Significance
Cell–cell adhesion is essential for embryonic development, tissue morphogenesis, and tissue repair, as well as for tumor invasion and metastasis. Thus, it is of fundamental importance to identify the molecular factors that affect this process. Here we demonstrate that O-mannosylation, an essential posttranslational protein modification, is crucial for the formation of adherens junctions between cells of the early mouse embryo, with O-mannosylation–deficient embryos dying at the morula-to-blastocyst transition. Moreover, we identified O-mannosyl glycans on E-cadherin, the major cell–cell adhesion protein of embryos, and demonstrated that these glycans are crucial for E-cadherin–mediated cell adhesion. Because O-mannosylation is a conserved feature of the classical cadherins, this protein modification most likely affects many more biological processes than previously thought.
Keywords: POMT2, mouse preimplantation development, protein-glycosylation, O-glycans, POMT1
Abstract
In recent years protein O-mannosylation has become a focus of attention as a pathomechanism underlying severe congenital muscular dystrophies associated with neuronal migration defects. A key feature of these disorders is the lack of O-mannosyl glycans on α-dystroglycan, resulting in abnormal basement membrane formation. Additional functions of O-mannosylation are still largely unknown. Here, we identify the essential cell–cell adhesion glycoprotein epithelial (E)-cadherin as an O-mannosylated protein and establish a functional link between O-mannosyl glycans and cadherin-mediated cell–cell adhesion. By genetically and pharmacologically blocking protein O-mannosyltransferases, we found that this posttranslational modification is essential for preimplantation development of the mouse embryo. O-mannosylation–deficient embryos failed to proceed from the morula to the blastocyst stage because of defects in the molecular architecture of cell–cell contact sites, including the adherens and tight junctions. Using mass spectrometry, we demonstrate that O-mannosyl glycans are present on E-cadherin, the major cell-adhesion molecule of blastomeres, and present evidence that this modification is generally conserved in cadherins. Further, the use of newly raised antibodies specific for an O-mannosyl–conjugated epitope revealed that these glycans are present on early mouse embryos. Finally, our cell-aggregation assays demonstrated that O-mannosyl glycans are crucial for cadherin-based cell adhesion. Our results redefine the significance of O-mannosylation in humans and other mammals, showing the immense impact of cadherins on normal as well as pathogenic cell behavior.
Protein O-mannosylation is a vital protein modification that is evolutionarily conserved across eukaryotes (1). In humans, defects in this modification result in a heterogeneous group of congenital muscular dystrophies (CMDs, α-dystroglycanopathies). The most severe of these disorders, Walker–Warburg syndrome, is characterized by CMD associated with brain malformations of various degrees, ocular abnormalities, and, most often, fatal outcome during the first year of life (2). In contrast, milder disorders such as limb-girdle muscular dystrophy, in which neither the brain nor the eyes are affected, may not present until adulthood (2). The key pathological feature of these diseases is the lack of O-mannosyl glycans on α-dystroglycan (α-DG), an integral component of the dystrophin–glycoprotein complex (2, 3). In the absence of these glycans, binding of α-DG to its extracellular matrix ligands (e.g., laminin) is abolished, and, consequently, basement membranes are fragmented (3–5). In addition to α-DG, O-mannosyl glycans constitute up to 30% of total O-linked carbohydrates in the mammalian brain (6, 7). However, to date only a few other proteins [including CD24 (8), PTPRZ1 (9), neurofascin 186 (10), neurocan, and versican (11)] have been shown to undergo O-mannosylation.
Synthesis of O-mannosyl glycans is initiated in the endoplasmic reticulum by the transfer of mannose from dolichol monophosphate-activated mannose (Dol-P-Man) to serine or threonine residues on membrane and secretory proteins (1). In mammals, this reaction is catalyzed by a heteromeric complex of the protein O-mannosyltransferase 1 (POMT1) and 2 (POMT2) (12). We previously showed that Pomt1-null mice display embryonic lethality during postimplantation development, between embryonic day (E)7.5 and E9.5 because of abnormal glycosylation and maturation of α-DG (4). Similarly, knockdown of POMTs in zebrafish and Drosophila melanogaster leads to severe developmental defects that are largely attributable to dysfunctional α-DG (13, 14). Although these animal models have been very helpful in elucidating the role of α-DG–linked O-mannosyl glycans, they have not revealed other biological functions of this protein modification.
In both animals and humans, adhesive interactions between neighboring cells are essential for embryogenesis as well as for tissue morphogenesis and renewal (15). Adherens junctions are sites of cell–cell contact where cell-surface receptors of the cadherin family mediate adhesion (16). Classical cadherins are conserved among vertebrates and invertebrates (17). These plasma-membrane glycoproteins share a conserved cytoplasmic domain, a single-pass transmembrane domain, and an ectodomain containing five extracellular cadherin (EC) domains (18). Located on opposing cells, cadherin ectodomains form calcium-dependent homophilic interactions whereby they mediate cell–cell contact (18). The classical cadherin family comprises multiple members, including epithelial cadherin (E-cad, CDH1), neuronal cadherin (N-cad, CDH2), and retinal cadherin (R-cad, CDH4), each of which shows a distinct tissue-specific distribution pattern (16). E-cad plays a critical role in the epithelial–mesenchymal transition during embryogenesis; E-cad–knockout mice die before implantation because of the lack of a functional trophectodermal cell layer at the blastocyst stage (19). Cell–cell adhesion is crucial not only for development but also for tissue morphogenesis and for the invasiveness of human cancer cells (20). Thus, it is particularly important to assess, on a molecular level, the factors that affect this process.
In the present study we demonstrate that protein O-mannosylation is essential for the formation of adherens junctions in the preimplantation embryo. Embryos deficient for O-mannosylation die during the morula-to-blastocyst transition because of impaired blastomere adhesion. We identify E-cad as an O-mannosylated glycoprotein and show that this modification is essential for cadherin-mediated cell adhesion. Our identification of functionally relevant O-mannosyl glycans on cadherins is expected to provide further insights into the molecular pathologies of α-dystroglycanopathies.
Results and Discussion
Impaired O-Mannosylation Results in Preimplantation Lethality of the Mouse Embryo.
