Abstract
BACKGROUND:
The allocation of research resources should favor conditions responsible for the greatest disease burden. This is particularly important in pediatric populations, which have been underrepresented in clinical research. Our aim was to measure the association between the focus of pediatric clinical trials and burden of disease and to identify neglected clinical domains.
METHODS:
We performed a cross-sectional study of clinical trials by using trial records in ClinicalTrials.gov. All trials started in 2006 or after and studying patient-level interventions in pediatric populations were included. Age-specific measures of disease burden were obtained for 21 separate conditions for high-, middle-, and low-income countries. We measured the correlation between number of pediatric clinical trials and disease burden for each condition.
RESULTS:
Neuropsychiatric conditions and infectious diseases were the most studied conditions globally in terms of number of trials (874 and 847 trials, respectively), while intentional injuries (5 trials) and maternal conditions (4 trials) were the least studied. Clinical trials were only moderately correlated with global disease burden (r = 0.58, P = .006). Correlations were also moderate within each of the country income levels, but lowest in low-income countries (r = .47, P = .03). Globally, the conditions most understudied relative to disease burden were injuries (–260 trials for unintentional injuries and –160 trials for intentional injuries), nutritional deficiencies (–175 trials), and respiratory infections (–171 trials).
CONCLUSIONS:
Pediatric clinical trial activity is only moderately associated with pediatric burden of disease, and least associated in low-income countries. The mismatch between clinical trials and disease burden identifies key clinical areas for focus and investment.
Keywords: clinical trials, burden of disease, pediatric research
What’s Known on This Subject:
Fewer clinical trials are performed in children compared with other patient populations. It is unknown how well existing pediatric clinical trials are aligned with the needs of children, both in high-income countries and globally.
What This Study Adds:
There is only moderate correlation between clinical trial activity and pediatric burden of disease, with certain conditions substantially underrepresented in the current research portfolio. Our findings provide a benchmark for prioritizing conditions for study, analyzing gaps, and identifying funding priorities.
Children are underrepresented as participants in clinical research.1–3 The inequity is manifest in the overall number of clinical trials performed in children as well as the number of randomized trials, drug trials, and publications that focus on pediatric populations.1,4,5 In reducing these disparities, research organizations and policy makers have the opportunity to prioritize investment in trials to address areas of greatest clinical burden. Previous work has shown that funding from the National Institutes of Health for all patient populations in the United States is only modestly correlated with the burden of disease.6,7 However, it is unknown how well existing pediatric clinical trials are aligned with the needs of children, both in high-income countries and globally. Clinical trials have been previously used as a proxy of clinical research activity in comparisons of research and disease burden.1,8,9 We sought to conduct the first study measuring the correspondence between pediatric clinical research and disease burden. Because of the variable impact of diseases across economic conditions, we examine the association between pediatric research and disease burden separately for more and less developed nations.
Methods
Study Design
We performed a cross-sectional study of the relationship between the number of pediatric clinical trials and the burden of disease among children. The conditions examined were the 21 primary conditions in the World Health Organization (WHO) global burden of disease taxonomy with additional analyses focusing on the 15 subcategories of infectious and parasitic diseases.10
Pediatric Clinical Trial Activity
Pediatric clinical research activity was measured by using the National Library of Medicine’s trials registry ClinicalTrials.gov. The ClinicalTrials.gov registry is the largest of the available trial registries, containing as many as 86% of all registrations.11,12 Since the International Committee of Medical Journal Editors instituted a policy in 2005 requiring prospective registration of all trials as a prerequisite for publication, registration of clinical trials has become standard practice.13,14 We identified all interventional trials with a start date on or after January 1, 2006, and registered by May 2, 2012. Pediatric trials were defined as those enrolling only or primarily participants <18 years of age (midpoint of the age range for enrolled participants was <18 years).1 Trials that were terminated or withdrawn were excluded (n = 279). Trials that focused on interventions targeting improvements in health care delivery at the provider or health care facility level were excluded (eg, interventions to enhance the use of computerized order entry by clinicians; n = 32).
All trials were individually reviewed to identify the medical condition under study, which was then mapped to 1 of the 21 WHO conditions. Trials studying conditions not represented in the WHO taxonomy (eg, food allergies) or interventions pertaining to multiple conditions (eg, antibiotic prophylaxis for immunocompromised patients) were classified separately.
