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 | 2, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 3 | 3, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 8 | II, VII, II, I, IV, III, II, III, I, III, III, I | |
| 7 | F | 4/11 | 77 | 15 | 27, 28, 19, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 | 2, 1, 2, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1 | <1, 0, 1, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0 | 19a | I, I, II, II, V, III, III, II, II, III, II, II, II |
| 8 | M | 11/2 | 41 | 3 | 39, 40, 41 | 1, 1, 1 | II, II, II | ||
| 9 | M | 21/5 | 12 | 3 | 41, 42, 43 | 1, 1, 1 | III, III, II | ||
| 10 | F | 16/8 | 49 | 2 | 44, 45 | 1, 1 | I, II | ||
| 11 | M | 11/9 | 48 | 3 | 46, 47, 48 | 1, 1, 1 | II, II, I | ||
| 12 | M | 1/6 | 37 | 6 | 49, 41a, 50, 51, 52, 53 | 1, 1, 1, 1, 1, 1 | 41a | VIII, II, VII, III, II, II | |
| 13 | M | 5/9 | 51 | 5 | 52a, 54, 55, 56 | 1, 2, 1, 1 | 0, 1, 0, 0 | 52a | II, III, II, II |
| 14 | F | 8/11 | 33 | 1 | 39 | 1 | II | ||
| 15 | M | 18/1 | 50 | 1 | 57 | 1 | I | ||
| 16 | F | 6/7 | 41 | 1 | 52 | 1 | VIII | ||
| 17 | F | 5/10 | 48 | 7 | 58, 59, 60, 61, 62 | 2, 1, 1, 1, 2 | 9, 0, 0, 0, 6 | II, V, I, III, II | |
| 18 | M | 4/10 | 40 | 4 | 63, 64, NT, 65 | 1, 1, 1, 1 | I, II, III, I | ||
| 19 | M | 22/6 | 28 | 2 | 66, 67 | 1, 1 | IV, III | ||
| 20 | F | 3/8 | 38 | 9 | 68, 69, 70, 71, 72, 73 | 1, 2, 2, 1, 2, 1 | 0, 11, 1, 0, 2, 0 | 72a | III, II, I, V, III, II |
| 21 | M | 1/2 | 47 | 7 | 74, 75, 76, 77, 78, 79, 70 | 1, 1, 1, 1, 1, 1, 1 | I, III, V, III, II, II, I | ||
| 22 | F | 5/8 | 16 | 5 | 80, 81, 82, 83 | 1, 1, 1, 2 | 0, 0, 0, 2 | 83a | II, II, II, VII, II |
| 23 | M | 2/3 | 40 | 7 | 84, 85, 86, 87, 88, 89 | 1, 2, 1, 1, 1, 1 | 0, 2, 0, 0, 0, 0 | III, II, III, II, II, I | |
| 24 | M | 20/2 | 19 | 2 | 90, 91 | 1, 1 | III, III | ||
| 25 | M | 3 | 17 | 2 | 92, 93 | 1, 1 | II, III | ||
| 26 | M | 9/7 | 23 | 3 | 94, 95, 96 | 1, 1, 1 | II, II, II | ||
| 27 | M | 5/6 | 14 | 5 | NT, 97, 98, 99, 100 | 1, 1, 1, 1, 1 | III, V, II, II, III | ||
| 28 | M | 20/9 | 72 | 27 | 101, 102, 103, 104, 105 | 23, 1, 1, 1, 1 | 72, 0, 0, 0, 0 | 101a | I (20)-II (2)-IV (1), I, I, I, II |
| 29 | M | 2/5 | 25 | 3 | 106, 107, 108 | 1, 1, 1 | VII, VI, I | ||
| 30 | M | 6/5 | 20 | 4 | 109, 110, 111 | 2, 1, 1 | 11, 0, 0 | I, I, I |
1A and 1B represent clone 1 subtypes. Lower case letters represent OMP variants. Boldface type represents cross-colonizing strains.
M, male; F, female.
Total number of isolates.
NT, nontypeable.
Serotypes and biotypes.
Four (2.1%) of the 188 isolates were serotype f, with three of them belonging to the same clone, despite being isolated from three different patients. The remaining 184 strains, from 27 patients, were nontypeable. The most frequent biotype was biotype II (30.3%), followed by biotypes I (27.7%), III (17%), V (10.6%), VI (6.4%), VII (3.7%), VIII (2.7%), and IV (1.6%). Removing duplicate strains (considering only the first isolate of each clone), the most frequent biotype was II (36.5%), followed by biotypes III (25.2%), I (21.7%), V (6.1%), VI (3.5%), VII (3.5%), IV (1.7%), and VIII (1.7%). Six (5.2%) of the 115 H. influenzae clones obtained as defined by the PFGE profile (see below) showed biotype changes in successive isolations in the same patient.
PFGE profiles.