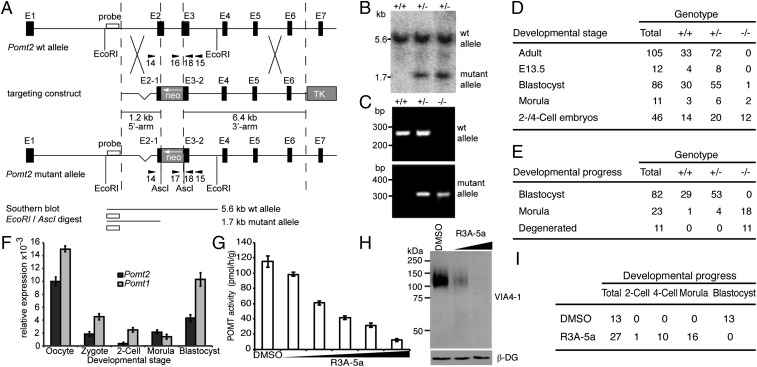
Our previous work showed that deletion of mouse Pomt1 results in embryonic lethality after implantation because of impaired basement-membrane formation (4). To address further the role of O-mannosyl glycans in vivo, we generated mice deficient for Pomt2 (Fig. 1A). By homologous recombination in ES cells, we created a mutant allele encoding a truncated POMT2 with a frameshift mutation after amino acid T100 and a premature stop after four additional amino acids (Fig. 1 A and B and Fig. S1). Targeted ES cells were used to produce chimeric mice that transmitted the mutant allele. Heterozygous Pomt2+/− mice developed normally and were indistinguishable from their WT littermates. However, intercrosses between heterozygous Pomt2+/− mice did not yield homozygous Pomt2−/− offspring (Fig. 1D). The observed 1:2 ratio of WT to heterozygous offspring indicated inheritance of a recessive embryonic lethal trait (Fig. 1D). Analysis of embryos at different developmental stages revealed homozygous Pomt2−/− embryos at the expected Mendelian distribution at the two-cell, four-cell, and morula stages but not at the blastocyst stage (Fig. 1 C and D). When embryos isolated at the two-cell stage were cultivated in vitro under conditions conducive to blastocyst formation, Pomt2−/− embryos either displayed morphologies indicative of degeneration or were arrested at the morula stage. Surprisingly, unlike Pomt1−/− embryos, Pomt2−/− embryos failed to form blastocysts (Fig. 1E). The observed phenotypic variations could be caused by differences in POMT activity, given that low enzymatic activity was detected for human and zebrafish POMT2 in the absence of POMT1 (13, 21). In addition, compared with the levels of the Pomt2 maternal mRNA during the transition from oocyte to morula, the levels of Pomt1 mRNA were strikingly high (Fig. 1F) and most likely helped Pomt1−/− null embryos overcome the bottleneck of early preimplantation lethality observed in our Pomt2−/− mutants. Such variations in early transcript levels also could explain why lethality of Pomt2−/− null mice on a different genetic background was not evident until postimplantation development (22).
Fig. 1.
Loss of O-mannosyl glycan biosynthesis in mouse embryos. (A) Schematic representation of the Pomt2 genomic locus, targeting construct, and mutant Pomt2 allele after homologous recombination. Arrowheads indicate PCR primers. NEO, neomycin resistance gene; TK, Herpes simplex virus thymidine kinase gene. (B) Southern blot analysis of EcoRI/AscI-digested genomic DNA from targeted ES cells. Pomt2 WT (5.6 kb) and mutant (1.7 kb) alleles were detected. (C) PCR-based genotyping of preimplantation embryos. PCR products of Pomt2 WT (+/+), heterozygous (+/−), and homozygous (−/−) embryos are shown. (D and E) Genotypes and developmental stages/states of embryos (D) and in vitro-cultured embryos (E) derived from heterozygous Pomt2+/− intercrosses. (F) Relative levels of the Pomt1 and Pomt2 mRNAs during early development, as determined by quantitative RT-PCR and normalized to Ppia. Maternal Pomt1 and Pomt2 transcripts were present at high levels in oocytes but were degraded in zygotes and two-cell embryos. (G) Effect of increasing levels of R3A-5a on in vitro activity of POMT. Mouse-liver membranes were used as the source of enzyme. Reactions were supplemented with DMSO or 25 µM, 50 µM, 100 µM, 200 µM, and 400 µM R3A-5a. Mean values of three independent experiments are shown. (H) Western blot of α-DG isolated from MDCK cells cultured in the presence of 12.5 µM and 50 µM R3A-5a. The VIA4-1 monoclonal antibody was used to determine the O-mannosylation state of α-DG. β-DG levels also were assessed to confirm that protein expression and loading were equal across samples. (I) Developmental progress of WT embryos cultivated in the presence of 50 µM of the inhibitor R3A-5a.
To rule out bias that quantitative differences in Pomt transcript levels might introduce in our functional analyses of O-mannosylation during preimplantation development, we established a method to inhibit POMT enzymatic activity directly in early embryos. Rhodanine-3-acetic acid derivatives such as compound 5a (R3A-5a) are highly specific inhibitors of fungal protein O-mannosyltransferases (23–25). We found that the enzymatic activity of mammalian POMTs also is blocked selectively by R3A-5a, both in vitro and in vivo. Over a wide range of concentrations, R3A-5a application resulted in a linear decrease of POMT in vitro activity, whereas enzymatic activity of the enzyme Dol-P-Man synthase was not affected (Fig. 1G and Fig. S2A). In Madin–Darby canine kidney (MDCK) cells cultured in the presence of up to 50 µM inhibitor, O-mannosylation of the known target protein α-DG vanished, as demonstrated by Western blotting with antibody VIA4-1, which is specific for an uncharacterized O-mannosidically linked carbohydrate epitope on α-DG (Fig. 1H). At the concentrations used, neither cytotoxicity nor inhibitory effects on cell proliferation were observed (Fig. S2 B and C), and protein N-glycosylation was unaffected (see Fig. 4C). Because R3A-5a specifically inhibited the enzymatic activity of POMTs in living cells, we cultivated two-cell–stage embryos in its presence. Although mock-treated embryos developed normally and proceeded to the blastocyst stage (Fig. 1I), embryos treated with R3A-5a exhibited developmental arrest during the morula-to-blastocyst transition (Fig. 1I). The observed phenotype is similar to that of Pomt2−/− embryos (see Fig. 3A), confirming that O-mannosyl glycans play a crucial role during preimplantation development.
