Significance
Pepducins are a class of biologics that allosterically control G protein-coupled receptor (GPCR) activity, but very little is known about their mode of action. Here, we report that ATI-2341, a pepducin targeting the C-X-C chemokine receptor type 4 (CXCR4), functions as a biased ligand, favoring Gαi activation over Gα13. Moreover, contrary to the natural CXCR4 agonist, stromal cell-derived factor-1α, ATI-2341 does not promote β-arrestin recruitment. In addition to revealing the selective signaling underlying ATI-2341 effects on hematopoietic cell mobilization, the study shows that pepducins are powerful tools offering perspectives for studying GPCR functional selectivity that could impact the development of drugs with fewer side effects.
Keywords: BRET, lipid-anchored peptide, beta-arrestin, protein–protein interaction, cell signaling
Abstract
Short lipidated peptide sequences derived from various intracellular loop regions of G protein-coupled receptors (GPCRs) are named pepducins and act as allosteric modulators of a number of GPCRs. Recently, a pepducin selectively targeting the C-X-C chemokine receptor type 4 (CXCR4) was found to be an allosteric agonist, active in both cell-based assays and in vivo. However, the precise mechanism of action of this class of ligands remains poorly understood. In particular, given the diversity of signaling effectors that can be engaged by a given receptor, it is not clear whether pepducins can show biased signaling leading to functional selectivity. To explore the ligand-biased potential of pepducins, we assessed the effect of the CXCR4 selective pepducin, ATI-2341, on the ability of the receptor to engage the inhibitory G proteins (Gi1, Gi2 and Gi3), G13, and β-arrestins. Using bioluminescence resonance energy transfer-based biosensors, we found that, in contrast to the natural CXCR4 ligand, stromal cell-derived factor-1α, which promotes the engagement of the three Gi subtypes, G13 and the two β-arrestins, ATI-2341 leads to the engagement of the Gi subtypes but not G13 or the β-arrestins. Calculation of the transduction ratio for each pathway revealed a strong negative bias of ATI-2341 toward G13 and β-arrestins, revealing functional selectivity for the Gi pathways. The negative bias toward β-arrestins results from the reduced ability of the pepducin to promote GPCR kinase-mediated phosphorylation of the receptor. In addition to revealing ligand-biased signaling of pepducins, these findings shed some light on the mechanism of action of a unique class of allosteric regulators.
Pepducins represent a class of molecules that regulate the activity of G protein-coupled receptors (GPCRs). These lipid-modified peptides are derived from the amino acid sequences of one of the four intracellular loops of a target GPCR (1). Although the precise mode of action is not completely understood, it is believed that pepducins bind to their target receptors and allosterically modulate their signaling activity (2, 3). Pepducins have been identified for several GPCRs, including the protease-activated receptors PAR1 (1, 4–10), PAR2 (1, 11–13), and PAR4 (6, 9), the formyl peptide receptor-2 (14), the melanocortin type 4 receptor (1), the sphingosine 1-phosphate receptor 3 (15), and the C-X-C chemokine receptor type 1, 2 (CXCR1, CXCR2) (16), and 4 (CXCR4) (2, 17, 18). They have been found to act as allosteric agonists as well as negative or positive allosteric modulators. However, in most cases, their activity was assessed for only one or a few signaling pathways engaged by the receptors.
One of the receptors for which pepducins were developed is CXCR4. This receptor, expressed in many tissues including hematopoietic and circulating cells, is a coreceptor for the entry of HIV (19, 20) and controls many physiological functions, including cell migration, mobilization, and retention of polymorphonuclear neutrophils (PMNs) and hematopoietic stem cells, as well as progenitor cells (HSPCs) in the bone marrow niche (21–23). It has also been found to play an important role in tumor progression, angiogenesis, and metastasis of a variety of cancers (24–26). A CXCR4 antagonist, AMD-3100 (Mozobil), is used to mobilize HSPCs from the bone marrow for transplantation of leukemic patients (27).
A pepducin derived from the first intracellular loop of CXCR4 (ATI-2341) was found to be an allosteric agonist on the chemotactic response elicited on a human T-lymphoblastic leukemia cell line (CCRF-CEM cells) that endogenously expresses CXCR4 (18). This activity of ATI-2341 was confirmed in fresh human PMNs as well as in vivo for their ability to promote the mobilization of PMNs and HSPCs in the peripheral circulation of both mice and monkeys (18). Of note, unlike the clinically used CXCR4 antagonist AMD-3100 and stromal cell-derived factor-1α (SDF-1; also known as CXCL12) that promote the mobilization of lymphocytes in addition to PMNs and HSPCs (18, 28–30), ATI-2341 was without effect on the mobilization of lymphocytes (18), indicating that ATI-2341 may display functional selectivity.
When assessing the effect of ATI-2341 on the signaling activity of CXCR4, the pepducin was found to be an allosteric agonist, activating the inhibitory heterotrimeric G protein (Gi) to promote inhibition of cAMP production and induce calcium mobilization (18, 31). In recent years, many GPCRs have been shown to engage in promiscuous signaling activities involving more than one G protein subtype as well as G protein-independent signaling. More importantly, it was found that different ligands can selectively couple to a subset of the signaling pathways that can be engaged by a receptor. In some cases, a given ligand can even have opposite efficacies on two different signaling pathways: a concept known as “ligand-biased signaling” or “functional selectivity” (32–36). In addition to its coupling to Gi, CXCR4 has also been found to signal through the engagement and activation of G13 (37, 38) and β-arrestin2 (39, 40), both pathways being proposed to contribute to the chemotactic responses. These observations raise the question of whether the CXCR4-selective pepducin, ATI-2341, is an allosteric agonist on all of the signaling pathways identified for the chemokine receptor or whether it could show bias toward selective signaling pathways and thus be functionally selective.
To determine whether pepducins can display functional selectivity on CXCR4 signaling at the molecular level, we took advantage of bioluminescence resonance energy transfer (BRET)-based assays that allow the direct monitoring of the engagement and activation of proximal signaling effectors. More specifically, we compared the ability of ATI-2341 and the natural agonist of the receptor, SDF-1, to promote the engagement/activation of three Gi family members (Gi1, Gi2, and Gi3), G13, β-arrestin1, and β-arrestin2. We found that, whereas SDF-1 promotes the engagement of all of the signaling effectors, ATI-2341 selectively led to the functional engagement of the Gi family members and had no effect on G13 or the two β-arrestins. The lack of recruitment of β-arrestins results from the poor recruitment of G protein-coupled receptor kinases (GRKs) to the receptor because, in contrast to SDF-1 that stimulates the phosphorylation of CXCR4 by protein kinase C (PKC), GRK2/3, and GRK6, ATI-2341 promotes effective PKC-dependent phosphorylation of the receptor but minimal GRK2/3 recruitment, as well as minimal GRK6-dependent phosphorylation.
Taken together, our results demonstrate that the pepducin ATI-2341 is a functionally selective allosteric regulator of CXCR4 that activates Gi-dependent pathways without modulating G13 and β-arrestin pathways. These data indicate that ATI-2341 could have physiological actions that may differ from the natural ligand SDF-1 and the clinically used AMD-3100, raising the intriguing possibility that ATI-2341 may have distinct clinical properties. These findings also shed some light on the mechanism of action of pepducins and indicates that, similar to orthosteric ligands, these allosteric regulators can be functionally selective.
Results
CXCR4-Derived Pepducin ATI-2341 Promotes the Engagement and Activation of Gαi but Not Gα13.
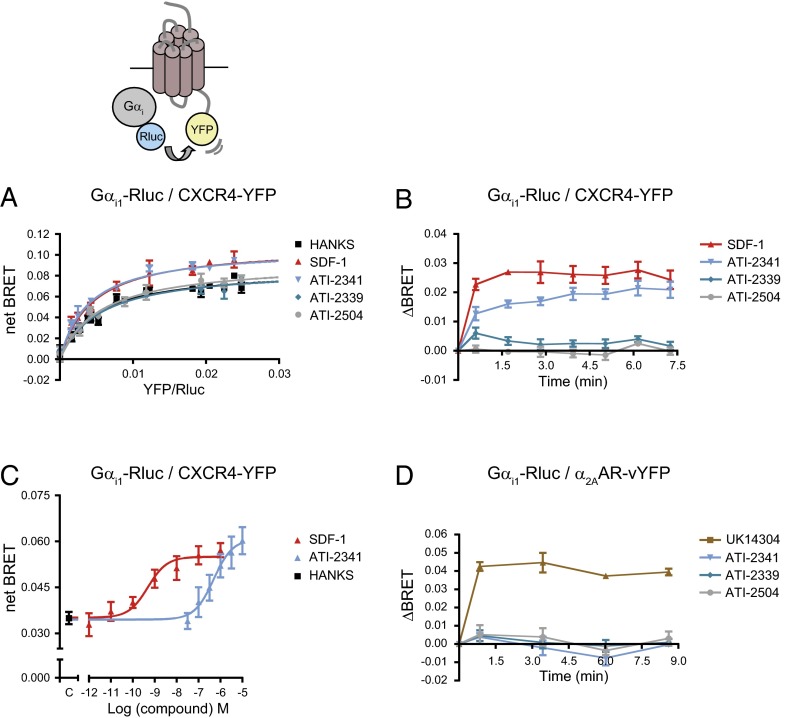
To dissect the signaling pathways engaged by ATI-2341, we first assessed the ability of this pepducin to promote the engagement and activation of its cognate G protein, Gi. For this purpose, the engagement of Gαi1 by CXCR4 was first determined by measuring BRET between Gαi1-Renilla luciferase (Rluc) and CXCR4-Yellow Fluorescent Protein (YFP). Similar to the endogenous ligand SDF-1, ATI-2341 induced a significant increase in the maximal BRET480-YFP signal as derived from BRET titration curves between Gαi1 and CXCR4 (Fig. 1A). In contrast, two negative control compounds, ATI-2339 [a related pepducin with a modified sequence missing the last three amino acids of ATI-2341 that is inactive in CXCR4-stimulated second messenger assays (18)] and ATI-2504 [a nonlipidated analog of ATI-2341 (18)] (both sequences are shown in Table S1), had no effect (Fig. 1A). The BRET increase was rapid, reaching its maximum at the earliest time point measured (1 min) and remaining constant for at least 7.5 min, a kinetic profile very similar to the one observed for SDF-1 (Fig. 1B). Similar results were observed for Gαi1, Gαi2, and Gαi3 when assessing BRET400-GFP2 between CXCR4-green fluorescent protein (GFP) 2 and the Gαi1/2/3-RlucII constructs (Fig. S1), indicating that the results are independent of the BRET configuration used (BRET480-YFP vs. BRET400-GFP2) and that the three Gαi subunits can be engaged by the pepducin-activated CXCR4. Although the efficacy reached is similar for both compounds (Fig. 1C), the potency of ATI-2341 was significantly lower than that of SDF-1 (EC50 values: ATI-2341, 533 ± 91 nM vs. SDF-1, 0.53 ± 0.19 nM), in agreement with the potencies previously observed in second messenger assays (18). The selectivity of ATI-2341 for CXCR4 was confirmed using BRET partners derived from a different GPCR signaling system, α2-adrenergic engagement of Gi (41). In contrast to the known α2-adrenergic agonist, UK14304, ATI-2341 did not promote an increase in BRET between α2AAR-venusYFP (vYFP) and Gαi1-Rluc (Fig. 1D).