Because some trials may have been registered in national or regional registries and not included in the ClincialTrials.gov registry, we also queried the International Trial Research Platform to assess the completeness of the data collected using the ClinicalTrials.gov registry.15 This platform combines data from 14 primary registries in addition to ClinicalTrials.gov. We selected discreet diseases among conditions with the highest global pediatric disease burden that could readily be identified with a text word search and identified all trials pertaining to these diseases in the registry platform. The diseases examined were malaria, prematurity, unipolar depression, iron-deficiency anemia, and congenital heart disease, representing 528 (10%) of all trials in our study sample (63% from high-income, 13% from middle-income, and 24% from low-income countries). We identified 89 pediatric trials not included in ClinicalTrials.gov and not analyzed in this study (61% from high-income, 12% from middle-income, and 27% from low-income countries), which suggests that overall, ∼83% of pediatric trials are recorded in ClinicalTrials.gov (84% of high-income, 84% of middle-income, and 81% of low-income trials).
Pediatric Global Burden of Disease
Data on global disease burden were obtained from the most recent Global Burden of Disease study conducted by the WHO.10 This study quantifies the health effects of diseases and injuries by age, gender, and country using national data and information collected through global health programs. Estimates include disability-adjusted life-years (DALYs), which is a composite measure that accounts for both years of life lost due to premature death and years lived in states of less than full health as a result of a disease or injury.16 Disease burden data are available for each of the 21 WHO disease and injury categories comprising the global burden of disease taxonomy. Estimates are also presented by country income level, with countries grouped as high-, middle-, or low-income based on their gross national product in 2004.
Data on burden of disease estimates are presented in predefined age categories and we combined DALYs for persons aged 0 to 4 years, 5 to 14 years, and three-fifths of 15 to 19 years to approximate DALYs for persons 0 to 17 years.
Trial Data Extraction and Classifications
For every trial, we extracted whether the trial was randomized, the unit of randomization (participant or cluster), number of participants enrolled (or estimated number for ongoing trials), type of intervention (eg, drug, behavioral, or procedural), names of countries with study sites, and number of participating countries and study sites.
Trials were classified as conducted primarily in high-, middle-, or low-income countries based on the income level of the countries representing the largest proportion of study sites. Countries were assigned to income levels according to the WHO categorization, which is based on a country’s gross national income per capita.10 The high-income level includes a total of 53 countries (income per capita of US$10 066 or more), the middle-income level 93 countries (income per capita of US$826 to $10 065), and the low-income level 59 countries (income per capita of US$825 or less). If a trial had equal proportions of sites in ≥2 income groups, the trial was assigned to the higher-income level (n = 21).
Data Analysis
Descriptive analyses characterized distribution of clinical research activity and global burden of disease across the 3 country income groups. Research activity was assessed in terms of the number of clinical trials and the number of participants represented in each of the 21 WHO conditions. Clinical trials were examined in terms of total number of trials, randomized controlled trials (RCTs), and randomized drug trials. All results and analyses pertaining to the number of participants were based on randomized trials that did not use cluster randomization. For low-income countries, where infectious diseases account for half the total disease burden, we analyzed separately the association between clinical trials and the burden attributable to the 16 specific diseases comprising this group. Student’s t test was used to compare mean number of study sites and participants across income groups.
Correlations between clinical trials and burden of disease were evaluated at a global level and for each of the income categories using Spearman’s rho test. To estimate the expected number of trials, we performed generalized γ regression analysis with a log link from a log transformation of disease burden. SAS software, version 9.3 (SAS Institute, Inc, Cary, NC) was used for all statistical analyses.
Results
Trial Characteristics
We identified 5373 pediatric clinical trials studying a medical condition. Of these, 3771 were RCTs and 1526 of the RCTs studied a drug intervention. A total of 1 466 689 children were studied in noncluster RCTs. Most trials were conducted in countries exclusively within 1 of the 3 income categories. Only 366 trials included study sites from multiple income levels, with 296 trials (5.5%) recruiting in high- and middle-income countries, 49 trials (0.9%) conducted in high- and low-income countries, and 65 trials (1.2%) in middle- and low-income countries. Trials conducted primarily in high-income countries tended to include a higher mean number of study sites (8 in high-income countries vs 4 in middle- and 2 in low-income countries, P < .001 for both comparisons) but trials recruiting primarily in low-income countries recruited on average a higher number of participants (1138 in low-income countries vs 315 in high- and 466 in middle-income countries, P < .001 for both comparisons).
