Significance
One of the most critical and complex steps of the protein synthesis elongation cycle is the coupled translocation of messenger (m)RNA and the A- and P-site transfer (t)RNAs through the ribosome, catalyzed by the elongation factor EF-G. This step involves large-scale molecular movements in the ribosome, including rotational movements of the body and head of the 30S subunit. Previously, structures have been obtained for trapped intermediates containing a single tRNA. Here, we report the cryo-EM structure of an intermediate trapped with both tRNAs. This structure represents a previously missing link in understanding the mechanism of translocation, revealing that the ribosome uses two distinct molecular ratchets, involving both intra- and intersubunit rotational movements, to drive the synchronous movement of tRNAs and mRNA.
Keywords: ratcheting, chimeric hybrid state, ribosome dynamics, translocation mechanism, fusidic acid
Abstract
During protein synthesis, coupled translocation of messenger RNAs (mRNA) and transfer RNAs (tRNA) through the ribosome takes place following formation of each peptide bond. The reaction is facilitated by large-scale conformational changes within the ribosomal complex and catalyzed by elongtion factor G (EF-G). Previous structural analysis of the interaction of EF-G with the ribosome used either model complexes containing no tRNA or only a single tRNA, or complexes where EF-G was directly bound to ribosomes in the posttranslocational state. Here, we present a multiparticle cryo-EM reconstruction of a translocation intermediate containing two tRNAs trapped in transit, bound in chimeric intrasubunit ap/P and pe/E hybrid states. The downstream ap/P-tRNA is contacted by domain IV of EF-G and P-site elements within the 30S subunit body, whereas the upstream pe/E-tRNA maintains tight interactions with P-site elements of the swiveled 30S head. Remarkably, a tight compaction of the tRNA pair can be seen in this state. The translocational intermediate presented here represents a previously missing link in understanding the mechanism of translocation, revealing that the ribosome uses two distinct molecular ratchets, involving both intra- and intersubunit rotational movements, to drive the synchronous movement of tRNAs and mRNA.
During protein synthesis the ribosome iteratively incorporates new amino acids delivered by aminoacylated transfer RNAs (tRNA) into the growing polypeptide chain in a manner specified by the codons in a messenger RNAs (mRNA). This elongation cycle is controlled by the two translocational GTPases elongation factors (EF)-Tu and EF-G. Following EF-Tu–dependent delivery of aminoacyl-tRNA to the A site and peptide bond formation, the ribosome adopts a pretranslocational state containing a peptidyl A-site tRNA and a deacylated P-site tRNA. In the subsequent translocation reaction, the interplay between the ribosome and elongation factor EF-G shifts the tRNAs from the A and P sites to the P and E sites, respectively. In each of these binding sites a tRNA contacts both ribosomal subunits and interacts with the 30S and 50S subunits via its anticodon-stem loop (ASL) and acceptor arm, respectively (1). Partial tRNA movement can occur before the EF-G–dependent translocation step, involving spontaneous and reversible movement of the tRNA acceptor arms relative to the large ribosomal subunit, which leads to a shift from classic A/A and P/P binding states into intersubunit A/P and P/E hybrid states (where the first and second letters indicate tRNA contacts on the small and large subunits, respectively) (2–4).
A remarkable feature of translocation is the precise coupling of movement of the tRNAs together with the bound mRNA (designated as the tRNA2•mRNA module), so that the mRNA advances by exactly one codon on the ribosome. Translocation is associated with large-scale conformational changes within the ribosomal complex, which includes rotation and back-rotation between the two subunits (during which the small subunit rotates counterclockwise and clockwise relative to the large subunit, viewing the ribosome from the solvent side of the small subunit) (5–7), and an additional forward and reverse swiveling movement of the 30S head (an autonomous domain of the 30S subunit, which can rotate around an axis roughly orthogonal to the axis of intersubunit rotation) (6, 8–13). Structural studies have suggested that intersubunit rotation within the pretranslocational complex is coupled to tRNA hybrid state formation (14–18). EF-G–dependent movement of the tRNAs and mRNA on the 30S subunit then occurs during reversal of the intersubunit rotation (6, 7). Moreover, multiparticle cryo-EM and X-ray crystallography studies suggest that movement of the tRNAs relative to the 30S subunit occurs via additional intermediate tRNA binding states, which are formed upon the back rotation of the 30S-body/platform and a large swiveling movement of the 30S head (6, 10). One of the first implications of swiveling of the 30S subunit head came from the studies of Schuwirth et al. (19), who observed a constriction of 13 Å between head and body of the 30S subunit that would block passage of tRNA between the P and E sites. These authors suggested that rotation of the 30S head would allow movement of the tRNA ASL, and could correspond to an unlocking event during translocation. Although swiveling of the small subunit head has been observed in different ribosomal complexes with bound EF-G (or eEF2) (6, 8–13) or with bound tRNAs (18), it has not been observed directly in the context of an authentic translocation complex containing EF-G together with two tRNAs. Previous structural analysis of translocation used model complexes where EF-G was directly bound to either vacant ribosomes, to ribosomal complexes with one tRNA, or to complexes in the posttranslocational state (5, 6, 8, 10–13, 20–22). The present study describes a cryo-EM reconstruction more closely resembling an authentic translocation intermediate, in which EF-G•GTP was bound to a canonical pretranslocational ribosomal complex containing two tRNAs and mRNA, and stalled during the translocation reaction by the antibiotic fusidic acid (FA). The resulting sample was analyzed by means of multiparticle cryo-EM (23). The classification yielded only a single major population of 70S•EF-G•GDP•FA particles trapped in an intermediate state of the translocation reaction. In contrast to all previously described 70S•EF-G structures (5, 6, 10–13, 20–22), the resulting reconstruction directly visualizes two tRNAs bound to the ribosome in two different chimeric intrasubunit hybrid states. The data presented here show how reciprocal conformational changes within the ribosome coordinate the synchronous movement of the mRNA and bound tRNA pair.