Fig. 1.
ATI-2341 promotes the engagement of Gαi. (A) BRET titration curves were performed in cells cotransfected with a constant amount of Gαi1-Rluc and increasing amounts of CXCR4-YFP. Cells were stimulated with 200 nM SDF-1, 1 μM pepducins (ATI-2341, ATI-2339, ATI-2504), or vehicle (Hanks) for 5 min. BRET was measured following coel-h addition using the BRET480-YFP filter set. The curves shown are derived from individual titration curves that are representative of four independent experiments. The error bars represent SD from duplicate wells. (B) Kinetics of the ligand-promoted BRET between Gαi1-Rluc and CXCR4-YFP. BRET was measured at the indicated times following the addition of 200 nM SDF-1 or 1 μM pepducins. Data shown are the mean ± SEM of four independent experiments. (C) Effect of increasing concentrations of SDF-1 or ATI-2341 on the BRET between Gαi1-Rluc and CXCR4-YFP. BRET was measured 5 min following the addition of the ligands (EC50: ATI-2341, 533 ± 91 nM vs. SDF-1, 0.53 ± 0.19 nM). Data shown are the mean ± SEM of five independent experiments. (D) Kinetics of the ligand-promoted BRET between Gαi1-Rluc and α2AAR-vYFP. BRET was measured at the indicated times following the addition of 100 nM UK14304 or 1 μM pepducin. Data shown are the mean ± SEM of three independent experiments.
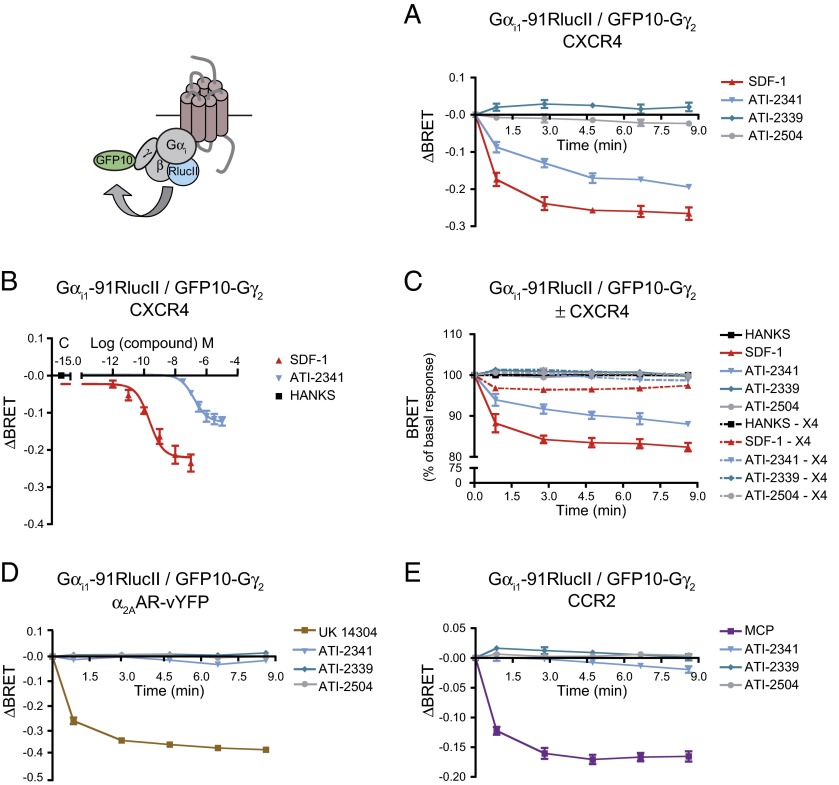
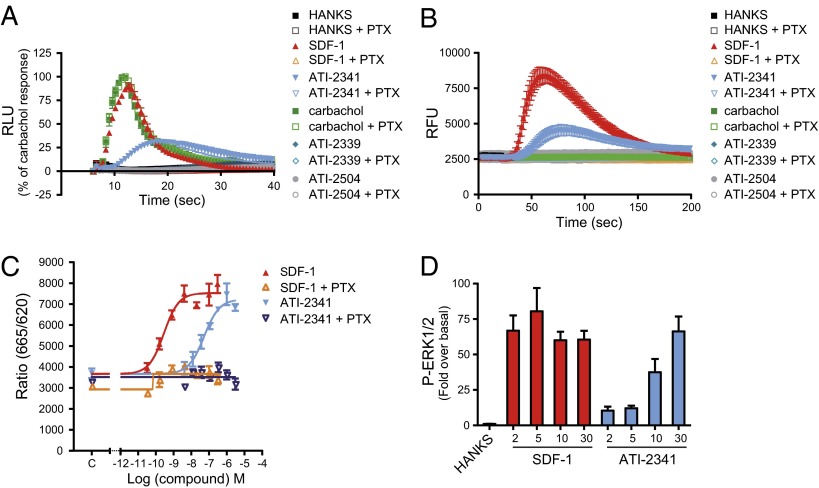
To determine whether this pepducin-promoted engagement of Gαi by CXCR4 results in the activation of the G protein, we used a BRET-based assay that monitors the separation of Gαi1 and Gγ2 that is promoted by agonist stimulation (41). Similarly to SDF-1, ATI-2341 promoted a rapid decrease in BRET400-GFP10 between Gαi1-RlucII and GFP10-Gγ2 in cells coexpressing the untagged CXCR4 and Gβ1 subunit (Fig. 2 A and B), confirming that the pepducin-promoted engagement of Gαi1 by CXCR4 results in its activation. The time required to reach the maximal efficacy was slightly increased for ATI-2341 relative to that of SDF-1 (t1/2: ATI-2341, 1.40 ± 0.23 min vs. SDF-1, 0.54 ± 0.15 min, respectively; *P < 0.05). Consistent with the engagement assay, two negative controls, ATI-2339 and ATI-2504, had no effect on the BRET between Gαi1-RlucII and GFP10-Gγ2. Further, ATI-2341 was without effect on the BRET signal in cells lacking CXCR4 (Fig. 2C), or in cells expressing the α2AAR or another chemokine receptor, the CCR2 (Fig. 2 D and E), confirming that the activation of Gαi1 by ATI-2341 necessitates the presence of CXCR4, thus establishing the selectivity of action of the pepducin. The ATI-2341–activated CXCR4 also promoted the activation of Gi2 and Gi3 (Fig. S2). Consistent with what was observed for the pepducin-promoted engagement of Gαi by CXCR4, the activation of Gi by ATI-2341 was found to be concentration-dependent and confirmed the lower potency of ATI-2341 compared with SDF-1 (EC50 values: ATI-2341, 208 ± 69 nM vs. SDF-1, 0.25 ± 0.06 nM). It should also be noted that the maximal amplitude of the pepducin-promoted BRET decrease is lower than that obtained with SDF-1 (Fig. 2B), indicating that ATI-2341 acts as a partial agonist on this pathway. Interestingly, this difference was not observed when considering the engagement of Gαi1, suggesting that the partial agonism results from an incomplete activation and not a reduced binding of Gi. To corroborate the pepducin-promoted Gi activation detected with the BRET-based biosensors in nonengineered cells, calcium measurements were performed in a HEK293T cell clone endogenously expressing CXCR4 as well as in the human lymphoblast-T cell line that endogenously expresses CXCR4, SUP-T1 cells (Fig. 3 A and B). In both cases, ATI-2341 and SDF-1 acted as agonists. Both the SDF-1 and pepducin responses were blocked by pretreatment with pertussis toxin (PTX), confirming the activation of the Gi pathway by ATI-2341 in cells natively expressing the receptor. Neither of the two control pepducins ATI-2339 and ATI-2504 promoted calcium mobilization. The ability of ATI-2341 to activate Gi was also confirmed by the complete inhibition of pepducin-stimulated ERK1/2 activity following inactivation of Gαi with PTX (Fig. 3C). ATI-2341 was also found to activate ERK1/2 in the HEK293T cells endogenously expressing CXCR4 (Fig. 3D).