Pediatric Clinical Trial Activity and Disease Burden
The distribution of clinical trial activity and burden of disease across countries in the 3 income levels is presented in Table 1. High-income countries are the primary sites of participant recruitment for 79% of all pediatric-focused clinical trials with low-income countries represented primarily in 7% of trials. By contrast, only a fraction of the total disease burden is borne by high-income countries (2%) with low-income countries sustaining the majority of disease burden (73%). This discrepancy between research activity and disease burden is similar when examining clinical trials limited to RCTs, drug RCTs, and participants.
TABLE 1.
Number of Pediatric Trials and Participants and Pediatric Burden of Disease by Country Income Level
| All Trials (N = 5373) | RCTs (n = 3771) | Drug RCTs (n = 1526) | Randomized Participants (n = 1 466 689) | Burden of Disease in DALYs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | %a,b | n | % | |
| High-income countries | 4261 | 79 | 2882 | 76 | 1176 | 77 | 889 448 | 61 | 13 569 772 | 2 |
| Middle-income countries | 746 | 14 | 568 | 15 | 225 | 15 | 260 879 | 18 | 147 338 773 | 25 |
| Low-income countries | 366 | 7 | 321 | 8 | 125 | 8 | 316 362 | 21 | 439 382 261 | 73 |
Excluding subjects enrolled in trials with cluster randomization.
Enrollment figures missing for 5 trials: 1 in middle-income level and 2 each in high- and low-income levels.
Correlation Between Clinical Trials and Disease Burden
Clinical trials and disease burden according to condition are shown in Table 2. Neuropsychiatric conditions represent the condition that is most studied globally in terms of number of trials (874 trials and 103 134 randomized participants) and nonrespiratory infections and parasitic diseases are the most common condition in terms of numbers of participants (847 trials and 636 471 randomized participants), followed by conditions arising in the perinatal period (523 trials and 179 933 randomized participants). Maternal conditions (4 trials and 324 randomized participants), intentional injuries (5 trials and 5479 randomized participants), and nonmalignant neoplasms (9 trials and 42 randomized participants) are the least studied conditions. The 4 trials studying maternal conditions were conducted in the United States on management of adolescent postpartum depression, pregnancy prevention among adolescents, and the integration of a program to reduce sexually transmitted diseases with prenatal care among adolescents.
TABLE 2.
Number of Pediatric Trials and Participants and Pediatric Global Burden of Disease According to Condition
| Condition | All Trials, n (N = 4619) | RCTs, n (N = 3246) | Drug RCTs, n (N = 1369) | Randomized Participants, na,b (N = 1 342 049) | Global Burden of Disease in DALYs (Rank) |
|---|---|---|---|---|---|
| Neuropsychiatric conditions | 874 | 642 | 267 | 103 134 | 48 940 526 (5) |
| Nonrespiratory infections and parasitic diseases | 847 | 637 | 188 | 636 471 | 182 759 532 (1) |
| Perinatal conditions | 523 | 430 | 152 | 179 933 | 126 420 651 (2) |
| Malignant neoplasms | 473 | 123 | 77 | 65 199 | 4 110 685 (14) |
| Respiratory diseases | 366 | 290 | 186 | 70 856 | 10 691 264 (8) |
| Respiratory infections | 247 | 211 | 82 | 127 579 | 77 264 822 (3) |
| Digestive diseases | 208 | 137 | 74 | 16 757 | 7 695 304 (9) |
| Diabetes mellitus | 138 | 117 | 49 | 14 935 | 542 738 (20) |
| Congenital anomalies | 129 | 89 | 53 | 10 406 | 24 256 090 (7) |
| Musculoskeletal diseases | 128 | 86 | 38 | 11 189 | 2 489 737 (17) |
| Nutritional deficiencies | 106 | 93 | 14 | 42 306 | 27 844 589 (6) |
| Unintentional injuries | 101 | 76 | 26 | 10 064 | 52 837 234 (4) |
| Cardiovascular diseases | 96 | 67 | 32 | 13 497 | 6 683 866 (11) |
| Genitourinary diseases | 96 | 62 | 43 | 6751 | 1 888 187 (18) |
| Sense organ diseases | 77 | 50 | 20 | 7500 | 6 024 722 (12) |
| Skin diseases | 67 | 42 | 29 | 3531 | 1 064 523 (19) |
| Oral conditions | 63 | 55 | 16 | 12 062 | 2 532 862 (16) |
| Endocrine disorders | 62 | 29 | 22 | 3654 | 3 682 728 (15) |
| Nonmalignant neoplasms | 9 | 3 | 1 | 422 | 372 642 (21) |
| Intentional injuries | 5 | 5 | 0 | 5479 | 7 130 242 (10) |
| Maternal conditions | 4 | 2 | 0 | 324 | 5 270 155 (13) |
Excluding subjects enrolled in trials with cluster randomization.
