Significance
Flies use fermenting fruit as a food source and a site for oviposition. One of the main metabolites of fermentation is ethanol. When provided with different choices in the laboratory, female flies prefer to lay their eggs on food supplemented with ecologically relevant concentrations of ethanol. We show that different subsets of dopaminergic neurons have opposing effects on oviposition preference. We propose that in the wild, this may be a mechanism by which flies choose oviposition sites that are optimal for offspring fitness and survival and that this choice is highly dependent on context.
Abstract
The neural circuits that mediate behavioral choice evaluate and integrate information from the environment with internal demands and then initiate a behavioral response. Even circuits that support simple decisions remain poorly understood. In Drosophila melanogaster, oviposition on a substrate containing ethanol enhances fitness; however, little is known about the neural mechanisms mediating this important choice behavior. Here, we characterize the neural modulation of this simple choice and show that distinct subsets of dopaminergic neurons compete to either enhance or inhibit egg-laying preference for ethanol-containing food. Moreover, activity in α′β′ neurons of the mushroom body and a subset of ellipsoid body ring neurons (R2) is required for this choice. We propose a model where competing dopaminergic systems modulate oviposition preference to adjust to changes in natural oviposition substrates.
In nature, rotting fruit is the social hub for the fruit fly Drosophila melanogaster. Flies use fermenting fruit as a food source (1) and a site for oviposition (2). The choice of a suitable oviposition substrate is an ecologically important decision with a direct impact on species fitness. However, other than having a clear preference for fermenting fruit, how females choose oviposition sites in nature is largely unknown.
One of the main metabolites of fermentation is ethanol, which is present in ripe fleshy fruits (3). Although ethanol concentrations in the fruit are rather low [≤5% (vol/vol)] (4), plumes containing ethanol vapor can act as long-distance signals to attract flies to rotting fruit (3, 5). When given the choice, female flies prefer to lay their eggs on media containing low concentrations of ethanol (up to 5%) (6), which leads to enhanced fitness of the developing larva and the adult fly.
D. melanogaster’s resistance to ethanol toxicity may have evolved to allow inhabitation of ethanol-containing environments (7). For example, adult flies allowed to mate on ethanol-containing media improve mating success and fecundity (8). Although rearing larvae on food containing relatively high ethanol concentrations delays development and decreases survival (9–11), larvae reared on low concentrations of ethanol develop into heavier adults (7, 12). This weight increase may be a result of D. melanogaster larvae metabolizing ethanol and using it as a food source (12). Ingestion of ethanol during the larval stage has additional benefits, such as protection from natural parasites such as endoparasitoid wasps (13).
Studies on the neural circuits underlying the oviposition program and choice of oviposition substrates have been initiated only recently in D. melanogaster (14, 15). Ethanol is a particularly intriguing stimulus for oviposition preference, because it has, depending on concentration, both beneficial and detrimental effects on developing larvae. In flies, the function of dopaminergic neurons has been implicated in responses to both rewarding and aversive stimuli (16–19), making it a candidate neuromodulator to signal the beneficial and detrimental effects of ethanol. Dopamine signaling has also been implicated in other innate behaviors required for survival, such as food consumption, sex, and social interaction (20–22). Although the circuitry for ethanol oviposition preference is unknown, both dopaminergic and mushroom body (MB) signaling is required for flies to remember ethanol as a reward (23).
We report that females show concentration-dependent preference for oviposition on food containing ethanol, with highest preference for food substrates containing the most ecologically beneficial concentrations of ethanol. This simple behavior relies on functional dopamine neurons, with different subsets regulating oviposition site preference in opposite ways. Our data also suggest that dopaminergic innervation of higher-order brain regions, including specific subsets of the MB and ellipsoid body (EB) neurons modulate the decision to lay eggs on ethanol.
Results
Flies Show Oviposition Preference for Ethanol-Containing Media.
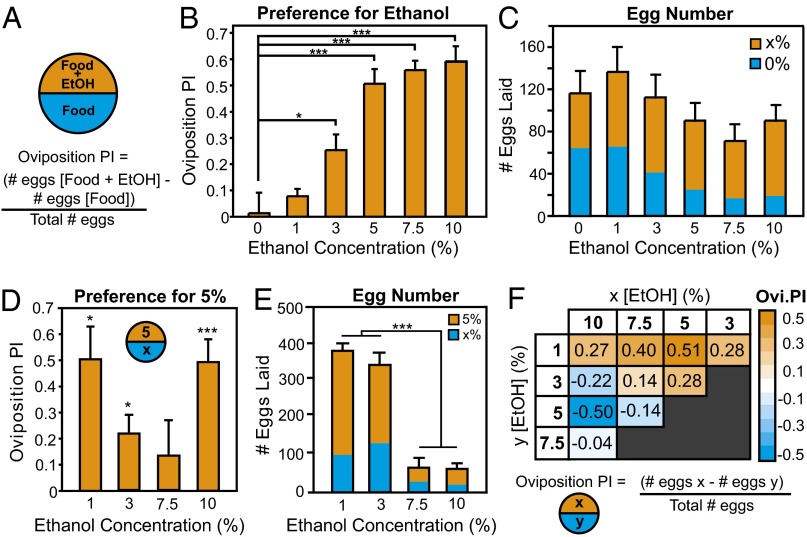
To measure oviposition preference, we used a simple assay in which flies choose between ethanol-containing food and regular food (Fig. 1A). We tested preference for concentrations of ethanol ranging from 0% to 10% and found that flies preferred substrates containing ethanol at all concentrations tested (Fig. 1B). There was a trend toward lower total numbers of eggs laid on plates containing higher concentrations of ethanol (Fig. 1C) known to be detrimental to larval development. Although high concentrations of ethanol (>5%) have negative effects on larval development (11) and decrease fecundity (8), females still preferred to lay eggs on food containing high ethanol concentrations over food lacking ethanol. To determine the optimal ethanol concentration for egg-laying, we presented flies with an arena containing six different concentrations of ethanol ranging from 0% to 10% (Fig. S1A). Similar to our two-choice assay, flies preferred to lay eggs on higher ethanol concentrations (Fig. S1B).