Fig. 2.
ATI-2341-promoted Gαi engagement results in Gi activation. (A) Kinetics of the ligand-promoted BRET between Gαi1-RlucII and GFP10-Gγ2. BRET was measured after coel-400a addition, using the BRET400-GFP2/10 filter set, at the indicated times following the addition of 500 nM SDF-1 or 1 μM pepducins (ATI-2341, ATI-2339, and ATI-2504). Data shown are the mean ± SEM of three independent experiments. (B) Effect of increasing concentrations of SDF-1 or ATI-2341 on the BRET between Gαi1-RlucII and GFP10-Gγ2 in cells cotransfected with the untagged Gβ subunit and CXCR4. BRET was measured 5 min following the addition of the ligands (EC50 values: ATI-2341, 208 ± 69 nM vs. SDF-1, 0.25 ± 0.06 nM). Data shown are the mean ± SEM of at least four independent experiments. (C) Kinetics of the ligand-promoted BRET between Gαi1-RlucII and GFP10-Gγ2 in cells cotransfected with (solid lines) or without (dotted lines) CXCR4 and with the untagged Gβ subunit. BRET was measured after coel-400a addition, using the BRET400-GFP2/10 filter set, at the indicated times following the addition of 500 nM SDF-1 or 1 μM pepducins. Data shown are the mean ± SEM of three independent experiments. (D and E) Kinetics of the ligand-promoted BRET between Gαi1-RlucII and GFP10-Gγ2 in cells cotransfected with the untagged Gβ subunit and α2AAR (D) or CCR2 (E). BRET was measured after coel-400a addition, using the BRET400-GFP2/10 filter set, at the indicated times following the addition of 1 μM pepducins and 100 nM UK14304 (D) or 200 nM MCP (E). Data shown are the mean ± SEM of three to four independent experiments.
Fig. 3.
ATI-2341 promotes second messengers activation in a Gi-dependent manner. (A) HEK293T cells stably expressing obelin and preincubated overnight in the presence (open symbols) or absence (filled symbols) of 100 ng/mL PTX were injected into wells containing 500 nM SDF-1, 3 μM pepducins (ATI-2341, ATI-2339, ATI-2504), 100 μM carbachol, or vehicle (Hanks). Luminescence was measured each 600 ms for 34 s after injection. Data were normalized to carbachol-stimulated maximal calcium response. Data are the mean ± SEM of three independent experiments. (B) SUP-T1 cells, preincubated overnight in the presence (open symbols) or absence (filled symbols) of 100 ng/mL PTX, were loaded with calcium 4 dye and incubated with 500 nM SDF-1, 3 μM pepducins, 100 μM carbachol, or vehicle (Hanks). Fluorescence intensity was measured each 1.5 s for 170 s after injection. Data are the mean ± SEM of four independent experiments. (C) Effect of increasing concentrations of SDF-1 or ATI-2341 on ERK1/2 activation, in the absence (filled triangles) or presence (open triangles) of 100 ng/mL PTX in cells stably expressing human CXCR4. Data shown are the mean ± SEM of three independent experiments. (D) Kinetics of the ligand-promoted ERK1/2 phosphorylation in HEK293T cells stimulated with 500 nM SDF-1 or 3 μM ATI-2341 at the indicated times (0–30 min). Data were expressed as fold over basal. Data shown are the mean ± SEM of at least three independent experiments.
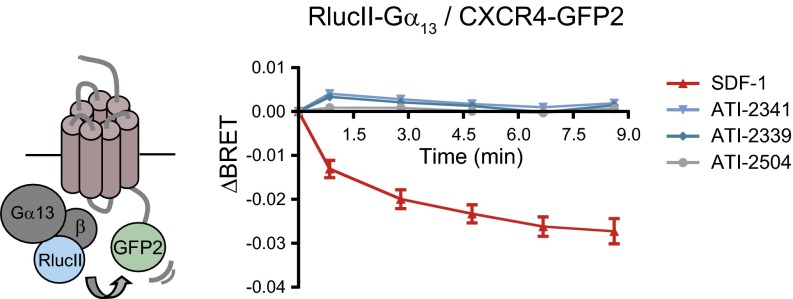
Because several studies indicate that CXCR4 mediates some of its actions through Gα13 (37, 38), we also assessed the ability of ATI-2341 to promote CXCR4 engagement of Gα13 by measuring BRET400-GFP2 between RlucII-Gα13 and CXCR4-GFP2. Using this biosensor, we previously showed that RlucII-Gα13 interacts specifically with CXCR4-GFP2 and that SDF-1 promotes a decrease in the BRET signal, reflecting the functional engagement of Gα13 by the receptor (38). In contrast to the BRET decrease promoted by SDF-1, ATI-2341 was without effect on the RlucII-Gα13/CXCR4-GFP2 BRET signal (Fig. 4). This result suggests that the pepducin cannot promote the engagement of Gα13. Taken together, these data indicate that ATI-2341 acts as a biased ligand, favoring Gαi proteins over Gα13.
Fig. 4.
ATI-2341 acts as a biased ligand favoring Gαi over Gα13. Kinetics of ligand-promoted BRET between RlucII-Gα13 and CXCR4-GFP2. BRET was measured after coel-400a addition, using the BRET400-GFP2/10 filter set, at the indicated times following the addition of 200 nM SDF-1, 1 μM ATI-2341, 1 μM ATI-2339, or 1 μM ATI-2504. Data shown are the mean ± SEM of three independent experiments.
ATI-2341 Is a Weak Partial Agonist of β-Arrestin Recruitment.
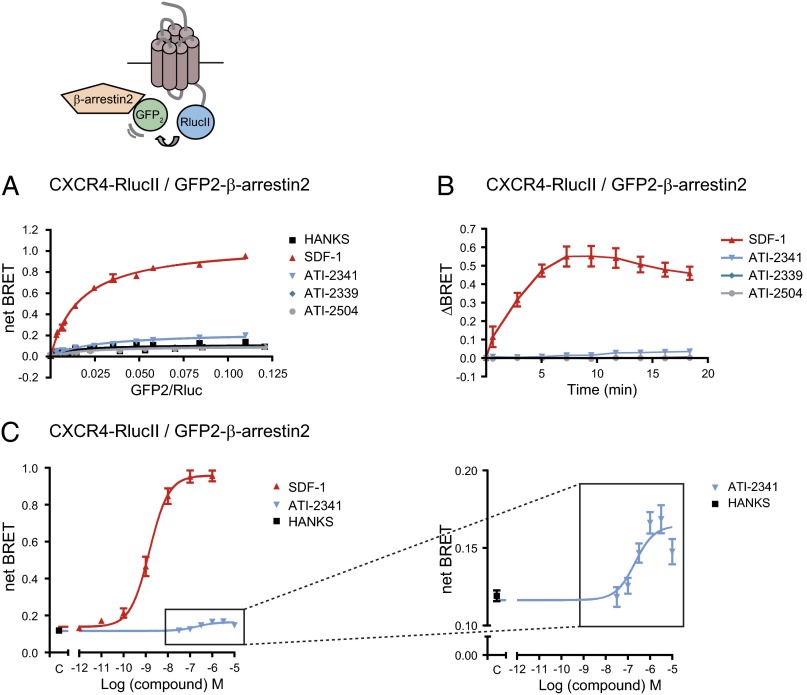
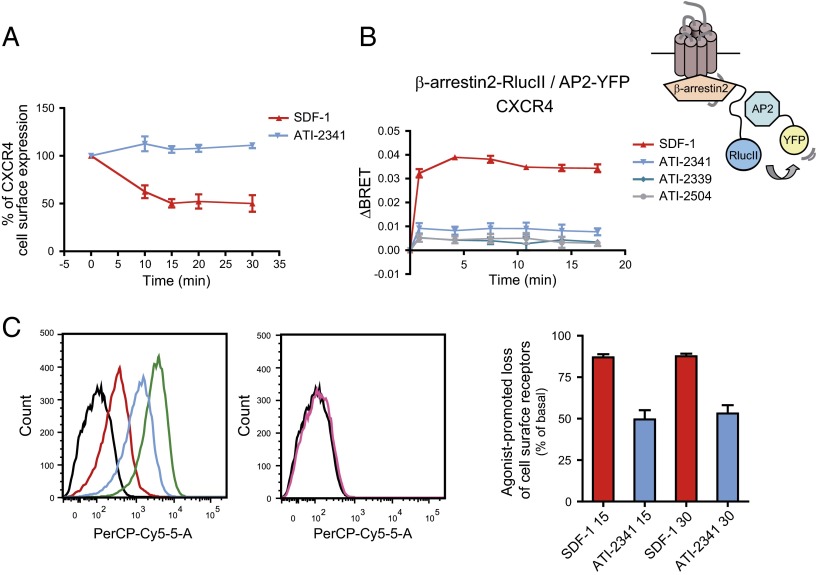
Given the key role of β-arrestins in G protein signaling arrest and initiation of G protein-independent signaling, we next monitored the effect of pepducins on the recruitment of β-arrestin2 to CXCR4. In the absence of ligand, BRET titration curves revealed a very weak BRET signal between CXCR4-RlucII and GFP2–β-arrestin2, characteristic of the very weak affinity of β-arrestins for inactive receptors (Fig. 5A). Stimulation with SDF-1 led to a large and saturable BRET increase representing the progressive association of CXCR4-RlucII with GFP2–β-arrestin2. In contrast, ATI-2341 promoted only a very small increase in the BRET signal whereas the two control pepducins, ATI-2339 and ATI-2504, had no effect. The SDF-1–promoted recruitment of β-arrestin2 to CXCR4 was very rapid, with a half time of 2.2 ± 0.7 min (Fig. 5B). In contrast, the very weak increase in BRET between CXCR4-RlucII and GFP2–β-arrestin2 promoted by ATI-2341 was detectable only after a stimulation of 7.5 min and yielded a half time of 9.4 ± 1.2 min. The recruitment of β-arrestin2 to CXCR4 induced by SDF-1 was dose-dependent, with an EC50 of 1.8 ± 0.3 nM (Fig. 5C) whereas the weak ATI-2341–promoted β-arrestin2 recruitment revealed an EC50 of 273.5 ± 78.6 nM (Fig. 5C, Inset), relatively similar to the values determined for the ATI-2341–promoted Gi engagement (Fig. 1C) and activation (Fig. 2B). To verify that the weak recruitment of β-arrestin2 induced by ATI-2341 to CXCR4 was not due to the BRET configuration used, we monitored β-arrestin2 recruitment to the receptor in the reverse orientation (i.e., β-arrestin2–RlucII and CXCR4-GFP2) or with BRET480-YFP using β-arrestin2–RlucII and CXCR4-YFP. As shown in Fig. S3, although a robust time-dependent β-arrestin2 recruitment could be observed for these two BRET configurations upon SDF-1 stimulation, no ATI-2341–promoted BRET could be detected, confirming that the pepducin is, at best, a very weak activator of the β-arrestin2 pathway. Similar results were obtained for β-arrestin1 (Fig. S4). Given the poor ATI-2341–promoted β-arrestin recruitment, the ability of the pepducin to induce receptor endocytosis was verified in HEK293T cells heterologously expressing an engineered receptor that allows monitoring both the cell surface and the total number of receptor. The receptor is tagged at its N terminus to an HA epitope and at its C terminus with vYFP. Dual FACS analysis allows one to assess the cell-surface receptor reflected by the HA immunoreactivity as a function of the total receptor number reflected by the vYFP fluorescence. As shown in Fig. 6A, ATI-2341 did not promote any significant endocytosis of the receptor compared with the 50% endocytosis promoted by SDF-1, consistent with the very weak recruitment of β-arrestin promoted by ATI-2341 compared with SDF-1. This lack of endocytosis is also consistent with the marginal ATI-2341–promoted BRET observed between β-arrestin2–RlucII and β2adaptin-YFP (Fig. 6B) used as a sensor of the β-arrestin– and clathrin-dependent endocytosis (42). The ligand-promoted loss of cell surface receptor has also been tested in the SUP-T1 cells endogenously expressing CXCR4 (Fig. 6C). FACS analysis using a monoclonal antibody directed against the extracellular domain of the human CXCR4 (Clone 12G5; BioLegend) revealed that ATI-2341 promoted a significant loss of cell-surface CXCR4 (49 ± 6%) that was much smaller than the loss promoted by SDF-1 (87 ± 2%). Although it is difficult to determine the fraction of the loss in cell-surface expression that results from β-arrestin–dependent endocytosis (β-arrestin independent down-regulation could also contribute), the smaller loss observed following ATI-2341 treatment is consistent with the reduced β-arrestin engagement promoted by the pepducin.
