Abstract
Serogroup C Neisseria meningitidis belonging to the electrophoretic type (ET) ET-15, a variant of ET-37, is endemic in Canada. Like other serogroup C ET-37 meningococci, the endemic ET-15 strains are usually found to carry the serotype and serosubtype antigens of 2a:P1.5,2. In 2001, a sudden increase in the number of cases of serogroup C meningococcal disease in Quebec, Canada, was caused by an antigenic variant of the ET-15 strain. This antigenic variant carries the unique serosubtype marker of P1.7,1. Strains of C:2a:P1.7,1 meningococci were not isolated in Canada in large numbers prior to 2001, and the characteristics of these meningococcal strains linked to an outbreak in Quebec, Canada, are described in the present study.
Neisseria meningitidis is a gram-negative obligate human pathogen that is a leading cause of septicemia and meningitis. A number of surface markers have been described for the meningococci (20), but the most useful structures for monitoring the epidemiology of infection are the polysaccharide capsules, and the outer membrane proteins (OMPs) (14). Based on the serological specificity of the capsule, 13 different serogroups of meningococci have been described (35). Of the five different classes of OMPs expressed by meningococci, two, PorB and PorA proteins, are routinely used for epidemiological studies (10). Two classes (classes 2 or 3, being mutually exclusive) of PorB proteins that carry the serotype antigens are produced by meningococci. Serosubtype antigens of meningococci are found on their class 1 PorA OMP, and both the serotype and serosubtype antigens are usually determined by a panel of monoclonal antibodies reacting with bacterial whole cells.
In Canada, invasive meningococcal disease (IMD) is a notifiable disease and surveillance is carried out in all provinces and territories by local public health officials and the provincial and territorial public health laboratories. Health Canada's Division of Disease Surveillance, Center for Infectious Disease Prevention and Control, and the National Microbiology Laboratory (NML) coordinates the national data and provides laboratory support in strain characterization (29).
Over the last several decades, the epidemiology of IMD in Canada has shifted gradually, with the most significant impact due to the spread of the electrophoretic type (ET) ET-15 clone to a worldwide distribution (13). First detected and identified in Canada in 1986, C:2a:P1.5,2 ET-15 meningococci were identified by multilocus enzyme electrophoresis as a variant of the ET-37 clonal complex, which has been known as a hypervirulent clone of meningococci that causes severe disease among adolescents and young adults (2, 38). Serogroup C meningococci belonging to the ET-15 clone have the same antigenic formula (C:2a:P1.5,2) as members of the serogroup C ET-37 clonal complex (37) but has a unique fumarase housekeeping gene (36) that imparts to the organisms a different fumarase allele (allele 2) different from that found in the ET-37 clonal complex (allele 1).
In 2001, there was an increase in IMD activity in many provinces in Canada due mostly to an increase in cases due to serogroup C meningococci (19). Although almost all serogroup C meningococci that caused IMD in 2001 belonged to the ET-15 clonal complex, the strains that caused disease in the different provinces appeared as antigenic variants of the common or original C:2a:P1.2,5 ET-15/ET-37 clonal complex (31, 32).
In the province of Quebec, Canada, active immunization with the serogroup C-polysaccharide vaccines had been used successfully to control outbreaks of group C meningococcal disease (12, 15, 18). Province-wide vaccination campaigns had been carried out in the early 1990s (6). After the province-wide vaccination campaign in the early 1990s, cases of IMD due to group C meningococci in Québec have decreased tremendously (6, 7). However, in January 2001, there was a sudden increase in IMD cases due to group C meningococci in Québec and early in the investigation, the NML has identified a unique variant of the serogroup C, ET-15 meningococci presenting with the antigenic formula of C:2a:P1.7,1. We describe the phenotypic and genetic characterization of this unique variant of serogroup C ET-15 meningococci that caused IMD almost exclusively in Québec.
MATERIALS AND METHODS
Identification, serogrouping, and typing of meningococci.
N. meningitidis recovered from patients with suspected IMD was routinely confirmed by biochemical tests, and the serogroup was determined by bacterial agglutination with rabbit serogrouping antisera at the Laboratoire de santé publique du Québec (21). Further genetic and phenotypic testing was done at the NML; these tests included serotyping and serosubtyping done by whole-cell enzyme-linked immunosorbent assay (ELISA) with monoclonal antibodies (1), multilocus enzyme electrophoresis by using a previously described method (25), multilocus sequence typing (MLST) according to the method of Maiden et al. (16), and sequencing of porB and porA genes according to methods described by Sacchi et al. (22, 23).