Results
Sample Preparation and Multiparticle Classification.
To analyze the mechanism of translocation, EF-G, GTP, and the antibiotic FA were incubated with Escherichia coli 70S ribosomes in the pretranslocational state carrying tRNAfMet and Val-tRNAVal in the P and A sites, respectively. FA specifically traps EF-G upon ribosome binding and GTP hydrolysis as it prevents complete conversion from the GTP to the GDP conformation, which is required for release of EF-G•GDP from the ribosome (6, 10, 22). The resulting sample was subjected to multiparticle classification to solve the problem of potential conformational heterogeneity within a prepared cryo-EM specimen, which can be caused by substoichometric complex formation (23). However, the multiparticle classification of the cryo-EM dataset yielded only a single population of 279,309 70S•EF-G•GDP•FA particle images, which were used for a final cryo-EM reconstruction giving a resolution of 6.8 Å (Fig. 1 and Fig. S1). The resulting cryo-EM map shows the complex of a 70S ribosome together with EF-G bound to the factor-binding site, mRNA, and two tRNAs (Fig. 1). At this subnanometer resolution the reconstruction displays clearly visible secondary structural features, such as rod-shaped α-helical elements. This finding provided the basis for generating pseudoatomic models based on 3D coordinates of ribosomal (11), mRNA (10), tRNA (10, 22), and EF-G (11) X-ray structures.
Fig. 1.
Overview of the canonical TIPOSTcomplex. (A and B) Cryo-EM density of the ligands ap/P- and pe/E-tRNAs (green and orange), mRNA (magenta), and EF-G (red) in surface representation within the 70S complex [50S (blue) and 30S (yellow)] (A) or with a mesh representation of both subunits together with docked models of the 30S [16S (yellow) and S-proteins (gray)] and 50S [23S and 5S (blue) and L-proteins (orange)] (B). (C) View from the intersubunit space onto the 30S subunit in the presented authentic TIPOST complex. Elements are colored according to their structural displacement compared with the classic conformation (30) upon a common 50S alignment.
Overall Conformation of the Ribosome.
Although the composition of our specimen, which contains EF-G•GDP•FA and two tRNAs, is similar to that of the posttranslocational 70S•EF-G•GDP•FA complex, which was used for X-ray crystallography (22), remarkable large-scale differences can be observed by comparing the ribosomal conformations of the two complexes. Foremost among these structural differences is the conformation of the 30S ribosomal subunit and its orientation relative to the 50S subunit. In contrast to the X-ray structure of the posttranslocational 70S•EF-G•GDP•FA complex in a classic unrotated and unswiveled ribosomal state (22), the 30S head domain of the present complex is swiveled by ∼18°, whereas the body/platform domain has undergone a moderate rotation of ∼2.5° (Fig. 1C). These movements cause a displacement of peripheral elements of the 30S head over distances of up to ∼36 Å compared with a ribosome in the unrotated state (22) (Fig. 1C). The conformation of the present complex bears overall similarity to recently described 70S•EF-G complexes designated as translocation intermediate (TIPOST) (6) or GDPNP-II (10) characterized by an intermediate (∼5°) body/platform rotation and large (∼18°) head swivel. However, the latter complexes contained only a single tRNA (6, 10), whereas the present complex contains two tRNAs, more closely resembling an authentic translocation intermediate (TIPOST). In this view, the TIPOST state is adopted after formation of the preceding TIPRE state, which was observed with a single bound tRNA (6, 12, 13). This TIPRE to TIPOST transition would include a back-rotation of the body/platform region from ∼7–9° to ∼2.5° and an additional swiveling movement of the 30S head domain from ∼5–7.5° to ∼18°. In agreement with the X-ray structures of 70S•EF-G complexes (10), the present structure also shows that the swiveling movement of the 30S head is accompanied by a lateral movement of the tip of Helix H38 (also called the A-site finger) and leads to formation of a new intersubunit contact formed between S19 and the tip of the A-site finger (SI Results and Discussion and Fig. S2).
Structural Characterization of the tRNA Binding Sites.