Fig. 5.
ATI-2341 promotes a weak recruitment of β-arrestin to CXCR4. (A) BRET titration curves were performed in cells cotransfected with a constant amount of CXCR4-RlucII and increasing amounts of GFP2–β-arrestin2. Cells were stimulated with 500 nM SDF-1, 1 μM pepducins (ATI-2341, ATI-2339, or ATI-2504), or vehicle (Hanks) for 10 min before BRET was measured following coel-400a addition using the BRET480-YFP filter set. The curves shown are derived from individual titration curves that are representative of three independent experiments. The error bars represent the mean ± SD from duplicate wells. (B) Kinetics of the ligand-promoted BRET between CXCR4-RlucII and GFP2–β-arrestin2. BRET was measured at the indicated times following the addition of 500 nM SDF-1 or 1 μM pepducins. Data shown are the mean ± SEM of four independent experiments. (C) Effect of increasing concentrations of SDF-1 or ATI-2341 on the BRET between CXCR4-RlucII and GFP2–β-arrestin2. BRET was measured 10 min following the addition of ligands (EC50: ATI-2341, 273.5 ± 78.6 nM vs. SDF-1, 1.8 ± 0.3 nM). (Inset) The y axis scale enlargement for better visibility. Data shown are the mean ± SEM of seven independent experiments.
Fig. 6.
ATI-2341 promotes a weak CXCR4 internalization. (A) Cell-surface expression of HA-CXCR4-vYFP is measured by flow-cytometry. vYFP emission represents CXCR4 total expression, and HA-Alexa Fluor 647 emission represents CXCR4 plasma membrane expression. The relative cell-surface expression (ratio Alexa/vYFP median emissions) is calculated for the selected cell population and is expressed as a percentage of the untreated condition. Data shown are the mean ± SEM of at least three independent experiments. (B) Kinetics of the ligand-promoted BRET between β-arrestin2–RlucII and β2-adaptin–eYFP in cells stably expressing β2-adaptin–eYFP transfected with β-arrestin2–RlucII and the untagged CXCR4. BRET was measured after coel-h addition, using the BRET480-YFP filter set, at the indicated times following the addition of 500 nM SDF-1 or 1 μM pepducins. Data shown are the mean ± SEM of three independent experiments. (C) Cell-surface expression of endogenous CXCR4 in SUP-T1 cells was assessed by flow-cytometry analysis. Cells were incubated with Hanks alone (green line) or stimulated with 500 nM SDF-1 (red line) or 1 μM ATI-2341 (blue line) for 30 min at 37 °C and stained with PerCP-Cy5.5-conjugated 12G5. The black line and the pink line in histogram plots represent unstained and isotype control-stained cells (PerCP/Cy5.5 Mouse IgG2a, k isotype control antibody), respectively (Left and Center). Data shown are representative of five independent experiments. The agonist-promoted loss of cell surface receptors (15 and 30 min stimulation) was calculated for the selected cell population and is expressed as a percentage of loss of CXCR4 cell-surface expression compared with the untreated condition (Right). Data shown are the mean ± SEM of at least three independent experiments.
Taken together with the results obtained for Gαi engagement and Gi activation, these data suggest a strong bias of ATI-2341 toward the Gi pathway vs. β-arrestins. To quantify this bias, we used a method (43) based on the Black and Leff operational model (44). We first determined τ and KA values using this model. τ (τ = [Rt]/KE), which describes the ligand’s efficacy toward specific pathways, is defined by the ratio of the total receptor density ([Rt]) over the general equilibrium dissociation constant (KE). KA is defined as the ligand equilibrium dissociation constant of the ligand-receptor complex. Using these parameters, the transduction ratios (τ/KA) and the transduction coefficients [Log (τ/KA)] for SDF-1 and ATI-2341 were deduced for each signaling pathway. To determine the relative ability of SDF-1 and ATI-2341 to activate a given pathway, we calculated the ΔLog (τ/KA), ΔLog (τ/KA) = Log (τ/KA)LIGAND − Log (τ/KA)SDF-1, for each of the pathways considered (Gαi engagement and β-arrestin2 recruitment to CXCR4). Finally, to quantify the ATI-2341 bias for Gαi engagement vs. β-arrestin2 recruitment, we calculated the ΔΔLog (τ/KA) as a difference between the ΔLog (τ/KA) values obtained for ATI-2341 for both signaling pathways, ΔΔLog (τ/KA) = ΔLog (τ/KA)SDF-1/ATI-2341(Gαi) − ΔLog (τ/KA)SDF-1/ATI-2341(β-arrestin2). The bias factor is defined as the anti-Log value of the ΔΔLog (τ/KA). The calculation of a bias factor allows a direct comparison of the relative effectiveness of ATI-2341 for each pathway in a system-independent manner, thus allowing one to distinguish a real bias from a difference that could result from partial agonism toward a less amplified pathway. As shown in Table 1, the ΔΔLog (τ/KA) comparing Gαi1 vs. β-arrestin2 engagement of 1.38 corresponds to a bias factor of 24 (i.e., anti-Log of 1.38) that indicates that ATI-2341 promotes the engagement of Gi 24 times more effectively than β-arrestin recruitment. The ΔLog (τ/Ka) calculated for ATI-2341 pepducin toward β-arrestin2 is significantly different from the ΔLog (τ/Ka) obtained for the pepducin toward the engagement of Gαi, indicating a significant bias of the pepducin toward Gαi vs. the β-arrestin pathway.
Table 1.
Data describing bias of ATI-2341 for Gαi engagement and β-arrestin2, GRK2, or GRK3 recruitment to CXCR4
| Engagement/recruitment | τ | KA | Log (τ/KA) ± SEM | ΔLog (τ/KA)† ± SEM | ΔΔLog (τ/KA) ± SEM | Bias |
| Gαi engagement | ||||||
| SDF-1 | 83.09 | 9.14E−08 | 8.90 ± 0.13 | 0.00 ± 0.18 | ||
| ATI-2341 | 25.03 | 1.75E−05 | 6.20 ± 0.14 | −2.70 ± 0.19 | ||
| β-arrestin2 recruitment | ||||||
| SDF-1 | 358.90 | 7.12E−07 | 8.70 ± 0.02 | 0.00 ± 0.03 | 0.00 ± 0.19 | 1.0 |
| ATI-2341 | 0.04 | 8.42E−07 | 4.62 ± 0.37 | −4.08*± 0.37 | 1.38 ± 0.42 | 24.0 |
| GRK2 recruitment | ||||||
| SDF-1 | 716.60 | 1.43E−06 | 8.71 ± 0.07 | 0.00 ± 0.09 | 0.00 ± 0.21 | 1.0 |
| ATI-2341 | 0.04 | 1.10E−06 | 4.62 ± 0.32 | −4.09**± 0.32 | 1.40 ± 0.38 | 24.9 |
| GRK3 recruitment | ||||||
| SDF-1 | 697.00 | 2.13E−06 | 8.51 ± 0.05 | 0.00 ± 0.06 | 0.00 ± 0.20 | 1.0 |
| ATI-2341 | 0.12 | 5.57E−06 | 4.33 ± 0.27 | −4.19**± 0.28 | 1.49 ± 0.34 | 30.8 |
Log (τ/KA), ΔLog (τ/KA), and bias factor values were calculated as described in Materials and Methods for Gαi engagement and β-arrestin2, GRK2, or GRK3 recruitment to CXCR4. *P < 0.05 and **P < 0.005 in comparison with the ΔLog (τ/KA) obtained for Gαi engagement.
