Significance
The bacterium Pseudomonas aeruginosa is an opportunistic pathogen of humans and is the leading cause of death in patients with cystic fibrosis (CF). We sequenced the genomes of P. aeruginosa isolated from respiratory tracts of patients with CF to investigate general patterns of adaptation associated with chronic infection. Selection imposed by the CF lung environment has had a major influence on genomic evolution and the genetic characteristics of isolates causing contemporary infection. Many of the genes and pathways implicated in adaptive evolution within the host had obvious roles in the pathogenic lifestyle of this bacteria. Genome sequence data indicated that an epidemic strain, with increased virulence and multidrug resistance, has spread between clinics in the United Kingdom and North America.
Keywords: virulence, multidrug resistance, redox balance
Abstract
Pseudomonas aeruginosa is an opportunistic pathogen of humans and is a major cause of morbidity and mortality in patients with cystic fibrosis (CF). Prolonged infection of the respiratory tract can lead to adaptation of the pathogen to the CF lung environment. To examine the general patterns of adaptation associated with chronic infection, we obtained genome sequences from a collection of P. aeruginosa isolated from airways of patients with CF. Our analyses support a nonclonal epidemic population structure, with a background of unique, recombining genotypes, and the rare occurrence of successful epidemic clones. We present unique genome sequence evidence for the intercontinental spread of an epidemic strain shared between CF clinics in the United Kingdom and North America. Analyses of core and accessory genomes identified candidate genes and important functional pathways associated with adaptive evolution. Many genes of interest were involved in biological functions with obvious roles in this pathosystem, such as biofilm formation, antibiotic metabolism, pathogenesis, transport, reduction/oxidation, and secretion. Key factors driving the adaptive evolution of this pathogen within the host appear to be the presence of oxidative stressors and antibiotics. Regions of the accessory genome unique to the epidemic strain were enriched for genes in transporter families that efflux heavy metals and antibiotics. The epidemic strain was significantly more resistant than nonepidemic strains to three different antibiotics. Multiple lines of evidence suggest that selection imposed by the CF lung environment has a major influence on genomic evolution and the genetic characteristics of P. aeruginosa isolates causing contemporary infection.
The ubiquitous, Gram-negative bacterium Pseudomonas aeruginosa can be found free-living in a wide range of environments or in association with various animal and plant species. P. aeruginosa is also an opportunistic pathogen of humans and is the most common species isolated from the respiratory tracts of adult patients with cystic fibrosis (CF). Chronic endobronchial infections caused by P. aeruginosa are found in up to 80% of adult patients with CF (1, 2) and are associated with increased morbidity and mortality (3). Once established in the lung, P. aeruginosa infections are notoriously difficult to eradicate and are highly resilient to repeated rounds of intensive, prolonged antibiotic treatment.
There is growing evidence that the often decades-long interaction between the host and P. aeruginosa during chronic infection results in adaptation of the pathogen to the CF lung environment (4). The CF lung is an inhospitable place characterized by osmotic and oxidative stress, limited oxygen, high concentrations of antibiotics, and constant assault by the host immune system. Although the hurdles posed by these factors are dynamic and fluctuate through time, they are likely the main drivers of adaptive evolution of bacteria in CF airways. Studies have documented some repeatable patterns of adaptation to the CF lung environment such as loss of motility associated with growth as an unattached biofilm or microcolony (5), reduced virulence factor expression (6), presumably an adaptation to escape detection by the host immune system, and increased activity of efflux pumps (7) associated with resistance to many antibiotics used in treatment.
The genetic changes responsible for these adaptations have recently begun to be investigated. Evidence from longitudinal studies suggests that a large number of candidate genes can accrue substitutions during adaptation of P. aeruginosa (6–9). Any given infection involves only a small subset of those substitutions, and most virulence-related phenotypes are not observed uniformly (10), making it difficult to say with confidence which genetic changes are most commonly associated with adaptation to the CF lung. A more comprehensive view therefore requires the comparison of a larger sample of diverse clinical isolates. To this end, we obtained whole-genome sequence data from a collection of P. aeruginosa isolated from the airways of patients with CF to investigate general patterns of adaptation associated with chronic infection. We summarize the broad-scale patterns of genomic variation and identify the most likely genetic targets of adaptation causing contemporary infection in our isolates. Our collection includes nonepidemic strains and a transmissible, epidemic strain recently reported within North America (1). Infection with an epidemic strain confers a poorer prognosis than infection with a nonepidemic strain, such as reduced lung function (11) and increased risk of death or lung transplant (1). Our comparative analyses of genomic variation identified some unique genomic features that distinguish the epidemic strain from nonepidemic strains.
Results and Discussion
Isolate Sources, Genome Sequencing, and Alignment.