The observed structural changes of the ribosome have a unique influence on the interactions between the ribosome and bound tRNAs. In each of the three classic binding states a tRNA interacts with a specific set of rRNA and ribosomal protein residues, which are distributed between both ribosomal subunits (1). In the trapped intermediate structure described here, the CCA acceptor ends of the tRNAs are fully translocated from the P and A sites to the E and P sites, respectively, on the 50S subunit (Fig. 1B). However, the tRNA ASLs have moved to an intermediate state of translocation relative to the 30S subunit. Interactions between the ASLs of the Val-tRNAVal and tRNAfMet and the A- and P-site elements of the 30S body are disrupted and replaced by contacts with 16S rRNA P- and E-site residues A790 and U788/A695, respectively (Fig. S3). At the same time, the ASL of tRNAfMet maintains its characteristic P-site contacts with A1229, G1338, and A1339 within the 30S head, as it moves to follow the large ∼18° swiveling movement of the 30S head (Fig. S3). The latter movement results in a chimeric hybrid binding state (the pe/E state; where p and e indicate contact between the ASL and the P site of the 30S head and E site of the 30S body/platform; and E indicates contact of the acceptor end with the 50S E site) in which the ASL of tRNAfMet is held between the P site of the 30S head and the E site of the 30S body, whereas its CCA acceptor end is bound to the 50S E site, as observed in previous 70S•EF-G complexes containing a single tRNA (6, 10). Meanwhile, contacts between the ASL of the A-site Val-tRNAVal and the A-site elements of the 30S body are disrupted, as the ASL had moved during TIPOST state formation. This process results in formation of an additional chimeric hybrid state (the ap/P state) in which the ASL is positioned between the A site of the 30S head and the P site of the 30S body, whereas its acceptor end is bound to the 50S P site (Fig. S3). This observed network of ribosome–tRNA interactions is distinct from the posttranslocational 70S•EF-G•GDP•FA complex in which the tRNAs are bound in their classic P/P and E/E binding states (22), and to rotated pretranslocational ribosome complexes with tRNAs bound in intersubunit A/P and P/E hybrid states (14–18) (Fig. S3). Therefore, the Val-tRNAVal and tRNAfMet bound in the complex presented here occupy a pair of tRNA binding states that can be understood as chimeric intrasubunit ap/P and pe/E hybrid states, which is consistent with related single-molecule studies (24). Although the pe/E binding state is similar to that observed previously for trapped ribosome•EF-G intermediate complexes containing a single tRNA (6, 10), the ap/P tRNA represents a previously unobserved binding state whose functional role was predicted by a recently suggested model for canonical translocation (6).
Within the present FA-stalled intermediate, the tRNA2•mRNA module has already moved relative to the 50S subunit and the 30S body/platform domain, in agreement with a toe-print signal, which indicated full mRNA-translocation for a similar FA-trapped ribosomal complex (25). This observation is consistent with the results of presteady-state kinetics experiments, which showed that GTP hydrolysis by EF-G precedes movement of the tRNA2•mRNA module (26). Movement then occurs independent of and parallel to the release of Pi from the ribosome-bound EF-G•GDP•Pi complex. However, as shown here, translocation is not completed in this FA-stalled state because the 30S head still maintains its characteristic pretranslocational A and P site contacts with the ap/P- and pe/E-tRNAs (Fig. S3). The kinetic model of Savelsbergh et al. (26) does not account for the occurrence of multiple steps of mRNA and tRNA movement. In light of the important role of 30S head rotation, it seems likely that this is a consequence of basing this model on a fluorescence quenching assay to detect mRNA movement. As discussed elsewhere (9), quenching may occur when the mRNA is moved into contact with the head or body of the 30S subunit, or alternatively when the 30S subunit head or body rotates into contact with the mRNA during their respective reverse rotations; these different possibilities render the interpretation of the quenching results ambiguous. Thus, the quenching assay may not report on the final steps of translocation.
EF-G Contacts the ap/P-tRNA, mRNA, and the 30S Head.
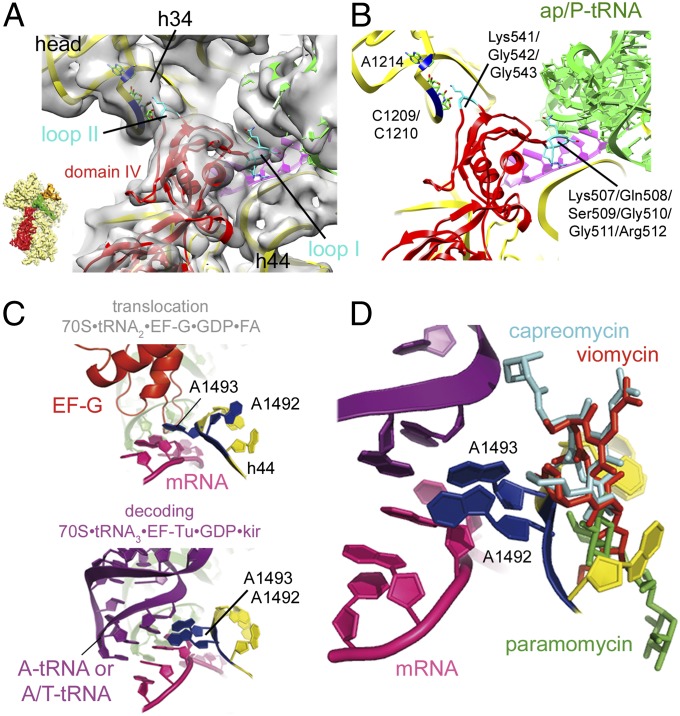
