Significance
Induction of tolerance to self-antigens in developing T cells depends on medullary thymic epithelial cells (mTECs), whose development, in turn, requires signals from mature single-positive (SP) thymocytes, a bidirectional interdependence termed cross-talk. We have probed the mechanism that underlies this requirement for cross-talk. Strikingly, selective deletion in thymic epithelial cells of TNF receptor-associated factor 3 (TRAF3), an inhibitor of nonclassical NF-kB signaling, resulted in development of normal numbers of fully mature mTECs in the complete absence of SP thymocytes. Thus, mTEC development can, in fact, occur in the absence of cross-talk with SP thymocytes, and the role of thymocytes is in overcoming TRAF3-imposed inhibition. We conclude that TRAF3 plays a central role in regulation of mTECs by imposing requirements for T cell cross-talk.
Abstract
Induction of self-tolerance in developing T cells depends on medullary thymic epithelial cells (mTECs), whose development, in turn, requires signals from single-positive (SP) thymocytes. Thus, the absence of SP thymocytes in Tcra−/− mice results in a profound deficiency in mTECs. Here, we have probed the mechanism that underlies this requirement for cross-talk with thymocytes in medullary development. Previous studies have implicated nonclassical NF-κB as a pathway important in the development of mTECs, because mice lacking RelB, NIK, or IKKα, critical components of this pathway, have an almost complete absence of mTECs, with resulting autoimmune pathology. We therefore assessed the effect of selective deletion in TEC of TNF receptor-associated factor 3 (TRAF3), an inhibitor of nonclassical NF-κB signaling. Deletion of TRAF3 in thymic epithelial cells allowed RelB-dependent development of normal numbers of AIRE-expressing mTECs in the complete absence of SP thymocytes. Thus, mTEC development can occur in the absence of cross-talk with SP thymocytes, and signals provided by SP T cells are needed to overcome TRAF3-imposed arrest in mTEC development mediated by inhibition of nonclassical NF-κB. We further observed that TRAF3 deletion is also capable of overcoming all requirements for LTβR and CD40, which are otherwise necessary for mTEC development, but is not sufficient to overcome the requirement for RANKL, indicating a role for RANKL that is distinct from the signals provided by SP thymocytes. We conclude that TRAF3 plays a central role in regulation of mTEC development by imposing requirements for SP T cells and costimulation-mediated cross-talk in generation of the medullary compartment.
A major role of the thymus is the generation of a functional T-cell repertoire that is broadly responsive to foreign antigens but is self-tolerant. Through their role in exposing developing thymocytes to a spectrum of self-antigens, the stromal cells of the thymus are integral to this tolerization. Of particular importance in this process are the epithelial cells comprising the thymic medulla, the region of the thymus where thymocytes selected into the CD4 and CD8 single-positive (SP) lineages reside before emigrating to the periphery (reviewed in refs. 1–3). The importance of thymic medullary epithelial cells (mTECs) in the maintenance of self-tolerance is illustrated by the destructive autoreactivity that results from disruption of mTEC development (reviewed in ref. 4).
Just as mTECs have a central role in shaping the developing T-cell repertoire, thymocytes, in turn, are vital to the development and maintenance of the mTEC compartment, a bidirectional interaction that has been termed cross-talk (5–7). A number of recent reports have characterized the CD4 SP thymocyte–stromal cell interactions that are critical for mTEC development (5–11). However, the mechanism that enforces the requirement for SP thymocytes in mTEC development has not been fully identified.
We therefore addressed the signaling requirements that mediate the cross-talk required for mTEC development. Previous studies have implicated nonclassical NF-κB as a pathway important in the development of mTECs. It has been shown that mice lacking RelB, NIK, or IKKα, components of the nonclassical NF-κB pathway, have an almost complete absence of mTECs and exhibit resulting autoimmune pathology (12–16). Engagement of TNF receptor (TNFR) family members including CD40, LTβR, and RANK has been shown to activate nonclassical NF-κB signaling via a pathway regulated by several members of the TRAF family of adaptor/ubiquitin ligase proteins. TRAF3 has a unique role in inhibiting nonclassical NF-κB signaling in resting cells (17), and it has been demonstrated that deletion of TRAF3 in B cells results in constitutive activation of the alternative NF-κB pathway (18, 19). We therefore tested the possibility that TRAF3-mediated inhibition of alternative NF-κB is responsible for the failure of mTEC development in the absence of signals from SP thymocytes. We made the striking observation that deletion of TRAF3 in thymic epithelium is sufficient to allow RelB-dependent mTEC development in the complete absence of TCRαβ SP thymocytes. TRAF3 deletion is also capable of overcoming all requirements for LTβR and CD40 during mTEC development, but is not sufficient to overcome the requirement for RANKL, indicating an essential role for RANKL that is distinct from the signals provided by SP thymocytes. Together, these results demonstrate that mTECs can develop in the complete absence of SP thymocytes and that TRAF3 plays a critical role in imposing the requirement for cross-talk with SP thymocytes, thus linking the appearance of mature thymocytes to the development of the thymic medullary environment necessary for imposing self-tolerance.
Results
TEC-Specific Deletion of Traf3 Overcomes All Requirements for Cross-Talk with SP Thymocytes in mTEC Development.