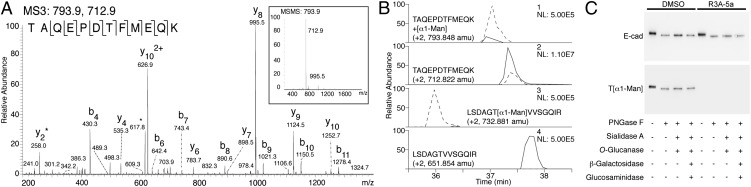
Fig. 4.
Detection of O-mannosyl glycans on E-cad. (A and B) E-cad–derived glycopeptides were analyzed by a combination of LC-MS/MS sequencing after CID and specific demannosylation using α-mannosidase as recently described (ref. 33 and SI Materials and Methods). The EC4-derived peptide TAQEPDTFMEQK was found to be modified with a single O-linked mannose residue. (A) MS3 analysis of the hexosylated peptide TAQEPDTFMEQK. (Inset) CID of a doubly charged peptide (m/z = 793.9) identified a dominant fragment ion (m/z = 712.9) formed by a neutral loss of the mass of a hexose. This fragment ion was selected for additional fragmentation leading to β and γ ions caused by backbone fragmentation of a peptide, allowing its identification. (B) Enzymatic demannosylation of the E-cad peptide TAQEPDTFMEQK (lanes 1 and 2) and a synthetic O-mannosylated control peptide LSDAGT(α1-Man)VVSGQIR (lanes 3 and 4). Peptides were treated without (dashed line) and with (solid line) α-mannosidase and were analyzed by LC-MS. Extracted-ion chromatograms of the mannosylated peptides (lanes 1 and 3) show that intensity decreased significantly upon α-mannosidase treatment. Consequently, signal intensities of the demannosylated peptides (lanes 2 and 4) increased. NL, normalized intensity level (counts per second). (C) Western blotting of affinity-purified E-cad following treatment of MDCK cells with 50 µM R3A-5a or mock treatment (DMSO), using the E-cad antibody DECMA-1 and the T[α1-Man]–specific antibody. Glycans were removed by treatment with glycosidases as indicated (for details see Fig. 2A).
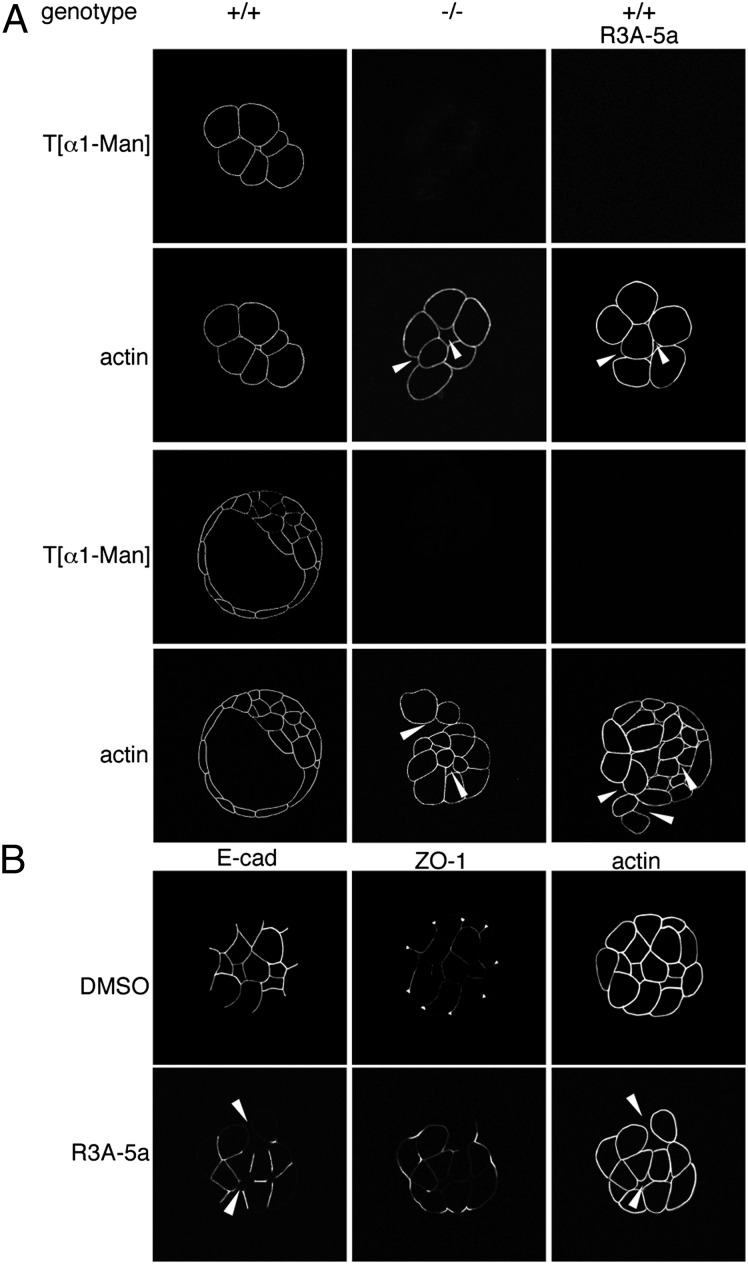
Fig. 3.
Characterization of O-mannosylation impaired embryos. (A) Whole-mount immunofluorescence analyses of embryos from intercrosses of Pomt2+/− mice and of WT embryos treated with R3A-5a. Morula- and blastocyst-stage embryos were analyzed with the T[α1-Man]–specific antibody and phalloidin. O-mannosyl glycans were not detectable in Pomt2−/− or inhibitor-treated embryos. Arrowheads indicate sites of impaired blastomere adhesion. Genotypes of the individual embryos shown were determined by PCR analysis as in Fig. 1, following microscopy. (B) Whole-mount immunofluorescent analysis of adherens and tight junctions in WT embryos after R3A-5a or mock (DMSO) treatment. Morula-stage embryos stained with E-cad– or ZO-1–directed antibodies or with phalloidin are shown. Arrowheads indicate diminished E-cad staining at sites of reduced blastomere attachment.