Fig. 1.
Flies prefer to lay their eggs on ethanol-containing food. (A) Cartoon of egg-laying assay. (B) Given the choice between food with and without ethanol, flies preferred to lay eggs on ethanol [ANOVA: F(5,71) = 25.36, P < 0.0001; n = 13–16; Tukey’s post hoc comparisons: for 0% vs. 3%, P = 0.04; for 0% vs. 5%, 7.5%, or 10%, P < 0.0001]. (C) The total number of eggs laid is not significantly affected by the presence of ethanol [ANOVA: F(5,66) = 1.46, P = 0.22; n = 11–13]. (D) Flies preferred to lay their eggs on 5% ethanol over 1% [Wilcoxon: χ2(1,8) = 6.05, P = 0.01], 3% [χ2(1,8) = 6.05, P = 0.01], or 10% [χ2(1,18) = 16.31, P < 0.0001] ethanol. There was no preference between 5% and 7.5% ethanol [χ2(1,10) = 0.48, P = 0.49]. (E) The number of eggs laid on ethanol concentrations lower than 5% was significantly greater than those laid on concentrations higher than 5% [ANOVA: F(3,26) = 249.45]. (F) Oviposition preference depends on the context of the oviposition substrate. Two-choice tests between 1%, 3%, 5%, 7.5%, and 10% ethanol suggest that concentrations near 5% ethanol are preferred. Preference indices for all pairwise comparisons are summarized. Detailed data are shown in Fig. S2. Bars on graphs represent means ± SEM. *P < 0.05; **P < 0.001; ***P < 0.0001.
Oviposition Preference Depends on the Context of the Egg-Laying Substrate.
The above results show that Drosophila females prefer to lay their eggs on ethanol concentrations that could potentially be toxic to developing larvae. However, oviposition decisions in Drosophila are also dependent on the surrounding environment onto which first-instar larvae can migrate (24). Thus, flies may lay eggs on potentially toxic ethanol concentrations only when low concentrations of ethanol beneficial to young larvae are found nearby. To test this, we provided flies with two-choice tests between various combinations of ethanol concentrations. Given a choice between 5% and 1% or 5% and 10%, flies showed a strong preference for 5% ethanol (Fig. 1D). Weaker preference for 5% ethanol was obtained when flies were given choices of 3% and 5% or 7.5% and 5% (Fig. 1D). In addition, flies reduced overall egg-laying when given choices of 5% and 7.5% ethanol or 5% and 10% ethanol (Fig. 1E). Given a choice between various ethanol concentrations ranging from 3% to 10% and 1% ethanol, flies always preferred the higher concentrations (Fig. S2 A–C). However, when presented with choices between either 3% and 10% or 5% and 10% (Fig. 1D and Fig. S2 D–F), flies chose the lower ethanol concentrations. Thus, it seems that 3–5% ethanol may be an optimal oviposition substrate. When choosing between 3% and 5%, however, flies preferred 5% (Fig. 1D and Fig. S2 D–F), suggesting that 5% is the highest concentration of ethanol that may be beneficial.
Taken together, these results suggest that flies evaluate the context of the egg-laying environment to define their preference, which is highest at concentrations that are beneficial to larvae. However, their preference is highly sensitive to ethanol concentrations present in nearby substrates. These results also show that flies can clearly discriminate between subtle differences in ecologically beneficial ethanol concentration. For example, flies show a significant preference for 3% over 1% and 5% over 3% ethanol food (Fig. 1F and Fig. S2 A–F), but no significant preference for 5% over 7.5% or 7.5% over 10% ethanol-containing food (Fig. 1F and Fig. S2 G–L).
Flies Do Not Show Positional Preference for Ethanol.
Despite the strong drive to lay eggs on a suitable substrate, the flies’ choice to dwell on this substrate is uncorrelated. For example, whereas flies prefer to lay eggs on a food containing either acetic acid or lobeline, they show positional avoidance of the same substrates (15, 25). To test whether this was also the case for ethanol, we tested the relationship between oviposition and positional preference (0% vs. 5%; Fig. S3A). Flies preferred to be on ethanol-containing food only while laying most of their eggs, within the first 50 min of the assay (Fig. S3B), but showed no positional preference afterward (Fig. S3C). Thus, flies show neither positional preference nor avoidance for ethanol-containing food, and the drive to lay eggs on ethanol can be dissociated from positional preference.
Flies Use Smell and Taste to Determine Oviposition Preference.
Flies show preference for the odor of ethanol (Fig. S4A). However, although removing antennae eliminated olfactory preference, it did not eliminate oviposition preference for 5% ethanol, suggesting that other sensory modalities are involved (Fig. S4 B–D). Surprisingly, abolishing the ability to taste altogether or in subsets of chemoreceptor neurons (using pox neuro alleles) (26) resulted in oviposition aversion for ethanol-containing food (Fig. S4E). Removing the ability to smell and taste decreased, but did not abolish, this aversion, suggesting that another sensory modality may also be involved (Fig. S4F). Clearly, the sensory input leading to ethanol preference is complex and requires more study.
Dopamine Affects Oviposition Preference for Ethanol.