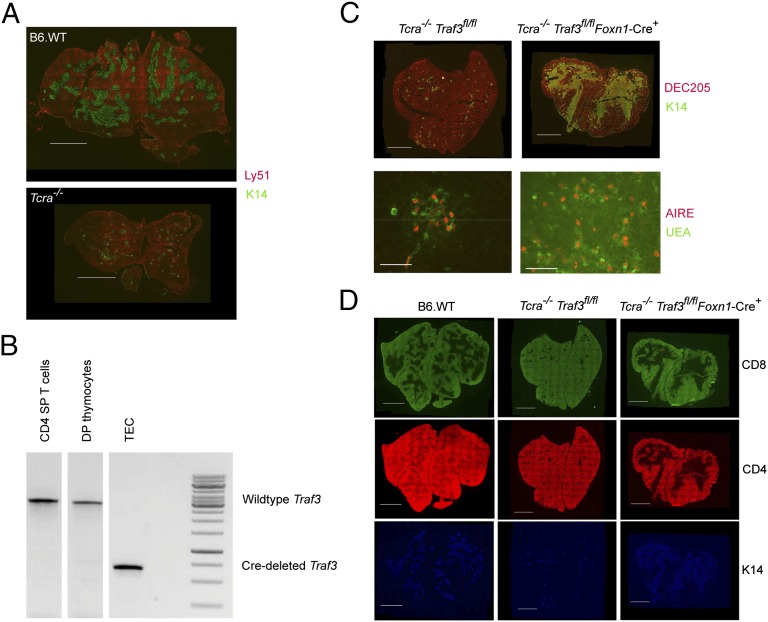
Previous work has documented the importance of cross-talk between SP thymocytes and epithelial cells in establishing normal thymic medullary structure (5, 7–9). This requirement is demonstrated in Fig. 1A, where absence of SP thymocytes in Tcra−/− mice results in a profound deficiency in formation of the thymic medulla as evidenced by the paucity of keratin 14-staining mTECs. However, the mechanisms mediating the requirement for this cross-talk have not been defined. The alternative NF-κB pathway is known to be critical to mTEC development as evidenced by the almost complete absence of mTECs in mice lacking components of this signaling pathway (12–16). Recently, the adaptor protein TRAF3 has been identified as a critical negative regulator of alternative NF-κB signaling, where it has been proposed to function in part by facilitating the targeting of NIK for proteasomal destruction (17). We hypothesized that the presence of TRAF3 in mTECs inhibits NF-κB signaling, preventing mTEC development, and ensuring that mTEC development depends on signals from SP thymocytes that release mTECs from TRAF3-mediated inhibition. To determine whether removal of TRAF3 would overcome the requirement for signals from SP thymocytes in supporting mTEC development, we generated mice in which Traf3 is deleted selectively in TEC by crossing Traf3fl/fl (19) mice to mice expressing TEC-specific FoxN1-Cre (20) (Fig. 1B). We then examined whether deletion of Traf3 in TEC could circumvent the need for all signals provided by SP thymocytes to promote a normal medullary region. We generated Traf3fl/fl Foxn1-Cre+ mice on a Tcra−/− background, in which T-cell development is arrested at the double positive (DP) stage and mature SP thymocytes are completely absent. Tcra−/− mice have extremely small mTEC regions and a marked decrease in mTECs as detected by immunohistology. Thymic sections from Tcra−/− Traf3fl/fl and Tcra−/− Traf3fl/fl Foxn1Cre+ mice were compared by staining with markers for mTECs (K14) and cortical TECs (cTECs) (DEC205) (Fig. 1C). Inactivation of Traf3 resulted in a striking change in the Tcra−/− Traf3fl/fl Foxn1Cre+ thymus, generating large and confluent medullary areas. Inactivation of Traf3 thus allows robust mTEC development in the complete absence of SP thymocytes. mTECs are a heterogeneous population consisting of UEA+MHCIIloCD80lo and UEA+MHCIIhiCD80hi cells. A subpopulation of the UEA+MHCIIhiCD80hi cells are AIRE+ and have a critical role in the induction of thymic tolerance (reviewed in ref. 21). Expression of AIRE was apparent in the reconstituted Tcra−/− Traf3fl/fl Foxn1Cre+ thymic medulla, indicating that mTEC development included generation of the AIRE+ mTEC subpopulation (Fig. 1C). These large mTEC regions present in the Tcra−/− Traf3fl/fl Foxn1Cre+ were devoid of thymocytes, indicating that, similar to wild-type medullary regions, DP thymocytes that have not received a TCR signal are unable to access the medulla (Fig. 1D).
Fig. 1.
TEC-specific deletion of Traf3 rescues mTEC development in Tcra−/− mice. (A) Thymic cryosections from B6 WT and Tcra−/− mice were stained with anti-Ly51 (red) and anti-K14 (green). (B) Deletion of conditional Traf3 allele is specific to thymic epithelial cells in Traf3fl/flFoxn1Cre+ mice. CD4+ splenocytes and thymocytes from Traf3fl/flFoxn1Cre+ mice were prepared by staining and sorting for CD4+ TCRβ+ and CD4+CD8+ cells, respectively. Thymic stromal cells were prepared as described in Materials and Methods and then stained with anti-CD45 and anti-EpCAM. After first gating on CD45neg cells, EpCAM+ epithelial cells were collected. Results are from PCR analysis using primers that distinguish between the intact Traf3 allele (∼900 bp) and the Traf3 allele that has undergone Cre-mediated recombination (∼350 bp) (19) (B and C) Thymic cryosections from B6 WT, Tcra−/− Traf3fl/fl, and Tcra−/− Traf3fl/fl Foxn1-Cre+ mice were stained with a combination of anti-keratin 14 (green) and anti-DEC205 (red) (C Upper); UEA (green) and AIRE (red) (C Lower); or anti-keratin 14 (blue), anti-CD8 (green), and anti-CD4 (red) (D), and imaged. [Scale bars: 2 mm for all figures with the exception of lower panels of C (AIRE and UEA staining) where bars are 0.2 mm.]