On the basis of PFGE patterns, with SmaI as the restriction enzyme, we obtained 112 different profiles from 188 isolates. Three strains were not digested with SmaI but were digested with Bsp120 I. The same number was assigned to identical restriction patterns obtained from different strains. PFGE patterns with coefficients of similarity greater than 85% were considered to define a particular clone. For instance, PFGE patterns 1A and 1B (Table 1) were considered to be same clone, clone 1, according to the criteria of Tenover et al. (31). We also tested the isolates with the other restriction enzyme (Bsp120 I), obtaining the same clonal distribution (Fig. 1, 2, and 3). In some cases, strains with the same PFGE profile had a variation in their OMP profiles; hence, we designated them with lowercase letters next to the numbers of the PFGE pattern (Table 1; see Fig. 5).
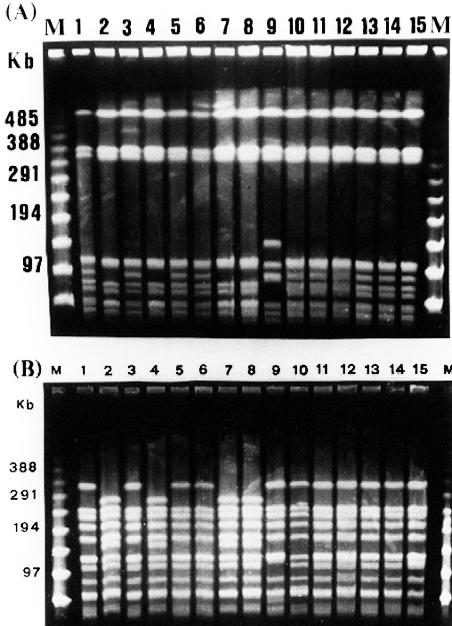
FIG. 1.
PFGE fingerprints of 15 strains from patient 1 in chronological order. The patient harbors three patterns: pattern 1A (lanes 1, 3, 5, 6, 10, 11, 12, 13, 14, and 15), pattern 1B (lanes 2, 4, 7, and 8), and pattern 2 (lane 9). Pattern 1B strains showed the same biotype (VI) as six strains (lanes 1, 3, 5, 6, 11, and 15) of pattern 1A and one strain of pattern 2 (lane 9). (A) SmaI PFGE fingerprints; (B) Bsp120 I (ApaI) PFGE fingerprints. M is the molecular size marker (PFGE marker).
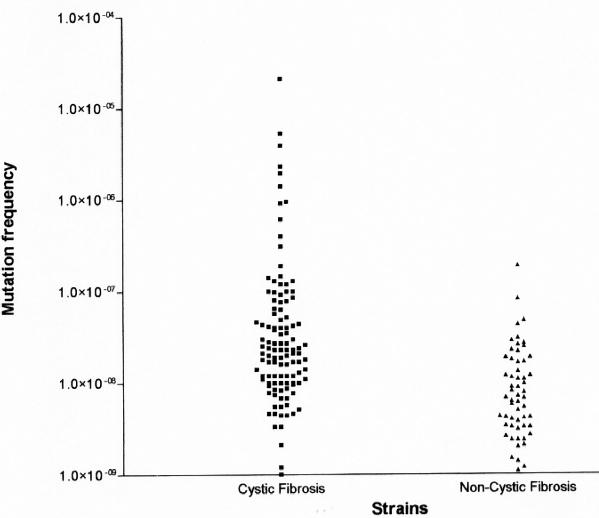
FIG. 5.
Mutation frequency of the CF and non-CF H. influenzae strains for rifampin resistance. Each dot represents the mean mutation frequency calculated from 3 experiments for 1 strain. Most strains in both groups yielded mutation frequencies to rifampin resistance of <10−7. Strains with mutation rates of >10−7 were considered to display a hypermutable phenotype.
Persistence.
The 115 H. influenzae PFGE patterns persisted for an average of 2.5 months (range, 1 to 80 months). Thirteen PFGE patterns (11.3%) persisted for 3 or more months in their respective patients. In several patients, H. influenzae strains with indistinguishable PFGE patterns persisted for a long period of time (Table 1). Figures 1, 2, and 3 show the colonization course in three selected patients: 1, 3, and 28. In 24 patients (80%), the first strain of H. influenzae was isolated only once and was replaced by other strains in subsequent cultures. In 27 patients (90%), two or more different strains were isolated over the period of study.
