Significance
Aneuploidy, denoting cells with an abnormal number of chromosomes, is a common phenomenon in cancer. Another common finding in cancer cells is chromosomal instability, a condition in which cells change their chromosomal content at a high rate. It is clear that chromosomal instability can lead to aneuploidy, but whether the opposite is true has been much debated in the field of cancer biology. The concept that aneuploidy automatically triggers chromosomal instability has been propagated in the scientific literature in recent years. Here, we show that aneuploidy does not, on its own, lead to chromosomal instability, even when cells acquire chromosome alterations typical of cancer. This has important implications for understanding the role of aneuploidy in cancer development.
Keywords: neoplasia, Down syndrome, Edwards syndrome, Patau syndrome
Abstract
Constitutional aneuploidy is typically caused by a single-event meiotic or early mitotic error. In contrast, somatic aneuploidy, found mainly in neoplastic tissue, is attributed to continuous chromosomal instability. More debated as a cause of aneuploidy is aneuploidy itself; that is, whether aneuploidy per se causes chromosomal instability, for example, in patients with inborn aneuploidy. We have addressed this issue by quantifying the level of somatic mosaicism, a proxy marker of chromosomal instability, in patients with constitutional aneuploidy by precise background-filtered dual-color FISH. In contrast to previous studies that used less precise methods, we find that constitutional trisomy, even for large chromosomes that are often trisomic in cancer, does not confer a significantly elevated rate of somatic chromosomal mosaicism in individual cases. Constitutional triploidy was associated with an increased level of somatic mosaicism, but this consisted mostly of reversion from trisomy to disomy and did not correspond to a proportionally elevated level of chromosome mis-segregation in triploids, indicating that the observed mosaicism resulted from a specific accumulation of cells with a hypotriploid chromosome number. In no case did the rate of somatic mosaicism in constitutional aneuploidy exceed that of “chromosomally stable” cancer cells. Our findings show that even though constitutional aneuploidy was in some cases associated with low-level somatic mosaicism, it was insufficient to generate the cancer-like levels expected if aneuploidy single-handedly triggered cancer-like chromosomal instability.
Somatic aneuploidy is almost ubiquitous in solid tumors and is very common in hematological malignancies (1). It is also found in various normal healthy tissues, such as the liver (2) and the central nervous system (3). A number of mechanisms have been described as causative in the generation of aneuploid somatic cells. For instance, it is well known that merotelic kinetochore attachments can lead to anaphase lagging, which in turn may lead to aneuploidy (4). Multiple processes that predispose to merotelic attachments have been described; for example, supernumerary centrosomes that cause merotely through a transient multipolar phase in cell division (5, 6). Another process that can lead to aneuploidy is defective sister chromatid cohesion (1, 7). In hepatocytes, the primary generative mechanism of aneuploidy is termed the ploidy conveyor, referring to a process by which polyploidization is followed by multipolar mitosis and aneuploidy (2). In addition, aneuploidy has in itself been suggested to cause chromosomal instability (8–10). Studies in lower eukaryotes have shown that certain karyotypes indeed predispose cells to genomic instability (11), although whether this holds true in humans is still debated.
Human in vitro systems in which extra copies of chromosomes have been introduced into cell lines have been shown to have dramatic effects on overall transcription patterns (12, 13). However, this type of study has produced conflicting results with respect to chromosomal instability, implying that the effects seen might be dependent on the cell line chosen to study (14, 15). Another approach to studying the connection between aneuploidy and chromosomal instability in humans is using constitutional aneuploidy syndromes as a model. Cells from patients with these syndromes provide a good experimental system for studying the effects of aneuploidy on overall genome stability on representative human material. Such cells typically only have a single or a limited set of stem-line chromosome aberrations compared with tumor cell lines, which typically harbor a multitude of genetic lesions, as well as a cancer phenotype. The few earlier studies performed on patients with constitutional aneuploidy have shown high frequencies of additional somatic aneuploidy (somatic mosaicism) in peripheral blood lymphocytes (16, 17), which may be interpreted as support for an immediate causal connection between aneuploidy and chromosomal instability. However, these studies are inconsistent, as healthy controls in one study showed a higher frequency of aneusomy than aneuploid cases in the other. In addition, both studies showed a prevalence of aneusomic cells in normal control cases that was remarkably high and, thus, was incompatible with earlier reports using golden standard cytogenetic techniques (18). An explanation for the very high frequencies previously reported might be the noise inherent in the single-probe FISH methodology on which these studies were based. To clarify the potential association between constitutional and somatic aneuploidy, and thereby address whether aneuploidy in itself by necessity triggers chromosomal instability, we here used a dual-color FISH approach, allowing a highly precise estimate of aneuploidy even when present at a low prevalence level.