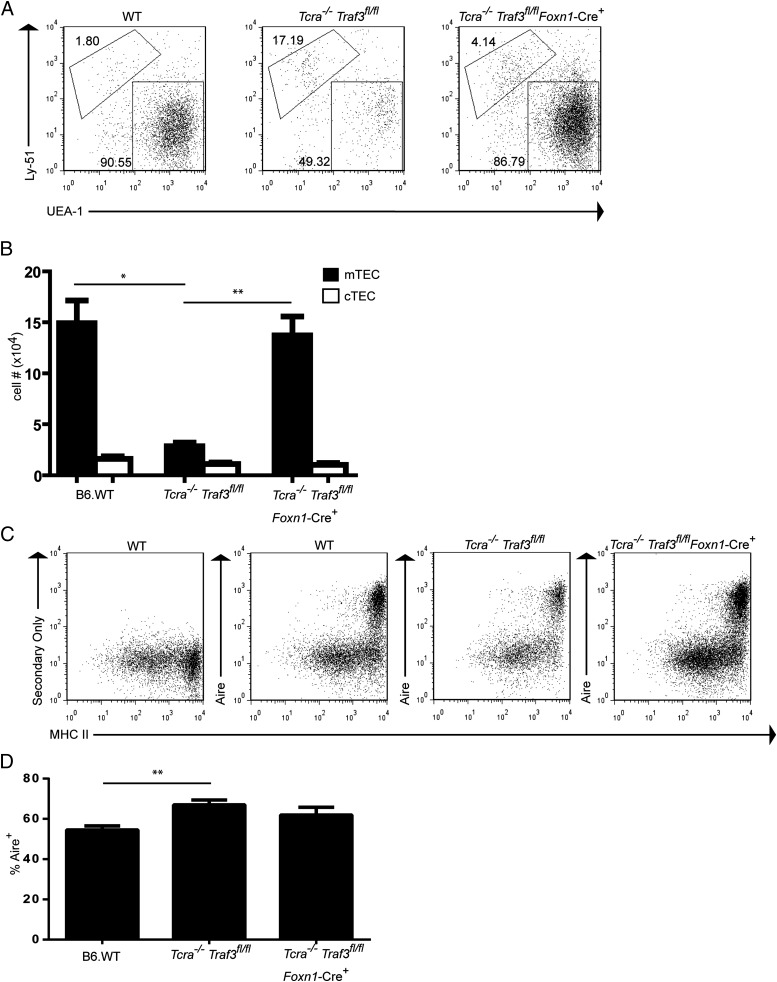
The impact of Traf3 deletion on TECs in Tcra−/− thymus was further assessed by flow cytometry, identifying thymic epithelial cells (CD45negMHCII+) as mTECs (UEA+) or cTEC (UEAnegLy51+). Flow cytometric examination of TEC populations in Tcra−/− Traf3fl/fl (Traf3 intact) and Tcra−/− Traf3fl/fl Foxn1Cre+ (Traf3 deleted) mice revealed that Traf3 deletion in TEC fully restored mTEC numbers in the Tcra−/− to wild-type levels, paralleling the immunohistochemical finding (Fig. 2 A and B). Expression of AIRE in the small number of mTECs in Tcra−/− Traf3fl/fl (Traf3 intact) thymus was similar to that found in WT thymus, indicating that AIRE expression can proceed in this residual mTEC population independent of cross-talk with SP thymocytes (Fig. 2 C and D), consistent with previous findings in RAG-deficient mice (22). Analysis of AIRE expression further demonstrated that the proportion of AIRE+ mTECs in Tcra−/− Traf3fl/fl Foxn1Cre+ thymus was equivalent to that in WT mice, indicating that Traf3 deletion restored this parameter of mTEC differentiation and restoring total mTEC numbers (Fig. 2 C and D). Inactivation of Traf3 thus overcomes the requirement for signals normally provided by SP thymocytes in mTEC development.
Fig. 2.
Comparable numbers of mature mTECs in B6 WT and Tcra−/− Traf3fl/fl Foxn1-Cre+ mice. (A and B) Thymic stromal cells from 3- to 5-wk-old mice were prepared as described in Materials and Methods and stained with anti-CD45, UEA-1, anti-Ly51, and anti-MHCII. (A) After first gating on CD45neg, MHCII+ cells, mTECs are identified as UEA+ and cTEC as UEAnegLy51+. Representative flow cytometry dot plots for each strain are shown. (B) After gating on CD45neg, MHCII+ cells, numbers of mTECs (UEA+) and cTEC (UEAneg, Ly51+) were calculated for each group. Data shown are mean ± SE for ≥3 mice per strain. (*P < 0.05; **P < 0.01). (C and D) Thymic stromal cells from B6 WT, Tcra−/− Traf3fl/fl, and Tcra−/− Traf3fl/fl Foxn1-Cre+ mice were prepared from 4- to 5-wk-old mice and stained with anti-CD45, anti-EpCAM, UEA-1, anti-MHCII, and anti-AIRE. After first gating on CD45neg, EpCAM+ cells, mTECs were identified as UEA+ and then examined for expression of MHCII and AIRE. (C) Representative flow cytometry dot plots for each strain are shown. (D) The percent AIRE+ cells in the MHCIIhi mTEC population for each strain are shown. Data shown are mean ± SE for ≥6 mice per strain.