The reconstruction of the present complex displays well-defined cryo-EM density for EF-G, which binds to the factor-binding site and places its domain IV into the ribosomal A site (Fig. 2). In this position, domain IV establishes contacts with the ap/P-tRNA, mRNA and h34 of 16S rRNA in the swiveled head (Fig. 2). Thus, the structure unifies separate structural observations coming from other 70S•EF-G complexes, in which either the domain IV–codon-anticodon interaction (22) or the domain IV–h34 contact were observed (6, 10). Interestingly, there are certain similarities in comparing the two contacts between EF-G and the two RNA helices, h34 and the mRNA codon–ap/P-tRNA anticodon helix (codon-anticodon helix). In each case, one of two protruding loops at the tip of domain IV (loop I or loop II) intercalates into the minor groove of the codon-anticodon helix or h34 of 16S rRNA, respectively. Remarkably, at the apical tips of both loops two neighboring glycine residues, which are 100% conserved among bacteria [Gly510/Gly511 and Gly542/Gly543 (E. coli nomenclature) for loop I and loop II, respectively] facilitate the tight turns required for their intercalation into the RNA minor grooves (Fig. 2B). Including all EF-G residues within 5 Å of mRNA and the ap/P-tRNA, the loop I region of EF-G buries a surface area of ∼1,000 Å2; in contrast, the loop II region buries an area of only ∼390 Å2 (Fig. 2). Accordingly, the loop I–codon-anticodon helix interaction may act as a robust “doorstop” to prevent back-slippage of the ap/P-tRNA and mRNA, whereas the less extensive loop II–h34 contact may help to stabilize the orientation of the 30S head. The latter interaction can explain why binding of EF-G leads to transient protection of h34 against chemical modification during the translocation reaction (27). In this regard the present structure reveals an important function of EF-G and explains the overall shape of its domain IV. Like a hand with two protruding fingers, domain IV uses loop I and loop II to establish simultaneous protein–RNA minor groove interactions with the codon-anticodon RNA triplet, as well as with h34. These data reveal how EF-G stabilizes the synchronous movement of the ap/P-tRNA anticodon, its corresponding mRNA codon, and the associated swiveling of the 30S head in the course of translocation. In addition, in agreement with observations coming from a high-resolution X-ray structure of a 70S•EF-G complex (13) and related data from a 80S•eEF2 complexes at intermediate resolution (28), the present data suggest that EF-G might trigger conformational changes within the 30S decoding center (Fig. 2 C and D and Fig. S4). These changes may include reorientation of A1492 and lead to destabilization of its interaction with the minor groove of the codon-anticodon helix. Such an interpretation would be consistent with the results of presteady-state kinetics studies (29) and may explain the inhibitory effects of the antibiotics paromomycin and viomycin, which reduce the rate of translocation by about 160- and >10,000-fold, respectively (30). Both antibiotics bind around h44 and stabilize the flipped-out positions of A1492 and A1493 (31, 32) (Fig. 2D). In particular, the presence of viomycin prevents such a proposed reorientation, as it would sterically clash with a reoriented A1492 (31) (Fig. 2D). This suggested mechanism would be consistent with kinetic and FRET studies, both of which describe translocation intermediates that can be stalled by viomycin (33, 34). Factor-induced rearrangements within the small subunit-decoding center residues were also observed during eukaryotic translation initiation (35). In the context of these structural data (13, 28, 29, 33–35) the small subunit-decoding center may be seen as a general pivotal element of the ribosome, whose flexibility is precisely controlled by external factors to coordinate several steps during different phases of translation.
Fig. 2.
Contacts of EF-G domain IV. (A) Cryo-EM density of the presented TIPOST complex shown as transparent gray surface together with docked models of the 30S, mRNA, ap/P-tRNA, and EF-G (colored as in Fig. 1) in the indicated view (Left). (B) Cartoon representation of the canonical TIPOST complex highlighting the interactions between loop I and loop II within EF-G domain IV and the minor grooves of the mRNA codon–tRNA anticodon helix, as well as h34, respectively. (C) Cartoon representation of the A1492 and A1493 (blue) within h44 (yellow) in their tentative positions during translocation (suggested by the present authentic TIPOST) (Upper) and upon domain closure during decoding (observed in a 70S•tRNA3•EF-Tu•GDP•kir complex) (43) (Lower). (D) Cartoon representation of A1492 and A1493 as shown in C, Lower, together with the antibiotics paramomycin (green) (32), viomycin (red), and capreomycin (cyan) (31).
tRNA Compaction.