O-Mannosyl Glycans Are Present at the Blastomere Surface.
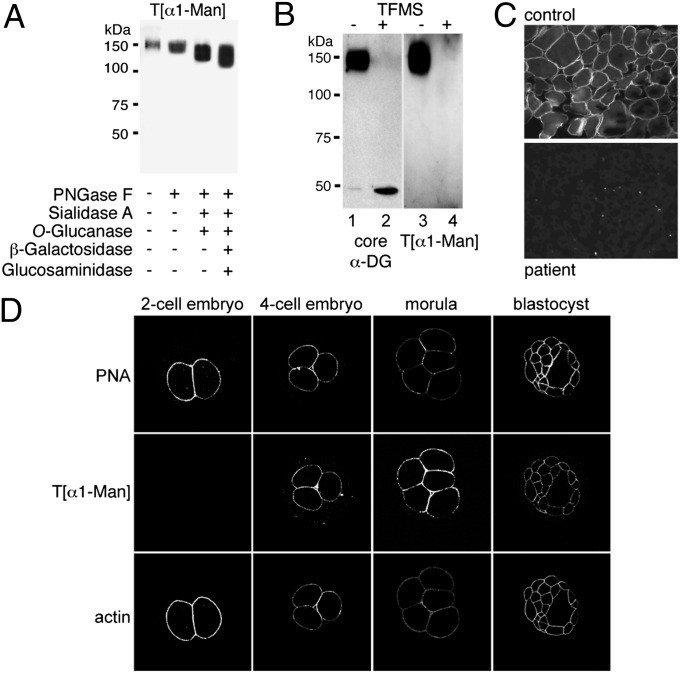
Collectively, the preimplantation lethality of Pomt2−/− and inhibitor-treated embryos suggested the presence of functionally important O-mannosyl glycans during early embryonic development. Therefore, we generated an antibody that is directed against a threonine O-mannosyl–conjugated epitope (T[α1-Man]) and specifically detects O-mannosylated proteins, such as skeletal-muscle α-DG, in immunoblots (Fig. 2A and Fig. S3 B and C). The T[α1-Man]–specific antibody retained its immunoreactivity after N-linked glycans were removed from α-DG by treatment with peptide-N-glycosidase F (PNGase F), after core 1 and core 3 O-linked oligosaccharides were eliminated by O-glucanase and, moreover, after the dominant O-mannosyl glycan structure Siaα3Galβ1–4GlcNAcβ1–2Man-Ser/Thr was trimmed back to a single mannose residue (Fig. 2A and Fig. S3A). However, complete chemical deglycosylation of α-DG with trifluoromethanesulfonic acid (TFMS) abolished the immunoreactivity of the T[α1-Man]–specific antibody (Fig. 2B). Upon incubation with the T[α1-Man]–specific antibody, cross-sections of control human skeletal muscle showed strong staining of the sarcolemma, but this staining was absent in muscle sections from a POMT1-deficient patient (Fig. 2C) (21), confirming the specificity of the antibody. Use of this diagnostic tool for whole-mount immunofluorescence analyses of early mouse embryos revealed that O-mannosyl glycans are present at the blastomere surface in four-cell embryos and throughout the following stages (Fig. 2D and Fig. S3D). In contrast, O-mannosylated α-DG was not detected (Fig. S3E). Consistent with very low Pomt2 transcript levels in two-cell embryos (Fig. 1F), O-mannosyl glycans were below the limit of detection at this developmental stage (Fig. 2D). In conclusion, the T[α1-Man]–specific antibody revealed O-mannosyl glycans on blastomeres of early mouse embryos.
Fig. 2.
Occurrence of O-mannosyl glycans in preimplantation embryos. (A–C) Characterization of anti–T[α1-Man] antibodies. In A and B, Western blots were probed with anti–T[α1-Man] or antibodies directed against the protein core of α-DG (core αDG) as indicated. (A) α-DG–enriched glycoprotein fractions were treated with glycosidases as indicated. N-linked glycans were removed using PNGase F, and sialylated core 1 and core 3 O-linked oligosaccharides were removed by combined treatment with sialidase A and O-glucanase. Further trimming of the O-mannosyl glycan core structure Galβ1–4GlcNAcβ1–2Man-Ser/Thr was achieved by treatment with β-galactosidase and N-acetyl-β-glucosaminidase. For further details, see Fig. S3A. (B) α-DG–enriched glycoprotein fractions were treated with nonaqueous TFMS to remove attached glycans fully. Identical blots were probed. (C) Immunofluorescence staining of skeletal muscle cross-sections from an unaffected control (Upper) and a POMT1-deficient patient with Walker–Warburg syndrome (Lower) (21). Anti–T[α1-Man] immunoreactivity coinciding with the localization of O-mannosylated α-DG is observed at the sarcolemma of control muscle cells but not in the sarcolemma of the patient. (D) Detection of O-mannosyl glycans during preimplantation development. Embryos at different developmental stages were analyzed by whole-mount immunofluorescence with anti–T[α1-Man]. O-mannosyl glycans emerge at the blastomere surface at the four-cell stage. The blastomere surface was visualized using PNA, and cortical actin was visualized by staining with phalloidin.
Cell–Cell Adhesion Is Affected in POMT-Deficient Embryos.
To elucidate further the role of O-mannosyl glycans in the morula-to-blastocyst transition, we characterized POMT-deficient embryos in greater detail. Specifically, we monitored blastocyst formation by two-cell–stage embryos cultivated in vitro. Whole-mount immunofluorescence analyses of Pomt2−/− and R3A-5a–treated embryos revealed a lack of immunoreactivity of the T[α1-Man]–specific antibody, verifying the loss of the O-mannosyl epitopes (Fig. 3A). At the four-cell stage, the POMT-deficient embryos were morphologically indistinguishable from WT embryos. However, after further development, the Pomt2−/− and inhibitor-treated morulae appeared to be disorganized, and blastomere size was extremely variable (Fig. 3A). Most strikingly, we observed that individual cells were detached from the embryo and that blastomere attachment was perturbed overall (Fig. 3A). Thus, cell–cell adhesion is significantly affected in the absence of O-mannosylation.