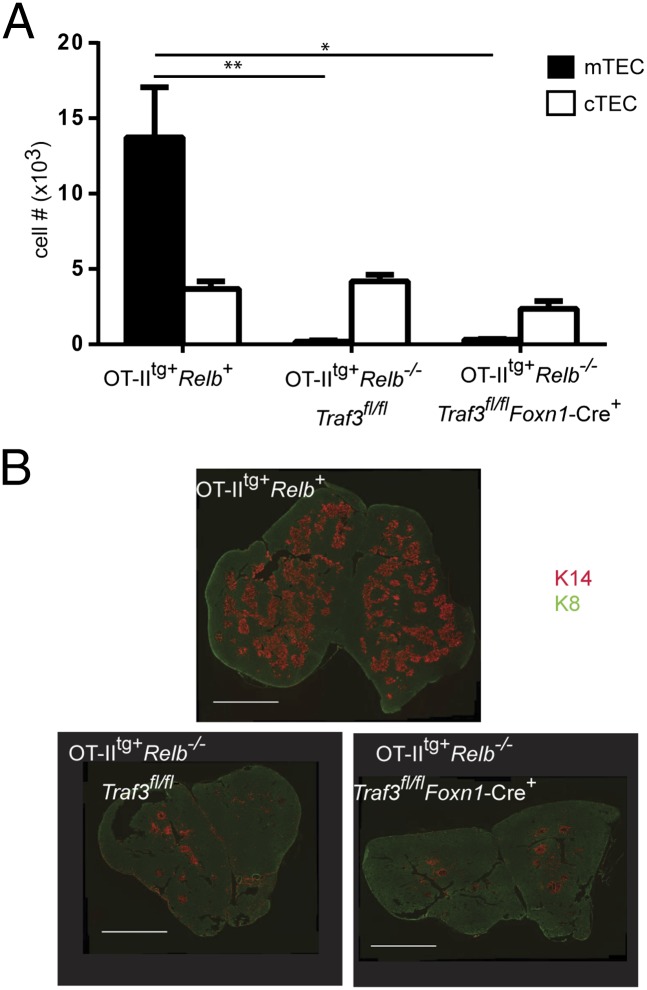
To determine whether the effect of Traf3 deletion is mediated through the alternative NF-κB pathway, we tested the effect of Traf3 deletion in mice lacking RelB, which are therefore incapable of signaling through alternative NF-κB. Mice expressing the OT-II TCR transgene were used to avoid the confounding effects of multiorgan inflammation that occur in Relb−/− mice expressing a normal T-cell repertoire (13). OTIItg+ Relb−/− (Traf3 intact) mice revealed a dramatic defect in mTECs, consistent with previous reports (12). When assessed either by flow cytometry or immunohistochemistry, deletion of Traf3 in Relb−/− TEC had no effect on this defect (Fig. 3). Thus, the ability of Traf3 inactivation to drive mTEC development is strictly RelB dependent. Collectively, these findings indicate that mTECs are fully capable of differentiation and expansion in the complete absence of signals from SP thymocytes, but that this capability is blocked by TRAF3-dependent inhibition of alternative NFκB signaling.
Fig. 3.
Specific deletion of Traf3 fails to rescue mTEC development in Relb−/− mice. Mice expressing the OT-II TCR transgene were used to avoid the confounding effects of multiorgan inflammation that occur in Relb−/− mice expressing a normal T-cell repertoire. (A) Thymic stromal cells from 6- to 8-wk-old mice were prepared as described in Materials and Methods and stained with anti-CD45, UEA-1, anti-Ly51, anti-MHCII, and anti-EpCAM. After gating on CD45neg, MHCII+ and/or EpCAM+ cells, numbers of mTECs (UEA+), and cTEC (UEAneg, Ly51+) were calculated for each group. Data shown are mean ± SE for ≥3 mice per strain (*P < 0.05; **P < 0.01). (B) Thymic cryosections from OT-IItg+ Relb+, OT-IItg+ Relb−/− Traf3fl/fl, and OT-IItg+ Relb−/− Traf3fl/fl Foxn1-Cre+ mice were stained with a combination of anti-keratin 14 (red) and anti-keratin 8 (green). (Scale bars: 2 mm.)
TEC-Specific Deletion of Traf3 Overcomes the Requirement for LTβR and CD40 in mTEC Development.