We take a population genomics approach to study the evolution of the opportunistic pathogen, P. aeruginosa. Our study is unique in using whole-genome sequence data to examine the cross-sectional genome diversity of P. aeruginosa at the population and intrastrain level. Isolates were sampled from the sputum of 24 different patients attending CF clinics in six cities in Ontario, Canada (ref. 1, SI Appendix, Table S1). Clinics were located within 520 km of each other, so this sample represents a geographically restricted population. Reference-guided mapping may skew the detection of variation in genomic content, so draft genomes were assembled de novo (average of 69-fold sequence coverage) from whole-genome sequence data for the 24 Ontario isolates. To place the Ontario population in the evolutionary context of other members of the species, eight previously sequenced genomes were also included in our analyses (four from non-CF sources; SI Appendix, Table S2). A 32-genome global alignment was produced and locally collinear blocks of conserved sequence were identified. Genomic regions possessed by all 32 isolates were classified as core genome, and those occurring in only some isolates were classified as accessory genome. The size of the core genome was 5.35 Mb, or 83.5% of the average full P. aeruginosa genome (6.41 Mb). When restricted to genes only, 74.6% were considered core. Summing all nonredundant, accessory regions gives a total of 6.89 Mb, but each isolate contains an average of only 1.06 Mb (15.4%) of this accessory genome pool.
Nucleotide Polymorphism.
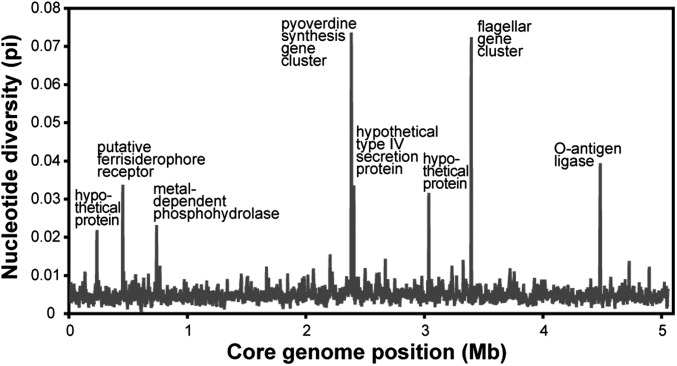
The core genome alignment was 5.06 Mb in length and contained 137,941 (2.7%) variable nucleotide positions. Core nucleotide diversity of the Ontario population (π = 0.0041, SE = 1.5 × 10−5) was 79% of the total for all isolates (π = 0.0052, SE = 1.6 × 10−5), confirming that much of the worldwide biodiversity can be present within a limited geographical region (12). Nucleotide diversity was fairly even along the core with only a few hotspots of polymorphism (Fig. 1). The most divergent region contained genes for the synthesis and uptake of iron-scavenging siderophores (pyoverdine), which are important for virulence and are known to be under diversifying selection (13, 14). Other divergent regions included genes for production of flagella or lipopolysaccharides (O-antigen), both of which elicit host immune response and are likely to be under diversifying selection (15). These results suggest that these clinical P. aeruginosa isolates have been evolving in close association with human hosts for long enough to leave imprints of diversifying selection on genomic polymorphism.
Fig. 1.
Nucleotide diversity (π) along the core genome alignment. Plotted values are means from sliding windows of 4 kb in length with a step size of 2 kb.
Phylogeny of Isolates.
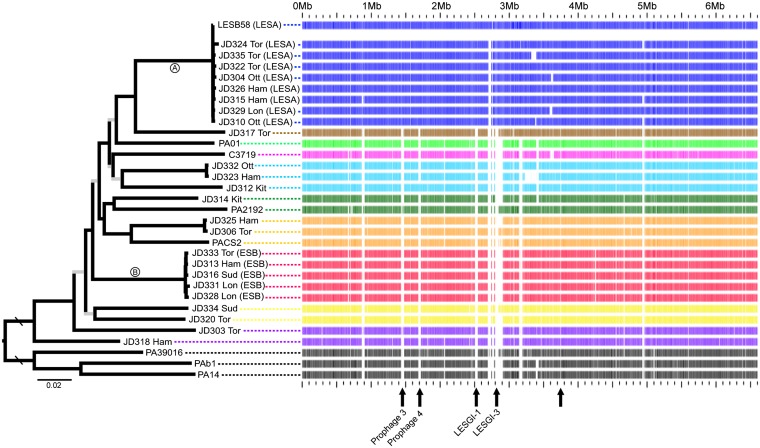
Our sampling scheme was designed to allow detailed analyses of our focal population (Ontario) and to place these isolates within the broader phylogenetic framework of the global species. The established P. aeruginosa multilocus sequence typing (MLST) (16) system was applied to the 24 Ontario isolates; a total of 14 sequence types were identified, half of which were previously undescribed (SI Appendix, Table S3). The MLST system covers only 0.18% of the genome and the loci have low levels of nucleotide variation, providing limited power for inferring phylogenetic relationships. Instead, we constructed a maximum likelihood phylogeny from the core genome alignment (Fig. 2 and SI Appendix Fig. S1). One notable feature of this phylogeny is the two well-supported clades (A and B) that correspond to transmissible, epidemic strains of P. aeruginosa (see next section), a designation supported by the tight phylogenetic clustering of isolates within clades. The core-genome nucleotide diversity within clades A and B is only 2.5% and 3.0%, respectively, of that in the entire sample.