Cell–cell adhesion plays a critical role in the morula-to-blastocyst transition. At the morula stage, intercellular contacts are maintained by the formation of cadherin-based adherens junctions. Tight junctions then are established between the outer blastomeres to provide a seal, allowing the blastocyst cavity to form (26). To determine whether O-mannosyl glycans have an effect on these cell junctions, we analyzed R3A-5a–treated embryos for the distribution of E-cad, the integral component of the adherens junctions of blastomeres (19), and of zonula occludens 1 (ZO-1), a peripheral protein of tight junctions (27). As shown in Fig. 3B, inhibitor-treated embryos largely retained E-cad staining basolaterally but not at sites of reduced cellular adhesion or in blastomeres that had detached from the embryo (Fig. 3B, E-cad). In addition, ZO-1 did not show its typical punctate localization but was distributed diffusely along the lateral membrane of outer cells (Fig. 3B, ZO-1). Thus, the failure of the morula-to-blastocyst transition in O-mannosylation–deficient embryos appears to be caused by defects in the molecular architecture of cell–cell contacts, including the adherens and tight junctions. Interestingly, in mouse embryos the loss of E-cad has been shown to result in preimplantation lethality and abnormal tight junctions (28, 29). This phenotype is highly similar to that of O-mannosylation–deprived embryos, suggesting that the defects in cell–cell adhesion are related to abnormal posttranslational O-mannosylation of E-cad.
E-Cad Is a Target of Protein O-Mannosylation.
Vertebrate E-cad is an N-glycosylated type I classical cadherin (30). Our results suggested that E-cad also undergoes O-mannosylation. Using a combination of α-mannosidase treatment and peptide sequencing by MS after collision-induced dissociation (CID), we were able to prove directly that O-linked mannose is present on ectodomain EC4 of E-cad (Fig. 4 A and B and Fig. S4). A previous crystal structure analysis of E-cad had identified at least nine additional O-linked monosaccharide modifications of EC2–4 whose exact identity remained unknown (Fig. S4B) (31). O-mannosylation of these sites is clearly indicated by our finding that blocking O-mannosylation in MDCK cells caused a significant decrease in the molecular mass of the E-cad that remained after N-linked glycans were removed (Fig. 4C). O-mannosylation of these sites is supported further by a study of Vester-Christensen et al. that is published in this issue (32). Using a human breast cancer cell line with simplified O-mannosyl glycans, these authors show that cadherins are major O-mannosylated glycoproteins and that O-mannosylation sites are highly conserved among cadherins (32). Most interestingly, treatment with glycosidases that trim back extended O-mannosyl glycans did not affect the molecular mass of E-cad (Fig. 4C), suggesting that the O-linked mannose residues are not elongated further. Very recently, we also demonstrated that single O-linked mannoses are attached to noncanonical heart cadherin from rabbit skeletal muscle (33). Why O-linked mannose residues are elongated on α-DG (Fig. 2A) but not on E-cad (Fig. 4C) is currently unclear. Strikingly, O-mannosyl glycosites on cadherins are present in β-strands whereas O-linked mannoses on α-DG are located in disordered regions (32). Whether distinct structural features of the target proteins influence the action of other glycosyltransferases on O-linked mannoses remains to be investigated in the future.
O-Mannosyl Glycans Directly Affect Cadherin-Mediated Cell Adhesion.
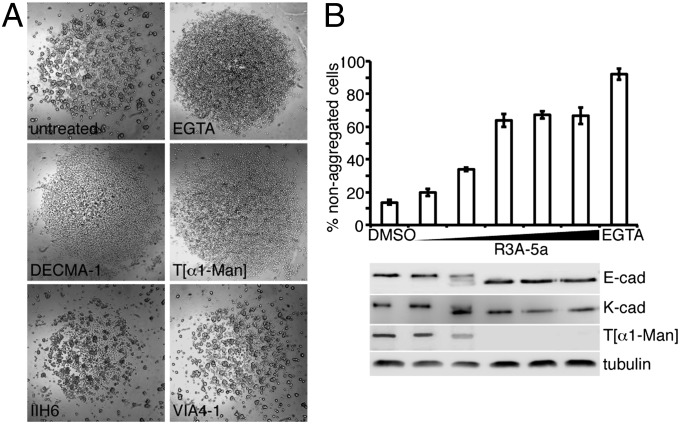
Our data suggest that O-mannosylation plays a unique role in cadherin function. Cell-aggregation assays are elegant tools with which to study the functionality of cell–cell adhesion complexes (34). Therefore, we used MDCK cells, which express high endogenous levels of E-cad (35), to test the effects of the O-mannosyl glycan–specific antibodies on cell aggregation. Slow-aggregation assays were performed in microtiter plates in which the experiments could be carried out with minute amounts of reagent. Single cells were seeded on semisolid agar, incubated under cell-culture conditions for 1 d, and evaluated for aggregate formation by microscopy. The IIH6 and VIA4-1 antibodies, which are specific for O-mannosylated α-DG, had no effect on cell adhesion, even at the highest concentration tested (Fig. 5A and Fig. S5). In contrast, our T[α1-Man]–specific antibody inhibited cadherin-based aggregation to the same extent as a function-blocking E-cad antibody (Fig. 5A and Fig. S5). It was demonstrated previously that aggregation of MDCK cells is mediated predominantly by E-cad and kidney cadherin (K-cad, CDH6) (36). K-cad also was identified as an O-mannosylated protein by Vester-Christensen et al. (32). To quantitate the effect of impaired O-mannosylation on MDCK aggregation, we used fast-aggregation assays (34). MDCK cells were grown in the presence of R3A-5a to block O-mannosylation. Single cells were prepared using a cadherin-preserving procedure, and cell aggregation was determined as detailed in Materials and Methods. Notably, inhibiting O-mannosylation by applying increasing amounts of R3A-5a negatively affected cell–cell aggregation. This gradual decline was accompanied by a decrease in O-mannosylation of E-cad (Fig. 5B, E-cad and T[α1-Man]) as well as K-cad (Fig. 5B, K-cad). Taken together, these results strongly indicate that O-mannosylation is crucial for cadherin function.
