Abstract
Seven cases of disseminated infection due to Dipodascus capitatus are reported. Infections occurred in a hematological unit of a tertiary hospital during a period of 5 years. Five cases were refractory to antifungal therapy. Antifungal susceptibility testing of seven isolates was performed, and strains were typed by PCR fingerprinting with the core sequence of phage M13 and by random amplification of polymorphic DNA with two primers, Ap12h and W-80A. A very short range of MICs of each antifungal agent was observed. The MICs of amphotericin B ranged between 0.50 and 2 μg/ml. Strains were susceptible in vitro to flucytosine and susceptible (dose-dependent) to fluconazole and itraconazole. Voriconazole exhibited an activity in vitro comparable to that of itraconazole. Typing techniques allowed seven additional isolates of D. capitatus neither geographically nor temporally related to be classified into two different genomic patterns. The genomic type of the seven strains from the hematological unit was identical regardless of typing technique utilized. It would indicate that the seven cases of disseminated infection could be related epidemiologically.
CASE REPORTS
Case 1.
The patientin case 1 was a 55-year-old female with an acute lymphoblastic leukemia, pre-B type, experiencing her second relapse. During a severe neutropenia episode, she became febrile and lethargic. Computed tomography and a cytochemical study of cerebrospinal fluid (CSF) showed no pathological findings. The patient worsened and died in spite of empirical antibacterial treatment along with conventional amphotericin B (AMB) treatment. Cultures of CSF yielded Dipodascus capitatus growth. The postmortem examination revealed mycotic nodules disseminated in all organs, including central nervous system.
Case 2.
The patient in case 2 was a 76-year-old male with a relapse of an acute myeloblastic leukemia. Severe neutropenia developed, the patient became febrile, and blood cultures were obtained. Fever persisted in spite of empirical antibiotic treatment with ceftazidime, amikacin, and vancomycin. Three days after antibacterial treatment, skin nodules appeared and a chest radiograph revealed new bilateral lung infiltrates. Conventional AMB, 1 mg/kg of body weight/day, was added to his treatment regimen. The patient remained febrile and died 5 days later. D. capitatus grew on all blood cultures.
Case 3.
The patient in case 3 was a 42-year-old male with a relapse of an acute myeloblastic leukemia, type M2, who had been diagnosed 5 years before. Eight days after the chemotherapy was finished, he became febrile and neutropenic. Blood cultures were taken and yielded Escherichia coli and viridans group Streptococcus that responded to treatment with meropenem plus vancomycin. Three days later his fever reappeared, and it was accompanied by tachypnea, diarrhea, and stupor. Blood cultures were drawn, chest radiography revealed new bilateral infiltrates, and conventional AMB was added to his treatment regimen. The patient died of septic shock. Blood cultures yielded D. capitatus.
Case 4.
The patient in case 4 was a 44-year-old female with a B prolymphocytic leukemia with disease progression in spite of fludarabine and CHOP (cyclophosphamide, hydroxydaunomycin, vincristine [oncovin], and prednisone) treatment. Pentostatin-prednisone was administered but caused severe hematological toxicity. The patient developed pancytopenia, fever, and lung consolidation, and empirical antibacterial treatment and conventional AMB, 1 mg/kg/day, were administered in combination with granulocyte-macrophage colony-stimulating factor. The patient died of septic shock. D. capitatus grew from respiratory samples and blood cultures.
Case 5.
The patient in case 5 was a 76-year-old female who was diagnosed with acute myeloblastic leukemia. During the administration of chemotherapy, the patient became febrile and responded to treatment with piperacillin-tazobactam plus amikacin. Leptotrichia buccalis was isolated from blood cultures. While she was receiving the antibacterial agents, she became febrile again and a lung consolidation was seen upon chest radiography. Other blood cultures were performed, vancomycin was added to her treatment regimen with no response, and the patient was asked to join a double-blind clinical trial for febrile neutropenia of suspected fungal etiology. The clinical trial compared treatments with caspofungin and liposomal AMB. The patient accepted and was included in the clinical trial. The patient seemed to improve the first 2 days but suddenly worsened, showing jaundice, tachypnea, and pleural effusions. Fungal growth from blood cultures was observed. The antifungal (yet-unknown) dosage was increased. Two days later, the fungus was identified as D. capitatus, the patient was removed from the clinical trial, and treatment with liposomal AMB at 3 mg/kg/day was started. Twenty-four hours later, the patient died with severe respiratory insufficiency.