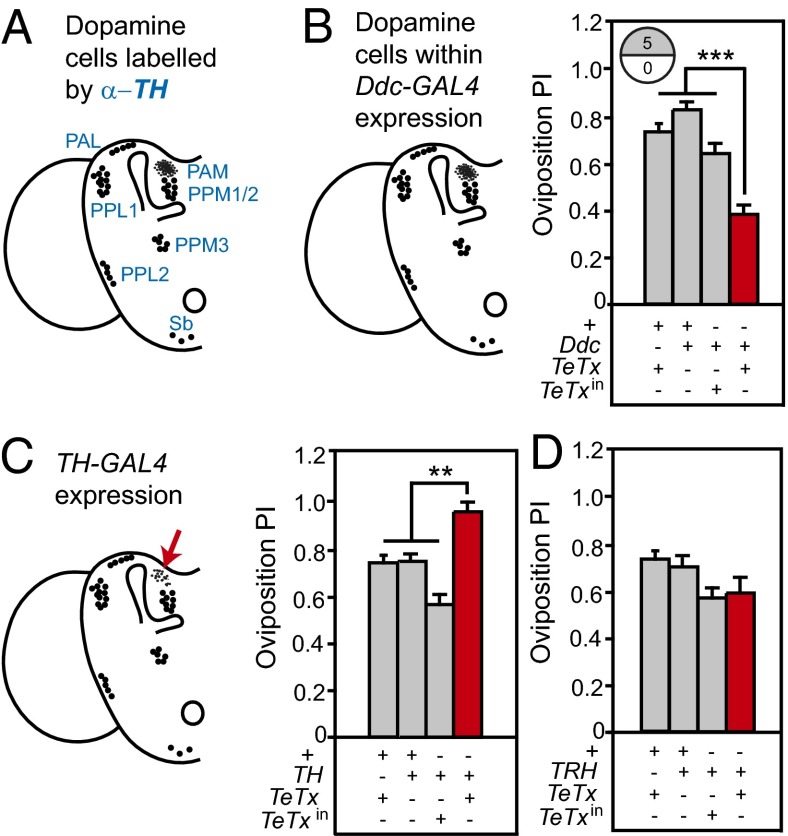
In the Drosophila central brain, subsets of dopaminergic cell bodies form discrete clusters (Fig. 2A). Each dopaminergic neuron sends extensive arborizations throughout the central brain, suggesting a complex modulatory function (27, 28) (Fig. S5A). To ask whether dopamine neurons play a role in oviposition preference for 5% ethanol, we used the GAL4/UAS system (29) to express tetanus toxin light chain (UAS-TeTx) to block synaptic transmission (30) in tyrosine hydroxylase (TH)- and Dopa decarboxylase (Ddc)-expressing neurons. Expression was targeted with either Ddc-GAL4 (31) (expressed in most dopaminergic and serotonergic neurons) or TH-GAL4 (32) (expressed in most dopaminergic neurons; Fig. S5B). Ddc-GAL4 and TH-GAL4 share expression with the exception of a subset of neurons termed the PAM neurons (33) (Fig. 2 B and C).
Fig. 2.
Activity of dopamine and not serotonin neurons affects oviposition preference. (A) Dopaminergic neuron cell body positions in one hemisphere of the adult central brain are marked based on anti-TH immunohistochemistry (27, 28, 31, 32). PAM cell number is underrepresented in the schematic (33). (B) Cells colabeled with anti-TH antibody and Ddc-GAL4 in the central brain (33). Disrupting neurotransmission in Ddc cells decreased oviposition preference [n = 17–21 per strain; ANOVA: F(3,73) = 47.51, P < 0.0001]. (C) Schematic of the TH-GAL4 expression pattern in the central brain (33). See Fig. S5 for more details. Arrow highlights that most PAM neurons do not express TH-GAL4. Disrupting neurotransmission in TH cells increased oviposition preference [n = 9–16 per strain; ANOVA: F(3,51) = 9.62, P < 0.0001]. (D) Disruption of transmission in serotonergic neurons with TRH-GAL4 did not disrupt oviposition preference [n = 19–21 per strain; ANOVA: F(2,76) = 4.23, P = 0.08; all Tukey’s comparisons to TRH/TeTx: P > 0.05]. Bars on graphs represent means ± SEM. *P < 0.05; **P < 0.001; ***P < 0.0001. Clusters of dopaminergic neurons are named based on their location in the brain: PAM, protocerebral anterior median; PPL, protocerebral posterior lateral; PPM, protocerebral posterior median; PAL, protocerebral anterior lateral; Sb, subesophageal ganglion.
Intriguingly, we found that blocking neurotransmission in Ddc-GAL4 neurons decreased preference (Fig. 2B), whereas the same manipulation in TH-GAL4 cells increased preference for 5% ethanol as an egg-laying substrate (Fig. 2C). The differences in the expression patterns of these GAL4 drivers are in (i) neurons producing serotonin (but not dopamine) and (ii) in most of the dopaminergic PAM neurons, which express Ddc-GAL4 but not TH-GAL4 (31, 32). Thus, our data lead to two alternate hypotheses: (i) dopamine suppresses oviposition preference, whereas serotonin enhances it; or (ii) nonoverlapping sets of dopaminergic neurons within the Ddc- and TH-GAL4 patterns affect oviposition preference in opposite ways. To discriminate between these possibilities, we expressed TeTx in serotonergic neurons using the tryptophan hydroxylase GAL4 driver (TRH-GAL4, 34). TRH-GAL4 expresses in nearly all serotonergic neurons including those expressing Ddc-GAL4, but not in dopaminergic neurons defined by TH-GAL4 expression (27, 31, 34). If serotonin were required for oviposition preference for ethanol, we expected that blocking TRH neurons would suppress the response. However, it did not affect oviposition preference (Fig. 2D), supporting the hypothesis that the reduced oviposition preference observed upon silencing Ddc-GAL4 neurons is attributable to a subset of dopaminergic neurons that do not express TH-GAL4.
Different Subsets of Dopamine Neurons Drive Competing Behavioral Responses.
Although Ddc-GAL4 and TH-GAL4 are coexpressed in most dopaminergic neurons, a large subset of dopaminergic neurons within the PAM neuronal cluster expresses Ddc-GAL4 but not TH-GAL4 (31–34) (Fig. 2 B and C; for definition of dopaminergic cell clusters see legend). These PAM neurons may thus function to enhance oviposition preference, while other dopaminergic neurons coexpressing Ddc-GAL4 and TH-GAL4 antagonize this effect. If so, blocking the activity of PAM neurons should suppress oviposition preference for 5% ethanol.