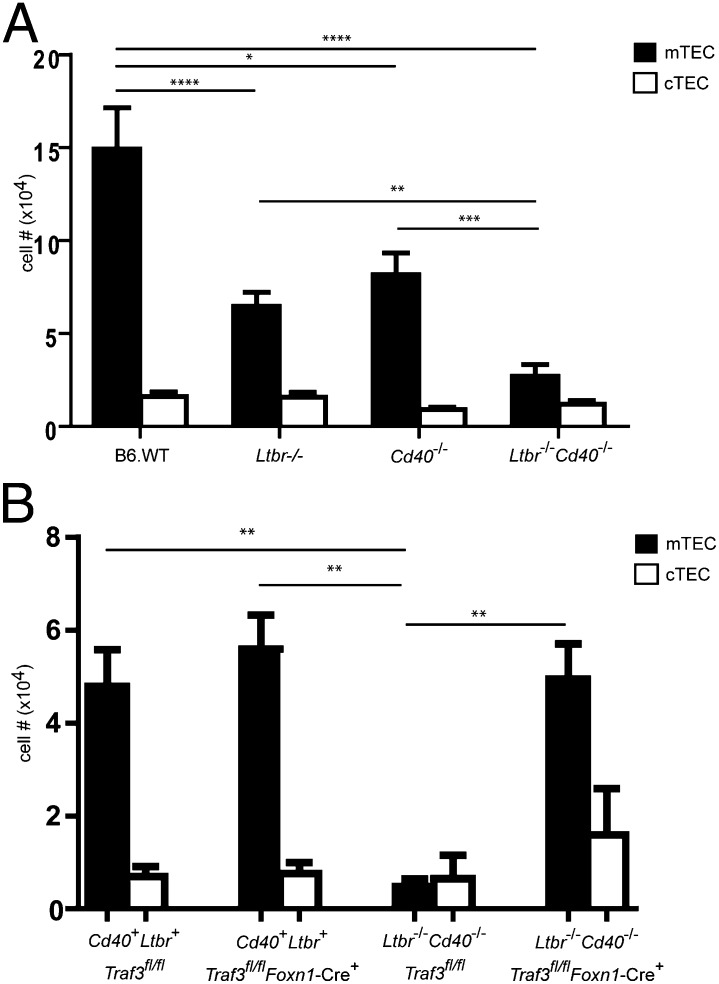
LTβR and CD40 signaling pathways have been shown to play a role in mTEC development. To better define the requirements for signaling through these pathways, we examined the medullary phenotype of Ltbr−/−, Cd40−/−, and double-deficient Ltbr−/−/Cd40−/− mice. As described previously, we observed significant reductions in UEA+ mTECs in Ltbr−/− and Cd40−/− mice relative to WT mice. Interestingly, mTECs were significantly more profoundly reduced when LTβR deficiency was combined with CD40 deficiency in Ltbr−/−/Cd40−/− mice (Fig. 4A). We tested the ability of Traf3 deletion to overcome the requirement in mTEC development for LTβR and CD40, receptors known to activate the alternative NF-κB pathway. Strikingly, TEC-specific deletion of Traf3 in Ltbr−/−/Cd40−/− mice reversed the profound defect in mTEC development and allowed reconstitution of mTEC numbers to wild-type levels (Fig. 4B). The proportions of mTECs that were MHCIIhi and MHCIIlo after Traf3 deletion in Ltbr−/−/Cd40−/− mice were not different from those in WT mice, indicating that reconstitution of mTEC numbers was accompanied by WT proportions of these mTEC subsets (Fig. S1A). In contrast, the number of cTEC was not significantly different among the genotypes tested. Traf3 deletion thus circumvents the requirements for LTβR and CD40 at all stages of development for generation of normal adult mTEC numbers.
Fig. 4.
Number of thymic medullary epithelial cells in Ltbr−/−/Cd40-/ mice is reduced relative to Ltbr−/− and Cd40−/− mice and is restored to wild-type levels with TEC-specific deletion of Traf3. (A) Thymic stromal cells from 3- to 5-wk-old B6 WT, Ltbr−/−, Cd40−/−, and Ltbr−/−/Cd40−/− mice were prepared as described in Materials and Methods and stained with anti-CD45, UEA-1, anti-Ly51, and anti-MHCII. After gating on CD45neg, MHCII+ cells, numbers of mTECs (UEA+), and cTEC (UEAneg, Ly51+) were calculated for each group. Data shown are mean ± SE for ≥3 mice per strain. (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). (B) Thymic stromal cells from 4- to 6-wk-old Ltbr+ Cd40+ Traf3fl/fl, Ltbr+ Cd40+ Traf3fl/flFoxn1-Cre+, LTbR-/ -Cd40−/−Traffl/fl, and Ltbr−/− Cd40−/− Traf3fl/fl Foxn1-Cre+ mice were prepared as described in Materials and Methods and stained with anti-CD45, UEA-1, anti-Ly51, and anti-MHCII. After gating on CD45neg, MHCII+ cells, numbers of mTECs (UEA+), and cTEC (UEAneg, Ly51+) were calculated for each group. Data shown are mean ± SE for ≥3 mice per strain. (**P < 0.01).
TEC-Specific Deletion of Traf3 Does Not Overcome the Requirement for RANKL in mTEC Development.
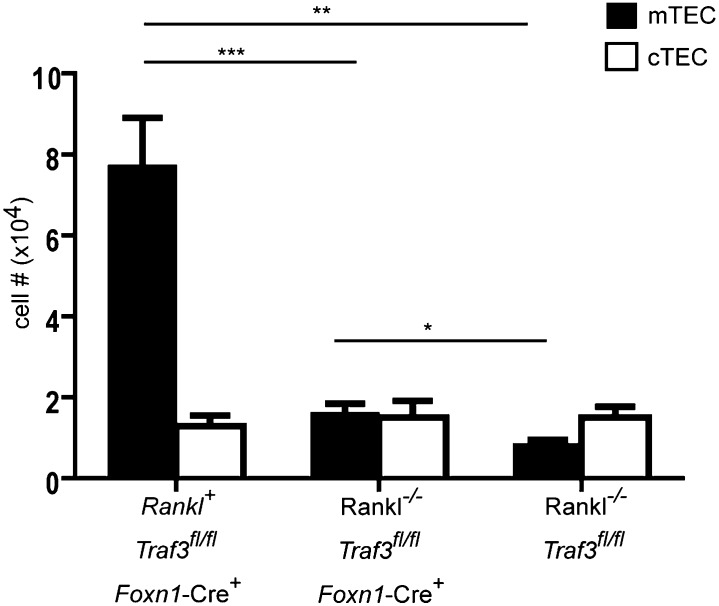
RANK–RANKL interactions have been reported to play an important role in mTEC development from early stages of thymic development, including embryonic and early postnatal stages that precede the appearance of SP thymocytes. It was therefore of particular interest to determine whether Traf3 deletion would overcome the requirement for RANK–RANKL in thymic development. Young Rankl−/− mice expressed a profound defect in mTEC development as reported (Fig. 5) (8, 23, 24). In contrast to our findings in Ltbr−/−/Cd40−/− and Tcra−/− mice where Traf3 deletion fully restored mTEC numbers, deletion of Traf3 in Rankl−/− mice did not restore medullary development to wild-type levels, but resulted in only minimal increase in mTEC numbers. The proportion of MHCIIhi mTECs was reduced in Rankl−/− mice and was not further influenced by Traf3 deletion (Fig. S1B). These findings indicate that there are essential functions of RANKL that are not overcome by Traf3 deletion.