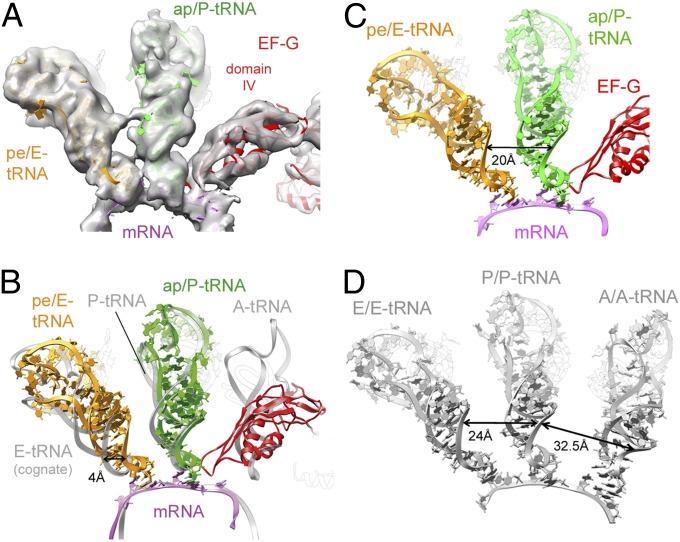
Comparison of the present tRNAs in their intrasubunit ap/P and pe/E hybrid states with tRNAs in classic A, P, and E sites using a 50S alignment (36) reveals a gap between the ASLs of the pe/E- and E-site tRNAs, placing the pe/E-tRNA ASL 4 Å closer to the P site (Fig. 3). The cognate E-site tRNA was selected for the present comparison because it directly interacts with the corresponding mRNA E site codon and creates at least one base pair of the codon-anticodon triplet (36, 37). However, the observed pe/E-tRNA superimposes closely on the pe*/E-tRNA bound to 70S•EF-G complex in an overall similar conformation (GDPNP-II) (10). Accordingly, the detected 4 Å gap indicates that the movement of the tRNA from the P to the E site is not fully completed during the FA-stalled TIPOST state formation. Apparently, a further movement of the tRNA must occur during the final step of translocation: that is, the release of EF-G•GDP and reverse rotation of the 30S subunit head. Such an interpretation would be compatible with the reported presteady-state kinetic model (26). Although the used biochemical system does not provide a resolution to describe such small-scale tRNA movements, the derived model indicates that translocation is completed by rearrangements within EF-G and the ribosome (26). Furthermore, we observe compaction of the tRNA pair during the translocation reaction. TIPOST formation moves the P-site tRNA ASL by about 20 Å from its classic position into the pe/E state (measuring the distance between their respective nucleotides at position 31). In contrast, the A-site tRNA moves by about 32 Å into the ap/P state and is thus pushed against the pe/E-tRNA ASL, presumably stabilized by its interaction with EF-G domain IV. Thus, the two tRNA ASLs move closer together, as indicated by the 20 Å distance between their apical base pairs and may even allow tRNA–tRNA contact in this state (Fig. 3A). In contrast, much larger equivalent distances of 32.5 Å and 24 Å are observed between adjacent tRNAs occupying classic A and P or P and E sites, respectively (Fig. 3D).
Fig. 3.
Ligand positions within the authentic TIPOSTcomplex. (A) Cryo-EM density for the ligands within the presented authentic TIPOST complex in a transparent surface representation together with docked models of EF-G (red), ap/P-tRNA (green), pe/E-tRNA (orange), and mRNA (magenta). (B) Superimposition of the ligands (as shown in A) together with tRNAs bound to classic A, P, and E sites (transparent gray) (23) upon a 50S alignment. (C and D) Ribbon representation of the ribosomal ligands within the present authentic TIPOST complex (C), and an unrotated ribosomal complex with classic A, P, and E-tRNAs (23) (D). Highlighted are the distances between the last base pair within ASL of bound tRNAs.
Ribosome–tRNA Interactions.
Interestingly, the present reconstruction shows very tight interactions between the 30S subunit and the two tRNAs that involve canonical P-site contacts in both cases. In the 30S platform, A790 contacts the “downstream” ap/P-tRNA, whereas around the 30S head G1338/A1339 contacts the “upstream” pe/E-tRNA (Fig. 4 A and B). Thus, the P-site interactions are partitioned between the two tRNAs in their observed transit states. Furthermore, the reconstruction suggests that both tRNAs maintain tight interactions with their corresponding mRNA codons (Fig. 4C). Close interaction between the pe/E-tRNA anticodon and mRNA codon created by three base pairs was also observed in the overall similar 70S•EF-G (GDPNP-II) structure with a single bound pe*/E-tRNA (10). Notably, our data suggest that consecutive codon–anticodon interactions are maintained in both chimeric ap/P and pe/E tRNA binding sites (Fig. 4C). The anticodon loop positions of the pe/E-and P-site tRNA closely overlap using a 30S-head alignment (Fig. 4D), suggesting that the interactions with G1338/A1339 provide the major contribution in shaping the binding pocket of the pe/E-tRNA anticodon loop.
Fig. 4.
Ribosome–tRNA interactions. (A) Cryo-EM density for the 30S and ligands within the presented authentic TIPOST complex in a transparent gray surface representation together with docked models of the 30S (yellow), ap/P-tRNA (green), pe/E-tRNA (orange), and EF-G (red). (B) Schematic of the 30S subunit and bound ligands [EF-G (red), ap/P-tRNA (green) and pe/E-tRNA (orange)] from the presented authentic TIPOST. (C) Cryo-EM density of the ligands within the presented authentic TIPOST (transparent gray surface) together with docked models of EF-G, pe/E-tRNA, ap/P-tRNA, and mRNA (colored as in A). (D) Superimposition of the ligands from the present authentic TIPOST complex with either tRNAs in classic A, P, and E-site (23) (transparent gray) upon a 30S head alignment.
Discussion
Coordinated Movement of tRNAs and mRNA.
During the translocation reaction, the ribosome—together with its bound ligands—must ensure stringent coupling of the movement of the two tRNAs together with their associated mRNA so that the mRNA advances by exactly one codon. The structure of the present authentic TIPOST complex reveals two tRNAs bound in chimeric intrasubunit ap/P and pe/E binding states trapped in an intermediate state of translocation. In these positions, both tRNAs appear to interact with their mRNA codons. We note that at the present resolution, it cannot be concluded with absolute certainty that the mRNA has moved along with the tRNAs and that the codon/anticodon densities represent canonical pairs. Nevertheless, a toe-printing study of an equivalent complex indicated that mRNA movement had already occurred in the FA-stalled state (25), although our structure demonstrates that translocation is not yet completed. Maintaining consecutive codon–anticodon interactions during translocation could explain how both tRNAs contribute to avoid a frameshift event.