Isolation of OMVs.
N. meningitidis sheds outer membrane vesicles (OMVs) or blebs during growth. OMVs in growth medium were isolated as described previously (11). Briefly, a strain was grown on a brain heart infusion plate with 1% horse serum for 6 to 8 h. The culture was transferred to 200 ml of tryptic soy broth in a 500-ml Wheaton bottle for growth at 37°C overnight to 24 h with shaking at the middle setting on a New Brunswick gyratory water bath shaker. The cells were removed by two centrifugation steps, each carried out at 10,000 × g for 20 min. The OMVs in the supernatant were then pelleted at 100,000 × g for 3 h, suspended in 0.5 to 1 ml of water containing 0.02% sodium azide, and stored at 4°C. Proteins and lipooligosaccharides (LOSs) in OMVs were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 10 and 16% gels, respectively. Proteins and LOSs in gels were visualized by staining with Coomassie blue R-250 or with silver nitrate.
Identification of LOS immunotype and genotype.
LOS immunotyping was done with both monoclonal and polyclonal antibodies to the LOS types L1, L2, L3,7,9, L8, and L10 by either indirect whole-cell ELISA (1) or immunoblot analysis after SDS-PAGE as previously described (27). Gels (16%) were used for the analysis of LOS on SDS-PAGE, and the LOSs were stained with silver.
The lgt (LOS glycosyltransferase) genes are responsible for biosynthesis of the LOS oligosaccharide chains. The lgt gene profile of N. meningitidis strains was determined as previously described (39).
RESULTS
Source, ET profile, serotype, and serosubtype characterization of the serogroup C meningococci isolated from IMD cases in the Province of Québec in 2001 and 2002.
A total of 77 strains of serogroup C meningococci were isolated from individual IMD cases in the Province of Quebec in 2001 (52 strains) and 2002 (25 strains). Of these strains, 52 were isolated from blood culture, 22 were isolated from cerebrospinal fluid, and 3 were isolated from knee and/or synovial fluids. A total of 69 strains were ET-15, 6 were ET-15 complex, and 2 were not ET-15 but belonged to other members of the ET-37 clonal complex.
Of the 77 serogroup C strains, 72 were serotype 2a and 5 strains (all isolated in 2001) were nonserotypeable. The most common serotype:serosubtype antigens combination was C:2a:P1.7,1. A total of 32 (61.5%) of the 52 strains isolated in 2001 and 22 (88%) of the 25 strains isolated in 2002 were typed as C:2a:P1.7,1. The endemic C:2a:P1.2,(5) strains made up of only 19% of the strains in 2001 and none in 2002, although two of the C:2a:P1.− strains isolated in 2002 could have the serosubtype antigens of P1.2,(5) if other methods of detection of the serosubtype antigens were used.
Of the five nonserotypeable group C strains isolated in 2001, four were found by DNA sequencing of their porB gene to be serotype 2a mutants similar to the strain found to cause a cluster of IMD cases in a community of men who have sex with other men in Toronto, Ontario, Canada (30). The other nonserotypeable strain (C:NT:P1.7,1) was found to be unrelated to serotype 2a since its variable region (VR) gene sequences showed very different VR types. The PorB VR types of this strain were determined to be VR1-C, VR2-F, VR3-B(a), and VR4-E according to the nomenclature scheme developed by Sacchi et al. (22). The VR1 region of this strain gave a nucleotide sequence identical to that found in strain B16B6 (B:2a:P1.5,2) accession number X67937. The nucleotide sequence of its VR3 region showed a 3-bp difference from that of strain M982 (B:9:P1.9) accession number X67938. Both the VR2 and VR4 regions of this strain showed <60% amino acid sequence homology to any of the VR2 and VR4 prototype sequences, respectively (22).
Two C:2a:P1.7,1 strains isolated in Québec in early 2001 were selected for both porB and porA gene sequencing to confirm their serotype 2a and serosubtype P1.7,1 identity. The PorB VR types of both strains gave typical serotype 2a nucleotide sequences in all four VR segments of their porB genes (VR1-C, VR2-Eb, VR3-2a, and VR4-C), showing 100% identity to that found in strain 94010 (C:2a:P1.5,2 [accession number U92907]). Sequencing part of the porA gene that encodes for the PorA VR1 and VR2 regions in both strains yielded PorA VR1 type 7d (equivalent to the P1.7 family, variant 7-4, according to the N. meningitidis PorA VR database [http://neisseria.org/nm/typing/pora/]) and PorA VR2 type 1 (equivalent to the P1.1 family). The PorA VR1 and VR2 regions in these two strains showed 100% identity to that of strain M978 (B:19,10:P1.7,1 [accession number U92938]).