To test this, we blocked neurotransmission with TeTx in patterns specified by three GAL4 lines that drive expression in various subsets of Ddc-expressing cells including the PAM neurons (transgenes HL5, HL7, and HL9) (33, 35). Blocking neurotransmission in PAM, PAL, PPM1/2, PPL2, and Sb neurons (HL5) decreased oviposition preference for 5% ethanol (Fig. S6 A and B). The same effect was observed upon silencing PAM neurons and subsets of PAL, PPM1/2, PPM3, PPL2, PPL1, and Sb neurons (HL7 and HL9) (Fig. S6 C and D). Because these drivers are expressed in PAM neurons, which is not the case for TH-GAL4, these results provide additional evidence that PAM neuron activity normally enhances oviposition preference.
To confirm this, we blocked activity specifically in PAM neurons by combining HL7-GAL4 (expressed in PAM neurons and some TH-GAL4–positive neurons) with the TH-specific GAL4-repressor line TH-GAL80 (36). This led to decreased oviposition preference (Fig. 3A and Fig. S7), indicating that the PAM neurons normally enhance oviposition preference for 5% ethanol.
Fig. 3.
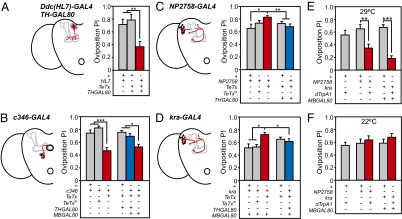
Dopaminergic neurons compete to affect oviposition preference. (A–D) Schematics represent dopamine-expressing cell bodies (black dots) and a subset expressing the particular GAL4 pattern (red dots) with their respective innervation patterns (red lines) for each GAL4 line; MB is drawn in gray (A, C, and D) and EB in black (B). Nondopaminergic expression is omitted in schematics. (A) Disrupting transmission in the HL7-expressing PAM neurons decreased preference [n = 9–19 per strain; ANOVA: F(2,42) = 9.33, P = 0.0004]. (B) Disrupting transmission in the PPM3 neurons (33) decreased preference [n = 12–18 per strain; ANOVA: F(2,49) = 20.65, P < 0.0001]. Eliminating expression of TH within the c346 expression pattern using THGAL80 reverted the behavior to control levels, whereas eliminating MB expression using MB-GAL80 did not. (C and D) Disrupting transmission in subsets of PPL1 neurons (17) increased preference [NP2758: n = 10–12 per strain; ANOVA: F(2,30) = 5.30, P = 0.01; kra: n = 20–21 per strain; ANOVA: F(2,59) = 3.98, P = 0.02]. Eliminating expression of TH within the NP2758 and kra expression patterns using TH-GAL80 reverted the behavior to control levels. (E and F) Activating the PPL1 neurons using dTrpA1 decreases oviposition preference at 29°C [n = 8–12 per strain; ANOVA: F(4,41) = 20.48, P < 0.0001] but not 22 °C [n = 6–15 per strain; ANOVA: F(4,49) = 1.07, P = 0.38]. Bars on graphs represent means ± SEM. *P < 0.05; **P < 0.001; ***P < 0.0001.
To identify which TH-expressing neurons normally suppress oviposition preference, we blocked neurotransmission with drivers that show expression in subsets of TH-expressing neurons. Indeed, blocking transmission in the PPM3 cluster (with GAL4 line c346) decreased oviposition preference (Fig. 3B). Thus, the PPM3 neurons normally enhance preference. In contrast, blocking transmission in a small subset of PPL1 neurons, the MB-MP1 neurons that project to the medial lobe and pedunculus of the MB [with GAL4 lines NP2758 and krasavietz (kra)], (17) increased oviposition preference for 5% ethanol (Fig. 3 C and D). Note that both NP2758 and kra are expressed in other TH-negative neurons, but the overlap in expression is believed to be limited to the MB-MP1 PPL1 neurons (17).
To confirm that PPL1 neurons suppress oviposition preference, we expressed the temperature-sensitive cation channel dTrpA1 (37) in the MB-MP1 PPL1 cells. This led to a significant decrease in oviposition preference for 5% ethanol (Fig. 3 E and F). Taken together, these results suggest that PAM and PPM3 neurons compete with PPL1 neurons by promoting and suppressing, respectively, oviposition preference for ethanol (Table S1). Notably, we also blocked neurotransmission in PAM neurons using an alternate GAL4 driver [R58E02 (19)] and did not find an effect on behavior suggesting that PAM cluster neurons that affect oviposition preference for ethanol may not be present within the R58E02 expression pattern.
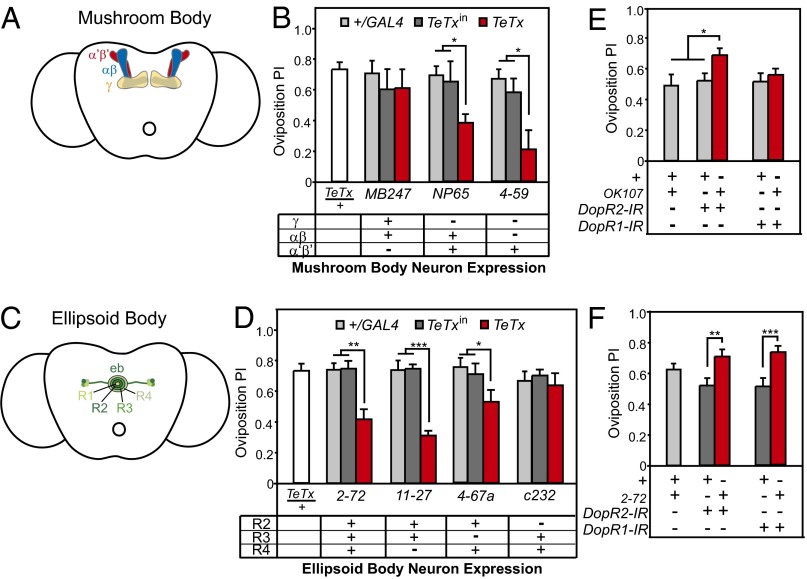
The MB and EB Mediate Oviposition Preference for Ethanol.