In addition, the present structure suggests how coupling of the movement of mRNA and tRNAs is promoted by the ribosome and EF-G during TIPOST formation. Around the ribosomal A site, synchronous movement of the mRNA codon and tRNA anticodon is mediated via interactions with a loop (loop I) that protrudes from the tip of domain IV of EF-G and inserts into the minor groove of the codon-anticodon helix (Figs. 2 and 4). A second loop (loop II) protruding from the tip of domain IV inserts into the minor groove of h34 of 16S rRNA in the head of the 30S subunit. In this way, domain IV of EF-G interconnects the downstream ap/P-tRNA and its A-site codon with the head of the ribosomal 30S subunit. If these interactions form before movement of the mRNA and tRNA ASLs, they would link the swiveling of the 30S head to movement of the tRNA2•mRNA module. Around the ribosomal E site, the 16S rRNA residues G1338 and A1339 of the 30S head appear to maintain a tight grip on the upstream pe/E-tRNA, leading to a precise positioning of the tRNA ASL (Fig. 4). Movement of the downstream ap/P-tRNA toward the pe/E-tRNA ASL then results in compaction of the tRNA pair (Fig. 3).
In agreement with our recent suggested model (6), the present work suggests that the ASLs of both tRNAs are moved from the A/P and P/E states within a rotated pretranslocated complex to the chimeric ap/P and pe/E states of the TIPOST. This movement is accompanied by a swiveling movement of the 30S head from ∼5–7.5° to ∼18° and the back rotation of the 30S body/platform from presumably ∼7–9° to the observed ∼2.5°. Key interactions for synchronization of head-swiveling with tRNA and mRNA movement appear to be G1338 and A1339 for the pe/E-tRNA and coupling of ap/P-tRNA and mRNA to h34 by domain IV of EF-G (Figs. 2 and 4). After the back rotation of the 30S body/platform and the EF-G–stabilized compaction of the tRNA pair, the ap/P-tRNA creates a tight interaction with the 16S rRNA residue A790 within the 30S body/platform (Fig. 4). Because of this interaction, the ap/P-tRNA and the 30S body/platform stabilize each other in the respective observed positions. This observation complements related findings coming from the X-ray structure of a 70S•EF-G complex (GDPNP-II) in an overall similar conformation (10). This X-ray structure showed intercalation of the universally conserved 16S rRNA bases C1397 and A1503 in the body of the 30S subunit between mRNA bases. By defining the first nucleotide of the P-site codon as +1, C1397 intercalates between positions +9 and +10 mRNA nucleotides and A1503 between −1 and −2; it was suggested that they could potentially act as molecular pawls to prevent slippage of the mRNA during reverse rotation of the 30S head. The ap/P-tRNA–A790 interaction may stabilize a specific conformation of the 30S body/platform after its back rotation from ∼7–9° to ∼2.5°. The EF-G–stabilized compaction of the tRNA pair may be coupled via the ap/P-tRNA–A790 contact to the positioning of the 30S body/platform, including the bases C1397 and A1503. By this mechanism, the positions of both intercalated bases (C1397 and A1503) relative to the mRNA would be stabilized after tRNA compaction and associated movement of the mRNA.
This sequence of events may also explain the process of programmed +1 frameshifts, which were shown to occur in vitro (38) and in vivo (39) and suppressed by frameshift-suppressor tRNAs that contain an extra base in their anticodon loops. Structural studies have demonstrated that such tRNAs use anticodons loops with a special architecture and may span a four-base mRNA quadruplet around the ribosomal A site, but still allow the formation of the regular minor groove geometry required for decoding (40). Accordingly, during translocation such a quadruplet codon may passively follow the frameshift-suppressor tRNA codon into the ribosomal ap/P-site stabilized by loop I within EF-G domain IV. The +1 frameshift can then be manifested by intercalation of C1397 between +10 and +11 and of A1503 between −1 and −2.
These structural observations may also rationalize why two tRNAs (or minimally, a P-site tRNA and an ASL bound to the A site) are required for effective translocation (41). The intrasubunit chimeric pe/E state state can be occupied by a single-bound tRNA as visualized by cryo-EM (6) and X-ray crystallography (10). If such a single-bound pe/E-tRNA maintained its tight interactions with the 30S head during the final steps of translocation (i.e., reverse rotation of the head and release of EF-G•GDP), it would be repositioned in the classic 30S P site rather than being moved into the posttranslocated 30S E-site in the absence of a second tRNA. However, reaccess to the classic 30S P site would be prevented by steric clash with an ap/P-tRNA, which is stabilized by interactions with A790 and possibly EF-G domain-IV.
Conclusion
The structure presented here represents a previously undescribed visualization of a translocation intermediate containing two tRNAs. This visualization suggests that EF-G stabilizes the swiveling movement of the 30S head, the synchronous movement of mRNA and tRNA through the A site, and compaction of the tRNA pair. The ribosome can be described as a bimodular molecular ratchet that can undergo intrasubunit (30S head swivel) and intersubunit rotations. The canonical P-site contacts (A790 within the body and G1338/A1339 within the head) may act as molecular pawls to couple these reciprocal rotations to the coordinated and synchronous movement of tRNAs and mRNA.