Two randomly selected C:2a:P1.7,1 strains isolated during the early part of the outbreak were also chosen for an MLST study to confirm the clonal type of the outbreak strain. Both strains gave the sequence type ST-11 by MLST, which is characteristic of the ET-15 and ET-37 clonal complex (of which ET-15 is a member).
Characterization of OMPs and LOSs in outer membrane vesicles and LOS immunotyping of 12 group C meningococcal strains from Québec province.
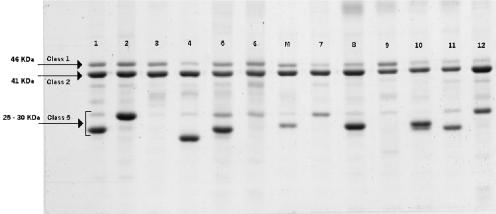
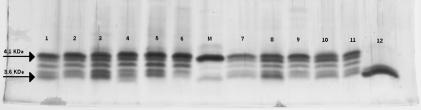
OMPs and LOSs present in outer membrane vesicles of six randomly selected C:2a:P1.7,1 as well as 6 C:2a:P1.5,2 strains were characterized by SDS-PAGE analysis as shown in Fig. 1 and 2, respectively. Among the 12 strains examined, all were shown to contain a 46-kDa class 1 and a major 41-kDa class 2 proteins. The sizes of their class 2 and class 1 proteins that contain the 2a serotype and P1.7,1 or P1.5,2 serosubtype epitopes, respectively, in these strains appeared to be the same between the strains. However, the sizes of class 5 proteins were variable (25 to 30 kDa) among these strains, even between strains showing the same serotype and serosubtype antigens. For their LOSs, 11 of the 12 strains showed five LOS components with a 4.1-kDa species as the major component. The remaining one (strain NML01-427) had a 3.6-kDa major component and a 3.7-kDa minor component but no 4.1-kDa component. Immunoblot analyses revealed that the 4.1-kDa major LOS components in the 11 strains reacted with an anti-L2 immunotype antiserum but not with an anti-L3 antibody. The 3.6-kDa major LOS component in strain NML01-427 was nontypeable and its minor 3.7-kDa LOS component reacted with an anti-L5 antiserum. The results of LOS immunotyping are summarized in Table 1.
FIG. 1.
SDS-10% PAGE analysis of OMPs prepared from serogroup C meningococcal strains isolated from IMD patients in Québec, Canada (lanes 1 to 6 were strains with antigens C:2a:P1.7,1; lanes 7 to 12 were strains with antigens C:2a:P1.5,2). Lane marked M is strain M986 (B:2a:P1.5,2). Arrows indicate the positions of class 1, 2, and 5 OMPs.
FIG. 2.
SDS-16% PAGE analysis of LOSs prepared from serogroup C meningococcal strains isolated from IMD patients in Québec, Canada (lanes 1 to 6 were strains with antigens C:2a:P1.7,1; lanes 7 to 12 were strains with antigens C:2a:P1.5,2). Lane marked M is strain M986 (B:2a:P1.5,2). Arrows indicate locations of 3.6- and 4.1-kDa components of LOS (28).
TABLE 1.
Major LOS immunotypes of 12 representative serogroup C ET-15 IMD isolates obtained from subjects in Québec, Canada, in 2000 and 2001
| Strain | Yr of isolation | Antigenic formula | Major LOS type |
|---|---|---|---|
| NML01-083 | 2001 | C:2a:P1.7, 1 | L2 |
| NML01-110 | 2001 | C:2a:P1.7,1 | L2 |
| NML01-111 | 2001 | C:2a:P1.7,1 | L2 |
| NML01-130 | 2001 | C:2a:P1.7,1 | L2 |
| NML01-147 | 2001 | C:2a:P1.7,1 | L2 |
| NML01-148 | 2001 | C:2a:P1.7,1 | L2 |
| NML00-215 | 2000 | C:2a:P1.5,2 | L2 |
| NML01-074 | 2001 | C:2a:P1.5,2 | L2 |
| NML01-082 | 2001 | C:2a:P1.5,2 | L2 |
| NML01-091 | 2001 | C:2a:P1.5,2 | L2 |
| NML01-388 | 2001 | C:2a:P1.5,2 | L2 |
| NML01-427 | 2001 | C:2a:P1.5,2 | Nontypeable |
lgt genes of C:2a:P1.7,1 meningococci.