To identify higher brain regions regulating oviposition preference, we surveyed brain structures innervated by the PAM, PPL1, and PPM3 dopaminergic neurons. PAM and PPL1 neurons innervate the MB (27). The MB consists of three major classes of neurons whose axonal branches occupy distinct lobes: the αβ, α′β′, and γ neurons (Fig. 4A) (38). PAM cluster neurons innervate different portions of the horizontal lobes, whereas the MB-MP1 neurons innervate an area of the MB peduncle occupied by the γ and αβ lobes (17, 27, 39).
Fig. 4.
The MB and EB are required for oviposition preference for ethanol. (A) The MB consists of three major classes of neurons whose axonal branches occupy distinct lobes elaborated by the αβ, α′β′, and γ neurons. (B) Disrupting neurotransmission in distinct subsets of MB neurons revealed that the α′β′ neurons mediate oviposition preference for ethanol [MB247: n = 10–11 per strain; ANOVA: F(2,30) = 0.52, P = 0.60; NP65: n = 9–11 per strain; ANOVA: F(2,28) = 7.61, P = 0.003; 4–59: n = 10–11 per strain; ANOVA: F(2,30) = 6.00, P = 0.007]. (C) The major contributions to the EB are the projections of R neurons that arborize as a ring. (D) Disrupting neurotransmission in R2 neurons decreased oviposition preference [2–72: n = 7–11 per strain; ANOVA: F(2,26) = 28.56, P < 0.0001; 11–27: n = 10–12 per strain; ANOVA: F(2,32) = 52.03, P < 0.0001; 4–67a: n = 12–15 per strain; ANOVA: F(2,32) = 52.03, P < 0.0001; c232: n = 10–14 per strain; ANOVA: F(2,35) = 1.62, P = 0.21]. (E) Decreasing DopR2 expression in the MB increases oviposition preference for ethanol [n = 18 per strain; ANOVA: F(2,51) = 7.63, P = 0.001]. (F) Decreasing DopR1 and DopR2 expression in the EB increases oviposition preference for ethanol [DopR1: n = 18 per strain; ANOVA: F(2,50) = 9.17, P = 0.0004; DopR2: n = 18 per strain; ANOVA: F(2,50) = 10.60, P = 0.001]. Bars on graphs represent means ± SEM. *P < 0.05; **P < 0.001; ***P < 0.0001.
To determine whether the MB mediates oviposition preference, we used TeTx to block neurotransmission in specific subsets of MB neurons using the GAL4 drivers MB247 (γ and αβ neurons), NP65 (αβ and α′β′ neurons), and 4–59 (α′β′ neurons) (23, 38). Blocking transmission using NP65 and 4–59, but not MB247, decreased oviposition preference (Fig. 4B). Because we observed no change in preference when blocking γ and αβ neurons, but saw a decrease in preference when silencing α′β′ neurons, we suggest that neurotransmission from these neurons is required for oviposition preference.
The remaining cluster of dopaminergic cells that affected oviposition preference, the PPM3 neurons, innervates the EB, a substructure of the central complex. c346-GAL4 expresses in PPM3 cells that project to the EB (33). The EB is organized in a concentric pattern of glomeruli with major contributions from the projections of large-field ring or R neurons (Fig. 4C) (40, 41). A subgroup of these, the R2/R4 EB neurons, mediate both ethanol-induced hyperactivity (33) and ethanol tolerance (42, 43).
We used TeTx to block synaptic transmission in different subsets of EB neurons and measured oviposition preference for ethanol. Different subsets of R neurons were targeted using the 2–72 (R2, R3, and R4), 11–27 (R2, R3), 4–67a (R2, R4), and c232 (R3, R4) (33, 41) GAL4 drivers. Blocking synaptic transmission in the 2–72, 11–27, 4–67a, but not c232 patterns decreased oviposition preference (Fig. 4D). Because 2–72, 11–27, and 4–67 all express in the R2 EB neurons (33), we deduce that these neurons are necessary for oviposition preference for ethanol and propose that the PPM3 dopaminergic neurons activate R2 EB neurons to promote oviposition preference for ethanol-containing food.
To test whether the MB and EB are downstream targets of dopamine signaling, we decreased expression of either D1-type dopamine receptors, DopR1 or DopR2, in the MB and EB. Decreasing expression of DopR2, but not DopR1, in the MB using RNA interference increased oviposition preference (44) (Fig. 4E). Decreasing expression of both DopR1 and DopR2 in the EB increased oviposition preference (Fig. 4F). This suggests that although the MB and EB are likely downstream targets of dopaminergic signaling, the role of dopamine signaling in mediating oviposition preference for ethanol is complex.
Discussion
This study characterizes D. melanogaster’s preferred concentration of ethanol for oviposition and uses this information to investigate neural circuits required for modulating this preference. We found that female flies are sensitive to subtle changes in ethanol concentration in the food substrate. Presumably, flies test the concentration of ethanol present in the food and then decide which substrate is most suitable for progeny fitness and survival. When flies are given a choice of food with or without ethanol, they always prefer to lay eggs on the ethanol substrates, including those containing potentially toxic concentrations. We speculate that this occurs because flies are able to evaluate the environment such that they only lay eggs on deleterious substrates when harmless alternatives are available for their mobile progeny to move toward. However, when the choice involves two different ethanol concentrations, flies prefer to lay eggs on the concentration of ethanol that is most beneficial (∼5%).