Enrollment figures missing for 5 trials: 1 each in infectious diseases, perinatal conditions, nutritional deficiencies, childhood cluster diseases, and sense organ diseases.
Clinical trials were only moderately correlated with global disease burden overall (r = 0.58, P = .006; Table 3). RCTs in particular were slightly more correlated (r = 0.64, P = .002) as were total number of randomized participants (r = 0.66, P = .001), whereas randomized drug trials were slightly less correlated (r = 0.47, P = .03). Correlations were also moderate within each of the 3 country income levels. Low-income countries had the lowest correlation overall (r = 0.47, P = .03) as well as based on RCTs (r = 0.47, P = .03) and randomized participants (r = 0.46, P = .04), with slightly higher values when considering randomized drug trials (r = 0.58, P = .006).
TABLE 3.
Correlation Between Number of Trials and Participants and Disease Burden Across Conditions
| All Trials | RCTs | Drug RCTs | Randomized Participants | |||||
|---|---|---|---|---|---|---|---|---|
| ra | P | ra | P | ra | P | ra | P | |
| World | 0.58 | .006 | 0.64 | .002 | 0.47 | .03 | 0.66 | .001 |
| High-income countries | 0.49 | .02 | 0.53 | .01 | 0.45 | .04 | 0.54 | .01 |
| Middle-income countries | 0.57 | .007 | 0.63 | .002 | 0.45 | .04 | 0.57 | .007 |
| Low-income countries | 0.47 | .03 | 0.47 | .03 | 0.58 | .006 | 0.46 | .04 |
Spearman’s rho.
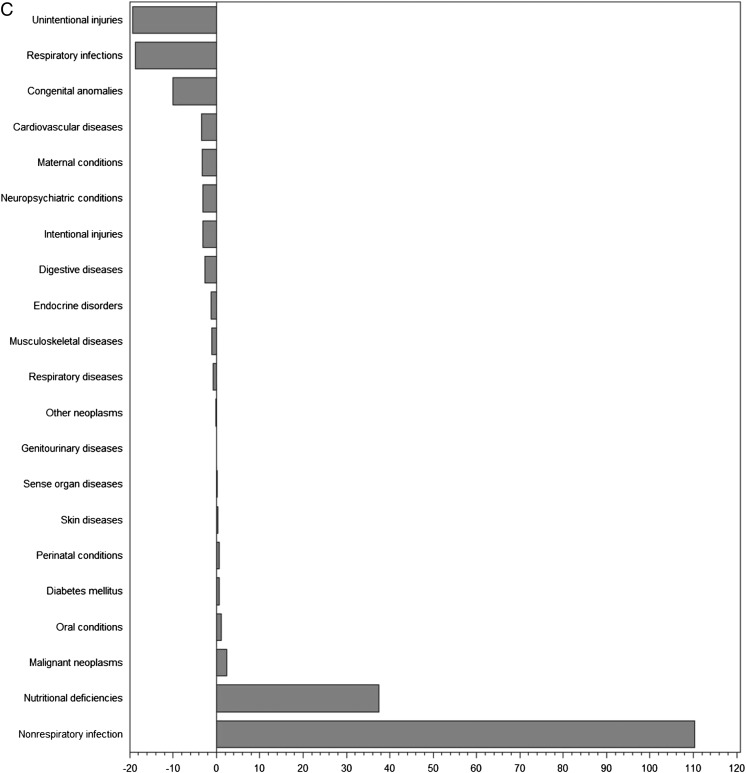
Infectious and parasitic diseases in particular were examined in greater detail in low-income countries and demonstrated good correlation between clinical research activity and disease burden. Correlations were high for clinical trials overall (r = 0.92, P < .001) as well as for RCTs (r = 0.94, P < .001), drug RCTs (r = 0.82, P < .001), and randomized participants (0.92, P < .001).