The reference agonist was SDF-1.
ATI-2341 Promotes PKC- but Not GRK6-Promoted Phosphorylation of CXCR4.
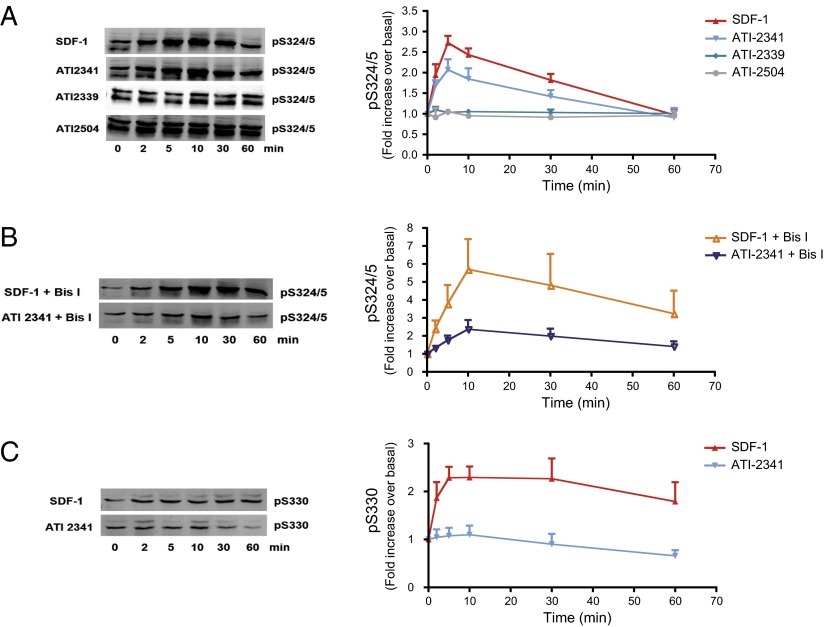
To assess the molecular mechanism underlying the negative bias toward β-arrestin recruitment, and given the role of receptor phosphorylation in the ligand-promoted recruitment of β-arrestin, we examined the SDF-1– and ATI-2341–promoted phosphorylation of the receptor. CXCR4 is phosphorylated by PKC and GRK6 on Ser-324 and -325 (Ser-324/5) in response to SDF-1 (45). To assess the relative contribution of these kinases to the SDF-1– and ATI-2341–promoted phosphorylation, the extent of phosphorylation was determined using an anti-phospho–Ser-324/5–specific antibody in the presence and absence of the broad-spectrum PKC inhibitor bisindolylmaleimide I (Bis I). In a previous study (45), we demonstrated that, in HEK293 cells, ∼60% of the SDF-1–promoted phosphorylation of Ser-324/5 was mediated by PKC (as determined by Bis I inhibition) and that PKCδ but not PKCα contributed to some but not all of this phosphorylation (using RNAi). This study also demonstrated that GRK6, but not GRK2, -3, or -5, mediated the remaining ∼40% of the SDF-promoted phosphorylation of Ser-324/5. Taken together, these data demonstrated that Bis I can be used to inhibit PKC-mediated phosphorylation of Ser-324/5 while having no effect on GRK6-mediated phosphorylation of Ser-324/5 or Ser-330. As previously described (45), we found that, in the absence of Bis I, SDF-1 promoted a rapid increase in Ser-324/5 phosphorylation, peaking at ∼5 min and returning to basal levels within 60 min (Fig. 7A). ATI-2341 also promoted the transient phosphorylation of Ser-324/5, albeit to a lower extent than SDF-1. The two control pepducins (ATI-2339 and 2504) were without effect on CXCR4 phosphorylation. In the presence of Bis I, the relative ATI-2341–promoted phosphorylation was considerably blunted compared with the SDF-1–stimulated phosphorylation (Fig. 7B), indicating that the ATI-2341–stimulated phosphorylation was predominantly PKC-dependent whereas GRK6 is the predominant kinase involved in the SDF-1–promoted phosphorylation of these residues. Consistent with this notion, SDF-1, but not ATI-2341, stimulated the phosphorylation of Ser-330 (Fig. 7C), a site that is solely phosphorylated by GRK6 in HEK293 cells as previously shown using Bis I and GRKs RNAis (45).
Fig. 7.
ATI-2341 promotes PKC- but not GRK6-dependent phosphorylation of CXCR4. HEK293 cells stably expressing FLAG-tagged CXCR4 were serum-starved for 6 h and then incubated without (A and C) or with (B) 2.5 μM of the PKC inhibitor Bis I for 30 min before stimulation with 100 nM SDF-1 or 3 μM ATI-2341 for 0–60 min. CXCR4 phosphorylation sites were detected using specific antibodies raised against either Ser-324/5 (A and B) or Ser-330 (C) in CXCR4. (Left) A representative immunoblot visualized using the ODYSSEY infrared imaging system from three to five individual experiments. (Right) Quantitative analysis of the fold increase ± SEM with SDF-1 or ATI-2341 from three to five independent experiments.
ATI-2341 Is a Weak Partial Agonist of GRK2 and GRK3 Engagement.
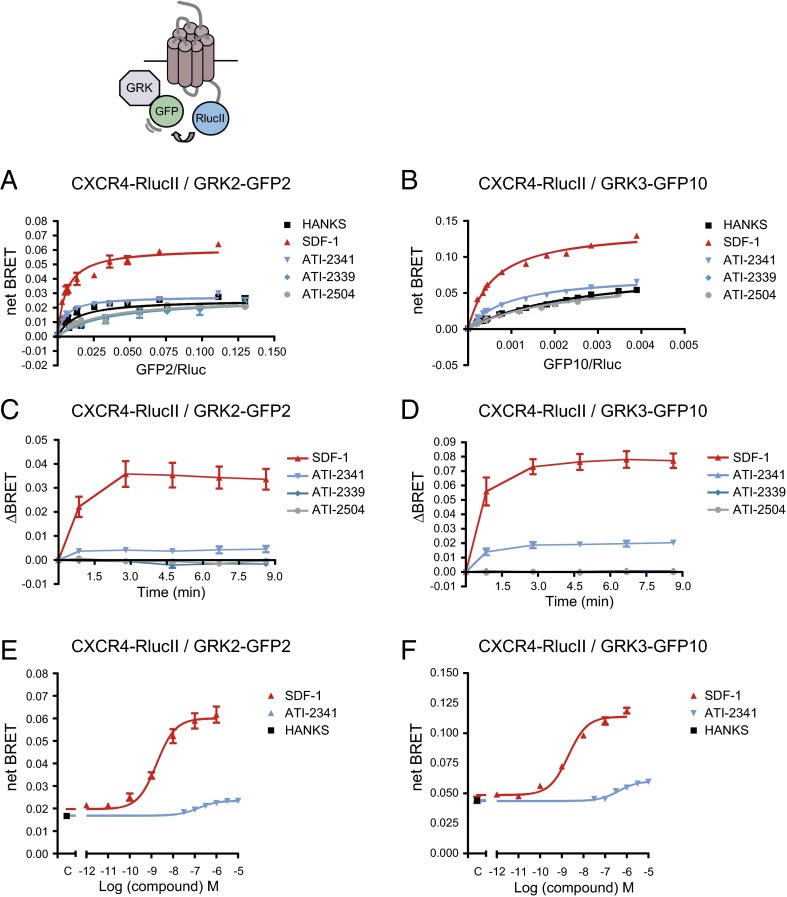
To assess the engagement of two other important kinases, GRK2 and -3, which play an important role in the recruitment of β-arrestin, we took advantage of BRET-based biosensors that directly monitor the recruitment of these kinases to the receptor. When monitoring BRET400-GFP2/10 between CXCR4-RlucII and either GRK2-GFP2 or GRK3-GFP10, a robust and time-dependent SDF-1–promoted increase in BRET was observed for the two kinases (Fig. 8 A–D). In contrast, ATI-2341 promoted only a very modest increase whereas the two control pepducins had no effect. Recruitment of GRK2 and GRK3 to CXCR4 were dose-dependent for both SDF-1 and ATI-2341, with EC50 values of 1.9 ± 0.3 nM and 2.0 ± 0.4 nM for SDF-1 and 154 ± 25 nM and 420 ± 43 nM for ATI-2341 for GRK2 and GRK3, respectively (Fig. 8 E and F).
Fig. 8.