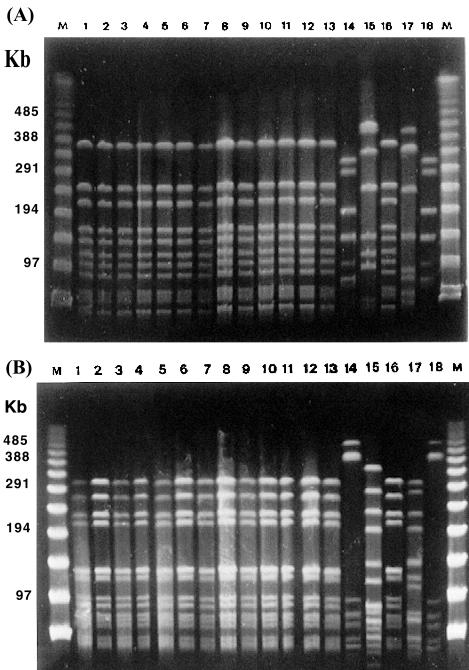
FIG. 2.
PFGE fingerprints of 18 strains from patient 3 in chronological order. The patient harbors four clones: clone 4 (lanes 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, and 16), clone 6 (lanes 14 and 18), clone 7 (lane 15), and clone 8 (lane 17). Clones 6 and 8 showed the same biotype (III). (A) SmaI PFGE fingerprints; (B) Bsp120 I (ApaI) PFGE fingerprints. M is the molecular size marker (PFGE marker). PFGE patterns of the same strains with Bsp120 I (ApaI) are shown in the second photo.
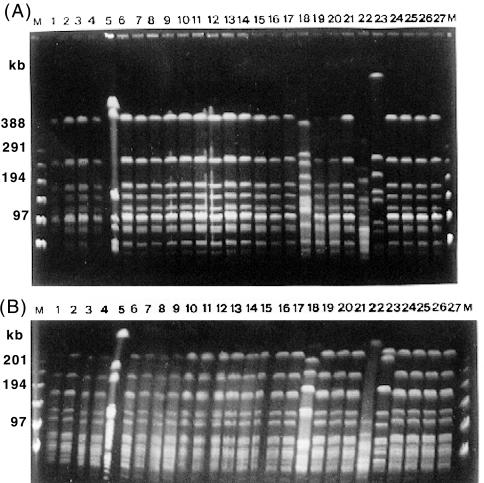
FIG. 3.
PFGE fingerprints of 27 strains from patient 28 in chronological order. The patient harbors five clones: clone 101 (lanes 1, 2, 3, 4, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 19, 20, 21, 24, 25, 26, and 27), clone 102 (lane 5), clone 103 (lane 18), clone 104 (lane 22), and clone 105 (lane 23). Three strains of clones 102 (lane 5), 103 (lane 18), and 104 (lane 22) and 20 strains (lanes 1, 2, 3, 4, 6, 7, 8, 9, 11, 12, 13, 14, 16, 17, 19, 20, 21, 24, 25, and 26) of clone 101 showed the same biotype (I). Clone 105 and two isolates of clone 101 (lanes 10 and 27) showed the same biotype (II). (A) SmaI PFGE fingerprints; (B) Bsp120 I (ApaI) PFGE fingerprints. M is the molecular size marker (PFGE marker).
Nine (7.9%) of the 114 clones (115 PFGE patterns) had OMP variations (Table 1 and Fig. 4) (27); the greatest number of OMP variants was found in patient 3. In patients 14, 15, and 16, with long follow-up periods (33 to 50 months), only one H. influenzae strain was isolated (Table 1).
FIG. 4.
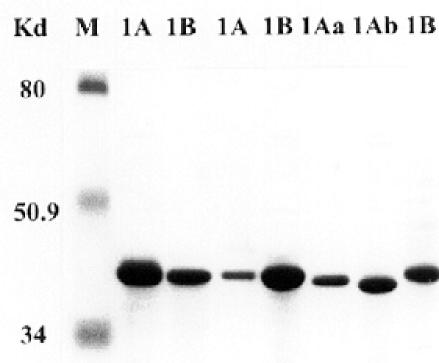
Variants of OMP profiles of seven H. influenzae strains belonging to two PFGE patterns (1A and 1B) from patient 1 isolated at different periods of time. Letters a and b indicate variations in the OMPs.
Cross-colonizing strains.
Five clones were isolated from more than one patient. Clone 19 was isolated from patients 6 and 7 the same month (Table 1), persisting one month in the last patient. Clone 39 colonized patients 8 and 14. Clone 41 was found in patients 8, 9, and 12. Clone 52 was obtained from patients 13, 16, and 20. Clone 70 (a type f isolate) colonized patient 20 for 1 month, and 2.5 years later, it appeared in patient 21 (Table 1). In total, 10 patients from the same hospital were cross-colonized with one or two strains from other patients, suggesting a patient-to-patient transmission. However, strains from patients sharing identical clones did not always present the same biotype. The biotype of clone 19 was IV in patient 6 but II in patient 7; the biotype of clone 52 was II in patient 12 but VIII in patient 16.
Antibiotic susceptibility.