Fig. 2.
(Left) Maximum likelihood phylogeny constructed from the core genome alignment (5.06 Mb; 137,941 SNPs). All internal branches have bootstrap percentages >70 except those shown in gray. The rooting branch is four times longer than shown. Isolates with names starting with JD were sampled from Ontario (Ham, Hamilton; Kit, Kitchener; Lon, London; Ott, Ottawa; Sud, Sudbury; Tor, Toronto). (Right) Example of a BLAST atlas with a LESA isolate as the reference genome. Coding genes in the LESB58 genome are indicated along the Upper row as blue bars. For every other genome, the presence/absence of the corresponding LESB58 gene is indicated by presence/absence of a bar. Arrows along the bottom indicate regions of genome that were identified as LESA specific; named regions correspond roughly with regions identified in ref. 19.
Another feature of the phylogeny is long terminal branches with a backbone of short internodes (Fig. 2 and SI Appendix, Fig. S1). Despite using genome-wide polymorphism data to construct the phylogeny, many internodes remain weakly supported. To investigate if this poor resolution was due to conflicting phylogenetic signals caused by recombination, recombinant regions of the core genome were identified (17) and removed in an isolate-specific manner. The maximum likelihood phylogeny built from this nonrecombinant core genome still had short, weakly supported internodes (SI Appendix, Fig. S1), indicating that recombination is not solely responsible for the poor resolution of the basal relationships.
An alternate explanation is rapid radiation and diversification among clades during adaptation to the CF lung environment. All but one of the ingroup isolates (PA01) originated from a patient with CF, in comparison to the well-supported outgroup clade from non-CF sources. A previous study of longitudinally sampled P. aeruginosa isolates (9) proposed that rates of adaptation are initially high and decline through time, a pattern commonly observed in laboratory evolution experiments (18). Evidence suggests that the initial burst of adaptation is driven by positive selection during colonization of the CF airways, whereas the subsequent prolonged period of persistence is characterized by purifying selection (6, 9). The structure of the phylogeny is consistent with this dynamic selection regime; however, increased genome sampling of isolates from environmental and non-CF sources is needed to confirm that these patterns are CF specific.
No evidence for phylogeographic structure was found. Closely related isolates were not sampled from the same geographic location, and isolates from Ontario were not exclusively monophyletic. Population differentiation was not detected between Ontario versus non-Ontario populations (coefficient of differentiation = 0.12, SE = 5.6 × 10−4), or between subpopulations (cities) within Ontario (0.005, SE = 0.001).
Intercontinental Spread of a Transmissible, Epidemic Strain.
Clade A was composed of the Liverpool epidemic strain (LES) (19) and eight isolates of a transmissible, epidemic strain from Ontario named “strain A” (1), representing the only case where an Ontario isolate was nearly identical to a non-Ontario isolate. These two strains have the same pulsed-field gel electrophoresis (PFGE) and MLST patterns and share characteristics of transmissibility and greater risks of negative clinical outcomes (1). Based on the high levels of genome-wide sequence similarity found here, we conclude that LES and epidemic strain A are equivalent and hereafter refer to them collectively as “Liverpool/epidemic strain A” (LESA). Our genomic data therefore confirm cross-infection of an epidemic strain within Ontario and, importantly, between the United Kingdom and North America.
It may be that LESA is not in fact an “epidemic” strain but is just highly prevalent in the surrounding environments. Two lines of evidence argue against this interpretation. First, it is extremely unlikely that the same high-frequency strain is found in both Ontario and the United Kingdom, especially given the large amount of genetic diversity found within Ontario, let alone on a global geographic scale (12). Second, in a collection of 65 P. aeruginosa isolates sampled from medical equipment and sinks from CF clinics and patients’ homes in Ontario (1), all were found to be nonepidemic.
Clade B consists of five isolates of an epidemic strain that have not been sampled outside of Ontario and were classified as Ontario “strain B” on the basis of their distinct PFGE profiles (1). We refer to this strain as “epidemic strain B” (ESB). Three additional isolates that were originally identified as strain B did not fall within the main ESB clade, demonstrating the superior utility of whole-genome analyses to distinguish phylogenetic relationships.
Adaptation to the CF Lung.
To identify genes putatively involved in adaptation to the CF lung, we examined the entire 32-genome sample to (i) quantify the average selective regime on core genes and (ii) establish the distribution and identity of accessory genome content.
Selection on core genes.
Confidently aligned core genes were analyzed individually to quantify the strength of selection using the relative rate of nonsynonymous to synonymous substitution (dN/dS = ω). Here, ω is a global parameter for each gene, and the average ω for 2,827 analyzed genes was 0.099 (SE = 0.002). Although this indicates a general background of purifying (negative) selection, there was substantial variation in the strength and direction of selection among core genes (ω range = 0–1.42; SI Appendix, Fig. S2). We investigated the characteristics of the top 5% of genes with the highest ω-values using gene ontology (GO) annotation and enrichment analyses. Three ontology classes were strongly represented in this top-ω sample: oxidoreductase activity, secretion, and heterocycle metabolism (SI Appendix, Table S4).