Fig. 5.
O-mannosyl glycans affect cadherin-based cell–cell adhesion. (A) Slow-aggregation assays. MDCK cells were aggregated overnight in the presence of an E-cad antibody (DECMA-1), a T[α1-Man]–specific antibody, or antibodies directed against carbohydrate epitopes that are O-mannosidically linked to α-DG (IIH6 and VIA4-1), as indicated. EGTA was used to block Ca2+-dependent, cadherin-mediated adhesion. (B) Quantitative summary of data from fast-aggregation assays. MDCK cells were aggregated in the presence of EGTA, DMSO, or increasing concentrations of R3A-5a (6.25–50 µM). The percentage of nonaggregated cells (fewer than four cells) was determined after a 30-min incubation. Mean values of three independent experiments are shown. Protein extracts were analyzed by Western blotting using the E-cad–specific antibody DECMA-1 (E-cad) and a K-cad–specific antibody (K-cad). O-mannosyl glycans on purified E-cad were detected by the T[α1-Man]–specific antibody. Tubulin served as the loading control.
In conclusion, our study identifies E-cad as O-mannosylated protein and establishes a functional link between O-linked glycans and cadherin-mediated cell–cell adhesion. Further, our findings clearly suggest that, in vivo, O-mannosylation of E-cad is highly relevant to the formation and/or maintenance of adherens junctions during the morula-to-blastocyst transition. Because multiple members of the cadherin family and other cell-adhesion molecules, as well as E-cad, are O-mannosylated (32), we cannot rule out completely the possibility that alternative mechanisms also might contribute to our findings. That blocking O-mannosylation did not noticeably affect the stability and/or cell-surface localization of E- and K-cad (Figs. 3B and 5B) suggests that O-mannosyl glycans may modulate cadherin structure. Unraveling the molecular role of this modification for cadherin function will be an exciting future challenge.
To date, the characterization of O-mannosyl glycan function was limited to α-DG–associated phenotypes because other POMT substrates were not known. However, recent glycomics efforts (7, 9) and the identification of additional O-mannosylated proteins (8–11, 32) indicated that O-mannosyl glycans contribute to more cellular processes than initially expected. Given the crucial role of cadherin-based cell–cell contacts in tissue morphogenesis and maintenance (37), our findings shed light on the molecular basis of some aberrations observed in CMDs. The most severe cases of O-mannosylation deficiencies, such as Walker–Warburg syndrome and muscle-eye-brain disease, are characterized by neuronal migration disorders that result in fatal brain and eye abnormalities (2). Corresponding animal models of O-mannosylation deficiency show highly disorganized neuroepithelia, which had been attributed to defects in the formation of the glia limitans, a basement membrane that is formed in a α-DG–dependent manner (22, 38). However, recent results suggest that these neuronal migration defects are explained only partially by α-DG loss of function. In zebrafish, severely perturbed lamination of the retina was observed in O-mannosylation–deficient (39) but not dystroglycan mutants (40). Interestingly, similarly disorganized retinal layering is manifested upon morpholino-mediated knockdown of R- and N-cad in zebrafish (41, 42), and perturbed lamination of the neocortex is found upon brain-specific deletion of N-cad (43). Thus, these CMD phenotypes most likely also are consequences of cadherin hypomannosylation. α-DG–deficient animal models and the T[α1-Man]–specific antibody will make it possible to unravel the impact of cadherins in α-dystroglycanopathies.
Given the fundamental role that cadherins play in morphogenetic processes during embryogenesis, in the differentiation and maintenance of tissue, and in cancer progression (20, 37), this study opens exciting possibilities for the significance of protein O-mannosylation in mammals and humans.
Materials and Methods
Animal Procedures.
Mice were bred in a pathogen-free mouse facility at the Gene Center, Ludwig Maximilian University, Munich, Germany. All animal procedures in this study were approved by the local Animal Welfare Committee (Regierung von Oberbayern) and were performed according to the German Animal Welfare Act and the European Communities Council Directive of November 24, 1986 (86/609/EEC). The targeting of Pomt2 in ES cells and the generation of Pomt2−/− mice is detailed in SI Materials and Methods.
Quantitative Expression Analysis.
Quantitative PCR was carried out using SYBR-Green Supermix (Bio-Rad). cDNA derived from 0.5 mouse embryo equivalents and gene-specific primers (100 nM each) of Pomt1Fwd/Pomt1Rev, Pomt2Fwd/Pomt2Rev, and PpiaFwd/PpiaRev were used. Relative expression of Pomt1 and Pomt2 was normalized to peptidylprolyl isomerase A (Ppia) expression using the ΔΔCT method (44). The primer sequences are listed in Table S1.
Inhibitor Treatment of Mouse Embryos and MDCK Cells.
Embryos at the two-cell stage were flushed from C57BL/6N donor females and incubated in M16 medium (Sigma). MDCK cells (American Type Culture Collection CRL-2936) were cultured under standard conditions. For inhibitor studies, culture medium was supplemented with 0.5% DMSO or R3A-5a (Biotrend) dissolved in DMSO for 3 d.
Generation of the T[α1-Man]–Specific Antibody.
The peptide CYAT(1α-Man)AV was synthesized using standard Fmoc chemistry and was coupled to maleimide-activated keyhole limpet hemocyanine (KLH; Pierce). KLH conjugates were used to immunize rabbits according to a standard protocol. The T[α1-Man]–specific polyclonal antibody was affinity purified using YAT(α1-Man)AV or YATAV, coupled to Affi-Gel(R) 10 (Bio-Rad).
Deglycosylation of Proteins and Peptides.