Expected Number of Trials
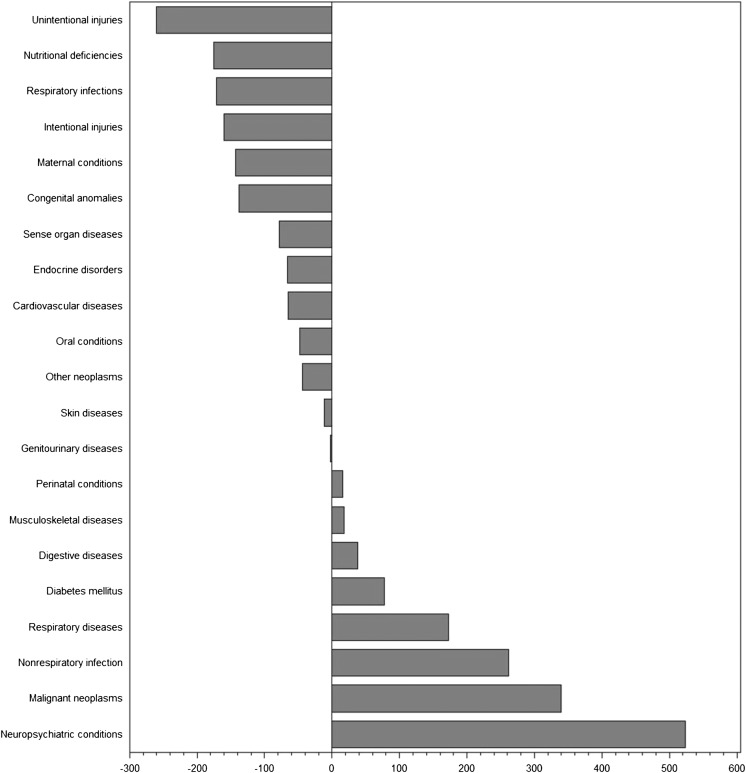
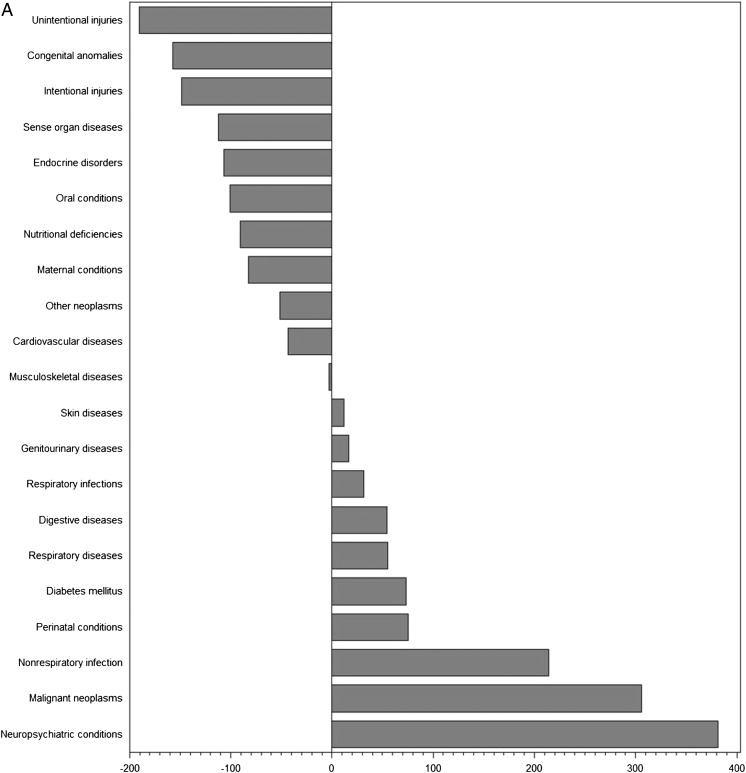
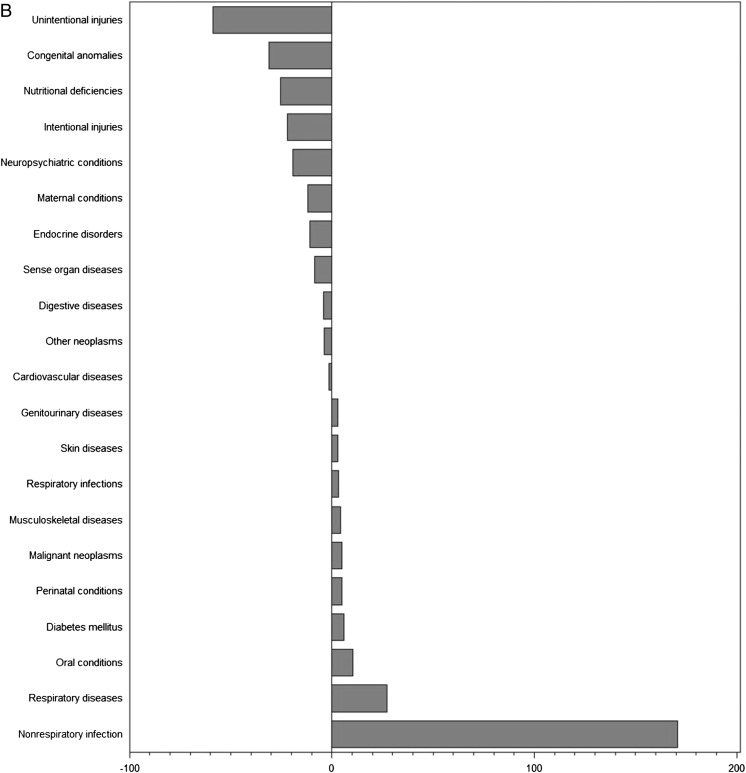
To further assess the proportionality between clinical trials and disease burden, we examined the actual and predicted number of trials as a function of burden of disease in DALYs (Figs 1 and 2 A–C, Supplemental Fig 3 A–D). The differences between expected and actual number of trials demonstrate that a number of conditions were substantially under- or overrepresented in clinical trials in countries in each of the income levels. Globally, the conditions most understudied relative to disease burden were injuries (–260 trials for unintentional injuries and –160 trials for intentional injuries), nutritional deficiencies (–175 trials), and respiratory infections (–171 trials). Conversely, neuropsychiatric conditions (+524 trials), malignant neoplasms (+340 trials), and nonrespiratory infections (+262 trials) represented the most overstudied conditions. For each of the country income levels, we identified a different set of conditions that was neglected or overstudied in relation to burden of disease.
FIGURE 1.
Differences between actual and expected pediatric clinical trials based on global burden of disease. For each condition, we show the difference between actual and expected number of trials based on the disease burden, with negative numbers indicating a deficit in trials and positive numbers excess trials. Generalized γ regression was used to calculate expected values.
FIGURE 2.
A, Differences between actual and expected pediatric clinical trials based on burden of disease in high-income countries. B, Differences between actual and expected pediatric clinical trials based on burden of disease in middle-income countries. C, Differences between actual and expected pediatric clinical trials based on burden of disease in low-income countries.
Discussion
There is substantial room for improvement in the match between allocation of clinical research resources and the worldwide impact of conditions. The correlation between pediatric clinical research activity and disease burden across conditions is only moderate among countries in all income levels, and least associated in low-income countries, where clinical research is sparse in general. High burden conditions are substantially understudied in countries in each of the income groups, including injuries, congenital anomalies, nutritional deficiencies, and respiratory infections. Clinical research in pediatric populations is unevenly distributed among countries at different income levels, with the majority of research conducted in high-income countries despite low-income countries accounting for the bulk of the global disease burden.
Previous studies have shown that the correlation between disease burden and research evidence as expressed by randomized trials or systematic reviews is low in sub-Saharan Africa and globally, respectively, whereas there is a better correlation with disease burden in countries with established market economies.8,17 Research agendas may be driven primarily by the needs of and diseases afflicting wealthy nations. However, in the United States, a mismatch between National Institutes of Health funding and burden of disease has persisted despite 1998 Institute of Medicine recommendations to improve funding priorities.6,7