Fig. 5.
TEC-specific deletion of Traf3 in Rankl−/− mice fails to reverse defect in mTEC development. Thymic stromal cells from 10- to 12-d-old mice were prepared as described in Materials and Methods and stained with anti-CD45, UEA-1, anti-Ly51, and anti-MHCII. After gating on CD45neg, MHCII+ cells, numbers of mTECs (UEA+), and cTEC (UEAneg, Ly51+) were calculated for each group. Data shown are mean ± SE for ≥4 mice per strain. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
The findings presented here provide insight into the processes that link development of thymic epithelial compartments with development of thymic T cells. The thymus provides an environment in which T lymphocytes develop through multiple stages. Positive selection, in which DP thymocytes expressing antigen-specific receptors are selected for survival and differentiation to SP thymocytes, occurs in the thymic cortex, which provides the environment and signals necessary for this stage of development. Positively selected thymocytes then traffic to the thymic medulla, where negative selection deletes potentially dangerous self-reactive cells. We have demonstrated here a unique role of TRAF3 in enforcing the requirement for SP thymocytes, and for SP-mediated signals, in medullary development. Our findings demonstrate that mTEC development can in fact occur in the complete absence of SP thymocytes but that this development is blocked by TRAF3 inhibition of alternative NF-κB signaling, and that the observed requirement for SP thymocytes is mediated by the need to counter TRAF3 inhibition in TEC, triggering a RelB-dependent pathway of mTEC development.
The mechanism(s) by which SP thymocytes promote mTEC development have been shown to include important roles for members of the TNFR family of receptors and ligands. In particular, CD40L, RANKL, and LTαβ have all been shown to be up-regulated on SP thymocytes relative to DN and DP thymocytes (8); mice lacking RANK–RANKL, LTαβ–LTβR, or CD40–CD40L interactions have mTEC compartment defects of varying severities (8, 11, 23–25). Our finding that Ltbr−/−/Cd40−/− mice have a profound mTEC defect more severe than either the Ltbr−/− or Cd40−/− mice is consistent with previous demonstrations of cooperation between different TNFR members in driving mTEC development, including cooperation between RANK and CD40 (24, 26), and RANK and LTβR(27). We found that the total number of mTECs is significantly decreased in Ltbr−/−/Cd40−/− mice relative to both the Ltbr−/− and Cd40−/− single knockout mice. Our findings are consistent with the recent report that combined stimulation of 2-dGUO-treated thymic lobes with agonist anti-LTβR antibody and CD40L synergized in the in vitro induction of mTECs (28), importantly extending these findings by demonstrating that LTβR and CD40L pathways, in fact, interact cooperatively during in vivo thymic development as evidenced by the severe reduction in mTEC numbers we observed in mice deficient in both pathways.
A feature shared by the TNFR family members expressed on mTECs, including CD40, LTβR, and RANK, is the capacity to activate RelB (29–31), a critical component of the nonclassical NF-κB pathway. The importance of this pathway in mediating development of a normal mTEC compartment is evidenced by the absence of UEA+ medullary epithelial cells in mice deficient for crucial components involved in the alternative NF-κB pathway including RelB, IKKα, and NIK (12–16). A role for classical NF-κB in the generation of a normal mTEC environment has also been proposed (24, 32). TRAF3 is an adaptor protein that negatively regulates nonclassical NF-κB signaling. In accordance with this role, it has been reported that TRAF3-deficient B cells have elevated amounts of NIK and increased processing of p100 to p52 (18). In addition, studies of mice with a B-cell–specific deletion of TRAF3 have established that TRAF3 is required for the maintenance of B-cell homeostasis (19, 33). We now show that TRAF3 has a critical role in mediating the requirement for thymocyte-mTEC cross-talk. Our demonstration that deletion of Traf3 in Tcra−/− mice allows the development of mTEC numbers comparable to wild-type mice indicates that TEC-specific deletion of Traf3 is, in fact, capable of allowing mTEC development in the complete absence of TCRαβ SP thymocytes. Additional experiments were informative in elucidating the role of TRAF3 in mediating aspects of mTEC development beyond those supported by SP thymocytes. We found that TEC-specific deletion of TRAF3 is able to compensate for the total absence of CD40–CD40L and LTβR–LTα/β interactions, two pathways important for mTEC development. Thus, Traf3 deletion overcomes the requirements for these interactions at all stages of thymic development necessary for generation of a normal adult mTEC population. In contrast, Traf3 deletion did not reverse the defect that results from RANKL deficiency. It is known that RANK–RANKL signaling plays a role in early mTEC development, including the embryonic and early postnatal stages at which SP thymocytes are absent or are present in small numbers. RANKL expressed on LTi and Vγ5+ DETC cells appears, for example, to play a critical role in early mTEC development (23, 34). Our findings reported here indicate that, if RANKL expressed by SP T cells plays a role in mTEC development, this function is bypassed by inactivation of Traf3. In contrast, our findings indicate that the role of RANKL on cells other than SP T cells is not overcome by FoxN1-Cre–mediated Traf3 deletion. More extensive studies of the requirements for RANKL by defined cell populations and at different stages of development will be required to fully elucidate the function of RANK–RANKL interactions.