ATI-2341 promotes a weak recruitment of GRK2 and GRK3 to CXCR4. BRET titration curves were performed in cells cotransfected with a constant amount of CXCR4-RlucII and increasing amounts of GRK2-GFP2 (A) or GRK3-GFP10 (B). Cells were stimulated with 500 nM SDF-1, 1 μM pepducins (ATI-2341, ATI-2339, and ATI-2504), or vehicle (Hanks) for 5 min. BRET was measured following coel-400a addition using the BRET400-GFP2/10 filter set. The curves shown are derived from individual titration curves that are representative of three (A) or four (B) independent experiments. The error bars represent the mean ± SD from duplicate wells. Kinetics of the ligand-promoted BRET between CXCR4-RlucII and GRK2-GFP2 (C) or GRK3-GFP10 (D). BRET was measured at the indicated times following the addition of 500 nM SDF-1 or 1 μM pepducins. Data shown are the mean ± SEM of five (C) or three (D) independent experiments. Effect of increasing concentrations of SDF-1 or ATI-2341 on the BRET between CXCR4-RlucII and GRK2-GFP2 (E) or GRK3-GFP10 (F). BRET was measured 5 min following the addition of the ligands (EC50 of 1.9 ± 0.3 nM and 2.0 ± 0.4 nM for SDF-1 and 154 ± 25 nM and 420 ± 43 nM for ATI-2341 for GRK2 and GRK3, respectively). Data shown are the mean ± SEM of three independent experiments.
These data suggest a negative bias of ATI-2341 toward the GRK recruitment and GRK-mediated phosphorylation of the receptor relative to the Gi engagement and activation. To quantify this bias, we calculated the transduction ratio for ATI-2341– and SDF-1–promoted GRK2 and GRK3 recruitment and compared it with the Gαi engagement to determine the bias factor. As shown in Table 1, the calculated bias factors indicate that ATI-2341 promotes the engagement of Gαi1 24.9 and 30.8 times more effectively than the recruitment of GRK2 and GRK3, respectively. The ΔLog (τ/Ka) calculated for ATI-2341 pepducin toward GRK2 and GRK3 were significantly different from the ΔLog (τ/Ka) obtained for the pepducin toward the engagement of Gαi, indicating a significant bias of the pepducin toward Gαi vs. GRK2 and GRK3 pathways.
GRK2 and GRK3 Are Involved in the Recruitment of β-Arrestin to CXCR4.
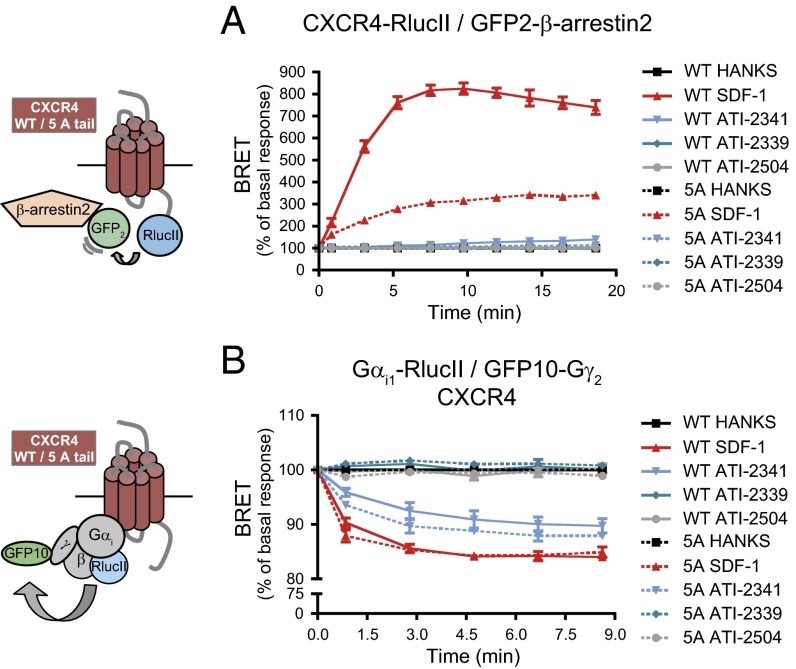
To confirm that the blunted ATI-2341–promoted recruitment of GRK2 and -3 could explain the negative bias of ATI-2341 toward β-arrestin vs. Gi, we assessed the ability of a mutant form of CXCR4 lacking the major GRK2 and -3 phosphorylation sites, CXCR4 5A tail (45), to recruit β-arrestin2 and activate Gi in response to SDF-1 and ATI-2341. By measuring BRET400-GFP2 between β-arrestin2–GFP2 and either CXCR4 5A tail-RlucII or wild-type CXCR4-RlucII, we observed that mutation of the GRK2/3 phosphorylation sites greatly inhibited both the robust SDF-1– and modest ATI-2341–promoted recruitment of β-arrestin2 (Fig. 9A), confirming that GRK2 and -3 play a central role in β-arrestin2 recruitment. In contrast, mutation of the GRK2/3 phosphorylation sites did not significantly affect the ability of either SDF-1 or ATI-2341 to activate Gi, as assessed by monitoring BRET400-GFP10 between Gαi1-RlucII and GFP10-Gγ2 (Fig. 9B).
Fig. 9.
β-arrestin recruitment to CXCR4 is dependent on GRK2 and GRK3. (A) Kinetics of the ligand-promoted BRET between wild type (WT, solid lines) or 5A tail mutant (5A, dotted lines) form of CXCR4-RlucII and GFP2-β-arrestin2. BRET was measured after coel-400a addition, using the BRET480-YFP filter set, at the indicated times following the addition of 500 nM SDF-1, 1 μM pepducins, or vehicle (Hanks). Data shown are the mean ± SEM of three independent experiments. BRET values are expressed as the percentage of basal response (Hanks buffer alone). (B) Kinetics of the ligand-promoted BRET between Gαi1-RlucII and GFP10-Gγ2 in cells cotransfected with the untagged Gβ subunit, and WT or 5A tail mutant form of CXCR4. BRET was measured after coel-400a addition, using the BRET400-GFP2/10 filter set, at the indicated times following the addition of 500 nM SDF-1 or 1 μM pepducins. Data shown are the mean ± SEM of three independent experiments. BRET values are expressed as the percentage of basal response (Hanks).
Discussion
Although pepducins were already known to allosterically modulate the function of GPCRs, both in cells (1, 2, 5, 9, 17) and in vivo (6, 18, 46), whether pepducins equally modulate all signaling pathways engaged by a receptor or can display functional selectivity by modulating only a subset of these pathways was unknown. The present study shows that the CXCR4-selective pepducin ATI-2341 displays clear functional selectivity, being biased in favor of Gi over β-arrestin and G13, two of the other downstream effectors being engaged by the receptor upon stimulation by its endogenous ligand SDF-1. The study also shows that the lack of engagement of β-arrestin by CXCR4 upon ATI-2341 stimulation results from an agonist-promoted phosphorylation pattern of the receptor that differs between pepducin and SDF-1.
In recent years, the notion that ligands can differentially modulate the activity of the various signaling pathways engaged by a single receptor has gained wide acceptance and is now known as functional selectivity or ligand-biased signaling (32, 33, 47–49). In many instances, ligands that are agonists for one pathway were found to be neutral antagonists or even inverse agonists for another pathway. Such functional selectivity is believed to result from the ability of different ligands to promote or stabilize distinct receptor conformations that in turn have different affinities for the various downstream effectors. Although this concept was first introduced for orthosteric ligands, examples of allosteric ligands acting as functionally selective modulators are emerging. In a recent study, a peptidomimetic derived from the second extracellular loop of the prostaglandin FP receptor was found to be a negative allosteric modulator of the G12/13-Rho signaling pathway while being a positive allosteric modulator of the Gq-PKC pathway (50). When characterizing pepducins that target the protease activated receptor 1 (PAR1), Cisowski et al. (5) found that a pepducin derived from the third intracellular loop fully inhibited cell motility, calcium mobilization, and ERK1/2 activation promoted by thrombin. In contrast, an intracellular loop 1 pepducin fully blocked cell motility but only partially inhibited calcium mobilization and had no effect on ERK1/2 activation. However, no study to date had assessed the ability of allosteric agonists such as ATI-2341 to display functional selectivity.
Using BRET-based biosensors that directly monitor the engagement of CXCR4 effectors by the receptor, we clearly showed a bias of the allosteric agonist ATI-2341 toward the engagement and the activation of Gi vs. either the engagement of Gα13 or the recruitment of β-arrestins. To exclude the possibility that the apparent bias could result from partial agonism that would be differentially detected by the different assays, the relative transduction ratios (τ/KA) and bias factors were calculated using a method based on the operational model of receptor activation (43). This method was selected over other approaches that use the affinities derived from radioligand binding assays to determine the KA (51) because it has been found impractical to measure accurate binding affinities using pepducins (2). The bias factor calculated for Gi vs. β-arrestin2 recruitment, using SDF-1 as the reference compound, indicates that ATI-2341 is 24.0 times more efficient in promoting Gi engagement over β-arrestin recruitment, revealing a strong bias. The observation that SDF-1 had very similar relative transduction ratios for Gi and β-arrestin2 engagement indicates that it does not have a strong bias for one pathway over the other and justifies its use as the reference compound to calculate the bias of the pepducin. No formal bias factor could be calculated for the G13 pathway because the very weak signal generated by the pepducin prevented the calculation of a KA. However, the absence of Gα13 engagement strongly suggest a negative bias toward this pathway for the pepducin.