Protocols for the partial enrichment of α-DG from skeletal muscle and protein extraction from MDCK cells are detailed in SI Materials and Methods. Five micrograms of α-DG–enriched fractions from porcine skeletal muscle and affinity-purified E-cad were enzymatically deglycosylated using the GLYCOPRO deglycosylation kit (Prozyme) and the PRO-LINK extender kite (Prozyme) or were chemically deglycosylated using the GlycoProfil kite (Sigma). Deglycosylated and mock-treated samples were analyzed by Western blotting using standard procedures (SI Materials and Methods). E-cad–derived glycopeptides were treated with α(1-2,3,6)-mannosidase (Jack Bean; Prozyme) and were analyzed by LC-MS as detailed in SI Materials and Methods.
Whole-Mount Immunofluorescence Analyses.
Embryos were fixed in PBS containing 2% (wt/vol) paraformaldehyde for 30 min, permeabilized in Tris-buffered saline (TBS), pH 7.4, containing 0.5% Triton X-100, and blocked in TBS containing 0.02% Tween-20 (TBS-T) and 2% (wt/vol) BSA. Rat monoclonal E-cadherin antibody (DECMA-1) (1:100; Sigma), polyclonal anti-ZO1 (1:100; Zymed), and polyclonal anti–T[α1-Man] (1:20; this study) were applied in TBS-T containing 2% (wt/vol) BSA. Secondary antibodies used were Alexa Fluor 488-conjugated anti-rat (1:2,500; Molecular Probes), Alexa Fluor 568-conjugated anti-rabbit (1:2,000; Molecular Probes), rhodamine-conjugated Arachis hypogaea agglutinin (PNA) (200 µg/µL; Vector Laboratories), and Dy-647–conjugated phalloidin (1:40; Dyomics). Embryos were analyzed on a Nikon A1Rsi confocal microscope.
Cell-Aggregation Assays.
Slow-aggregation assays were performed in the presence of monoclonal DECMA-1, IIH6 (Millipore), VIA4-1, polyclonal T[α1-Man]–specific antibodies, and EGTA as described (34). For fast-aggregation assays (34) E-cad was reconstituted overnight in the presence of DMSO (0.5%) or R3A-5a. The extent of cell aggregation was assessed in the presence of DMSO, R3A-5a, or EGTA by counting the number of nonaggregated cells (fewer than four cells). Results are expressed as a percentage of the mean relative to the total number of cells present before aggregation.
Supplementary Material
Acknowledgments
We thank I. Hagen and T. Spruss for glycopeptide synthesis and immunization of rabbits; F. Habermann for technical advice on whole-mount immunofluorescence analysis; I. Breloy and L. Popolo for plasmids; A. Metschies for excellent technical assistance; P. Renner for expert animal care; and the Nikon Imaging Center Heidelberg core facility for support. S.S. and M.L. received financial support from the Deutsche Forschungsgemeinschaft (STR-443/2, LO 1807/1-1). S.S. and M.F.B. received financial support from research contract ’Glykobiologie/Glykomik‘ of the Baden-Württemberg Stiftung.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 20858.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316753110/-/DCSupplemental.
References
- 1.Lommel M, Strahl S. Protein O-mannosylation: Conserved from bacteria to humans. Glycobiology. 2009;19(8):816–828. doi: 10.1093/glycob/cwp066. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey C, Foley AR, Clement E, Muntoni F. Dystroglycanopathies: Coming into focus. Curr Opin Genet Dev. 2011;21(3):278–285. doi: 10.1016/j.gde.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Barresi R, Campbell KP. Dystroglycan: From biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119(Pt 2):199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 4.Willer T, et al. Targeted disruption of the Walker-Warburg syndrome gene Pomt1 in mouse results in embryonic lethality. Proc Natl Acad Sci USA. 2004;101(39):14126–14131. doi: 10.1073/pnas.0405899101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michele DE, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418(6896):417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 6.Chai W, et al. High prevalence of 2-mono- and 2,6-di-substituted manol-terminating sequences among O-glycans released from brain glycopeptides by reductive alkaline hydrolysis. Eur J Biochem. 1999;263(3):879–888. doi: 10.1046/j.1432-1327.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- 7.Stalnaker SH, et al. Glycomic analyses of mouse models of congenital muscular dystrophy. J Biol Chem. 2011;286(24):21180–21190. doi: 10.1074/jbc.M110.203281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleckmann C, et al. O-glycosylation pattern of CD24 from mouse brain. Biol Chem. 2009;390(7):627–645. doi: 10.1515/BC.2009.044. [DOI] [PubMed] [Google Scholar]
- 9.Trinidad JC, Schoepfer R, Burlingame AL, Medzihradszky KF. N- and O-glycosylation in the murine synaptosome. Mol Cell Proteomics. 2013. 10.1074/mcp.M113.030007. Available at www.mcponline.org/content/early/2013/07/01/mcp.M113.030007.long. [DOI] [PMC free article] [PubMed]
- 10.Pacharra S, Hanisch FG, Breloy I. Neurofascin 186 is O-mannosylated within and outside of the mucin domain. J Proteome Res. 2012;11(8):3955–3964. doi: 10.1021/pr200996y. [DOI] [PubMed] [Google Scholar]
- 11.Pacharra S, et al. The lecticans of mammalian brain perineural net are O-mannosylated. J Proteome Res. 2013;12(4):1764–1771. doi: 10.1021/pr3011028. [DOI] [PubMed] [Google Scholar]
- 12.Manya H, et al. Demonstration of mammalian protein O-mannosyltransferase activity: Coexpression of POMT1 and POMT2 required for enzymatic activity. Proc Natl Acad Sci USA. 2004;101(2):500–505. doi: 10.1073/pnas.0307228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avsar-Ban E, et al. Protein O-mannosylation is necessary for normal embryonic development in zebrafish. Glycobiology. 2010;20(9):1089–1102. doi: 10.1093/glycob/cwq069. [DOI] [PubMed] [Google Scholar]