Materials and Methods
Pretranslocational ribosomal complexes were prepared carrying an mRNA, a tRNAfMet, and a Val-tRNAVal in the P and A site, respectively. Such pretranslocational complexes were incubated with EF-G in the presence of FA and GTP. The obtained complex was flash-frozen in liquid ethane. Subsequently, cryo-EM data were collected using a Tecnai G2 Polara Microscope (FEI) and scanned using a D8200 Primscan drum scanner (Heidelberger Druckmaschinen). Digitized data were processed using multiparticle classification protocols (23) implemented with SPIDER (42). Further details are presented in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank J. Buerger for his assistance during the cryo-EM data collection, Lucas Horan for the double-mutant ribosome construct, and D. Ermolenko for critical discussions. This work was supported by a grant from the Federation of European Biochemical Societies Long-Term Fellowship (to D.J.F.R.); National Institutes of Health Grants GM-17129 and GM-59140 (to H.F.N.); and Deutsche Forschungsgemeinschaft Grants FOR 1805 and SFB 740 (to C.M.T.S.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the EMDB database, www.ebi.ac.uk/pdbe/emdb/ (accession no. EMDB-5775), and the Protein Data Bank, www.ebi.ac.uk/pdbe/ (accession nos. 3j5n and 3j5o).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320387110/-/DCSupplemental.
References
- 1.Yusupov MM, et al. Crystal structure of the ribosome at 5.5 A resolution. Science. 2001;292(5518):883–896. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]
- 2.Moazed D, Noller HF. Intermediate states in the movement of transfer RNA in the ribosome. Nature. 1989;342(6246):142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard SC, Kim HD, Gonzalez RL, Jr, Puglisi JD, Chu S. tRNA dynamics on the ribosome during translation. Proc Natl Acad Sci USA. 2004;101(35):12893–12898. doi: 10.1073/pnas.0403884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munro JB, Altman RB, O’Connor N, Blanchard SC. Identification of two distinct hybrid state intermediates on the ribosome. Mol Cell. 2007;25(4):505–517. doi: 10.1016/j.molcel.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank J, Agrawal RK. A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature. 2000;406(6793):318–322. doi: 10.1038/35018597. [DOI] [PubMed] [Google Scholar]
- 6.Ratje AH, et al. Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. Nature. 2010;468(7324):713–716. doi: 10.1038/nature09547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ermolenko DN, Noller HF. mRNA translocation occurs during the second step of ribosomal intersubunit rotation. Nat Struct Mol Biol. 2011;18(4):457–462. doi: 10.1038/nsmb.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spahn CM, et al. Domain movements of elongation factor eEF2 and the eukaryotic 80S ribosome facilitate tRNA translocation. EMBO J. 2004;23(5):1008–1019. doi: 10.1038/sj.emboj.7600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Z, Noller HF. Rotation of the head of the 30S ribosomal subunit during mRNA translocation. Proc Natl Acad Sci USA. 2012;109(50):20391–20394. doi: 10.1073/pnas.1218999109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Lancaster L, Donohue JP, Noller HF. Crystal structures of EF-G–ribosome complexes trapped in intermediate states of translocation. Science. 2013;340(6140):1236086. doi: 10.1126/science.1236086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pulk A, Cate JH. Control of ribosomal subunit rotation by elongation factor G. Science. 2013;340(6140):1235970. doi: 10.1126/science.1235970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tourigny DS, Fernández IS, Kelley AC, Ramakrishnan V. Elongation factor G bound to the ribosome in an intermediate state of translocation. Science. 2013;340(6140):1235490. doi: 10.1126/science.1235490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Feng S, Kumar V, Ero R, Gao YG. Structure of EF-G–ribosome complex in a pretranslocation state. Nat Struct Mol Biol. 2013;20(9):1077–1084. doi: 10.1038/nsmb.2645. [DOI] [PubMed] [Google Scholar]
- 14.Agirrezabala X, et al. Visualization of the hybrid state of tRNA binding promoted by spontaneous ratcheting of the ribosome. Mol Cell. 2008;32(2):190–197. doi: 10.1016/j.molcel.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julián P, et al. Structure of ratcheted ribosomes with tRNAs in hybrid states. Proc Natl Acad Sci USA. 2008;105(44):16924–16927. doi: 10.1073/pnas.0809587105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer N, Konevega AL, Wintermeyer W, Rodnina MV, Stark H. Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. Nature. 2010;466(7304):329–333. doi: 10.1038/nature09206. [DOI] [PubMed] [Google Scholar]
- 17.Budkevich T, et al. Structure and dynamics of the mammalian ribosomal pretranslocation complex. Mol Cell. 2011;44(2):214–224. doi: 10.1016/j.molcel.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agirrezabala X, et al. Structural characterization of mRNA-tRNA translocation intermediates. Proc Natl Acad Sci USA. 2012;109(16):6094–6099. doi: 10.1073/pnas.1201288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuwirth BS, et al. Structures of the bacterial ribosome at 3.5 A resolution. Science. 2005;310(5749):827–834. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