Case 6.
The patient in case 6 was a 59-year-old female with acute myeloblastic leukemia secondary to chronic lymphocytic leukemia. Induction therapy caused severe febrile neutropenia. Blood cultures were drawn and produced no growth, but she responded to empirical antibiotic therapy. At day 18 of neutropenia, fever reappeared and two other blood cultures were taken, both of which yielded D. capitatus growth. The patient's central catheter was removed, and conventional AMB treatment was started. The patient's leukocyte counts recovered soon after, and she eventually recovered completely from fungal infection and reached complete bone marrow remission of the leukemia. Secondary prophylaxis with the polyene was administered for the other chemotherapy cycles, and D. capitatus did not reappear. The patient is still alive (5 years later).
Case 7.
The patient in case 7 was a 75-year-old female who was diagnosed with acute leukemia, type M5. After the first cycle of chemotherapy, the patient was severally neutropenic. On the fifth day of neutropenia the patient became febrile and tachypneic. Chest radiography showed opacity of the whole left lung with pleural effusion. Blood cultures were drawn; conventional antibacterial (meropenem plus amikacin plus vancomycin) treatment was started but without any improvement, so liposomal AMB was added. Twenty-four hours after amphotericin was started, blood cultures became positive, yielding fungus growth. The fungus was described at the preliminary as possibly having arthroconidia, so oral flucytosine (5FC) was added and the patient's Hickman catheter was removed. The patient began to improve slowly. Two weeks later, respiratory function was completely recovered, and chest X-rays showed a great improvement. The patient recovered from this episode, but she died later on because of leukemia progression.
Identification of isolates and susceptibility testing.
The clinical strains were subcultured on 4% malt extract-0.5% yeast extract agar (MEYA). After 10 days at 30°C, colonies were whitish, glassy, and funiculose with a smooth expanding zone. Microscopic examination revealed hyphae and conidiophores creeping or ascending, profusely branched at acute angles with conidiogenous cells which formed long, cicatrized rachids on which the conidia were borne. Conidia were cylindrical to clavate, with a rounded apex and flat base. Arthroconidia and endoconidia were often also present. The isolates were identified by routine physiological tests including the lack of urease and lack of growth on a medium containing d-xylose as a carbon source (27).
The isolates were sent to the Mycology Reference Laboratory of National Center for Microbiology of Spain for susceptibility testing. Strains were labeled as Table 1 shows. The susceptibility testing followed strictly the National Committee for Clinical Laboratory Standards (NCCLS) recommendations for microdilution procedure but included minor modifications described previously (17, 23). Briefly, the susceptibility testing included RPMI supplemented with 2% glucose as assay medium, inoculum size of 105 CFU/ml, flat-bottom trays, and spectrophotometric reading. All microplates were wrapped with a film sealer to prevent the medium from evaporating, attached to an electrically driven wheel inside the incubator, agitated at 350 rpm, and incubated at 30°C for 48 h. Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were used as quality control strains.
TABLE 1.