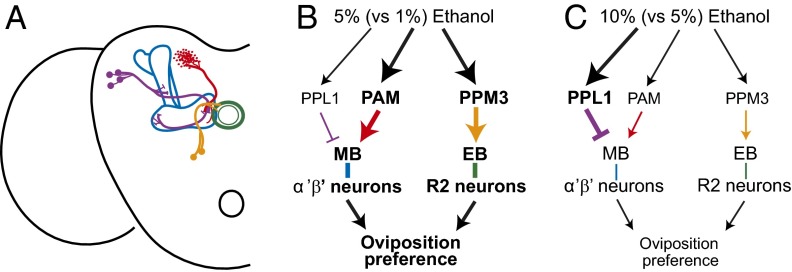
Our data suggest that ethanol is sensed through multiple sensory systems, and this information is relayed through distinct sets of dopaminergic neurons (Fig. 5A). The PAM and PPM3 neurons promote oviposition preference for 5% ethanol, whereas the MB-MP1 PPL1 neurons suppress the response (Fig. 5B). A combination of anatomical and behavioral data has shown that the PAM and PPL1 dopaminergic inputs are relayed to the MB, whereas the PPM3 inputs to the EB. These signals are then transmitted by the MB α′β′ neurons and the EB R2 neurons (Fig. 5B). We propose that 5% ethanol activates PAM and PPM3 neurons which, in turn, activate MB α′β′ and EB R2 neurons, respectively, leading to increased preference for ethanol (Fig. 5B). PPL1 neurons, on the other hand, may inhibit MB α′β′ neurons, thus resulting in reduced oviposition preference. The latter model is supported by the fact that activation of PPL1 neurons leads to strongly reduced preference.
Fig. 5.
A model for the neural circuitry mediating oviposition preference for ethanol. (A) A schematic representation of one hemisphere of the Drosophila central brain depicting innervation of the MB (blue) and EB (green) by dopaminergic neurons of the PAM (red), PPL1 (purple), and PPM3 (yellow) clusters. (B) Sensory information about ethanol is gated through dopaminergic neurons. The PAM (red) and PPM3 (yellow) neurons promote oviposition preference, whereas the PPL1 neurons (purple) inhibit preference. Dopaminergic input is relayed to the MB via the PAM and PPL1 neurons and to the central complex EB via the PPM3 neurons. The output response is relayed through the MB α′β′ neurons and EB R2 neurons. Sensory information about 5% ethanol activates the PAM and PPM3 neurons, which activate the MB α′β′ neurons and EB R2 neurons, respectively, leading to an increase in oviposition on ethanol. Sensory information about 5% ethanol simultaneously activates PPL1 neurons, which inhibit the MB α′β′ neurons, resulting in a decrease in oviposition on ethanol. When given choices of low ethanol concentrations (for example 5% vs. 0%) activation of the PAM neurons would have a stronger effect on oviposition behavior than activation of PPL1 neurons, resulting in increased preference for ethanol 5%. (C) When given choices that include detrimental ethanol concentrations (for example 10% vs. 5%), we hypothesize that activation of the PPL1 neurons has a stronger effect on oviposition relative to PAM neurons, resulting in a decrease in oviposition preference for the higher ethanol concentration. See Table S1 for detailed expression data.
In Drosophila, the innervation of the MB by dopaminergic neurons is crucial for choice behavior (16). This innervation is complex and can convey information regarding both aversive and appetitive stimuli. The PPL1 neurons signal punishment in the context of shock-conditioned odor memory (35, 45) but also play a role in modulating sugar-conditioned odor memory (17). The PAM neurons strongly signal reward for odor memory (19), although a small subset of these neurons have been reported to signal punishment (39, 45). Our results concur with studies in which the MB-MP1 PPL1 neurons signal an aversive stimulus, whereas the PPM3 and PAM neurons signal an appetitive stimulus. Unlike electric shock or sugar, however, ethanol acts as both an aversive and appetitive stimulus (23). We propose that recognition of ecologically beneficial ethanol concentrations would induce an appetitive response through activation of PAM and PPM3 neurons (Fig. 5B), whereas detrimentally high ethanol concentrations would induce an aversive response through preferential activation of PPL1 neurons, inhibition of MB function, and ultimately decreased oviposition preference for ethanol (Fig. 5C). Thus, the balance between appetitive and aversive responses would be dependent on ethanol concentration and context to optimize progeny fitness and survival (see the legend to Fig. 5 for more details).
Unexpectedly, we found that decreasing DopR2 levels in the MB or decreasing DopR1 or DopR2 levels in the EB increased oviposition preference for ethanol. This may be attributable to an inhibitory role of the D1-type receptors in MB and EB neurons, compensatory responses to other dopamine receptors, or dopamine receptors having different behavioral effects within subpopulations of MB and EB neurons. Clearly, the role of dopamine receptors in regulating ethanol preference is complex.
How varying concentrations of ethanol activate or inhibit dopaminergic neurons remains to be investigated. Because both olfaction and taste affect oviposition preference for ethanol, integration of multiple sensory inputs likely plays a role. Because innate olfactory preference for ethanol is octopamine-dependent, and a subset of octopaminergic neurons mediates PAM neuron activation required for appetitive memory, octopamine may play a role in the olfactory response (46, 47). Neurons expressing neuropeptide F (NPF) or its receptor may also be involved. NPF signaling shifts oviposition preference to higher ethanol concentrations in the presence of parasitic wasps (48), resulting in higher survival (48). It would be intriguing to test whether NPF signaling acts through dopaminergic neurons to affect oviposition preference similarly to how it affects expression of food-associated memory (17).
Our data suggest that integration of multiple sensory inputs may happen in the MB and EB, both of which have known roles in decision-making. Output from the MB α′β′ neurons and EB neurons have been implicated in olfactory memory consolidation and visual pattern memory (23, 49–53). Because activity of dopamine neurons and MB α′β′ neurons are required for both oviposition preference for ethanol and ethanol-reward memory in the fly (23), our results raise the possibility that competing dopamine responses may also modulate memory for ethanol reward. Thus, a simple oviposition preference assay might be useful to identify circuits involved in more complex ethanol preference behaviors such as ethanol-reward memory. We speculate that circuits that evolved for female flies to evaluate an oviposition substrate as beneficial to the fitness of their progeny may be more generally required to evaluate appetitive and aversive stimuli. Interestingly, similar neural principles can be applied to motivational control of behavior in mammals, where dopamine neurons can also transmit signals related to rewarding and nonrewarding experiences (54).