GO information is available for only a subset of genes in the genome, so more comprehensive manual annotation of predicted function or biological role was performed. The list of the top 50 ω-ranked annotated genes contained many genes with reduction/oxidation (redox), regulatory, secretion, or transport functions (Table 1). The most common class, redox, comprised 36% of the top 50 genes, a proportion significantly greater than that for the full reference genome (Fisher’s exact, P < 0.0001).
Table 1.
Functional categorization of the top 50 core genes with the highest relative rates of nonsynonymous substitution (with automated or manual annotation)
| Class | No. of genes | Examples of genes and functional roles |
| Redox | 18 | Nitrous oxide reductase (nosY) |
| Regulation of denitrification (nirQ) | ||
| Ethanol oxidation (erbR, eraS) | ||
| Glutathione S-transferases | ||
| Epoxide hydrolase | ||
| Oxidases | ||
| Dehydrogenases | ||
| Oxidoreductase | ||
| Dioxygenase | ||
| Transport | 7 | MFS transporters |
| ABC transporters | ||
| Multidrug efflux protein (norA) | ||
| Secretion | 5 | Type III secretion system (pscQ) |
| Phospholipases | ||
| Exotoxin (exoY) | ||
| Regulatory | 7 | Two-component response regulators |
| Transcriptional regulators | ||
| RNA polymerase sigma-70 factor | ||
| Hydrolysis | 8 | Beta-lactamases |
| Hydrolases | ||
| Miscellaneous | 5 | Kinases |
| Transferases |
Redox genes of interest included those with roles in the dentrification pathway (nosY and nirQ) and antioxidation (glutathione S-transferases, epoxide hydrolase). Other genes were involved in type-III secretion of exotoxins (exoY and pscQ), a key bacterial virulence mechanism that has been demonstrated in P. aeruginosa (20). The annotated gene with the second highest ω was plcR, a regulator of phospholipase C secretion, which is a determinant of virulence and pathogenesis in numerous bacteria (21). The annotated gene with the highest ω was erbR, which encodes a response regulator of biofilm formation, ethanol oxidation, and drug resistance (22). Signatures of elevated nonsynonymous rates in genes with these particular molecular functions and biological processes suggest they are involved in adaptation to the CF lung environment.
Characteristics of the accessory genome.
Gain and loss of accessory genome regions via horizontal transfer determines the evolution of genome content. Horizontal acquisition of novel genetic elements is often associated with adaptation to new niches or lifestyles (23), so the nonessential, accessory genome of P. aeruginosa may contain adaptive factors that contribute to successful colonization and persistence within CF lungs. We visualized the location, distribution, and relative sizes of accessory genome regions by constructing BLAST atlases (example in Fig. 2). Accessory genome content was commonly, but not always, shared among isolates that were closely related in the core phylogeny.
Gene identification predicted 3,299 ORFs within the 4.4 Mb of analyzed accessory genome sequence. Compared with the entire genome, the accessory genome was significantly enriched for numerous functional roles (SI Appendix, Table S5). The hypothesis that the accessory genome helps P. aeruginosa adapt to a diversity of environments, particularly the CF lung, was supported by many of the significantly overrepresented biological processes, such as pathogenesis, antibiotic metabolism, and transmembrane transport. The top molecular function and biological process were both oxidation/reduction, further suggesting that genes with redox functionality have played an important role in the evolution of clinical P. aeruginosa strains.
Oxidative Stress and Redox Balance.
How might selection on P. aeruginosa genes with redox functions be associated with an adaptive benefit in the CF lung? The CF airway is characterized by multiple disparate sources of oxidative stress (Fig. 3), three of which are described below.
Fig. 3.
Diagram of the proposed functional relationships between sources of oxidative stress, regulation of NO, and candidate pathways identified in this study.
Oxidative burst by host.
The host’s immune response includes the production of several reactive oxygen species (ROS) and reactive nitrogen intermediates (RNIs). The oxidative burst by activated macrophages releases nitric oxide (NO) and superoxide, creating oxidative stress that functions to protect the host’s lung from infection (24). Lungs of patients with CF also have reduced levels of the major pulmonary antioxidant, glutathione (25).
Anaerobic respiration.
The thick, viscous mucus of the CF lung has reduced oxygen permeability, and growth as a biofilm further reduces the oxygen availability to bacterial cells. Under such conditions, P. aeruginosa respires anaerobically (26) using nitrate as the terminal electron acceptor, which produces NO as a toxic intermediate.
Antibiotic exposure.