- 14.Haines N, Seabrooke S, Stewart BA. Dystroglycan and protein O-mannosyltransferases 1 and 2 are required to maintain integrity of Drosophila larval muscles. Mol Biol Cell. 2007;18(12):4721–4730. doi: 10.1091/mbc.E07-01-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halbleib JM, Nelson WJ. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20(23):3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 16.Meng W, Takeichi M. Adherens junction: Molecular architecture and regulation. Cold Spring Harb Perspect Biol. 2009;1(6):a002899. doi: 10.1101/cshperspect.a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hulpiau P, van Roy F. Molecular evolution of the cadherin superfamily. Int J Biochem Cell Biol. 2009;41(2):349–369. doi: 10.1016/j.biocel.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Brasch J, Harrison OJ, Honig B, Shapiro L. Thinking outside the cell: How cadherins drive adhesion. Trends Cell Biol. 2012;22(6):299–310. doi: 10.1016/j.tcb.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci USA. 1994;91(17):8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4(2):118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 21.Lommel M, et al. Correlation of enzyme activity and clinical phenotype in POMT1-associated dystroglycanopathies. Neurology. 2010;74(2):157–164. doi: 10.1212/WNL.0b013e3181c919d6. [DOI] [PubMed] [Google Scholar]
- 22.Hu H, et al. Conditional knockout of protein O-mannosyltransferase 2 reveals tissue-specific roles of O-mannosyl glycosylation in brain development. J Comp Neurol. 2011;519(7):1320–1337. doi: 10.1002/cne.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orchard MG, et al. Rhodanine-3-acetic acid derivatives as inhibitors of fungal protein mannosyl transferase 1 (PMT1) Bioorg Med Chem Lett. 2004;14(15):3975–3978. doi: 10.1016/j.bmcl.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 24.Argyros R, et al. A phenylalanine to serine substitution within an O-protein mannosyltransferase led to strong resistance to PMT-inhibitors in Pichia pastoris. PLoS ONE. 2013;8(5):e62229. doi: 10.1371/journal.pone.0062229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arroyo J, et al. Functional and genomic analyses of blocked protein O-mannosylation in baker’s yeast. Mol Microbiol. 2011;79(6):1529–1546. doi: 10.1111/j.1365-2958.2011.07537.x. [DOI] [PubMed] [Google Scholar]
- 26.Fleming TP, et al. Molecular maturation of cell adhesion systems during mouse early development. Histochemistry. 1994;101(1):1–7. doi: 10.1007/BF00315824. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, et al. Zonula occludens-1 (ZO-1) is involved in morula to blastocyst transformation in the mouse. Dev Biol. 2008;318(1):112–125. doi: 10.1016/j.ydbio.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kan NG, et al. Gene replacement reveals a specific role for E-cadherin in the formation of a functional trophectoderm. Development. 2007;134(1):31–41. doi: 10.1242/dev.02722. [DOI] [PubMed] [Google Scholar]
- 29.Ohsugi M, Larue L, Schwarz H, Kemler R. Cell-junctional and cytoskeletal organization in mouse blastocysts lacking E-cadherin. Dev Biol. 1997;185(2):261–271. doi: 10.1006/dbio.1997.8560. [DOI] [PubMed] [Google Scholar]
- 30.Pinho SS, et al. Modulation of E-cadherin function and dysfunction by N-glycosylation. Cell Mol Life Sci. 2011;68(6):1011–1020. doi: 10.1007/s00018-010-0595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison OJ, et al. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure. 2011;19(2):244–256. doi: 10.1016/j.str.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vester-Christensen MB, Halim A, Joshi HJ, Steentoft C, Bennett EP, et al. (2013) Mining the O-mannose glycoproteome reveals cadherins as major O-mannosylated glycoproteins. Proc Natl Acad Sci USA 110:21018–21023. [DOI] [PMC free article] [PubMed]
- 33. Winterhalter PR, Lommel M, Ruppert T, Strahl S (2013) O-glycosylation of the non-canonical T-cadherin from rabbit skeletal muscle by single mannose residues. FEBS Lett 587(22):3715–3721. [DOI] [PubMed]
- 34.Boterberg T, Bracke ME, Bruyneel EA, Mareel MM. Cell aggregation assays. Methods Mol Med. 2001;58:33–45. doi: 10.1385/1-59259-137-X:033. [DOI] [PubMed] [Google Scholar]
- 35.Stewart DB, Barth AI, Nelson WJ. Differential regulation of endogenous cadherin expression in Madin-Darby canine kidney cells by cell-cell adhesion and activation of beta -catenin signaling. J Biol Chem. 2000;275(27):20707–20716. doi: 10.1074/jbc.M000467200. [DOI] [PubMed] [Google Scholar]
- 36.Jia L, Liu F, Hansen SH, Ter Beest MB, Zegers MM. Distinct roles of cadherin-6 and E-cadherin in tubulogenesis and lumen formation. Mol Biol Cell. 2011;22(12):2031–2041. doi: 10.1091/mbc.E11-01-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6(8):622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, et al. Cellular and molecular characterization of abnormal brain development in protein o-mannose N-acetylglucosaminyltransferase 1 knockout mice. Methods Enzymol. 2010;479:353–366. doi: 10.1016/S0076-6879(10)79020-0. [DOI] [PubMed] [Google Scholar]
- 39.Thornhill P, Bassett D, Lochmüller H, Bushby K, Straub V. Developmental defects in a zebrafish model for muscular dystrophies associated with the loss of fukutin-related protein (FKRP) Brain. 2008;131(Pt 6):1551–1561. doi: 10.1093/brain/awn078. [DOI] [PubMed] [Google Scholar]
- 40.Gupta V, et al. The zebrafish dag1 mutant: A novel genetic model for dystroglycanopathies. Hum Mol Genet. 2011;20(9):1712–1725. doi: 10.1093/hmg/ddr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Babb SG, et al. Zebrafish R-cadherin (Cdh4) controls visual system development and differentiation. Dev Dyn. 2005;233(3):930–945. doi: 10.1002/dvdy.20431. [DOI] [PubMed] [Google Scholar]
- 42.Erdmann B, Kirsch FP, Rathjen FG, Moré MI. N-cadherin is essential for retinal lamination in the zebrafish. Dev Dyn. 2003;226(3):570–577. doi: 10.1002/dvdy.10266. [DOI] [PubMed] [Google Scholar]
- 43.Kadowaki M, et al. N-cadherin mediates cortical organization in the mouse brain. Dev Biol. 2007;304(1):22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.