Many of the diseases in less developed countries have a substantial prevalence in more developed countries where they are well studied, raising the question of whether the best strategy would be to apply evidence derived from trials conducted in more developed nations to populations in less developed countries and vice versa.18 The determinants of disease as well as the contexts for prevention and treatment are sometimes similar, but other times they can be vastly different in low- and middle-income countries, limiting the adoption of recommendations developed in high-income settings.19,20 As an example, the ministry of health in Ghana recently considered adding several pediatric medications to its national drug program based on recommendations from the WHO Essential Medicines Program.21 In the process, they reviewed the evidence supporting their use and found that even when there was high-quality evidence on the benefits of the drugs, few trials had been conducted in Africa, and little of the existing evidence could be extended to Ghana due to differences in practice standards, issues of feasibility, and resource implications. Organophosphate and other pesticide exposures provide another example of the imperative to consider local context for therapeutic options. These poisonings occur at particularly high rates in low-income nations, and their treatment is fraught with challenges around access to health care not encountered in high-income countries, necessitating entirely different treatment approaches.22
Noncommunicable diseases such as asthma, diabetes, depression, and cancers, are now the greatest contributors to death and disability worldwide and yet have been particularly neglected compared with infectious diseases in low- and middle-income countries.10,19,23 Our results highlight this disparity, showing much higher correlation between clinical trials and disease burden for infectious diseases than other conditions. Increasing clinical research activity around infectious conditions in less developed nations may not only benefit local populations but also shed light on prevention and treatment strategies in more developed countries where many important infectious diseases are rare.19,24 This would require building a strong, unbiased research agenda in less developed nations because there is currently evidence that in some cases, trials performed in these countries are subject to several biases, including selective reporting bias, because it is even more difficult to publish a negative result when the trial comes from a less developed country.18
For countries in each of the income groups, we identified a different set of conditions that require greater focus based on the distribution of pediatric disease burden. Other factors, such as the absolute number of trials addressing a condition, may also be relevant. Only 4 trials addressed pregnancy prevention and management among adolescents even though birth control, psychosocial needs, and the prevention of sexually transmitted diseases are entirely different in this patient population than in older women. Similarly, intentional injuries, which include self-inflicted injuries and child maltreatment, are studied in only 5 trials despite the unique circumstances that must be considered in the prevention and management of these injuries. Additional factors may also be important in evaluating research investments and funding decisions, including indirect benefits of certain types of research, political and social priorities, research quality, and potential for knowledge implementation.25