Susceptibility testing results of seven D. capitatus isolates causing disseminated infection in a hematological unit of a tertiary hospital
| Case no. | Strain | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|---|
| AMB | SFC | FLC | ITC | VRC | ||
| 1 | CNM-CL-3914 | 0.50 | 0.50 | 16.0 | 0.25 | 0.50 |
| 2 | CNM-CL-3924 | 0.50 | 0.25 | 32.0 | 0.25 | 0.50 |
| 3 | CNM-CL-3922 | 1.0 | 0.25 | 32.0 | 0.25 | 0.25 |
| 4 | CNM-CL-3913 | 2.0 | 0.25 | 32.0 | 0.50 | 0.25 |
| 5 | CNM-CL-3923 | 1.0 | 0.25 | 32.0 | 0.25 | 0.25 |
| 6 | CNM-CL-3943 | 1.0 | 0.25 | 32.0 | 0.25 | 0.50 |
| 7 | CNM-CL-3945 | 1.0 | 0.25 | 32.0 | 0.12 | 0.25 |
The antifungal agents used in the study were as follows: AMB (Sigma-Aldrich Química S.A., Madrid, Spain), 5FC (Sigma-Aldrich Química), fluconazole (FLC) (Pfizer S.A, Madrid, Spain), itraconazole (ITC) (Janssen S.A., Madrid, Spain), and voriconazole (VRC) (Pfizer S.A.); AMB, FLC, VRC, and ITC were dissolved in 100% dimethyl sulfoxide (Sigma-Aldrich Química). The MICs were determined at 24 and 48 h. MICs were obtained by measuring the absorbance at 530 nm with an MRXII reader (Dynatech, Cultek, Madrid, Spain). For AMB the MIC endpoints were defined as the lowest drug concentration exhibiting reduction in growth of 90% or more compared with that of the control growth. For 5FC and azole drugs the MIC endpoint was defined as the lowest drug concentration exhibiting a reduction in growth of 50% (10).
Molecular typing studies.
A single colony of each strain was used to inoculate 25 ml of yeast extract-peptone-dextrose (Oxoid Unipath, Madrid, Spain). The fungal mass was blot-dried, frozen with liquid nitrogen, and then ground to powder using a pestle and mortar. After RNase treatment, the samples were treated with proteinase K (Sigma-Aldrich Química), and the DNA was purified again by extraction with phenol-chloroform and ethanol precipitation.
Two techniques were used for molecular typing, the random amplification of polymorphic DNA (RAPD) method and PCR fingerprinting. Two RAPD primers were selected for genotyping, AP12h (5′-CGGCCCCTGT-3′) and W-80A (5′-TGACCCCGGC-3′). These primers had a G+C content ranging from 50 to 80% and did not contain palindromic sequences. The PCR fingerprinting technique was performed with the core sequence of phage M13 (5′-GAGGGTGGCGGTTCT-3′) as a single primer. The reaction mixtures included 10 mM Tris HCl (pH 8)-50 mM KCl as buffer; a 200 mM concentration (each) of dATP, dTTP, dCTP, and dGTP; 2.5 mM MgCl2 (3.5 mM in the case of PCR fingerprinting); 1 μM primers; 2.5 U of Taq polymerase (AmpliTaq; Applied Biosystems, Madrid, Spain); and 50 ng of template DNA in a final volume of 50 μl. The thermal cycler (GeneAmp PCR System 2700, Applied Biosystems) was set at two different programs: (i) 40 cycles of 1 min of denaturation at 95°C, 1 min of annealing at 36°C, and 2 min of primer extension at 72°C in the case of RAPD analysis; and (ii) 30 cycles of 20 s at 95°C, 2 min at 50°C, and 20 s at 72°C with a final extension of 6 min at 72°C for PCR fingerprinting. A control with all the reagents except DNA was included in each set of experiments. Typing techniques were performed a least two times on separate days. Band patterns were electrophoresed through 1.2% agarose gels (Pronadisa, Madrid, Spain), stained with ethidium bromide (Sigma Aldrich Química), and photographed under UV light. PCR profiles were analyzed visually and letters or numbers indexed them, and even a single mismatch led to a different letter or number code.
Seven other clinical isolates of D. capitatus were used as control organisms in molecular typing studies. All strains were recovered during a period of 8 years (1995 to 2002) from six different Spanish hospitals. Each clinical isolate represented a unique isolate from a patient.
Susceptibility testing results.
The susceptibility results for D. capitatus strains are displayed in Table 1. A very short range of MICs of each antifungal agent was observed. The MICs of AMB ranged between 0.50 and 2 μg/ml. For three strains the MIC of AMB was 1 μg/ml, and for one strain the MIC of AMB was 2 μg/ml. Patients in cases 6 and 7 responded to treatment with AMB, but it should be noted that no significant differences in MICs of AMB were seen between strains causing infections that were refractory to antifungal treatment and isolates that responded to therapy. According to document M27-A2 of the NCCLS (17), the seven strains were susceptible in vitro to 5FC, exhibiting MICs between 0.25 and 0.50 μg/ml. However, FLZ and ITC MICs for each isolate were placed in the susceptible-dose-dependent category of the NCCLS. With regard to VRC, this agent exhibited an activity in vitro comparable with that of ITC, with MICs that ranged between 0.25 and 0.50 μg/ml.