Taken together, the findings reported here highlight a previously unappreciated capability of mTECs to develop and expand in the absence of SP thymocytes and without signals from CD40 and LTβR. It is TRAF3 that blocks this mTEC development and imposes the requirement for SP-TEC cross-talk. In the presence of TRAF3, the signaling that mediates negative selection of SP thymocytes is also necessary for formation of the medulla where negative selection occurs.
Materials and Methods
Mice.
C57BL/6 (B6) mice were obtained from the Frederick Cancer Research Facility and maintained at Bioqual. B6.Cd40−/− were obtained from The Jackson Laboratory and maintained at Bioqual. Ltbr−/− (35), Foxn1-Cre+ (20), Traf3fl/fl (19), OT-II TCRtg (36), Relb−/− (13), Rankl−/− (37), and Tcra−/− (38) mice have been described. Protocols for animal care and use were conducted consistent with the Guide for the Care and Use of Laboratory Animals of National Institutes of Health (39). Experimental protocols were approved by the Institutional Animal Care and Use Committee of the National Institutes of Health.
Preparation of Thymic Stromal Cells for Flow Cytometric Analysis.
Thymic stromal cell preparations were made by using methods modified from those reported by Gray et al. (40). Following release of thymocytes by gentle teasing of the thymus, thymic fragments were digested with Collagenase/Dispase at 0.25% wt/vol plus DNase 1 at 0.125% wt/vol (Roche) in four sequential incubations at 37 °C. Reactions were stopped by addition of FCS to 20%. For TEC analysis, single-cell suspensions were stained with anti-CD45.2 (104; BD), anti-Ly51 (BP-1; BD), anti-MHC class II (M5-114; Ebiosciences) and/or anti-EpCAM (G8.8; Biolgend), and UEA-1 (Vector). Dead cells were excluded with propidium iodide staining. Intracellular staining for AIRE was performed by using FoxP3 fix/permeabilization (eBioscience), rat anti-AIRE (clone 5H12; a generous gift from H. Scott, Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia; ref. 41) and anti-rat IgG2c-PE (clone 2C-8F1; Southern Biotechnology Associates). Gating for TEC analysis included all MHC class II positive (high and low) cells.
Immunohistology.
Sections (6 µm) of OCT-embedded frozen tissue were air dried for 15 min and then incubated 2 h with optimal dilutions of the primary rat monoclonal anti-keratin K8 (developed by Philippe Brulet and Rolf Kemler and obtained from the Developmental Studies Hybridoma Bank at the University of Iowa) and rabbit polyclonal anti-keratin 14 (K14) (Covance Research Products); anti-DEC205 (NLDC-145; Abcam) and UEA-1 FITC; anti–Ly51-PE (BD) and UEA-1 FITC; anti-AIRE (M-300; Santa Cruz Biotechnologies) and UEA-1 FITC; or anti-CD4 PE (BD), anti–CD8 FITC (BD), and anti-K14. Tissues were washed, and after an amplification step (anti–rat-Alexa 488 for K8, anti–rabbit-Alexa 546, or Alexa 633 for K14; anti-rabbit-Alexa 546 for AIRE; streptavidin Alexa 546 for DEC205; or biotinylated anti-PE followed by streptavidin Alexa 546 for Ly51), were mounted on microscope slides and imaged by using a Zeiss axiovert 200M inverted epifluorescence microscope equipped with appropriate fluorescence filters (Chroma Technologies), a motorized scanning stage, a 10× plan-apochromat (N.A. 0.45) objective lens, and a Photometrics CoolsnapES CCD camera (Roper Scientific). Sequential images were acquired by using the multidimensional mosaix algorithm in AxioVision software (Carl Zeiss MicroImaging). The resultant matrix of images, covering the entire area of the thymus section, was aligned and stitched by using AxioVision software and finally converted to a single large TIFF file. In preparing figures for display, a copy of each TIFF file was rescaled and compiled by using Adobe Photoshop.
PCR on Sorted Thymic and Splenic Subpopulations.
Genomic DNA was prepared from sorted splenic CD4+ T cells (CD4+H57+), CD4 SP thymocytes (CD4+CD8−), and TEC (CD45neg, EpCAM+IA/IE+). PCR analysis to detect the excision of exons 1 and 2 of the Traf3 gene was done as described by using primers U7 (5′-GTT ACA ATG AAG TTC TGC CAC-3′) and BT6 (5′-ATG CAA CGA GTG ATG AGG TT-3′) (19).
Statistics.
The Student t test (unpaired, two-tailed) was used to determine P values.
Supplementary Material
Acknowledgments
We thank Dr. Alfred Singer, Prof. Eric Jenkinson, and Prof. Graham Anderson for critical reading of the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314859111/-/DCSupplemental.