The three lgt loci were first examined in the six C:2a:P1.7,1 strains by PCR with flanking primers as described before (39). No size variation was observed in the PCR products from them; the sizes of the PCR products were 3.3 kb for lgt-1, 1.0 kb for lgt-2, and 3.2 kb for lgt-3. The PCR products were then subjected to digestions with four restriction endonucleases AluI, HaeIII, MspI, and RsaI. Identical restriction patterns were observed for each enzyme from all six strains. The presence of the lgt genes in these six strains was further determined by PCR with internal primers specific for each lgt gene, and the gene products were confirmed by dot DNA hybridization with gene-specific probes (39). The results from the two methods were consistent. All six C:2a:P1.7,1 strains were found to have three genes lgtA, lgtB, and lgtH at locus lgt-1 and genes lgtF and lgtG at loci lgt-2 and lgt-3, respectively. On the basis of this genetic organization of their lgt genes, the C:2a:P1.7,1 strains appeared to belong to the LOS genotype 3 (VII-I-I) (39).
DISCUSSION
Most serogroup C IMD cases in North America, Europe, and Africa are due to strains belonging to the ET-37 clonal complex, known for its hypervirulent nature and association with both endemic and epidemic disease (37). The ET-37 clonal complex of N. meningitidis comprises a group of clonally related strains that may be covered by any one of the four serologically distinct capsule types of B, C, Y, and W135, which all have sialic acid as a component of their structures. The earliest isolate of N. meningitidis known to be ET-37 was a serogroup B strain recovered in 1917 in the United States (5). Outbreaks due to serogroup C meningococci of the ET-37 clonal complex were documented in the U.S. Army in the early 1960s (4).
In Canada, serogroup C disease was uncommon prior to mid-1980. For example, in a study carried out between 1977 and 1984, serogroup C strains accounted for only a small percentage (14 to 15%) of the IMD cases (33). Since it was first detected and identified in 1986, a unique genetic variant of serogroup C ET-37 meningococci, characterized as C:2a:P1.5,2 and belonging to ET-15 (2), has been responsible for causing not only an increase in the proportion of endemic IMD but also an increase in the number of IMD cases, including localized outbreaks in schools and other settings that affected mainly adolescents and young adults. The proportion of IMD cases due to serogroup C organisms in Canada as a whole increased from 25% in 1985 to 64% in 1992 (19). This clone of ET-15 meningococci has also been documented to cause more severe IMD, resulting in a higher case fatality rate compared to disease caused by serogroup B organisms (38) and also resulting in severe complications and postinfection sequelae (8). In Québec, local outbreaks due to this serogroup C ET-15 strain in 1991 and 1992 led to a province-wide immunization campaign, with about 1.6 million doses of the meningococcal polysaccharide vaccine administered; this program was carried out between December 1992 and March 1993 (6). The effectiveness of this mass vaccination campaign was evident from the drop in the incidence of serogroup C disease after the immunization, with disease incidence decreasing from 1.4 per 100,000 population in 1990 and 1992 (before the vaccination campaign) to 0.3 per 100,000 population in 1993 to 1998 (after the vaccination campaign) (7). However, beginning in January 2001, there was again a sudden increase in the number of IMD cases due to a unique antigenic variant (C:2a:P1.7,1) of the ET-15 serogroup C meningococci in Québec, and cases were reported in populations previously vaccinated in the mass immunization campaign of 1991 to 1992. At the same time, four other provinces in Canada had also reported increase in cases of IMD due to serogroup C meningococci (19, 31), including a cluster of cases that occurred in a community of men who have sex with men in Toronto, Ontario, Canada. These Toronto cases were caused by another unique genetic variant of the endemic serotype 2a ET-15 meningococci presenting with the nonserotypeable phenotype (C:NT:P1.2) due to a point mutation in its gene that encodes for the serotype 2a antigen (30).