Among the 188 H. influenzae strains, diminished ampicillin susceptibility was found in 29.2%, 23.9% produced β-lactamase, and 5.3% presented diminished susceptibility (MIC ≥ 2 μg/ml) to ampicillin (β-lactamase-negative ampicillin resistance [BLNAR] phenotype) (8). Considering only the first isolate of each one of the different 115 clones, the corresponding figures are 29.6% for reduced ampicillin susceptibility, 26.1% for β-lactamase production, and 3.5% for BLNAR (Table 2). All isolates were susceptible to cefotaxime, cefixime, and amoxicillin-clavulanate. Only one strain was β-lactamase positive and amoxicillin-clavulanate resistant.
TABLE 2.
MICs for H. influenzae isolated from CF patients, as determined by the Epsilon test
| Antibiotic | No. of isolatesa | MIC rangeb | MIC50b | MIC90b | Geometric mean |
|---|---|---|---|---|---|
| Ampicillin | 188 | 0.064-256 | 0.50 | 64 | 0.88 |
| 115 | 0.064-256 | 0.25 | 64 | 0.82 | |
| Amoxicillin-clavulanic acid | 188 | 0.064-8 | 0.5 | 2 | 0.55 |
| 115 | 0.064-4 | 0.5 | 2 | 0.47 | |
| Cefotaxime | 188 | 0.003-2 | 0.23 | 0.125 | 0.03 |
| 115 | 0.003-1 | 0.016 | 0.06 | 0.02 | |
| Chloramphenicol | 188 | 0.125-16 | 0.5 | 8 | 0.70 |
| 115 | 0.125-16 | 0.5 | 1.0 | 0.53 | |
| Cotrimoxazole | 188 | 0.012-64 | 4 | 64 | 1.8 |
| 115 | 0.012-64 | 4 | 32 | 0.90 | |
| Ciprofloxacin | 188 | 0.003-64 | 0.012 | 4 | 0.04 |
| 115 | 0.003-32 | 0.012 | 0.025 | 0.01 |
Two values are specified: 188 is the total number of isolates, and 115 is the number of PFGE patterns.
Expressed in micrograms/milliliter.
Regarding the ciprofloxacin susceptibility, the ciprofloxacin MIC was >1 μg/ml for 40 (21.3%) of the 188 H. influenzae isolates compared to 9 (7.8%) of the 115 first isolates of each PFGE pattern (P = 0. 002).
According to NCCLS breakpoints (20), chloramphenicol and amoxicillin-clavulanate exhibited susceptibility rates equal to or higher than 85%. The antibiotics with the lowest intrinsic activity (as measured by the MIC at which 90% of the isolates tested are inhibited [MIC90]) were trimethoprim-sulfamethoxazole and ampicillin, both of which had MIC90s of ≥32 μg/ml.
Eleven (36.7%) patients were colonized by multidrug-resistant H. influenzae clones (defined as resistance or diminished susceptibility to three or more antimicrobials of different antibiotic classes). The most common combinations of multidrug resistance were ampicillin, cefaclor, chloramphenicol, ciprofloxacin, and cotrimoxazole. Only three patients (5, 9, and 10) (Table 1) were colonized by totally antibiotic-susceptible strains.
Antibiotic resistance and H. influenzae persistence.
Of the 13 PFGE patterns (11.3%, 12 clones) that persisted for at least 3 months, 3 did not change antimicrobial susceptibility over time, 3 acquired additional antibiotic resistance markers, 3 lost at least one resistance, and 4 suffered apparent changes in sensitivity. The most frequently acquired resistance determinants were tetracycline, in six PFGE patterns, and kanamycin, in three PFGE patterns. Of the 12 clones that persisted for more than 3 months, 4 had diminished susceptibility to two or more antibiotics and persisted for 1,545 days (51.5 months; range, 15 to 80 months) on average, compared with a period of 291 days (9.7 months; range, 3 to 26 months) for the 8 other susceptible persistent clones (P = 0.0001, Fisher's exact test).
Twenty-three patients (76.7%) yielded at least three consecutive H. influenzae strains, irrespective of their clonality. In four patients (13.3%), no changes were observed in the antibiotic susceptibility patterns. In six patients (20%), the isolates increased their antibiotic resistance markers. In five patients (16.7%), the isolates diminished in antibiotic resistance. In three patients (10%), the strains initially increased in antibiotic resistance but it decreased further on. In two patients (6.7%), the strains initially lost resistance determinants, but they increased afterwards. In three patients (10%), there was a continuous fluctuation in antibiotic susceptibility. Older patients (patients 1 [37 years], 2 [18 years], and 28 [20 years at the beginning of the study]) were colonized with more strains resistant to different antibiotics such as ciprofloxacin (nalidixic acid), cotrimoxazole, chloramphenicol, ampicillin, and cefaclor.
Antibiotic susceptibility of CF patient strains versus non-CF patient control strains.