The lower ability of ATI-2341 vs. SDF-1 to promote the recruitment of β-arrestins to CXCR4 was observed for both β-arrestin2 and β-arrestin1 as well as a truncated version of β-arrestin1 that has an increased affinity for the receptor (Fig. S4B). Such negative bias toward the β-arrestin pathway may be due to the different phosphorylation profile promoted by the pepducin vs. SDF-1. Indeed, whereas SDF-1-promoted phosphorylation involves both PKC and GRKs, the preponderant CXCR4 phosphorylation promoted by ATI-2341 was due to PKC. In addition, the pepducin promoted only a very modest GRK2 and GRK3 recruitment compared with SDF-1, the calculated bias factor indicating that ATI-2341 is 24.9- and 30.8-fold more efficient at promoting Gαi engagement than GRK2 and GRK3 recruitment, respectively, whereas SDF-1 had similar relative transduction ratios for GRK2/3 and Gi. Although no antibody exists that selectively recognizes the CXCR4 sites phosphorylated by GRK2/GRK3, an antibody detecting the phosphorylation of a GRK6 site, Ser-330, further showed that, in contrast to SDF-1, ATI-2341 does not promote GRK6-mediated phosphorylation of CXCR4. The results presented herein and previous studies clearly showed that phosphorylation of CXCR4 by GRK2 and GRK3 promotes β-arrestin recruitment (45, 52). It can thus be suggested that the decreased propensity of ATI-2341 to favor GRK-mediated recruitment underlies the negative bias toward β-arrestins recruitment.
Given that pepducins are believed to act intracellularly by binding directly to the inner face of targeted GPCRs (1, 53), it may not be surprising that different subsets of downstream effectors than those promoted by the binding of SDF-1 are engaged. However, the precise mechanism by which ATI-2341 can selectively activate Gi remains unknown. Consistent with the notion that the action occurs at the level of the receptor and not Gi directly is supported by our observation that the pepducin promoted the direct engagement of Gαi to CXCR4 and that ATI-2341–induced activation of Gi was seen in cells expressing CXCR4 but not the Gi-coupled α2AAR or CCR2. Given that CXCR4 has been shown to form homodimers (54, 55), it can be hypothesized that pepducins might act by modulating dimerization. However, no effect of ATI-2341 could be detected on the BRET between CXCR4-Rluc and CXCR4-YFP (Fig. S5), indicating that the pepducin did not affect dimerization. The lack of effect of the pepducin is in contrast with the SDF-1–promoted increase in BRET associated with a change in conformation of the dimer (54). This finding further indicates that ATI-2341 induces conformational changes that are different from those promoted by SDF-1. When considering the BRET between CXCR4 and Gαi, both ATI-2341 and SDF-1 induced an increase in the maximal BRET without affecting the BRET50, suggesting that both ligands promoted a conformational rearrangement of a preexisting receptor–G protein complex rather than the recruitment of Gi to the receptor. These data are consistent with the notion that GPCRs may be precoupled to G protein, even before activation (41, 56, 57). Although no detailed structure activity relationship (SAR) was performed, the composition in amino acid of the pepducins plays an important role for their activity. Indeed, a pepducin lacking the last three amino acids of ATI-2341 was without activity and used as a negative control throughout the study. Another analog lacking the first four amino acids of ATI-2341 also showed a dramatically reduced ability to promote Gαi1 engagement (Fig. S6), indicating that residues both in N- and C-terminal position of the peptide play a role for activity. Also, a pepducin differing from ATI-2341 by a substitution of the position 1 methionine for a glycine and the substitution of the serine and methionine at position 9 and 10 by d-proline and a histidine, respectively, was more efficacious than ATI-2341 for both Gαi1 and β-arrestin engagements, suggesting a possible SAR.
A potentially important consequence of functional selectivity at GPCR is the different physiological responses that could be triggered by biased vs. balanced ligands. For instance, reduced ability of ATI-2341 to promote β-arrestin recruitment could result in attenuated desensitization and/or altered β-arrestin–dependent signaling pathways. In recent studies, whereas SDF-1 was found to promote mobilization of B and T lymphocytes, ATI-2341 did not lead to the mobilization of either lymphocyte type (18, 28, 58). However, the two drugs promote neutrophil and hematopoietic progenitor cell mobilization (18). Whether this functional difference is due to the biased signaling activity of ATI-2341 remains to be investigated. Interestingly, Gα13/Rho axis has recently been implicated in CXCR4-mediated chemotaxis and migration of metastatic breast cancer cells (38). Therefore, the observation that SDF-1 but not ATI-2341 leads to the engagement of Gα13 by CXCR4 indicates that the pepducin could provide a useful tool to assess the role of the different signaling pathways in CXCR4-mediated cell migration and mobilization.
Overall, our study demonstrates that the pepducin ATI-2341 is a biased allosteric agonist, favoring Gi over G13 and β-arrestin pathways. In addition to identifying a biased ligand for CXCR4, thus providing a useful tool for studying the functional consequences of such bias, our study clearly illustrates how BRET-based biosensors can be used to directly assess ligand-biased signaling and their molecular basis. The study also opens up the intriguing possibility that pepducins represent a generic approach to generating biased ligands that can selectively activate specific pathways.
Materials and Methods
Plasmids, Cell Culture and Transfections.
The plasmids used in this study are described in SI Materials and Methods. Transiently and stably transfected Human Embryonic Kidney 293 (HEK293T) cells as well as SUP-T1 cells were used in this study. Cell culture and transfection conditions are described in SI Materials and Methods.
Bioluminescence Resonance Energy Transfer Measurement.
BRET480-YFP and BRET400-GFP2/10 were used in this study (59) and experiments were performed as described in SI Materials and Methods.
Western Blotting.
The CXCR4 specific phosphorylation state of Ser-324/5 or Ser-330 was measured by Western blotting as previously described (45) (SI Materials and Methods).
Flow Cytometry.
To measure cell surface receptor expression, flow-cytometry experiments were performed on HEK293T and SUP-T1 cells using a LSR II flow-cytometer (BD Biosciences) and data were analyzed using the BD FACSDiva and FlowJo software (SI Materials and Methods).
Calcium Measurements.
Obelin biosensor was used as a calcium reporter (60) in HEK293T cells while calcium mobilization was measured using a calcium 4 dye in SUP-T1 cells (SI Materials and Methods).
MAPK Experiments.
Dose-response experiments were performed in HEK293 cells stably expressing human CXCR4 using the Cellul'erk kit (Cisbio). Kinetics experiments were performed in HEK293T cells endogenously expressing CXCR4 using the AlphaScreenSureFire kit (Perkin Elmer) (SI Materials and Methods).
Data and Statistical Analyzis.
Data analysis, statistical significance and bias factors were calculated using GraphPad Prism (GraphPad Software, Inc.) (SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank M. Lagacé for critical reading of the manuscript; M. Hogue, W. Stallaert, and A.-M. Schönegge for providing plasmids and cell lines; G. Dulude and D. Gagné for flow cytometry technical assistance; and C. Le Gouill, E. van der Westhuizen, and G. Piñeyro for helpful discussions. J.Q. was supported by a fellowship from the Fonds de la Recherche en Santé du Québec. M.B. holds a Canada Research Chair in Signal Transduction and Molecular Pharmacology. This work was supported by Anchor Therapeutics (M.B. and J.L.B.) and Canadian Institutes of Health Research Grant CIHR-11215 (to M.B.).
Footnotes
Conflict of interest statement: This study was performed as a collaboration between the academic laboratories of M.B. and J.L.B. and scientists at Anchor Therapeutics. Anchor Therapeutics is a biotechnology company that designs and develops pepducins as modulators of G protein-coupled receptors (GPCRs) for therapeutic purposes. Neither M.B., J.L.B., or members of their laboratories have financial interests related to Anchor Therapeutics. The study was partly supported by a grant from Anchor Therapeutics.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312515110/-/DCSupplemental.