The serogroup C strains causing IMD in Québec in 2001 were unique since no ET-37 or ET-15 meningococci with the serotype and serosubtype antigen combination of 2a:P1.7,1 have ever been reported in large numbers from IMD cases anywhere either in or outside of Canada. These C:2a:P1.7,1 strains were confirmed to produce typical class 1 and class 2 porins by SDS-PAGE analysis of their OMPs (Fig. 1). Since strains of serogroup C ET-15 meningococci with the serosubtype antigens of P1.7,1 have never been isolated in large numbers in our laboratory before, we sought to confirm the serotype and serosubtype antigenicity of these strains, as determined with monoclonal antibodies, by performing partial gene sequencing of the PorB and PorA proteins in two randomly selected isolates obtained in early 2001. Both isolates were confirmed by their porB gene sequence to be serotype 2a. DNA sequencing of the VR1 and VR2 regions of their PorA proteins indicated that they are of the VR type 7d and 1 (23). PorA protein with the 7d VR1 epitope was originally named VR1-7 (17), and strains with this antigen have been reported to react with the monoclonal antibody that recognizes the VR1-7 antigen (23).
The LOSs in clinical isolates, regardless of their OMP antigenic makeup of either C:2a:P1.7,1 or C:2a:P1.5,2, were found to be more heterogeneous compared to that of a typical laboratory strain (M986). Microheterogeneity of lipopolysaccharide or LOS is a well-known phenomenon. Prototype strains such as M986 have probably gone through numerous passages in the laboratory, and this may explain the lower degree of heterogeneity observed. Strains of C:2a:P1.7,1 meningococci were classified by their lgt gene profile as genotype 3. Although their genetic makeup may not be different from those of LOS genotype 2 (which allow them to synthesize the LOS L2 immunotype), they were found to have the LOS immunotype L2. The structural difference between L2 and L3 LOS immunotypes appears to depend on what is being substituted at the 3′ position of the heptose II unit. In L2 LOS, glucose is found to form the β-chain, whereas in L3 LOS, phosphoethanolamine replaces the β-chain glucose. Phosphoethanolamine when present may inhibit or block the function of the LgtG enzyme from adding the glucose to its heptose acceptor and hence the strain may present as L3 instead of L2 despite the possibility of the presence of a functional lgtG gene. However, since various mechanisms may be involved in the regulation, expression, and function of the different lgt genes, it may be too difficult to know for sure why strains which apparently have identical lgt gene profile may synthesize different LOS phenotype or immunotypes.
In order to understand the emergence of this antigenic variant of ET-15 group C meningococci, we examined our serotyping records of meningococcal strains from previous years and looked for serogroup C serotype 2a strains with the P1.7,1 PorA serosubtype phenotype. The first strain of C:2a:P1.7,1 ET-15 meningococcus isolated in the province of Québec prior to the observed increase in IMD cases due to this strain was from the cerebrospinal fluid of an 18-year-old female IMD case which happened in August 2000. Prior to this, there was only one other similar strain of C:2a:P1.7,1 ET-15 meningococcus observed in the previous 9 years from 1991 to 1999, and it was isolated from the blood of a 74-year-old female IMD case back in 1997. Besides these two strains, there were 11 other C:2a:P1.7,1 ET-15 meningococcal strains observed in Canada during the period from 1991 to 2000 from a total of 1212 serogroup C IMD isolates submitted to the NML from all of the different provincial and territorial public health laboratories across the country (NML, unpublished data).
In 2001, in addition to the 32 isolates from IMD cases in the province of Québec, there were also 7 other C:2a:P1.7,1 isolates recovered from individual IMD cases in the province of Ontario (6 isolates) and Alberta. Four of the six isolates from Ontario were recovered from IMD cases in the London, Ontario, Canada, area and the other two isolates were from other parts of the province. The single C:2a:P1.7,1 isolate recovered from the province of Alberta was from a subject who was a resident of the province of Quebec but developed IMD while visiting Alberta, Canada. In 2002, in addition to the 22 isolates recovered from the province of Québec, there were also another three C:2a:P1.7,1 isolates that were recovered from other provinces (one from Alberta and two from Ontario, including one isolate which was recovered from an IMD case in London, Ontario).
The antigenic variant of C:2a:P1.7,1 ET-15 meningococci is unlikely to have evolved from the C:2a:P1.5,2 strain by mutation of its porA gene. This is because amino acid sequences of the P1.5 and P1.7 epitope present in the VR1 of the PorA protein are very different, with the P1.7 amino acid sequence being twice as long as that of the P1.5 sequence, and they share only three identical amino acids. Similarly, the VR2 amino acid sequences of the P1.2 and P1.1 epitopes are also very different, with only 2 of the 15 and 13 amino acids, respectively, present in these two serosubtype antigens being identical (23).