We observed a significantly higher frequency of resistance to ampicillin (55 of 188 strains, 29.2%) and decreased susceptibility to ciprofloxacin (40 of 188 strains, 21.2%) among CF patients than among the patients of the control group (39 of 188 strains, 20.7 and 0%, respectively) (Table 3). Similarly, a significantly higher proportion of BLNAR (10 of 188 strains, 5.3%) and resistance to two or more antibiotics (62 of 188 strains, 32.9%) were found in the strains from the CF group than non-CF control strains (20 of 188 strains, 10.6%) (Table 3).
TABLE 3.
Antimicrobial susceptibility comparison between H. influenzae strains isolated from CF patients and controls without CF
| Parameter | Result (no. of strains) for:
|
Pa | |
|---|---|---|---|
| Control isolates (188) | CF isolates (188) | ||
| Fully antibiotic susceptible | 88 | 50 | 0.006 |
| Chloramphenicol resistant | 23 | 36 | 0.12 |
| Ciprofloxacin MIC > 1 μg/mlb | 0 | 40 | <0.0001 |
| Amoxicillin-clavulanic acid resistant | 1 | 4 | 0.37 |
| Cotrimoxazole resistant | 68 | 123 | 0.001 |
| β-Lactamase positive | 38 | 45 | 0.54 |
| BLNAR | 1 | 10 | 0.01 |
| Resistant to 2 or more antimicrobials | 20 | 62 | <0.0001 |
Fisher's exact test.
The usual ciprofloxacin MIC for H. influenzae is ≤0.03 μg/ml (23).
Frequency of hypermutation.
Of the H. influenzae strains isolated from CF patients, 18 (14.5%) exhibited a mutator phenotype compared with 1 (1.4%) from non-CF patients (P = 0.002). Ten patients (33.3%) were colonized by hypermutable strains over the study period. The mutation frequency distributions are shown in Fig. 5. The mean mutation frequency in the two groups (3.4 × 10−7 for 124 strains in the CF group and 1.22 × 10−8 for 71 strains in the non-CF group) significantly differed (P < 0.0001, Mann-Whitney U test). Two groups of isolates from CF patients were distinguished: a group of nonmutators, with a mean mutation frequency of 2.4 × 10−8 ± 2.5 × 10−8, and a group of mutators, with a mean mutation frequency of 2.2 × 10−6 ± 4.9 × 10−6 (Fig. 5). The mutation frequency of the only mutator strain in non-CF patients was 1.9 × 10−7; the remaining strains had a low mutation frequency (mean, 9.7 × 10−9 ± 1.4 × 10−8) (Fig. 5).
Of the 18 hypermutable strains, 16 presented resistance to one or more antibiotics (7 were multiple antibiotic resistant, 1 was resistant to ampicillin and cotrimoxazole, 5 were resistant to cotrimoxazole, 2 were resistant to tetracycline, and 1 was resistant to ampicillin) and 2 were fully antibiotic susceptible.
DISCUSSION
Several studies have previously investigated the epidemiology of long-term P. aeruginosa colonization in the respiratory tracts of patients with CF, but there are a limited number of studies dealing with H. influenzae (17, 18, 26, 28). We describe here the dynamics of H. influenzae colonization in 30 CF patients over a period of time (median, 40.8 months; range, 12 to 80 months) that is longer time than in the only previous study (18). Sequential isolates were obtained from 27 patients. Only in 20% of the patients was the same clone repeatedly recovered during the time of observation. This proportion was identical by PFGE (this study) or randomly amplified polymorphic DNA typing (18).
We observed that all biotypes are recognized among H. influenzae isolates from CF patients, as did other authors (17, 37). In our series, the order of biotype isolation by frequency (considering the first isolate of each PFGE clone) was II (36%), III (25%), and I (21%), followed by V, VI, VII, and VIII (in total, 17%). These proportions are almost identical to those found in The Netherlands, with the exception of biotype VIII, hyperrepresented in the series from Möller et al. (17). Like these authors, we have also observed biotype fluctuations among persistent and cross-colonizing clones. No apparent association between biotypes and OMP profiles was found, in accordance with previous observations (2). Nevertheless, as with biotypes, variations over time were observed in persisting clones (Table 1), corroborating the low power as a typing marker of this technique (34).
In our study, only the strains from three patients remained totally susceptible to the antimicrobials studied. We observed greater antibiotic susceptibility figures among the 115 first isolates of each PFGE pattern (114 clones) than among the total 188 H. influenzae isolates, suggesting that persistence increases the levels of resistance. The usual ciprofloxacin MIC for H. influenzae is ≤0.03 μg/ml (23). We have shown that H. influenzae strains for which the MIC was ≥0.12 μg/ml have target mutations (9). It is noteworthy that the number of strains for which the ciprofloxacin MIC is >1.0 μg/ml in our study increased from 7.8% in the 115 first isolates to 21.3% in all 188 isolates. The two most persistent clones (clone 1 from patient 1, which persisted for 80 months, and clone 101 from patient 28, which persisted for 72 months) presented a high MIC of ciprofloxacin (4).