Materials and Methods
Fly Stocks and Growth.
Flies were grown and maintained on standard cornmeal/molasses/yeast/agar medium in an incubator at 25 °C and 70% humidity. All experiments used wild-type Berlin flies that were collected 1 d after eclosion and tested 4–5 d after eclosion. All transgenic strains used were backcrossed for at least five generations to a w1118; Berlin strain. UAS-TeTx (active) and UAS-TeTxin (inactive) were provided by S. Sweeney (University of York, York, UK) and UAS-dTrpA1 by P. Garrity (Brandeis University, Waltham, MA). NP65 and c739 were provided by I. King (University of North Carolina at Chapel Hill, Chapel Hill, NC); 201Y, MB247, c232, OK348, Ddc-GAL4, TH-GAL4, TH-GAL80, HL5, HL7, and HL9 by F. Wolf (University of California, Merced, CA); dTRH by E. Kravitz (Harvard Medical School, Boston); NP2758 and kra-MBGAL80 by S. Waddell (University of Oxford, Oxford); 2–72, 4–59, 4–64, 4–67, and 11–27 are from the P[GAL4GawB] collection of U.H. (23, 33). Dopamine receptor UAS-RNAi–carrying flies were obtained from the Vienna Drosophila RNAi Center.
Two-Choice Oviposition Assay.
The oviposition assay was performed similarly to that described by Joseph et al. (15). Groups of 30 mated females and 7 males were placed (without anesthesia) in a 6-ounce round-bottom plastic bottle, topped with a two-choice food plate, and inverted. Flies were left in the dark at 25 °C and 70% humidity to lay eggs for 3 h. The food plate was removed and photographed for later analysis. The number of eggs laid for each choice test is listed in Table S2.
Statistical Analysis.
All data are presented as means ± SEM. Statistical analyses were performed using JMP 8.0. 2 (SAS Institute). Two-sample t tests and one-way ANOVAs, followed by Tukey’s two-sample post hoc comparisons, were performed when data were parametric. Post hoc comparisons are included in Table S3. Levene and Brown–Forsythe tests were used to confirm homogeneity of variance. Wilcoxon or Kruskal–Wallis tests were performed when data were nonparametric. Significance level was set to P < 0.05.
Supplementary Material
Acknowledgments
We thank F. Wolf (University of California Merced) for flies, advice, and discussions; E. Kravitz (Harvard Med School), S. Sweeney (University of York), P. Garrity (Brandeis University), and S. Waddell (University of Oxford) for flies; Z. Maung, S. Yong, and C. Wong for technical support; and the members of the U.H. laboratory for advice and comments on prior versions of the manuscript, especially R. Joseph, A. Devineni, J. Simon, and G. Shohat-Ophir. This work was funded by a grant from the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism (to U.H.), a Heart and Stroke Foundation of Canada Junior Personnel Fellowship (to K.R.K.), and the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320208110/-/DCSupplemental.
References
- 1.Ashburner M. Speculations on the subject of alcohol dehydrogenase and its properties in Drosophila and other flies. Bioessays. 1998;20(11):949–954. doi: 10.1002/(SICI)1521-1878(199811)20:11<949::AID-BIES10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Griffith LC, Ejima A. Multimodal sensory integration of courtship stimulating cues in Drosophila melanogaster. Ann N Y Acad Sci. 2009;1170:394–398. doi: 10.1111/j.1749-6632.2009.04367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley R. Fermenting fruit and the historical ecology of ethanol ingestion: Is alcoholism in modern humans an evolutionary hangover? Addiction. 2002;97(4):381–388. doi: 10.1046/j.1360-0443.2002.00002.x. [DOI] [PubMed] [Google Scholar]
- 4.Gibson JB, Oakeshott JG. Genetics of biochemical and behavioural aspects of alcohol metabolism. Aust N Z J Med. 1981;11(2):128–131. doi: 10.1111/j.1445-5994.1981.tb04218.x. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann AA, Parsons PA. Olfactory response and resource utilization in Drosophila – interspecific comparison. Biolog J Linnean Soc. 1984;22:43–53. [Google Scholar]
- 6.McKenzie JA, Parsons P. Alcohol tolerance – ecological parameter in relative success of Drosophila melanogaster and Drosphila simulans. Oecologia. 1972;10:373–388. doi: 10.1007/BF00345738. [DOI] [PubMed] [Google Scholar]
- 7.Devineni AV, Heberlein U. The evolution of Drosophila melanogaster as a model for alcohol addiction. Annu Rev Neurosci. 2013;36:121–138. doi: 10.1146/annurev-neuro-062012-170256. [DOI] [PubMed] [Google Scholar]
- 8.Bokor K, Pecsenye K. Differences in the effect of ethanol on fertility and viability components among laboratory strains of Drosophila melanogaster. Hereditas. 2000;132(3):215–227. doi: 10.1111/j.1601-5223.2000.00215.x. [DOI] [PubMed] [Google Scholar]
- 9.Geer BW, McKechnie SW, Heinstra PWH, Pyka MJ. Heritable variation in ethanol tolerance and its association with biochemical traits in Drosophila melanogaster. Evolution. 1991;45(5):1107–1119. doi: 10.1111/j.1558-5646.1991.tb04378.x. [DOI] [PubMed] [Google Scholar]
- 10.Ranganathan S, Davis DG, Hood RD. Developmental toxicity of ethanol in Drosophila melanogaster. Teratology. 1987;36(1):45–49. doi: 10.1002/tera.1420360107. [DOI] [PubMed] [Google Scholar]
- 11.McClure KD, French RL, Heberlein U. A Drosophila model for fetal alcohol syndrome disorders: Role for the insulin pathway. Dis Model Mech. 2011;4(3):335–346. doi: 10.1242/dmm.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geer BW, Heinstra PW, McKechnie SW. The biological basis of ethanol tolerance in Drosophila. Comp Biochem Physiol B. 1993;105(2):203–229. doi: 10.1016/0305-0491(93)90221-p. [DOI] [PubMed] [Google Scholar]
- 13.Milan NF, Kacsoh BZ, Schlenke TA. Alcohol consumption as self-medication against blood-borne parasites in the fruit fly. Curr Biol. 2012;22(6):488–493. doi: 10.1016/j.cub.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang CH, Belawat P, Hafen E, Jan LY, Jan Y-N. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science. 2008;319(5870):1679–1683. doi: 10.1126/science.1151842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph RM, Devineni AV, King IFG, Heberlein U. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc Natl Acad Sci USA. 2009;106(27):11352–11357. doi: 10.1073/pnas.0901419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waddell S. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 2010;33(10):457–464. doi: 10.1016/j.tins.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krashes MJ, et al. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139(2):416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marella S, Mann K, Scott K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron. 2012;73(5):941–950. doi: 10.1016/j.neuron.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C, et al. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488(7412):512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- 20.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn Sci. 2011;15(1):37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaus JG, et al. Who, what, where, when (and maybe even why)? How the experience of sexual reward connects sexual desire, preference, and performance. Arch Sex Behav. 2012;41(1):31–62. doi: 10.1007/s10508-012-9935-5. [DOI] [PubMed] [Google Scholar]