- 20.Valle M, et al. Locking and unlocking of ribosomal motions. Cell. 2003;114(1):123–134. doi: 10.1016/s0092-8674(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 21.Connell SR, et al. Structural basis for interaction of the ribosome with the switch regions of GTP-bound elongation factors. Mol Cell. 2007;25(5):751–764. doi: 10.1016/j.molcel.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Gao YG, et al. The structure of the ribosome with elongation factor G trapped in the posttranslocational state. Science. 2009;326(5953):694–699. doi: 10.1126/science.1179709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loerke J, Giesebrecht J, Spahn CM. Multiparticle cryo-EM of ribosomes. Methods Enzymol. 2010;483:161–177. doi: 10.1016/S0076-6879(10)83008-3. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Altman RB, Blanchard SC. Insights into the molecular determinants of EF-G catalyzed translocation. RNA. 2011;17(12):2189–2200. doi: 10.1261/rna.029033.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiegel PC, Ermolenko DN, Noller HF. Elongation factor G stabilizes the hybrid-state conformation of the 70S ribosome. RNA. 2007;13(9):1473–1482. doi: 10.1261/rna.601507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savelsbergh A, et al. An elongation factor G-induced ribosome rearrangement precedes tRNA-mRNA translocation. Mol Cell. 2003;11(6):1517–1523. doi: 10.1016/s1097-2765(03)00230-2. [DOI] [PubMed] [Google Scholar]
- 27.Matassova AB, Rodnina MV, Wintermeyer W. Elongation factor G-induced structural change in helix 34 of 16S rRNA related to translocation on the ribosome. RNA. 2001;7(12):1879–1885. [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor DJ, et al. Structures of modified eEF2 80S ribosome complexes reveal the role of GTP hydrolysis in translocation. EMBO J. 2007;26(9):2421–2431. doi: 10.1038/sj.emboj.7601677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khade PK, Joseph S. Messenger RNA interactions in the decoding center control the rate of translocation. Nat Struct Mol Biol. 2011;18(11):1300–1302. doi: 10.1038/nsmb.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peske F, Savelsbergh A, Katunin VI, Rodnina MV, Wintermeyer W. Conformational changes of the small ribosomal subunit during elongation factor G-dependent tRNA-mRNA translocation. J Mol Biol. 2004;343(5):1183–1194. doi: 10.1016/j.jmb.2004.08.097. [DOI] [PubMed] [Google Scholar]
- 31.Stanley RE, Blaha G, Grodzicki RL, Strickler MD, Steitz TA. The structures of the anti-tuberculosis antibiotics viomycin and capreomycin bound to the 70S ribosome. Nat Struct Mol Biol. 2010;17(3):289–293. doi: 10.1038/nsmb.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogle JM, Murphy FV, Tarry MJ, Ramakrishnan V. Selection of tRNA by the ribosome requires a transition from an open to a closed form. Cell. 2002;111(5):721–732. doi: 10.1016/s0092-8674(02)01086-3. [DOI] [PubMed] [Google Scholar]
- 33.Pan D, Kirillov SV, Cooperman BS. Kinetically competent intermediates in the translocation step of protein synthesis. Mol Cell. 2007;25(4):519–529. doi: 10.1016/j.molcel.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen B, Ticu C, Wilson KS. Intramolecular movements in EF-G, trapped at different stages in its GTP hydrolytic cycle, probed by FRET. J Mol Biol. 2010;397(5):1245–1260. doi: 10.1016/j.jmb.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 35.Weisser M, Voigts-Hoffmann F, Rabl J, Leibundgut M, Ban N. The crystal structure of the eukaryotic 40S ribosomal subunit in complex with eIF1 and eIF1A. Nat Struct Mol Biol. 2013;20(8):1015–1017. doi: 10.1038/nsmb.2622. [DOI] [PubMed] [Google Scholar]
- 36.Jenner LB, Demeshkina N, Yusupova G, Yusupov M. Structural aspects of messenger RNA reading frame maintenance by the ribosome. Nat Struct Mol Biol. 2010;17(5):555–560. doi: 10.1038/nsmb.1790. [DOI] [PubMed] [Google Scholar]
- 37.Feng S, Chen Y, Gao YG. Crystal structure of 70S ribosome with both cognate tRNAs in the E and P sites representing an authentic elongation complex. PLoS ONE. 2013;8(3):e58829. doi: 10.1371/journal.pone.0058829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hohsaka T, Taira H, Fukushima M, Sisido M. Effect of single base insertion into anticodon loop of frameshift suppressor tRNA. Nucleic Acids Res Suppl. 2001;(1):189–190. doi: 10.1093/nass/1.1.189. [DOI] [PubMed] [Google Scholar]
- 39.Pande S, Vimaladithan A, Zhao H, Farabaugh PJ. Pulling the ribosome out of frame by +1 at a programmed frameshift site by cognate binding of aminoacyl-tRNA. Mol Cell Biol. 1995;15(1):298–304. doi: 10.1128/mcb.15.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunham CM, et al. Structures of tRNAs with an expanded anticodon loop in the decoding center of the 30S ribosomal subunit. RNA. 2007;13(6):817–823. doi: 10.1261/rna.367307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joseph S, Noller HF. EF-G–catalyzed translocation of anticodon stem-loop analogs of transfer RNA in the ribosome. EMBO J. 1998;17(12):3478–3483. doi: 10.1093/emboj/17.12.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frank J, et al. SPIDER and WEB: Processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol. 1996;116(1):190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 43.Schmeing TM, et al. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science. 2009;326(5953):688–694. doi: 10.1126/science.1179700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.