A better understanding of which conditions are insufficiently addressed in the current research portfolio may provide guidance to organizations on how to allocate and prioritize available resources. Certain conditions, such as unintentional and intentional injuries, may be difficult to address in trials. These injuries result from road traffic accidents, drowning, poisoning, and fires. However, trials can address the prevention of these events as well as the medical management of the resulting injuries. Furthermore, heightened awareness of the limitations of medical research in certain areas may foster greater emphasis on other approaches, including national and global policies on road traffic safety interventions and legislation mandating universal child-resistant medication packaging.26
There are several limitations to our study. Some trials are not registered in ClinicalTrials.gov, and other registries may be used differentially according to country income group. However, our query of trials in the International Trial Research Platform revealed that ∼83% of pediatric trials are registered in ClinicalTrials.gov, and the proportion of trials missing in each income group mirrors the distribution among the trials studied. It is also possible that our system for classifying trials into country income categories underestimated the amount of research being conducted in low-income countries. To explore this possibility, we conducted an additional analysis in which trials were assigned to the low-income country group if any study site was in the low-income category. This contributed an additional 53 trials to the low-income group but did not substantially change the correlations between clinical trials and disease burden (r = 0.51 for correlation with all trials and with RCTs, and r = 0.56 for correlation with drug RCTs). We used DALYs as the measure of disease burden, which have been previously used to assess associations between research activity and disease burden, but it is possible that other approaches may have highlighted different conditions as over- or understudied.6,7,17 Finally, limitations inherent to the use of the trial registry include missing data and that we were unable to verify the completeness and accuracy of the information provided by investigators.
Conclusions
A greater number of children could derive benefit from clinical trials if the pediatric research agenda were more closely aligned with the disease burden in children, beginning with a greater focus on conditions that appear to be underrepresented in current clinical trials. Noncommunicable diseases and injuries in low-income countries, in particular, require additional prioritization. The use of ongoing and consistent accounting of the association between pediatric clinical research activity and disease burden could help optimize the rationale and efficient allocation of resources for pediatric clinical trials.
Supplementary Material
Acknowledgment
We thank Jeong Kim for her help with queries and data extraction from the International Trial Research Platform.