The MICs of the five antifungal agents for the two quality control organisms were consistent within two or three twofold dilutions. No differences were observed between MICs obtained by the NCCLS reference procedure and those achieved by the modified method used in this study (17).
Molecular typing results.
The molecular typing results are shown in Table 2. The seven isolates of D. capitatus were studied by three different genomic DNA analyses. In addition, the genomic profiles were compared with those of seven other D. capitatus isolates that acted as control organisms. These control isolates were not geographically or temporally related.
TABLE 2.
Molecular typing patterns of various D. capitatus isolatesa
| Strain | Specimen or isolation siteb | Pattern observed
|
Typing code | ||
|---|---|---|---|---|---|
| PCR fingerprinting M13 | RAPD Ap12h | RAPD W-80A | |||
| Control | |||||
| CNM-CL-3441 | OPE | I | A | a | I.A.a |
| CNM-CL-3500 | BAL fluid | II | B | b | II.B.b |
| CNM-CL-2026 | BAL fluid | I | A | a | I.A.a |
| CNM-CL-4176 | Blood | II | B | b | II.B.b |
| CNM-CL-3503 | Sputum | I | A | a | I.A.a |
| CNM-CL-3512 | Blood | II | B | b | II.B.b |
| CNM-CL-4622 | Skin | II | B | b | II.B.b |
| Study | |||||
| CNM-CL-3914 | CSF | II | B | b | II.B.b |
| CNM-CL-3924 | Blood | II | B | b | II.B.b |
| CNM-CL-3922 | Blood | II | B | b | II.B.b |
| CNM-CL-3913 | Blood | II | B | b | II.B.b |
| CNM-CL-3923 | Blood | II | B | b | II.B.b |
| CNM-CL-3943 | Blood | II | B | b | II.B.b |
| CNM-CL-3945 | Blood | II | B | b | II.B.b |
Seven D. capitatus isolates causing disseminated infection in a hematological unit of a tertiary hospital (study strains) and seven other control strains. Genomic profiles are indexed by letters or numbers.
Abbreviations: OPE, oropharyngeal exudate; BAL, bronchoalveolar lavage.
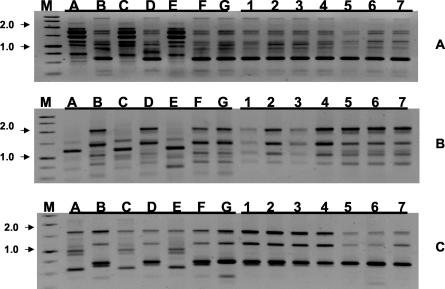
Patterns obtained with primer M13 are shown in Fig. 1. The discriminatory power was comparable for each of the three primers. The three primers yielded only two different patterns of bands. The same degree of discrimination was observed by combining the results obtained for all three primers. Strains belonging to the type I by PCR fingerprinting exhibited the type A by RAPD analysis Ap12h and the type a when primer W-80A was used. Strains grouped in type II with primer M13 belonged to type B by the Ap12h technique and type b by the W-80A method. Following that, the isolates from distinct geographic sites were grouped in two different genomic types. The profiles were reproducible between different DNA preparations from the same strain as well as between runs when samples were run a second or a third time. With regard to the seven strains that were isolated from different patients in a hematological unit of a tertiary hospital during a period of 5 years, the genomic patterns were identical regardless of the typing technique utilized. It would indicate that the seven cases of disseminated infection caused by D. capitatus could be related epidemiologically.
FIG. 1.
(A) PCR fingerprinting profiles obtained with primer M13. 1. (B) RAPD profiles obtained with primer Ap12h. (C) RAPD profiles obtained with primer W-80a. Lane M, molecular marker (sizes in kilobases) (1-kb ladder; Pharmacia, Madrid, Spain); lanes A to G, control strains according to Table 2; lanes 1 to 7, isolates causing disseminated infection in a hematological unit of a tertiary hospital according to Table 2.
Discussion.