A more likely explanation for the emergence of the C:2a:P1.7,1 strains is a recombination between the meningococcus of the C:2a:P1.5,2 strain with another meningococcus that has the serosubtype antigen of P1.7,1. Recombination has been described as a frequent event in meningococci and Neisseria species as a whole (34), and recombination has been described as a mechanism for exchanging either part or the entire porA gene, leading to genetic diversity, as well as novel antigenic variants (9). With regard to the donor source of the P1.7,1 antigens, serogroup B meningococci have been reported to have the P1.7,1 serosubtype antigens. For example, in the United States, it was found that 8.6% of 444 serogroup B meningococcus isolates examined between 1992 and 1998 contained this serosubtype antigen (24), whereas in Brazil 11% of 1,297 group B strains were found to have this PorA type (26). Although strains with nonserosubtypeable antigens of P1.7, 1 are not common in Canada, accounting for only ca. 2.3% of 301 serogroup B strains examined and another 2.7% expressed only either P1.1 or P1.7 antigens (3), it is not known from the present study how many of the nonserosubtypeable strains may actually have the PorA VR genotypes that may allow them to express the P1.7,1 epitopes.
To control the increase in IMD activity in 2001, the province of Québec launched another province-wide vaccination campaign against serogroup C meningococci. This vaccination campaign targeted those younger than 20 years old and included the use of the newly licensed serogroup C conjugated vaccine for those younger than 2 years old. As a result of this vaccination campaign, the number of serogroup C isolates from IMD cases in 2002 were much fewer compared to 2001 (25 case-strains in 2002 versus 52 case-strains in 2001), but what was remarkable was the age group of the group C IMD cases in 2002 versus 2001. In 2001 the majority of the cases were found among those ≤20 years old (35 of 52 cases or 67%), but in 2002 only 3 of 25 cases (12%) were in this age group, and 88% of the cases were in individuals ≥21 years old, with 6 subjects who were 21 to 30 years old, 7 subjects who were 31 to 40 years old, and 9 subjects who were ≥41 years old.
In summary, we have described here an increase in IMD cases in Québec, Canada, caused by a unique antigenic variant of the endemic C:2a:P1.5,2 ET-15 meningococci that expresses a new set of serosubtype antigens that may have been imported from serogroup B meningococci.
Nucleotide sequence accession numbers.
The partial sequences of porB and porA genes of two C:2a:P1.7,1 strains have been deposited in GenBank (National Center for Biotechnology Information) under accession numbers AY465900, AY465901, AY465902, and AY465903. The partial sequence of the porB gene of four serotype 2a mutants with the nonserotypeable phenotype shows identity to those described before (GenBank accession numbers AY234206 to AY234211). The partial sequence of the porB gene of the fifth nonserotypeable group C strain described in this study was assigned the GenBank accession number AY394654.
Acknowledgments
We thank Jan Stoltz, Averil Henderson, Shaun Tyler, Carole Gagnon, Denis Boudreau, Robert Lacas, and George Kao for their assistance in this study. NML's DNA Core Facility provided the DNA sequencing service.
This study was supported in part by Health Canada's Genomics R&D Fund.
REFERENCES
- 1.Abdillahi, H., and J. T. Poolman. 1987. Whole cell ELISA for typing Neisseria meningitidis with monoclonal antibodies. FEMS Microbiol. Lett. 48:367-371. [PubMed] [Google Scholar]
- 2.Ashton, F. E., J. A. Ryan, A. Borczuk, D. A. Caugant, L. Mancino, and D. Huang. 1991. Emergence of a virulent clone of Neisseria meningitidis serotype 2a that is associated with meningococcal group C disease in Canada. J. Clin. Microbiol. 29:2489-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashton, F. E., and D. A. Caugant. 2001. The panmictic nature of Neisseria meningitidis serogroup B during a period of endemic disease in Canada. Can. J. Microbiol. 47:283-289. [PubMed] [Google Scholar]