References
- 1.Anderson G, Lane PJ, Jenkinson EJ. Generating intrathymic microenvironments to establish T-cell tolerance. Nat Rev Immunol. 2007;7(12):954–963. doi: 10.1038/nri2187. [DOI] [PubMed] [Google Scholar]
- 2.Nitta T, Murata S, Ueno T, Tanaka K, Takahama Y. Thymic microenvironments for T-cell repertoire formation. Adv Immunol. 2008;99:59–94. doi: 10.1016/S0065-2776(08)00603-2. [DOI] [PubMed] [Google Scholar]
- 3.Manley NR, Richie ER, Blackburn CC, Condie BG, Sage J. Structure and function of the thymic microenvironment. Front Biosci (Landmark Ed) 2011;16:2461–2477. doi: 10.2741/3866. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher AL, Calder A, Hince MN, Boyd RL, Chidgey AP. The contribution of thymic stromal abnormalities to autoimmune disease. Crit Rev Immunol. 2011;31(3):171–187. doi: 10.1615/critrevimmunol.v31.i3.10. [DOI] [PubMed] [Google Scholar]
- 5.Shores EW, Van Ewijk W, Singer A. Disorganization and restoration of thymic medullary epithelial cells in T cell receptor-negative scid mice: Evidence that receptor-bearing lymphocytes influence maturation of the thymic microenvironment. Eur J Immunol. 1991;21(7):1657–1661. doi: 10.1002/eji.1830210711. [DOI] [PubMed] [Google Scholar]
- 6.van Ewijk W, Shores EW, Singer A. Crosstalk in the mouse thymus. Immunol Today. 1994;15(5):214–217. doi: 10.1016/0167-5699(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 7.Surh CD, Ernst B, Sprent J. Growth of epithelial cells in the thymic medulla is under the control of mature T cells. J Exp Med. 1992;176(2):611–616. doi: 10.1084/jem.176.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hikosaka Y, et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29(3):438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Irla M, et al. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29(3):451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 10.White AJ, et al. Lymphotoxin signals from positively selected thymocytes regulate the terminal differentiation of medullary thymic epithelial cells. J Immunol. 2010;185(8):4769–4776. doi: 10.4049/jimmunol.1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White AJ, et al. Sequential phases in the development of Aire-expressing medullary thymic epithelial cells involve distinct cellular input. Eur J Immunol. 2008;38(4):942–947. doi: 10.1002/eji.200738052. [DOI] [PubMed] [Google Scholar]
- 12.Burkly L, et al. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373(6514):531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 13.Weih F, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80(2):331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 14.Lomada D, Liu B, Coghlan L, Hu Y, Richie ER. Thymus medulla formation and central tolerance are restored in IKKalpha-/- mice that express an IKKalpha transgene in keratin 5+ thymic epithelial cells. J Immunol. 2007;178(2):829–837. doi: 10.4049/jimmunol.178.2.829. [DOI] [PubMed] [Google Scholar]
- 15.Kinoshita D, et al. Essential role of IkappaB kinase alpha in thymic organogenesis required for the establishment of self-tolerance. J Immunol. 2006;176(7):3995–4002. doi: 10.4049/jimmunol.176.7.3995. [DOI] [PubMed] [Google Scholar]
- 16.Kajiura F, et al. NF-kappa B-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J Immunol. 2004;172(4):2067–2075. doi: 10.4049/jimmunol.172.4.2067. [DOI] [PubMed] [Google Scholar]
- 17.Vallabhapurapu S, et al. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9(12):1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He JQ, et al. Rescue of TRAF3-null mice by p100 NF-kappa B deficiency. J Exp Med. 2006;203(11):2413–2418. doi: 10.1084/jem.20061166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie P, Stunz LL, Larison KD, Yang B, Bishop GA. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27(2):253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuklys S, et al. Stabilized beta-catenin in thymic epithelial cells blocks thymus development and function. J Immunol. 2009;182(5):2997–3007. doi: 10.4049/jimmunol.0713723. [DOI] [PubMed] [Google Scholar]
- 21.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 22.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2(11):1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 23.Rossi SW, et al. RANK signals from CD4(+)3(-) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007;204(6):1267–1272. doi: 10.1084/jem.20062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akiyama T, et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29(3):423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Boehm T, Scheu S, Pfeffer K, Bleul CC. Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTbetaR. J Exp Med. 2003;198(5):757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desanti GE, et al. Developmentally regulated availability of RANKL and CD40 ligand reveals distinct mechanisms of fetal and adult cross-talk in the thymus medulla. J Immunol. 2012;189(12):5519–5526. doi: 10.4049/jimmunol.1201815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mouri Y, et al. Lymphotoxin signal promotes thymic organogenesis by eliciting RANK expression in the embryonic thymic stroma. J Immunol. 2011;186(9):5047–5057. doi: 10.4049/jimmunol.1003533. [DOI] [PubMed] [Google Scholar]
- 28.Irla M, et al. Antigen recognition by autoreactive CD4⁺ thymocytes drives homeostasis of the thymic medulla. PLoS ONE. 2012;7(12):e52591. doi: 10.1371/journal.pone.0052591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coope HJ, et al. CD40 regulates the processing of NF-kappaB2 p100 to p52. EMBO J. 2002;21(20):5375–5385. doi: 10.1093/emboj/cdf542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novack DV, et al. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003;198(5):771–781. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dejardin E, et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17(4):525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 32.Akiyama T, et al. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science. 2005;308(5719):248–251. doi: 10.1126/science.1105677. [DOI] [PubMed] [Google Scholar]
- 33.Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28(3):391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Roberts NA, et al. Rank signaling links the development of invariant γδ T cell progenitors and Aire(+) medullary epithelium. Immunity. 2012;36(3):427–437. doi: 10.1016/j.immuni.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fütterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9(1):59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 36.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76(1):34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim N, Odgren PR, Kim DK, Marks SC, Jr, Choi Y. Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci USA. 2000;97(20):10905–10910. doi: 10.1073/pnas.200294797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philpott KL, et al. Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science. 1992;256(5062):1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 39. Committee on Care and Use of Laboratory Animals (1985) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85–23.
- 40.Gray DH, et al. Unbiased analysis, enrichment and purification of thymic stromal cells. J Immunol Methods. 2008;329(1-2):56–66. doi: 10.1016/j.jim.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Hubert FX, et al. A specific anti-Aire antibody reveals aire expression is restricted to medullary thymic epithelial cells and not expressed in periphery. J Immunol. 2008;180(6):3824–3832. doi: 10.4049/jimmunol.180.6.3824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.