Several classes of antibiotics appear to cause bacterial cell death by oxidative damage. The proposed central mechanism involves elevated levels of intracellular ROS (27) but the details of the mechanism are still under debate (28). Also, bacterial NO and oxidative stress interact to affect sensitivities to a wide spectrum of antibiotics (29). The interplay between these two phenomena requires further study, but they demonstrate a role for oxidative stress and redox balance in antibiotic resistance.
Taken together, these three lines of evidence suggest there can be strong selective pressure for adequate mitigation of oxidative insult and maintenance of proper redox balance within the bacterial cell (Fig. 3). In particular, the regulation of the free radical NO may be a central mediator of oxidative stress management. In P. aeruginosa, the accumulation of NO is prevented by reduction to nitrous oxide (N2O), which is itself an RNI and is further reduced to nontoxic dinitrogen gas. This pathway is regulated by nirQ, and the last step is performed by nosY (nitrous oxide reductase), both of which were on the list of genes with highest relative nonsynonymous rates.
We hypothesized that isolates from CF sources may be able to withstand greater concentrations of external NO than isolates from non-CF sources. Consistent with this expectation, minimum inhibitory concentrations (MICs) of NO (reduction product of NaNO2) varied among CF isolates from Ontario, with over half (15/24) (SI Appendix, Fig. S3) being equally or more resistant than the non-CF outgroup isolate, PA14. However, median MICs of CF and non-CF isolates (PA01 and PA14) did not differ significantly (Z = 0.55; P > 0.59). These results should be treated as preliminary, as laboratory assay conditions may not accurately simulate some aspects of the CF lung that affect NO resistance and the non-CF sample consisted of just two laboratory isolates. A stronger test requires more extensive sampling of environmental isolates from Ontario.
LESA.
Laboratory and clinical studies have revealed differences between epidemic and nonepidemic strains in drug resistance profiles, virulence in infection models, propensity for superinfection (colonization by a new strain of a patient already infected with another strain), and clinical outcomes for patients with CF (1, 10, 30–33). We investigated to what extent genes and genomic regions distinguish LESA from all other strains by analyzing (i) LESA divergence in core gene phylogenies and (ii) the functional characteristics of the LESA-specific accessory genome.
LESA divergence in core gene phylogenies.
Many nucleotide polymorphisms within the core genome phylogenetically differentiate LESA from other isolates (Fig. 2). To determine which genes contributed most to LESA divergence, we ranked core genes based on the relative length of the LESA branch and summarized the functional roles of the top 5% (SI Appendix, Table S6). The most represented molecular function and biological process was kinase activity and arginine metabolism, respectively. Adding manual annotation reinforced these results (SI Appendix, Table S7), but also showed that the 50 top-ranked, annotated genes were significantly overrepresented by redox functions [12 of 50 (24%), P < 0.001].
LESA-divergent genes of interest included several involved in arginine metabolism (aguB, spuF, and aotP) and two with roles in biofilm matrix formation (pelF and pelG). A gene required for the biosynthesis of pyrroloquinoline quinone (pqqE), a redox cofactor and free-radical scavenger, was also found. Two genes whose products are secreted by type-III systems were also discovered: exotoxin T (exoT) and a pore-forming hemolysin, which causes the lysis of host red blood cells, both of which contribute to virulence (34).
The importance of arginine metabolism can be explained by its multiple roles in several pathways relevant to CF lung adaptation. First, both bacteria and macrophages synthesize NO from arginine precursors. Second, P. aeruginosa preferentially catabolizes arginine (into ornithine) to provide energy for anaerobic growth in conditions that mimic the CF lung, a pathway previously implicated in adaptation during chronic infection (35, 36). Third, the synthesis of the polyamine putrescine from arginine precursors produces agmatine as an intermediate, which inhibits NO synthases of macrophages (37) and diminishes the macrophage-derived oxidative burst. An imbalance of arginine and polyamine metabolism in the CF lung has been implicated in the pathophysiology of CF lung disease (38). The many LESA-divergent genes with roles in utilization and transport of arginine, ornithine, agmatine, and putrescine (SI Appendix, Tables S6 and S7) suggest LESA may be particularly well adapted to this aspect of the CF lung environment.
Characteristics of the LESA-specific accessory genome.
We identified five broad regions of LESA-specific accessory genome (regions possessed by all LESA isolates but by no other isolates; Fig. 2), summing to 85 kb. We studied the functions of the 72 included ORFs and found that the two most overrepresented biological processes were protein secretion and cation transport (SI Appendix, Table S8). The largest LESA-specific accessory region was 30 kb in length and corresponded to a portion of a previously described genomic island in the LES (LESGI-3) (19). This region contained nine transport-related genes and three genes with homology to transcriptional regulators of heavy metal resistance operons (SI Appendix, Table S9). Many of the LESA-specific transporters were efflux pumps belonging to the major facilitator superfamily (MFS) and resistance–nodulation–division (RND) families, both of which have a wide substrate range, with affinities for binding and exporting sugars, metal ions, and multiple classes of antibiotics (39). These transporters actively pump the toxic compound out of the bacterial cell to avoid contact with target sites.