References
- 1.Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc Natl Acad Sci USA. 2002;99(2):643–648. doi: 10.1073/pnas.022460899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janz JM, et al. Direct interaction between an allosteric agonist pepducin and the chemokine receptor CXCR4. J Am Chem Soc. 2011;133(40):15878–15881. doi: 10.1021/ja206661w. [DOI] [PubMed] [Google Scholar]
- 3.O’Callaghan K, Kuliopulos A, Covic L. Turning receptors on and off with intracellular pepducins: New insights into G-protein-coupled receptor drug development. J Biol Chem. 2012;287(16):12787–12796. doi: 10.1074/jbc.R112.355461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boire A, et al. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120(3):303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Cisowski J, et al. Targeting protease-activated receptor-1 with cell-penetrating pepducins in lung cancer. Am J Pathol. 2011;179(1):513–523. doi: 10.1016/j.ajpath.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covic L, Misra M, Badar J, Singh C, Kuliopulos A. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat Med. 2002;8(10):1161–1165. doi: 10.1038/nm760. [DOI] [PubMed] [Google Scholar]
- 7.Kaneider NC, et al. ‘Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol. 2007;8(12):1303–1312. doi: 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubo S, et al. Distinct activity of peptide mimetic intracellular ligands (pepducins) for proteinase-activated receptor-1 in multiple cells/tissues. Ann N Y Acad Sci. 2006;1091:445–459. doi: 10.1196/annals.1378.087. [DOI] [PubMed] [Google Scholar]
- 9.Leger AJ, et al. Blocking the protease-activated receptor 1-4 heterodimer in platelet-mediated thrombosis. Circulation. 2006;113(9):1244–1254. doi: 10.1161/CIRCULATIONAHA.105.587758. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P, et al. Suppression of arterial thrombosis without affecting hemostatic parameters with a cell-penetrating PAR1 pepducin. Circulation. 2012;126(1):83–91. doi: 10.1161/CIRCULATIONAHA.112.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann R, et al. Met receptor tyrosine kinase transactivation is involved in proteinase-activated receptor-2-mediated hepatocellular carcinoma cell invasion. Carcinogenesis. 2009;30(9):1487–1496. doi: 10.1093/carcin/bgp153. [DOI] [PubMed] [Google Scholar]
- 12.Sevigny LM, et al. Interdicting protease-activated receptor-2-driven inflammation with cell-penetrating pepducins. Proc Natl Acad Sci USA. 2011;108(20):8491–8496. doi: 10.1073/pnas.1017091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sroussi HY, Lu Y, Villines D, Sun Y. The down regulation of neutrophil oxidative metabolism by S100A8 and S100A9: Implication of the protease-activated receptor-2. Mol Immunol. 2012;50(1-2):42–48. doi: 10.1016/j.molimm.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HY, et al. Activation of human monocytes by a formyl peptide receptor 2-derived pepducin. FEBS Lett. 2010;584(18):4102–4108. doi: 10.1016/j.febslet.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 15.Licht T, Tsirulnikov L, Reuveni H, Yarnitzky T, Ben-Sasson SA. Induction of pro-angiogenic signaling by a synthetic peptide derived from the second intracellular loop of S1P3 (EDG3) Blood. 2003;102(6):2099–2107. doi: 10.1182/blood-2002-12-3634. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal A, et al. Identification of a metalloprotease-chemokine signaling system in the ovarian cancer microenvironment: Implications for antiangiogenic therapy. Cancer Res. 2010;70(14):5880–5890. doi: 10.1158/0008-5472.CAN-09-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Callaghan K, et al. Targeting CXCR4 with cell-penetrating pepducins in lymphoma and lymphocytic leukemia. Blood. 2012;119(7):1717–1725. doi: 10.1182/blood-2011-04-347518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tchernychev B, et al. Discovery of a CXCR4 agonist pepducin that mobilizes bone marrow hematopoietic cells. Proc Natl Acad Sci USA. 2010;107(51):22255–22259. doi: 10.1073/pnas.1009633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi WT, An J. Biology and clinical relevance of chemokines and chemokine receptors CXCR4 and CCR5 in human diseases. Exp Biol Med (Maywood) 2011;236(6):637–647. doi: 10.1258/ebm.2011.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lusso P. HIV and the chemokine system: 10 years later. EMBO J. 2006;25(3):447–456. doi: 10.1038/sj.emboj.7600947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoggatt J, Pelus LM. Mobilization of hematopoietic stem cells from the bone marrow niche to the blood compartment. Stem Cell Res Ther. 2011;2(2):13. doi: 10.1186/scrt54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marquez-Curtis LA, Turner AR, Sridharan S, Ratajczak MZ, Janowska-Wieczorek A. The ins and outs of hematopoietic stem cells: Studies to improve transplantation outcomes. Stem Cell Rev. 2011;7(3):590–607. doi: 10.1007/s12015-010-9212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suárez-Álvarez B, López-Vázquez A, López-Larrea C. Mobilization and homing of hematopoietic stem cells. Adv Exp Med Biol. 2012;741:152–170. doi: 10.1007/978-1-4614-2098-9_11. [DOI] [PubMed] [Google Scholar]
- 24.Burger JA, Kipps TJ. CXCR4: A key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107(5):1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 25.Kryczek I, Wei S, Keller E, Liu R, Zou W. Stroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesis. Am J Physiol Cell Physiol. 2007;292(3):C987–C995. doi: 10.1152/ajpcell.00406.2006. [DOI] [PubMed] [Google Scholar]
- 26.Sun X, et al. CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29(4):709–722. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohty M, et al. The role of plerixafor in optimizing peripheral blood stem cell mobilization for autologous stem cell transplantation. Leukemia. 2011;25(1):1–6. doi: 10.1038/leu.2010.224. [DOI] [PubMed] [Google Scholar]
- 28.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184(3):1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devine SM, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112(4):990–998. doi: 10.1182/blood-2007-12-130179. [DOI] [PubMed] [Google Scholar]
- 30.Kean LS, et al. Significant mobilization of both conventional and regulatory T cells with AMD3100. Blood. 2011;118(25):6580–6590. doi: 10.1182/blood-2011-06-359331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roland J, et al. Role of the intracellular domains of CXCR4 in SDF-1-mediated signaling. Blood. 2003;101(2):399–406. doi: 10.1182/blood-2002-03-0978. [DOI] [PubMed] [Google Scholar]
- 32.Galandrin S, Oligny-Longpré G, Bouvier M. The evasive nature of drug efficacy: Implications for drug discovery. Trends Pharmacol Sci. 2007;28(8):423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Kenakin TP. Biased signalling and allosteric machines: New vistas and challenges for drug discovery. Br J Pharmacol. 2012;165(6):1659–1669. doi: 10.1111/j.1476-5381.2011.01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stallaert W, Christopoulos A, Bouvier M. Ligand functional selectivity and quantitative pharmacology at G protein-coupled receptors. Expert Opin Drug Discov. 2011;6(8):811–825. doi: 10.1517/17460441.2011.586691. [DOI] [PubMed] [Google Scholar]
- 36.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17(3):126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan W, Martin D, Gutkind JS. The Galpha13-Rho signaling axis is required for SDF-1-induced migration through CXCR4. J Biol Chem. 2006;281(51):39542–39549. doi: 10.1074/jbc.M609062200. [DOI] [PubMed] [Google Scholar]
- 38.Yagi H, et al. A synthetic biology approach reveals a CXCR4-G13-Rho signaling axis driving transendothelial migration of metastatic breast cancer cells. Sci Signal. 2011;4(191):ra60. doi: 10.1126/scisignal.2002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng ZJ, et al. beta-arrestin differentially regulates the chemokine receptor CXCR4-mediated signaling and receptor internalization, and this implicates multiple interaction sites between beta-arrestin and CXCR4. J Biol Chem. 2000;275(4):2479–2485. doi: 10.1074/jbc.275.4.2479. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277(51):49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 41.Galés C, et al. Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol. 2006;13(9):778–786. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- 42.Hamdan FF, et al. Unraveling G protein-coupled receptor endocytosis pathways using real-time monitoring of agonist-promoted interaction between beta-arrestins and AP-2. J Biol Chem. 2007;282(40):29089–29100. doi: 10.1074/jbc.M700577200. [DOI] [PubMed] [Google Scholar]
- 43.Kenakin T, Watson C, Muniz-Medina V, Christopoulos A, Novick S. A simple method for quantifying functional selectivity and agonist bias. ACS Chem Neurosci. 2012;3(3):193–203. doi: 10.1021/cn200111m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci. 1983;220(1219):141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- 45.Busillo JM, et al. Site-specific phosphorylation of CXCR4 is dynamically regulated by multiple kinases and results in differential modulation of CXCR4 signaling. J Biol Chem. 2010;285(10):7805–7817. doi: 10.1074/jbc.M109.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaneider NC, Agarwal A, Leger AJ, Kuliopulos A. Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat Med. 2005;11(6):661–665. doi: 10.1038/nm1245. [DOI] [PubMed] [Google Scholar]
- 47.Kenakin T, Christopoulos A. Signalling bias in new drug discovery: Detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12(3):205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- 48.Urban JD, Vargas GA, von Zastrow M, Mailman RB. Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology. 2007;32(1):67–77. doi: 10.1038/sj.npp.1301071. [DOI] [PubMed] [Google Scholar]
- 49.Azzi M, et al. Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA. 2003;100(20):11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goupil E, et al. A novel biased allosteric compound inhibitor of parturition selectively impedes the prostaglandin F2alpha-mediated Rho/ROCK signaling pathway. J Biol Chem. 2010;285(33):25624–25636. doi: 10.1074/jbc.M110.115196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajagopal S, et al. Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol. 2011;80(3):367–377. doi: 10.1124/mol.111.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orsini MJ, Parent JL, Mundell SJ, Marchese A, Benovic JL. Trafficking of the HIV coreceptor CXCR4: Role of arrestins and identification of residues in the c-terminal tail that mediate receptor internalization. J Biol Chem. 1999;274(43):31076–31086. doi: 10.1074/jbc.274.43.31076. [DOI] [PubMed] [Google Scholar]
- 53.Tsuji M, et al. FRET-based imaging of transbilayer movement of pepducin in living cells by novel intracellular bioreductively activatable fluorescent probes. Org Biomol Chem. 2013;11(18):3030–3037. doi: 10.1039/c3ob27445d. [DOI] [PubMed] [Google Scholar]
- 54.Percherancier Y, et al. Bioluminescence resonance energy transfer reveals ligand-induced conformational changes in CXCR4 homo- and heterodimers. J Biol Chem. 2005;280(11):9895–9903. doi: 10.1074/jbc.M411151200. [DOI] [PubMed] [Google Scholar]
- 55.Wu B, et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330(6007):1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jakubík J, Janíčková H, Randáková A, El-Fakahany EE, Doležal V. Subtype differences in pre-coupling of muscarinic acetylcholine receptors. PLoS ONE. 2011;6(11):e27732. doi: 10.1371/journal.pone.0027732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nobles M, Benians A, Tinker A. Heterotrimeric G proteins precouple with G protein-coupled receptors in living cells. Proc Natl Acad Sci USA. 2005;102(51):18706–18711. doi: 10.1073/pnas.0504778102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christopherson K, 2nd, Hromas R. Chemokine regulation of normal and pathologic immune responses. Stem Cells. 2001;19(5):388–396. doi: 10.1634/stemcells.19-5-388. [DOI] [PubMed] [Google Scholar]
- 59.Breton B, Lagacé M, Bouvier M. Combining resonance energy transfer methods reveals a complex between the alpha2A-adrenergic receptor, Galphai1beta1gamma2, and GRK2. FASEB J. 2010;24(12):4733–4743. doi: 10.1096/fj.10-164061. [DOI] [PubMed] [Google Scholar]
- 60.Campbell AK, Dormer RL. Studies on free calcium inside pigeon erythrocyte ‘ghosts’ by using the calcium-activated luminescent protein, obelin. Biochem Soc Trans. 1975;3(5):709–711. doi: 10.1042/bst0030709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.