The large proportion of strains with diminished susceptibility (MIC > 1.0 μg/ml) to ciprofloxacin (21.3%) in our study is noteworthy and the highest reported to date in the literature for H. influenzae from any geographical or clinical location. Note that the overall frequency of ciprofloxacin resistance in H. influenzae in Spain does not exceed 0.1% (16).
Interestingly, the rate of ampicillin resistance in our CF H. influenzae isolates, although higher than in The Netherlands (19), was almost identical (29.2%) to the average frequency of ampicillin resistance in Spain (30.1%) (16). BLNAR isolates from CF patients were more abundant among persistent strains (5.3%) than among the first isolated strains of each clone (3.5%).
In general, this study suggests that CF H. influenzae strains are much more antibiotic resistant than strains from non-CF patients. Resistance to two or more antimicrobials occurred in 62 (32.9%) of all H. influenzae isolates (Table 3) and 11 (36.7%) patients; analogous observations have made for other bacterial pathogens, such as P. aeruginosa, Stenotrophomonas maltophilia, and S. aureus (22, 25, 32, 33). The increased resistance rates in these patients have been associated with high and persistent consumption of antimicrobial agents (15). However, the effect of high rates of consumption on the frequency of antibiotic resistance is expected to be significantly amplified in the presence of hypermutable bacteria. All of these facts may have important therapeutic consequences.
Our measurements of mutation frequency corroborated with H. influenzae the presence of a significant proportion (14.5%) of hypermutable strains previously found in CF patients with Pseudomonas (19.5%) (22) and S. aureus (14.6%). It has been shown that hypermutation strongly correlates with antibiotic resistance (22), and this probably also occurs with H. influenzae. It is interesting that the mechanisms of resistance derived from mutational events in H. influenzae (such as ciprofloxacin and cotrimoxazole resistance) are more influenced by hypermutation than those mediated by horizontal gene transfer (such as β-lactamases). We cannot rule out the possibility that hypermutation may originate other selective advantages related to the adaptation and survival in the local conditions of the CF lung.
Mutation frequency (Fig. 5) was less polarized in H. influenzae than in Pseudomonas and similar to that in Staphylococcus, with a wide range of distribution. Van Schilfgaarde et al. (36) suggested that penetration of H. influenzae between epithelial cells in vivo might contribute to the persistence of this microorganism in CF patients in the presence of antibiotics and antibodies, despite the fact that the isolated bacteria are fully susceptible to these antibacterial activities in vitro. In addition to this, it does appear that the prolonged microbial colonization or infection that is characteristic of the CF lung results from defects in the innate or nonspecific immune system (10).
H. influenzae cross-colonization with five clones among 10 different patients was observed. Since these patients were from the same hospital, this could be due to patient-to-patient transmission and/or that these clones have a special ability to invade the respiratory tract. One of these five clones was serotype f. Nonencapsulated H. influenzae isolates are almost always from CF patients, although encapsulated serotype b may be occasionally isolated, most often from CF children less than 5 years of age. In our study, with the exception of four type f isolates, all H. influenzae isolates were nontypeable. To the best of our knowledge, this is one of the first CF studies to isolate capsulated type f strains. In other research from our laboratory (3), with different types of patients, 18 strains of serotype f were found in a total of 38 respiratory samples from 1996 to 2000, suggesting that serotype f strains often colonize the upper respiratory tract.
In summary, using PFGE typing, we characterized 115 genotypes among 188 isolates of H. influenzae in 30 CF patients over a long follow-up period ranging from 1 to 7 years. Although persistence of the same clone was only observed in nearly 12% of patients, chronic colonization with multiple H. influenzae clones was observed in the majority of patients. Antimicrobial resistance was associated with strain persistence over long periods of time. H. influenzae strains isolated from CF patients show a marked prevalence of hypermutable strains. These data may influence the antibiotic treatment of CF patients.
Acknowledgments
This work was supported by a research grant from the Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo, Madrid, Spain (95-98/0364).
We are grateful to E. Moguel for his technical assistance and to C. García (Hospital Lozano Blesa, Zaragoza) and J. C. Alados (Hospital Virgen de las Nieves, Granada) for their cooperation.