Enrichment of these types of efflux pumps may confer increased resistance to antibiotics. To test this prediction directly, we determined the MICs of three antibiotics with different mechanisms of action—the quinolone ciprofloxacin, the β-lactam ceftazidime, and the aminoglycoside gentamicin—for 48 clinical isolates from Ontario and two reference isolates. Resistance to each of the three antibiotics was significantly greater for LESA than non-LESA isolates (Table 2). These results lend experimental support to the idea that higher levels of efflux-mediated multidrug resistance may be a major factor contributing to the success of this epidemic strain.
Table 2.
Comparison of MICs of three antibiotics with different modes of action
| Antibiotic | Strain group | Median MIC (μg/mL) | Wilcoxon rank sum test |
|
| Z | Prob>|Z| | |||
| Ciprofloxacin | LESA | 4 | 1.97 | 0.049 |
| (Quinolone) | Non-LESA | 2 | ||
| Ceftazidime | LESA | 32 | 2.14 | 0.033 |
| (Beta-lactam) | Non-LESA | 12 | ||
| Gentamicin | LESA | 128 | 3.28 | 0.001 |
| (Aminoglycoside) | Non-LESA | 8 | ||
LESA isolates (n = 10) were significantly more resistant than non-LESA isolates (n = 40) to all three antibiotics. MICS, minimum inhibitory concentrations.
The abundance of LESA-specific transporters also suggested that LESA may have a greater ability to survive in harsh environments with high concentrations of cations or heavy metals. To test this hypothesis, we measured the MIC of NaCl, CuSO4, ZnCl2, and CoCl2 for the 24 sequenced Ontario isolates. Of the four tested cations, LESA isolates were significantly more resistant to only one, cobalt (P < 0.02, SI Appendix, Table S10), potentially due to the two LESA-specific genes in the cobalt–zinc–cadmium (CzcA) resistance family (SI Appendix, Table S9).
Our evolutionary genomic analyses of an epidemic P. aeruginosa strain focused on the most prevalent strain in our Ontario population, LESA. Other transmissible, epidemic strains are common in different regions of the world, such as Denmark (9) and Australia (40). When population-level genomic data become available for these epidemic strains, and similar analyses are performed, it will be very interesting to compare the results with those described here. The addition of other epidemic strains will help to determine if the characteristics of LESA observed here are shared among all epidemic strains or are simply strain specific.
Conclusions
We have demonstrated that P. aeruginosa has a nonclonal epidemic population structure, with a background of many unique, recombining genotypes and the rare occurrence of successful epidemic clones that spread to higher prevalence under certain conditions. We present unique genome sequence evidence that the most common epidemic strain in the United Kingdom is the same as that in North America. This highlights the importance of comprehensive surveillance of dominant, CF-associated strains that occur worldwide. Furthermore, it emphasizes the need for identifying the genetic factors that characterize epidemic strains of P. aeruginosa and similar pathogenic bacteria.
Resident P. aeruginosa can reproduce for hundreds of thousands of generations during a patient’s lifetime of chronic infection, adapting for survival and persistence in the CF lung. As opposed to longitudinal sampling of a single infection, we examined a broader sample of strains from a larger number of individual CF patients, allowing us to identify the trends that emerge from multiple independent bouts of adaptation to CF lungs. By analyzing the genomes of a diverse set of isolates that have successfully infected and persisted within CF lungs, we could infer the genes and functional pathways that were important for the evolution of this human pathogen. Consistent with a long history of host–pathogen interaction, the selection pressures imposed by the CF lung environment have had a major influence on the genomic evolution and genetic characteristics of clinically important isolates. Many of the highlighted candidate genes were involved in biological functions with obvious roles in this pathosystem, such as biofilm formation, transmembrane transport, pathogenesis, hemolysis, and secretion. However, the presence of oxidative stressors and antibiotics appear to be key factors that have driven the adaptive evolution of this pathogen within the host.
The evolution of generalized resistance to oxidative stress, whether the source is macrophages, anaerobic respiration, or antibiotics, would confer a strong adaptive benefit for survival in the CF lung. We hypothesize that bacteria can adapt simultaneously to these three similar selection pressures, in part, because of the interconnections between the underlying genetic pathways that are affected. For example, resistance to antibiotics may differ between anaerobic and aerobic conditions (41), and a regulatory connection exists between oxidation sensing and antibiotic resistance (42). This may explain why eliminating P. aeruginosa infections from the CF lung is so difficult. Survival in untreated CF lungs may require the ability to neutralize oxidative stressors, a characteristic that preadapts the bacteria to survive extended periods of antibiotic exposure during clinical treatment.
The molecular bases for transmissibility, colonization, and persistence of pathogens in the CF lung remain poorly understood. As studies of other CF airway pathogens continue to reveal additional candidate genes (e.g., Burkholderia) (43), a consensus will form regarding the functions and processes that are important for infection and survival. Of course, detailed genetic manipulations and tests in infection models are required to verify genes of particular importance. By identifying the key genetic pathways involved in bacterial adaptation to the CF lung, research on new therapeutic approaches can be targeted toward blocking the common adaptive routes taken by the pathogen.