- 4.Brundage, J. F., M. A. K. Ryan, B. H. Feighner, and F. J. Erdtmann. 2002. Meningococcal disease among United States military service members in relation to routine uses of vaccines with different serogroup-specific components, 1964-1998. Clin. Infect. Dis. 35:1376-1381. [DOI] [PubMed] [Google Scholar]
- 5.Caugant, D. A. 1998. Population genetics and molecular epidemiology of Neisseria meningitidis. APMIS 106:505-525. [PubMed] [Google Scholar]
- 6.De Wals, P., M. Dionne, M. Douville-Fradet, N. Boulianne, J. Drapeau, and G. De Serres. 1996. Impact of a mass immunization campaign against serogroup C meningococcus in the province of Québec, Canada. Bull. W. H. O. 74:407-411. [PMC free article] [PubMed] [Google Scholar]
- 7.De Wals, P., G. De Serres, and T. Niyonsenga. 2001. Effectiveness of a mass immunization campaign against serogroup C meningococcal disease in Québec. JAMA 285:177-181. [DOI] [PubMed] [Google Scholar]
- 8.Erickson, L., and P. De Wals. 1984. Complications and sequelae of meningococcal disease in Québec, Canada, 1990-1994. Clin. Infect. Dis. 26:1159-1164. [DOI] [PubMed] [Google Scholar]
- 9.Feavers, I. M., A. B. Heath, J. A. Bygraves, and M. C. J. Maiden. 1992. Role of horizontal genetic exchange in the antigenic variation of the class 1 outer membrane protein of Neisseria meningitidis. Mol. Microbiol. 6:489-495. [DOI] [PubMed] [Google Scholar]
- 10.Frasch, C. E., W. D. Zollinger, and J. T. Poolman. 1985. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev. Infect. Dis. 7:504-510. [DOI] [PubMed] [Google Scholar]
- 11.Gu, X. X., and C. M. Tsai. 1991. Purification of rough-type lipopolysaccharides of Neisseria meningitidis from cells and outer membrane vesicles in spent media. Anal. Biochem. 196:311-318. [DOI] [PubMed] [Google Scholar]
- 12.Guillemette, P., B. Fournier, and Y. Robert. 1991. A cluster of Neisseria meningitidis cases in Québec, January 1991. Can. Dis. Wkly. Rep. 17:295-296. [PubMed] [Google Scholar]
- 13.Jelfs, J., R. Munro, F. E. Ashton, and D. A. Caugant. 2000. Genetic characterization of a new variant within the ET-37 complex of Neisseria meningitidis associated with outbreaks in various parts of the world. Epidemiol. Infect. 125:285-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuipers, B., G. van den Dobbelsteen, E. Wedege, and L. Van Alphen. 2001. Serological characterization, p. 131-145. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal disease: methods and protocols. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 15.Lavigne, P., N. Boulianne, C. Fortin, H. Accache, and M. Douville-Fradet. 1992. Meningococcal infections in Québec, 1991-1992. Can. Commun. Dis. Rep. 18:113-116. [PubMed] [Google Scholar]
- 16.Maiden, M. C. J., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and R. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCguinness, B. T., P. R. Lambden, and J. K. Heckels. 1993. Class 1 outer membrane protein of Neisseria meningitidis: epitope analysis of the antigenic diversity between strains, implications for subtype definition and molecular epidemiology. Mol. Microbiol. 7:505-514. [DOI] [PubMed] [Google Scholar]
- 18.Peron, L., and C. Laferriere. 1991. Four cases of Neisseria meningitidis infection linked to frequenting a bar C Québec. Can. Dis. Wkly. Rep. 17:294-295. [PubMed] [Google Scholar]
- 19.Pollard, A. J., T. W. S. Tam, and the National Advisory Committee on Immunization. 2001. Advisory Committee statement on recommended use of meningococcal vaccines. Can. Commun. Dis. Rep. 27:2-36. [PubMed] [Google Scholar]
- 20.Poolman, J. T., P. A. van der Ley, and J. Tommassen. 1995. Surface structures and secreted products of meningococci, p. 21-34. In K. A. V. Cartwright, (ed.), Meningococcal disease. John Wiley & Sons, Inc., New York, N.Y.