D. capitatus de Hoog et al., 1986, is the holomorph as well as the sexual form (teleomorph) of Geotrichum capitatum [Trichosporon capitatum Diddens et Lodder, 1942; Blastoschizomyces capitatus (Didd. et Lodd.) Salkin et al., 1985] von Arx, 1977. This species belonging to the Ascomycota division has been isolated from environmental sources such as woods and poultry feces and is widely distributed in nature as a soil saprobe. In addition, D. capitatus is part of the normal flora of human skin and is frequently isolated from sputum and the digestive tract of healthy people. As with other opportunistic yeasts, the number of infections due to this species has increased during the past 2 decades, as a consequence of the rise of hosts presenting factors which predispose them to fungal infections such as cytotoxic chemotherapy, neutropenia, broad-spectrum antibiotic treatment, steroids, and invasive catheterization (5, 13, 14, 16, 18, 20-22, 25, 26).
D. capitatus has been involved in urinary tract infections (12), meningitis, osteomyelitis, endocarditis and disseminated infections (14, 16, 18). Systemic infection caused by D. capitatus resembles candidiasis in several major manifestations, but is usually fatal in neutropenic patients, despite the administration of systemic antifungal therapy (14). The digestive and respiratory tracts act as a portal of entry in some cases of deep infection, but contamination of venous catheter or prosthetic valves has been also reported (3, 22). It has been suggested that a number of cases have a nosocomial origin, emanating from a common source within the hospital environment (2) and exhibiting identical genomic DNA restriction profiles. In addition resistance to azole agents in vitro has been reported (8), and antifungal treatment with azole compounds at a low dosage has been described as a predisposing factor for systemic infections due to D. capitatus (14).
We report seven patients with D. capitatus deep infection that occurred in a hematological unit of a tertiary hospital during a period of 5 years. Five of them were refractory to antifungal treatment. In order to study the nature of antifungal refractivity and the clonality of such isolates, we studied the in vitro antifungal susceptibilities and we also performed molecular typing studies. All patients included in the study were afflicted with neutropenia caused by aggressive chemotherapy for hematological malignances and had a central catheter. None of them had received antifungal prophylaxis.
Our seven D. capitatus isolates showed quite homogeneous antifungal susceptibility results. Differences in AMB MICs were not clinically significant, so that the patient that carried the isolate for which the MIC was the highest, 2 μg/ml, responded to the treatment, but patients with strains for which the MICs were lower did not respond to AMB treatment. Although none of the patients had received antifungal prophylaxis before the clinical episode, MICs of FLC were quite high, from 16 to 32 μg/ml, placing them in the NCCLS category susceptible-dose-dependent. This fact confirms previous data suggesting that FLC does not have a strong activity against D. capitatus and that previous drug exposure is not necessary for the acquisition of a D. capitatus strain with some level of FLC resistance (1, 2).
There are not enough clinical data to assess the best treatment for D. capitatus in neutropenic patients. Although most of the MICs of AMB reported for D. capitatus isolates were low, there are different reports which suggest that this species could have reduced susceptibility to AMB and they would respond better to ITC (3). Unsuccessfully treatment with a high dose of liposomal AMB (7 mg/kg) in spite of susceptibility in vitro has been reported as well (5). But there are also reports that suggest that D. capitatus infections responded better with AMB regimens than with azole regimens (13). VRC exhibits an activity in vitro comparable to ITC, as had been reported earlier (9), and perhaps it may be useful in the treatment of such infection, but unfortunately, there are no clinical reports. Finally, all our D. capitatus isolates and perhaps all D. capitatus reported previously were susceptible in vitro to 5FC. It could be a good course of treatment as part of a combination antifungal therapy, in spite of the fact that it has a known bone marrow toxicity that makes this antifungal very poorly attractive for hematologists who are treating a neutropenic patient. Our seventh patient received 5FC combined with liposomal AMB, and the patient was cured though her neutrophil level did not recover during the fungal infection.
It could be argued that other factors along with antifungal therapy are involved in the outcome and severity of the D. capitatus infection episode. A central venous catheter may be colonized in the course of the fungemia, but also it may be the initial source of the fungal infection; the latter could be the situation of our sixth patient, and the removal of the catheter was an important complementary treatment (11). In addition, the course of neutropenia is another important fact in the outcome of D. capitatus-infected patients; for this reason, other adjuvant therapies to improve the phagocytic activity, colony stimulating factors, interferons, etc., have been combined with antifungal drugs with some success (5, 19).