REFERENCES
- 1.Barenkamp, S. J., R. S. Munson, Jr., and D. M. Granoff. 1981. Subtyping isolates of Haemophilus influenzae type b by outer membrane protein profiles. J. Infect. Dis. 143:668-676. [DOI] [PubMed] [Google Scholar]
- 2.Bilton, D., A. Pye, M. M. Johnson, J. L. Mitchell, M. Dodd, A. K. Webb, R. A. Stockley, and L. Hill. 1995. The isolation and characterization of non-typeable Haemophilus influenzae from the sputum of adult cystic fibrosis patients. Eur. Respir. J. 8:948-953. [PubMed] [Google Scholar]
- 3.Campos, J., F. Román, M. Pérez-Vázquez, B. Aracil, J. Oteo, and E. Cercenado. 2003. Antibiotic resistance and clinical significance of Haemophilus influenzae type F. J. Antimicrob. Chemother. 52:961-966. [DOI] [PubMed] [Google Scholar]
- 4.Campos, J., F. Román, M. Georgiou, C García, R. Gómez-Lus, R. Cantón, and H. Escobar, F. Baquero. 1996. Long-term persistence of ciprofloxacin-resistant Haemophilus influenzae in patients with cystic fibrosis. J. Infect. Dis. 174:1345-1347. [DOI] [PubMed] [Google Scholar]
- 5.Cerquetti, M., M. L. Ciofi degli Atti, G. Renna, A. E. Tozzi, M. L. Garlaschi, and P. Mastrantonio. 2000. Characterization of non-type B Haemophilus influenzae strains isolated from patients with invasive disease. J. Clin. Microbiol. 38:4649-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doern, G. V., E. H. Gerlach, J. H. Jorgensen, P. R. Murray, C. Thornsberry, and J. A. Washington, Jr.. 1991. Quality control limits for disk diffusion and broth microdilution susceptibility tests with Haemophilus test medium. Diagn. Microbiol. Infect. Dis. 14:485-493. [DOI] [PubMed] [Google Scholar]
- 7.Falla, T. J., D. W. Crook, L. N. Brophy, D. Maskell, J. S. Kroll, and E. R. Moxon. 1994. PCR for capsular typing of Haemophilus influenzae. J. Clin. Microbiol. 32:2382-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazagne, L., C. Delmas, E. Bingen, and H. Dabernat. 1998. Molecular epidemiology of ampicillin-resistant non-beta-lactamase-producing H. influenzae. J. Clin. Microbiol. 36:3629-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgiou, M., R. Muñoz, F. Román, R. Cantón, R. Gómez-Lus, J. Campos, and A. G. de la Campa. 1996. Ciprofloxacin-resistant Haemophilus influenzae strains possess mutations in analogous positions of GyrA and ParC. Antimicrob. Agents Chemother. 40:1741-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart, C. A., and C. Winstanley. 2002. Persistent and aggressive bacteria in the lungs of cystic fibrosis children. Br. Med. Bull. 61:81-96. [DOI] [PubMed] [Google Scholar]
- 11.Kerem, B., J. Rommens, J. Buchanan, D. Markewicz, T. Cox, A. Chakranarti, M. Buchwald, and L. Tsui. 1989. Identification of the cystic fibrosis gene: genetics analysis. Science 245:1873-1880. [DOI] [PubMed] [Google Scholar]
- 12.Kilian, M. 1976. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J. Gen. Microbiol. 93:9-62. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 14.Lee, J. J., and H. O. Smith. 1998. Sizing of the Haemophilus influenzae Rd genome by pulsed-field agarose gel electrophoresis. J. Bacteriol. 170:4402-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marco, F., J. García de Lomas, C. García-Rey, E. Bouza, L. Aguilar, C. Fernández-Mazarrasa, and The Spanish Surveillance Group for Respiratory Pathogens. 2001. Antimicrobial susceptibilities of 1,730 Haemophilus influenzae respiratory tract isolates in Spain in 1998-1999. Antimicrob. Agents Chemother. 45:3226-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Möller, L. V., H. Grasselier, J. Dankert, and L. van Alphen. 1996. Variation in metabolic enzyme activity of persistent Haemophilus influenzae in respiratory tracts of patients with cystic fibrosis. J. Clin. Microbiol. 34:1926-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moller, L. V., A. G. Regelink, H. Grasselier, J. E. Dankert-Roelse, J. Dankert, and L. van Alphen. 1995. Multiple Haemophilus influenzae strains and strain variants coexist in the respiratory tract of patients with cystic fibrosis. J. Infect. Dis. 172:1388-1392. [DOI] [PubMed] [Google Scholar]
- 19.Moller, L. V., A. G. Regelink, H. Grasselier, L. van Alphen, and J. Dankert. 1998. Antimicrobial susceptibility of Haemophilus influenzae in the respiratory tracts of patients with cystic fibrosis. Antimicrob. Agents Chemother. 42:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing; twelfth informational supplement, vol. 22, no. 1. M2-A7 and M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 21.O′Callaghan, C. H., A. Morris, S. M. Kirby, and A. H. Shingler. 1972. Novel method for detection of B-lactamases by using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliver, A., R. Cantón, P. Campo, F. Baquero, and J. Blázquez. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251-1254. [DOI] [PubMed] [Google Scholar]