Materials and Methods
Sources of isolates and genome sequencing methods are presented in SI Appendix. Methods for de novo genome assembly, multiple-genome alignment, core and accessory genome calculation, and phylogenetic reconstruction are described in SI Appendix. Assessment of polymorphism, population differentiation, core recombination, strength of selection, and gene ontology information were performed as described in SI Appendix. Methods for measuring minimum inhibitory concentrations are also described in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes for Health Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The draft genome sequences reported in this paper have been deposited in the DNA DataBank of Japan (DDBJ)/European Molecular Biology Laboratory/GenBank database, www.ncbi.nlm.nih.gov (accession nos. AWYJ00000000–AWZG00000000).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307862110/-/DCSupplemental.
References
- 1.Aaron SD, et al. Infection with transmissible strains of Pseudomonas aeruginosa and clinical outcomes in adults with cystic fibrosis. JAMA. 2010;304(19):2145–2153. doi: 10.1001/jama.2010.1665. [DOI] [PubMed] [Google Scholar]
- 2.LiPuma JJ. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev. 2010;23(2):299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser AR, Jain M, Bar-Meir M, McColley SA. Clinical significance of microbial infection and adaptation in cystic fibrosis. Clin Microbiol Rev. 2011;24(1):29–70. doi: 10.1128/CMR.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folkesson A, et al. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: An evolutionary perspective. Nat Rev Microbiol. 2012;10(12):841–851. doi: 10.1038/nrmicro2907. [DOI] [PubMed] [Google Scholar]
- 5.Sriramulu DD, Lünsdorf H, Lam JS, Römling U. Microcolony formation: A novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J Med Microbiol. 2005;54(Pt 7):667–676. doi: 10.1099/jmm.0.45969-0. [DOI] [PubMed] [Google Scholar]
- 6.Smith EE, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA. 2006;103(22):8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poole K. Efflux-mediated antimicrobial resistance. J Antimicrob Chemother. 2005;56(1):20–51. doi: 10.1093/jac/dki171. [DOI] [PubMed] [Google Scholar]
- 8.Cramer N, et al. Microevolution of the major common Pseudomonas aeruginosa clones C and PA14 in cystic fibrosis lungs. Environ Microbiol. 2011;13(7):1690–1704. doi: 10.1111/j.1462-2920.2011.02483.x. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, et al. Evolutionary dynamics of bacteria in a human host environment. Proc Natl Acad Sci USA. 2011;108(18):7481–7486. doi: 10.1073/pnas.1018249108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mowat E, et al. Pseudomonas aeruginosa population diversity and turnover in cystic fibrosis chronic infections. Am J Respir Crit Care Med. 2011;183(12):1674–1679. doi: 10.1164/rccm.201009-1430OC. [DOI] [PubMed] [Google Scholar]
- 11.Al-Aloul M, et al. Increased morbidity associated with chronic infection by an epidemic Pseudomonas aeruginosa strain in CF patients. Thorax. 2004;59(4):334–336. doi: 10.1136/thx.2003.014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirnay JP, et al. Pseudomonas aeruginosa population structure revisited. PLoS ONE. 2009;4(11):e7740. doi: 10.1371/journal.pone.0007740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takase H, Nitanai H, Hoshino K, Otani T. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect Immun. 2000;68(4):1834–1839. doi: 10.1128/iai.68.4.1834-1839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith EE, Sims EH, Spencer DH, Kaul R, Olson MV. Evidence for diversifying selection at the pyoverdine locus of Pseudomonas aeruginosa. J Bacteriol. 2005;187(6):2138–2147. doi: 10.1128/JB.187.6.2138-2147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raymond CK, et al. Genetic variation at the O-antigen biosynthetic locus in Pseudomonas aeruginosa. J Bacteriol. 2002;184(13):3614–3622. doi: 10.1128/JB.184.13.3614-3622.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jolley KA, Maiden MCJ. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marttinen P, et al. Detection of recombination events in bacterial genomes from large population samples. Nucleic Acids Res. 2012;40(1):e6. doi: 10.1093/nar/gkr928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dettman JR, et al. Evolutionary insight from whole-genome sequencing of experimentally evolved microbes. Mol Ecol. 2012;21(9):2058–2077. doi: 10.1111/j.1365-294X.2012.05484.x. [DOI] [PubMed] [Google Scholar]
- 19.Winstanley C, et al. Newly introduced genomic prophage islands are critical determinants of in vivo competitiveness in the Liverpool Epidemic Strain of Pseudomonas aeruginosa. Genome Res. 2009;19(1):12–23. doi: 10.1101/gr.086082.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat Rev Microbiol. 2009;7(9):654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmiel DH, Miller VL. Bacterial phospholipases and pathogenesis. Microbes Infect. 1999;1(13):1103–1112. doi: 10.1016/s1286-4579(99)00205-1. [DOI] [PubMed] [Google Scholar]