Another point to consider is the genotyping of the isolates. The two methods used for molecular typing of the seven D. capitatus isolates have been demonstrated to be useful in other fungal infections (6, 7, 15, 24). All seven strains, isolated during a period of 5 years in our hematology unit, were exactly identical with both typing methods. Unfortunately, both methods only discriminated among two molecular types for the control organisms. Previous typing of D. capitatus isolates by molecular methods showed that all the strains were clonally related (2) or showed two different patterns (4). This scenario may represent that D. capitatus is a more genetically homogeneous fungus or perhaps that the molecular methods applied are not discriminative enough. Whatever the case, the seven cases of disseminated infection could be related epidemiologically, but otherwise, infections occurred during a period of 5 years, and D. capitatus was not isolated from a common source within the hospital environment. Results obtained using these genotyping methods and this species of fungus should be interpreted cautiously and additional primer combinations may increase sensitivity of typing procedures.
REFERENCES
- 1.Buchta, V., P. Zak, A. Kohout, and M. Otcenasek. 2001. Case report. Disseminated infection of Blastoschizomyces capitatus in a patient with acute myelocytic leukaemia. Mycoses 44:505-512. [DOI] [PubMed]
- 2.D'Antonio, D., A. Mazzoni, A. Iacone, B. Violante, M. A. Capuani, F. Schioppa, and F. Romano. 1996. Emergence of fluconazole-resistant strains of Blastoschizomyces capitatus causing nosocomial infections in cancer patients. J. Clin. Microbiol. 34:753-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Antonio, D., R. Piccolomini, G. Fioritoni, A. Iacone, S. Betti, P. Fazii, and A. Mazzoni. 1994. Osteomyelitis and intervertebral discitis caused by Blastoschizomyces capitatus in a patient with acute leukemia. J. Clin. Microbiol. 32:224-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Antonio, D., F. Romano, A. Iacone, B. Violante, P. Fazii, E. Pontieri, T. Staniscia, C. Caracciolo, S. Bianchini, R. Sferra, A. Vetuschi, E. Gaudio, and G. Carruba. 1999. Onychomycosis caused by Blastoschizomyces capitatus. J. Clin. Microbiol. 37:2927-2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeMaio, J., and L. Colman. 2000. The use of adjuvant interferon-gamma therapy for hepatosplenic Blastoschizomyces capitatus infection in a patient with leukemia. Clin. Infect. Dis. 31:822-824. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Guerra, T., J. Martinez-Suarez, F. Laguna, and J. Rodriguez-Tudela. 1997. Comparison of four molecular typing methods for evaluating genetic diversity among Candida albicans isolates from human immunodeficiency virus-positive patients with oral candidiasis. J. Clin. Microbiol. 35:856-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enger, L., S. Joly, C. Pujol, P. Simonson, M. Pfaller, and D. R. Soll. 2001. Cloning and characterization of a complex DNA fingerprinting probe for Candida parapsilosis. J. Clin. Microbiol. 39:658-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff, A., L. Stockman, G. Roberts, D. Pincus, J. Pollack, and J. Marler. 1998. Comparison of RapID yeast plus system with API 20C system for identification of common, new, and emerging yeast pathogens. J. Clin. Microbiol. 36:883-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinel-Ingroff, A. 1998. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J. Clin. Microbiol. 36:198-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghannoum, M. A., A. S. Ibrahim, Y. Fu, M. C. Shafiq, J. E. Edwards, and R. S. Criddle. 1992. Susceptibility testing of Cryptococcus neoformans: a microdilution technique. J. Clin. Microbiol. 30:2881-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hachem, R., and I. Raad. 2002. Prevention and management of long-term catheter related infections in cancer patients. Cancer Investig. 20:1105-1113. [DOI] [PubMed] [Google Scholar]