- 23.Pérez-Vázquez, M., F. Román, M. C. Varela, R. Cantón, and J. Campos. 2003. Activities of 13 quinolones by three susceptibility testing methods against a collection of Haemophilus influenzae isolates with different levels of susceptibility to ciprofloxacin: evidence for cross-resistance. J. Antimicrob. Chemother. 51:147-151. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller, M. A., C. Wendt, R. J. Hollis, R. P. Wenzel, S. J. Fristschel, J. J. Neubauer, and L. A. Herwaldt. 1996. Comparative evaluation of an automated ribotyping system versus pulsed-field gel electrophoresis for epidemiological typing of clinical isolates of Escherichia coli and Pseudomonas aeruginosa from patients with gram-negative bacteremia. Diagn. Microbiol. Infect. Dis. 25:1-8. [DOI] [PubMed] [Google Scholar]
- 25.Prunier, A. L., B. Malbruny, M. Laurans, J. Brouard, J. F. Duhamel, and R. Leclerq. 2003. High rate of macrolide resistance in Staphylococcus aureus strains from patients with cystic fibrosis reveals high proportions of hypermutable strains. J. Infect. Dis. 187:1709-1716. [DOI] [PubMed] [Google Scholar]
- 26.Rajan, S., and L. Saiman. 2002. Pulmonary infections in patients with cystic fibrosis. Semin. Respir. Infect. 17:47-56. [DOI] [PubMed] [Google Scholar]
- 27.Regelink, A. G., D. Dahan, L. V. Moller, J. W. Coulton, P. Eijk, P. Van Ulsen, J. Dankert, and L. Van Alphen. 1999. Variation in the composition and pore function of major outer membrane pore protein P2 of Haemophilus influenzae from cystic fibrosis patients. Antimicrob. Agents Chemother. 43:226-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renders, N., L. Licciardello, C. Ijsseldijk, M. Sijmons, L. Van Alphen, H. Verbrugh, and A. van Belkum. 1999. Variable numbers of tandem repeat loci in genetically homogeneous Haemophilus influenzae strains alter during persistent colonization of cystic fibrosis patients. FEMS Microbiol. Lett. 173:95-102. [DOI] [PubMed] [Google Scholar]
- 29.Schmid, J., E. Voss, and D. R. Soll. 1990. Computer-assisted methods for assessing strain relatedness in Candida albicans by fingerprinting with the moderately repetitive sequence Ca3. J. Clin. Microbiol. 28:1236-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorensen, B., E. S. Falk, E. Wisloff-Nilsen, B. E. Bjorvatn, and B. E. Kristiansen. 1985. Multivariate analysis of Neisseria DNA restriction endonuclease patterns. J. Gen. Microbiol. 131:3099-3104. [DOI] [PubMed] [Google Scholar]
- 31.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valdezate, S., A. Vindel, A. Echeita, F. Baquero, and R. Cantón. 2002. Topoisomerase II and IV quinolone resistance-determining regions in Stenotrophomonas maltophilia clinical isolates with different levels of quinolone susceptibility. Antimicrob. Agents Chemother. 46:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valdezate, S., A. Vindel, L. Maiz, F. Baquero, H. Escobar, and R. Cantón. 2001. Persistence and variability of Stenotrophomonas maltophilia in cystic fibrosis patients, Madrid, 1991-1998. Emerg. Infect. Dis. 7:113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Alphen, L. 1992. Epidemiology and prevention of respiratory tract infections due to nonencapsulated Haemophilus influenzae. J Infect. Dis. 165(Suppl. 1):S177-S180. [DOI] [PubMed] [Google Scholar]
- 35.Van Belkum, A., B. Duim, A. Regelink, L. Moller, W. Quint, and L. Van Alphen. 1994. Genomic DNA fingerprinting of clinical Haemophilus influenzae isolates by polymerase chain reaction amplification: comparison with major outer-membrane protein and restriction fragment length polymorphism analysis. J. Med. Microbiol. 41:63-68. [DOI] [PubMed] [Google Scholar]
- 36.Van Schilfgaarde, M., P. Eijk, A. Regelink, P. van Ulsen, V. Everts, J. Dankert, and L. van Alphen. 1999. Haemophilus influenzae localized in epithelial cell layers is shielded from antibiotics and antibodies and antibody-mediated bactericidal activity. Microb. Pathog. 26:249-262. [DOI] [PubMed] [Google Scholar]
- 37.Watson, K. C., E. J. C. Kerr, and M. Baillie. 1988. Temporal changes in biotypes of Haemophilus influenzae isolated from patients with cystic fibrosis. J. Med. Microbiol. 26:129-132. [DOI] [PubMed] [Google Scholar]
- 38.Wong, K., M. C. Roberts, L. Owens, M. Fife, and A. L. Smith. 1984. Selective media for the quantification of bacteria in cystic fibrosis sputum. J. Med. Microbiol. 17:113-119. [DOI] [PubMed] [Google Scholar]