Glossary
- DALY
disability-adjusted life-year
- RCT
randomized controlled trial
- WHO
World Health Organization
Footnotes
Dr Bourgeois contributed to study conception and design, data acquisition, analysis and interpretation of data, and revision of the article; Dr Olson contributed to analysis and interpretation of data and revision of the article; Drs Ioannidis and Mandl contributed to study conception and design, interpretation of data, and revision of the article; and all authors gave final approval.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by a grant from the National Institute of Child Health and Human Development (1R21HD072382), National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Bourgeois FT, Murthy S, Pinto C, Olson KL, Ioannidis JP, Mandl KD. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics. 2012;130(2):285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen E, Uleryk E, Jasuja M, Parkin PC. An absence of pediatric randomized controlled trials in general medical journals, 1985–2004. J Clin Epidemiol. 2007;60(2):118–123 [DOI] [PubMed] [Google Scholar]
- 3.Thomson D, Hartling L, Cohen E, Vandermeer B, Tjosvold L, Klassen TP. Controlled trials in children: quantity, methodological quality and descriptive characteristics of pediatric controlled trials published 1948–2006. PLoS One. 2010;5(9):e13106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen E, Goldman RD, Ragone A, et al. Child vs adult randomized controlled trials in specialist journals: a citation analysis of trends, 1985–2005. Arch Pediatr Adolesc Med. 2010;164(3):283–288 [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Castaldi C, Silverstein M, Bauchner H. Child versus adult research: the gap in high-quality study design. Pediatrics. 2008;122(1):52–57 [DOI] [PubMed] [Google Scholar]
- 6.Gross CP, Anderson GF, Powe NR. The relation between funding by the National Institutes of Health and the burden of disease. N Engl J Med. 1999;340(24):1881–1887 [DOI] [PubMed] [Google Scholar]
- 7.Gillum LA, Gouveia C, Dorsey ER, et al. NIH disease funding levels and burden of disease. PLoS One. 2011;6(2):e16837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isaakidis P, Swingler GH, Pienaar E, Volmink J, Ioannidis JP. Relation between burden of disease and randomised evidence in sub-Saharan Africa: survey of research. BMJ. 2002;324(7339):702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kappagoda S, Ioannidis JP. Neglected tropical diseases: survey and geometry of randomised evidence. BMJ. 2012;345:e6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. The global burden of disease: 2004 update. Geneva: World Health Organization; 2008. Available at: www.who.int/evidence/bod. Accessed April 9, 2011
- 11.Estellat C, Ravaud P. Lack of head-to-head trials and fair control arms: randomized controlled trials of biologic treatment for rheumatoid arthritis. Arch Intern Med. 2012;172(3):237–244 [DOI] [PubMed] [Google Scholar]
- 12.Viergever RF, Ghersi D. The quality of registration of clinical trials. PLoS One. 2011;6(2):e14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeAngelis C, Drazen JM, Frizelle FA, et al. International Committee of Medical Journal Editors . Clinical trial registration: a statement from the International Committee of Medical Journal Editors. Med J Aust. 2004;181(6):293–294 [PubMed] [Google Scholar]
- 14.Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database—update and key issues. N Engl J Med. 2011;364(9):852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. International Clinical Trials Registry Platform (ICTRP). Available at: www.who.int. Accessed May 20, 2013
- 16.Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72(3):429–445 [PMC free article] [PubMed] [Google Scholar]
- 17.Swingler GH, Volmink J, Ioannidis JP. Number of published systematic reviews and global burden of disease: database analysis. BMJ. 2003;327(7423):1083–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panagiotou OA, Contopoulos-Ioannidis DG, Ioannidis JP. Comparative effect sizes in randomised trials from less developed and more developed countries: meta-epidemiological assessment. BMJ. 2013;346:f707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebrahim S, Pearce N, Smeeth L, Casas JP, Jaffar S, Piot P. Tackling non-communicable diseases in low- and middle-income countries: is the evidence from high-income countries all we need? PLoS Med. 2013;10(1):e1001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKee M, Stuckler D, Basu S. Where there is no health research: what can be done to fill the global gaps in health research? PLoS Med. 2012;9(4):e1001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinclair D, Gyansa-Lutterodt M, Asare B, Koduah A, Andrews E, Garner P. Integrating global and national knowledge to select medicines for children: the Ghana National Drugs Programme. PLoS Med. 2013;10(5):e1001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eddleston M, Juszczak E, Buckley NA, et al. Ox-Col Poisoning Study collaborators . Multiple-dose activated charcoal in acute self-poisoning: a randomised controlled trial. Lancet. 2008;371(9612):579–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lachat C, Otchere S, Roberfroid D, et al. Diet and physical activity for the prevention of noncommunicable diseases in low- and middle-income countries: a systematic policy review. PLoS Med. 2013;10(6):e1001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catalá-López F, García-Altés A, Alvarez-Martín E, Gènova-Maleras R, Morant-Ginestar C. Does the development of new medicinal products in the European Union address global and regional health concerns? Popul Health Metr. 2010;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghinai I, Hla TT, Smith R. Global health priorities and research funding. Lancet Infect Dis. 2013;13(8):653. [DOI] [PubMed] [Google Scholar]
- 26.Redelmeier DA, McLellan BA. Modern medicine is neglecting road traffic crashes. PLoS Med. 2013;10(6):e1001463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.