- 22.Beaudoin T, Zhang L, Hinz AJ, Parr CJ, Mah TF. The biofilm-specific antibiotic resistance gene ndvB is important for expression of ethanol oxidation genes in Pseudomonas aeruginosa biofilms. J Bacteriol. 2012;194(12):3128–3136. doi: 10.1128/JB.06178-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson RW, Vinatzer B, Arnold DL, Dorus S, Murillo J. The influence of the accessory genome on bacterial pathogen evolution. Mobile Genet Elements. 2011;1(1):55–65. doi: 10.4161/mge.1.1.16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 25.Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol (1985) 1993;75(6):2419–2424. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- 26.Schobert M, Jahn D. Anaerobic physiology of Pseudomonas aeruginosa in the cystic fibrosis lung. Int J Med Microbiol. 2010;300(8):549–556. doi: 10.1016/j.ijmm.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130(5):797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Imlay JA. Cell death from antibiotics without the involvement of reactive oxygen species. Science. 2013;339(6124):1210–1213. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gusarov I, Shatalin K, Starodubtseva M, Nudler E. Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science. 2009;325(5946):1380–1384. doi: 10.1126/science.1175439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCallum SJ, et al. Superinfection with a transmissible strain of Pseudomonas aeruginosa in adults with cystic fibrosis chronically colonised by P aeruginosa. Lancet. 2001;358(9281):558–560. doi: 10.1016/s0140-6736(01)05715-4. [DOI] [PubMed] [Google Scholar]
- 31.Salunkhe P, et al. A cystic fibrosis epidemic strain of Pseudomonas aeruginosa displays enhanced virulence and antimicrobial resistance. J Bacteriol. 2005;187(14):4908–4920. doi: 10.1128/JB.187.14.4908-4920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beaudoin T, Aaron SD, Giesbrecht-Lewis T, Vandemheen K, Mah TF. Characterization of clonal strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients in Ontario, Canada. Can J Microbiol. 2010;56(7):548–557. doi: 10.1139/w10-043. [DOI] [PubMed] [Google Scholar]
- 33.Carter MEK, et al. A subtype of a Pseudomonas aeruginosa cystic fibrosis epidemic strain exhibits enhanced virulence in a murine model of acute respiratory infection. J Infect Dis. 2010;202(6):935–942. doi: 10.1086/655781. [DOI] [PubMed] [Google Scholar]
- 34.Shafikhani SH, Engel J. Pseudomonas aeruginosa type III-secreted toxin ExoT inhibits host-cell division by targeting cytokinesis at multiple steps. Proc Natl Acad Sci USA. 2006;103(42):15605–15610. doi: 10.1073/pnas.0605949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoboth C, et al. Dynamics of adaptive microevolution of hypermutable Pseudomonas aeruginosa during chronic pulmonary infection in patients with cystic fibrosis. J Infect Dis. 2009;200(1):118–130. doi: 10.1086/599360. [DOI] [PubMed] [Google Scholar]
- 36.Palmer KL, Aye LM, Whiteley M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol. 2007;189(22):8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regunathan S, Piletz JE. Regulation of inducible nitric oxide synthase and agmatine synthesis in macrophages and astrocytes. Ann N Y Acad Sci. 2003;1009:20–29. doi: 10.1196/annals.1304.002. [DOI] [PubMed] [Google Scholar]
- 38.Grasemann H, et al. L-ornithine derived polyamines in cystic fibrosis airways. PLoS ONE. 2012;7(10):e46618. doi: 10.1371/journal.pone.0046618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saier MH, Jr, et al. Evolutionary origins of multidrug and drug-specific efflux pumps in bacteria. FASEB J. 1998;12(3):265–274. doi: 10.1096/fasebj.12.3.265. [DOI] [PubMed] [Google Scholar]
- 40.Armstrong DS, et al. Detection of a widespread clone of Pseudomonas aeruginosa in a pediatric cystic fibrosis clinic. Am J Respir Crit Care Med. 2002;166(7):983–987. doi: 10.1164/rccm.200204-269OC. [DOI] [PubMed] [Google Scholar]
- 41.Hill D, et al. Antibiotic susceptabilities of Pseudomonas aeruginosa isolates derived from patients with cystic fibrosis under aerobic, anaerobic, and biofilm conditions. J Clin Microbiol. 2005;43(10):5085–5090. doi: 10.1128/JCM.43.10.5085-5090.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H, et al. The Pseudomonas aeruginosa multidrug efflux regulator MexR uses an oxidation-sensing mechanism. Proc Natl Acad Sci USA. 2008;105(36):13586–13591. doi: 10.1073/pnas.0803391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lieberman TD, et al. Parallel bacterial evolution within multiple patients identifies candidate pathogenicity genes. Nat Genet. 2011;43(12):1275–1280. doi: 10.1038/ng.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.