- 12.Krcmery, S., M. Dubrava, and V. Krcmery. 1999. Fungal urinary tract infections in patients at risk. International J. Antimicrob. Agents 11:289-291. [DOI] [PubMed] [Google Scholar]
- 13.Krcmery, V., I. Krupova, and D. W. Denning. 1999. Invasive yeast infections other than Candida spp. in acute leukaemia. J. Hosp. Infect. 41:181-194. [DOI] [PubMed] [Google Scholar]
- 14.Martino, P., M. Venditti, A. Micozzi, G. Morace, L. Polonelli, M. P. Mantovani, M. C. Petti, V. L. Burgio, C. Santini, and P. Serra. 1990. Blastoschizomyces capitatus: an emerging cause of invasive fungal disease in leukemia patients. Rev. Infect. Dis. 12:570-582. [DOI] [PubMed] [Google Scholar]
- 15.Mellado, E., T. M. Diaz-Guerra, M. Cuenca-Estrella, V. Buendia, J. Aspa, E. Prieto, J. R. Villagrasa, and J. L. Rodriguez-Tudela. 2000. Characterization of a possible nosocomial aspergillosis outbreak. Clin. Microbiol. Infect. 6:543-548. [DOI] [PubMed] [Google Scholar]
- 16.Naficy, A. B., and H. W. Murray. 1990. Isolated meningitis caused by Blastoschizomyces capitatus. J. Infect. Dis. 161:1041-1042. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NCCLS document M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Ortiz, A. M., C. Sanz-Rodriguez, J. Culebras, B. Buendia, I. Gonzalez-Alvaro, E. Ocon, and R. de la Camara. 1998. Multiple spondylodiscitis caused by Blastoschizomyces capitatus in an allogeneic bone marrow transplantation recipient. J. Rheumatol. 25:2276-2278. [PubMed] [Google Scholar]
- 19.Pagano, L., G. Morace, E. Ortu-La Barbera, M. Sanguinetti, and G. Leone. 1996. Adjuvant therapy with rhGM-CSF for the treatment of Blastoschizomyces capitatus systemic infection in a patient with acute myeloid leukemia. Ann. Hematol. 73:33-34. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Sanchez, I., J. Anguita, P. Martin-Rabadan, P. Munoz, D. Serrano, A. Escudero, and T. Pintado. 2000. Blastoschizomyces capitatus infection in acute leukemia patients. Leukemia Lymphoma 39:209-212. [DOI] [PubMed] [Google Scholar]
- 21.Perfect, J. R., and W. A. Schell. 1996. The new fungal opportunists are coming. Clin. Infect. Dis. 22(Suppl. 2):S112-S118. [DOI] [PubMed] [Google Scholar]
- 22.Polacheck, I., I. F. Salkin, R. Kitzes-Cohen, and R. Raz. 1992. Endocarditis caused by Blastoschizomyces capitatus and taxonomic review of the genus. J. Clin. Microbiol. 30:2318-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez-Tudela, J. L., F. Martín-Díez, M. Cuenca-Estrella, L. Rodero, Y. Carpintero, and B. Gorgojo. 2000. Influence of shaking on antifungal susceptibility testing of Cryptococcus neoformans: a comparison of the NCCLS standard M27A medium, buffered yeast nitrogen base, and RPMI-2% glucose. Antimicrob. Agents Chemother. 44:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz-Diez, B., V. Martinez, M. Alvarez, J. Rodriguez-Tudela, and J. Martinez-Suarez. 1997. Molecular tracking of Candida albicans in a neonatal intensive care unit: long-term colonizations versus catheter-related infections. J. Clin. Microbiol. 35:3032-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryder, N. S. 1999. Activity of terbinafine against serious fungal pathogens. Mycoses 42(Suppl. 2):115-119. [PubMed] [Google Scholar]
- 26.Sanz, M. A., F. Lopez, M. L. Martinez, G. F. Sanz, J. A. Martinez, G. Martin, and M. Gobernado. 1996. Disseminated Blastoschizomyces capitatus infection in acute myeloblastic leukaemia. Report of three cases. Supp. Care Cancer 4:291-293. [DOI] [PubMed] [Google Scholar]
- 27.Yarrow, D. 1998. Methods for the isolation, maintenance and identification of yeasts. Elsevier Science, Amsterdam, The Netherlands.