- 21.Ringuette, L., M. Lorange, A. Ryan, and F. Ashton. 1995. Meningococcal infections in the province of Québec, Canada, during the period 1991 to 1992. J. Clin. Microbiol. 33:53-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacchi, C. T., A. P. S. Lemon, A. M. Whitney, C. A. Solari, M. E. Brandt, C. E. A. Melles, C. E. Frasch, and L. W. Mayer. 1998. Correlation between serological and sequence analyses of the PorB outer membrane protein in the Neisseria meningitidis serotyping scheme. Clin. Diagn. Lab. Immunol. 5:348-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacchi, C. T., A. P. S. Lemos, M. E. Brandt, A. M. Whitney, C. E. A. Melles, C. A. Solari, C. E. Frasch, and L. W. Mayer. 1998. Proposed standardization of Neisseria meningitidis PorA variable-region typing nomenclature. Clin. Diagn. Lab. Immunol. 5:845-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacchi, C. T., A. M. Whitney, T. Popovic, D. S. Beall, M. W. Reeves, B. D. Plikaytis, N. E. Rosentein, B. A. Perkins, M. L. C. Tondella, and L. W. Mayer. 2000. Diversity and prevalence of PorA types in Neisseria meningitidis serogroup B in the United States, 1992-1998. J. Infect. Dis. 182:1169-1176. [DOI] [PubMed] [Google Scholar]
- 25.Selander, R. K., D. A. Caugant, H. Ochman, J. M. Musser, M. N. Gilmour, and T. S. Whittam. 1986. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl. Environ. Microbiol. 51:873-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tondella M. L. C., T. Popovic, N. E. Rosentein, D. B. Lake, G. M. Carlone, L. W. Mayer, B. A. Perkins, and the Active Bacterial Core Surveillance Team. 2000. Distribution of Neisseria meningitidis serogroup B serosubtypes and serotypes circulating in the United States. J. Clin. Microbiol. 38:3323-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai, C. M., L. F. Mocca, and C. E. Frasch. 1987. Immunotype epitopes of Neisseria meningitidis lipopolysaccharide types 1 through 8. Infect. Immun. 55:1652-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai, C. M., W. H. Chen, and P. A. Balakonis. 1998. Characterization of terminal NeuNAcα 2-3Galβ 1-4GlcNAc sequence in lipooligosaccharides of Neisseria meningitidis. Glycobiology 8:359-365. [DOI] [PubMed] [Google Scholar]
- 29.Tsang, R. S. W., S. G. Squires, W. D. Zollinger, and F. E. Ashton. 2002. Distribution of serogroups of Neisseria meningitidis and antigenic characterization of serogroup Y meningococci in Canada, January 1, 1999 to June 30, 2001. Can. J. Infect. Dis. 13:391-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsang, R. S. W., L. Kiefer, D. K. S. Law, J. Stoltz, R. Shahin, S. Brown, and F. Jamieson. 2003. Outbreak of serogroup C meningococcal disease caused by a variant of Neisseria meningitidis serotype 2a ET-15 in a community of men who have sex with men. J. Clin. Microbiol. 41:4411-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsang, R. S. W., S. G. Squires, and T. W. S. Tam. 2003. Characterization of Neisseria meningitidis strains isolated from invasive meningococcal disease cases in Canada in 2001. Can. J. Microbiol. 49:633-638. [DOI] [PubMed]
- 32.Tyrrell, G. J., L. Chiu, M. Johnson, N. Chang, R. P. Rennie, and J. A. Talbot. 2002. Outbreak of Neisseria meningitidis, Edmonton, Alberta, Canada. Emerg. Infect. Dis. 8:519-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varughese, P. V., and A. O. Carter. 1983. Meningococcal disease in Canada and serogroup distribution. Can. Dis. Wkly. Rep. 9:177-180. [Google Scholar]
- 34.Vazquez, J. A., S. Berron, M. O'Rourke, G. Carpenter, E. Feil, N. H. Smith, and B. G. Spratt. 1995. Interspecies recombination in nature: a meningococcus that has acquired a gonococcal PIB porin. Mol. Microbiol. 15:1001-1007. [DOI] [PubMed] [Google Scholar]
- 35.Vedros, N. A. 1987. Development of meningococcal serogroups, p. 33-37. In N. A. Vedros (ed.), Evolution of meningococcal disease. CRC Press, Boca Raton, Fla.
- 36.Vogel, U., H. Claus, M. Frosch, and D. A. Caugant. 2000. Molecular basis for distinction of the ET-15 clone within the ET-37 complex of Neisseria meningitidis. J. Clin. Microbiol. 38:941-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, J. F., D. A. Caugant, G. Morelli, B. Koumare, and M. Achtman. 1993. Antigenic and epidemiologic properties of the ET-37 complex of Neisseria meningitidis. J. Infect. Dis. 167:1320-1329. [DOI] [PubMed] [Google Scholar]
- 38.Whalen, C. M., J. C. Hockin, A. Ryan, and F. Ashton. 1995. The changing epidemiology of invasive meningococcal disease in Canada, 1985 through 1992. JAMA 273:390-394. [PubMed] [Google Scholar]
- 39.Zhu, P., M. J. Klutch, M. C. Bash, R. S. W. Tsang, L. K. Ng, and C. M. Tsai. 2002. Genetic diversity of three lgt loci for biosynthesis of LOS in Neisseria species. Microbiology 148:1833-1844. [DOI] [PubMed] [Google Scholar]