- 22.Trezza V, Baarendse PJ, Vanderschuren LJ. The pleasures of play: Pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31(10):463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. A model for ethanol reward in Drosophila melanogaster. Nat Neurosci. 2011;14(5):612–619. doi: 10.1038/nn.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz NU, Zhong L, Bellemer A, Tracey WD. Egg laying decisions in Drosophila are consistent with foraging costs of larval progeny. PLoS ONE. 2012;7(5):e37910. doi: 10.1371/journal.pone.0037910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph RM, Heberlein U. Tissue-specific activation of a single gustatory receptor produces opposing behavioral responses in Drosophila. Genetics. 2012;192(2):521–532. doi: 10.1534/genetics.112.142455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boll W, Noll M. The Drosophila Pox neuro gene: Control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development. 2002;129(24):5667–5681. doi: 10.1242/dev.00157. [DOI] [PubMed] [Google Scholar]
- 27.Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: Anatomical and physiological heterogeneity. Front Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nässel DR, Elekes K. Aminergic neurons in the brain of blowflies and Drosophila: Dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res. 1992;267(1):147–167. doi: 10.1007/BF00318701. [DOI] [PubMed] [Google Scholar]
- 29.Brand AH, Manoukian AS, Perrimon N. Ectopic expression in Drosophila. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- 30.Martin JR, Keller A, Sweeney ST. Targeted expression of tetanus toxin: A new tool to study the neurobiology of behavior. Adv Genet. 2002;47:1–47. doi: 10.1016/s0065-2660(02)47001-0. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Chaney S, Roberts IJ, Forte M, Hirsh J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr Biol. 2000;10(4):211–214. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- 32.Friggi-Grelin F, et al. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54(4):618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 33.Kong EC, et al. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS ONE. 2010;5(4):e9954. doi: 10.1371/journal.pone.0009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS ONE. 2010;5(5):e10806. doi: 10.1371/journal.pone.0010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Claridge-Chang A, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139(2):405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sitaraman D, et al. Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci USA. 2008;105(14):5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454(7201):217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aso Y, et al. The mushroom body of adult Drosophila characterized by GAL4 drivers. J Neurogenet. 2009;23(1-2):156–172. doi: 10.1080/01677060802471718. [DOI] [PubMed] [Google Scholar]
- 39.Aso Y, et al. Three dopamine pathways induce aversive odor memories with different stability. PLoS Genet. 2012;8(7):e1002768. doi: 10.1371/journal.pgen.1002768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanesch U, Fishback KF, Heisenberg M. Neuronal architecture of the central complex in Drosophila melanogaster. Cell Tissue Res. 1989;257:343–346. [Google Scholar]
- 41.Renn SC, et al. Genetic analysis of the Drosophila ellipsoid body neuropil: Organization and development of the central complex. J Neurobiol. 1999;41(2):189–207. [PubMed] [Google Scholar]
- 42.Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron. 2000;28(1):261–271. doi: 10.1016/s0896-6273(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 43.Urizar NL, Yang Z, Edenberg HJ, Davis RL. Drosophila homer is required in a small set of neurons including the ellipsoid body for normal ethanol sensitivity and tolerance. J Neurosci. 2007;27(17):4541–4551. doi: 10.1523/JNEUROSCI.0305-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keleman K, et al. Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature. 2012;489(7414):145–149. doi: 10.1038/nature11345. [DOI] [PubMed] [Google Scholar]
- 45.Aso Y, et al. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol. 2010;20(16):1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider A, et al. Neuronal basis of innate olfactory attraction to ethanol in Drosophila. PLoS ONE. 2012;7(12):e52007. doi: 10.1371/journal.pone.0052007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burke CJ, et al. Layered reward signaling through octopamine and dopamine in Drosophila. Nature. 2012;492(7429):433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kacsoh BZ, Lynch ZR, Mortimer NT, Schlenke TA. Fruit flies medicate offspring after seeing parasites. Science. 2013;339(6122):947–950. doi: 10.1126/science.1229625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu CL, et al. Specific requirement of NMDA receptors for long-term memory consolidation in Drosophila ellipsoid body. Nat Neurosci. 2007;10(12):1578–1586. doi: 10.1038/nn2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu G, et al. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439(7076):551–556. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- 51.Connolly JB, et al. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274(5295):2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- 52.Pan Y, et al. Differential roles of the fan-shaped body and the ellipsoid body in Drosophila visual pattern memory. Learn Mem. 2009;16(5):289–295. doi: 10.1101/lm.1331809. [DOI] [PubMed] [Google Scholar]
- 53.Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during drosophila odor memory processing. Neuron. 2007;53(1):103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.