Results
The prevalence of somatic copy number alterations (somatic mosaicism) affecting whole chromosomes was assessed in fibroblasts from nine patients with a constitutionally abnormal chromosome number and two diploid control cases, as well as in two colorectal cancer cell lines (Table 1). The cancer cell lines were chosen to include one bona fide “chromosomally stable” (DLD1) and one “chromosomally unstable” (SW480) line (14, 19). As a measure of somatic aneuploidy, the aneusomy index (AI) was used, defined as the ratio of aneusomic cells to the total cell count for a certain chromosome and sample (14). In our dual-color approach, each chromosome was detected by two probes, and a cell was only included in the calculation of AI if its FISH signal configuration was completely concordant with either normal disomy or a whole chromosome aberration (monosomy, disomy, trisomy, etc.), thereby reducing confounding factors such as background signals and structural chromosome rearrangements. We chose chromosomes 2 and 17 as index chromosomes because they represent one large [chromosome 2; 10,004 transcript alignments, Genome Reference Consortium Human Build 37 (GRCh37)] and one relatively small (chromosome 17; 5,900 transcript alignments, GRCh37) chromosome. To validate the dual-color system, dual- and single-color FISH data for diploid control fibroblasts (F1 and F2, respectively) were compared with traditional chromosome banding estimates of low-level somatic variation. For this purpose, published data for peripheral lymphocytes and amniocytes (18) were retrieved and analyzed together with cytogenetic data from 14 karyotypically normal skin fibroblast cultures. Dual-color FISH and chromosome banding both estimated AI in normal cells to be ∼0.1%, with little variation between cases and chromosomes (Tables S1 and S2). In comparison, estimates by single-color FISH were, on average, ninefold higher and ranged from 0.5% to 2.4%. Thus, dual-color FISH provided an estimate more similar to standard cytogenetic techniques by substantially reducing false-positive scoring, and there was no evidence of underestimation.
Table 1.
Summary of cytogenetic findings and aneuploidy indices (AI) obtained by dual color FISH
| Case number | Phenotype | Stem line karyotype | Chromosome 2 AI, % | Chromosome 17 AI, % |
| F1 | Normal | 46,XY | 0.09 | 0.17 |
| F2 | Normal | 46,XY | 0.15 | 0.18 |
| D1 | Down syndrome | 47,XY,+21 | 0.20 | 0.20 |
| D2 | Down syndrome | 48,XXX,+21 | 0.40 | 0.20 |
| D3 | Down syndrome | 47,XX,+21 | 0.40 | 0.34 |
| P1 | Patau syndrome | 47,XY,+13 | 0.20 | 0.48 |
| E1 | Edwards syndrome | 47,XX,+18 | 0.20 | 0.70 |
| W1 | Fetal death | 47,XY,+8 | 0.15 | 0.34 |
| DT1 | Fetal death | 48,XY,+2,+21 | 0.43 | 0.31 |
| T1 | Triploidy | 69,XXX | 0.66 | 0.99 |
| T2 | Triploidy | 69,XXX | 1.25 | 1.8 |
| DLD1 | Colorectal cancer | 46,XY,dup (2)(p13p23) | 1.2 | 1.7 |
| SW480 | Colorectal cancer | 52–59 < 2n>,X,+X,-Y,t (1, 9)(q12;q11),+2,dic (2, 12)(q24;p13), | 4.7 | 2.4* |
| +del (3)(q10),+der(3)t (3, 3)(p21;q21),der(5)t (5, 20)(q15;p11), | ||||
| +der(7)t (7, 14)(q22;q22),+i (7)(q10),del (8)(p11), | ||||
| der (8, 9)(q10;q10),der(10)t (10, 12)(p13;q12)t (3, 12)(q21;q15), | ||||
| +11,del (12)(q14),+13,+i (17)(q10),del (18)(q12),add (19)(q13), | ||||
| +der(20)t (5, 20)(q15;p11)x2-3,+21[cp40] |
Data for chromosome 16.
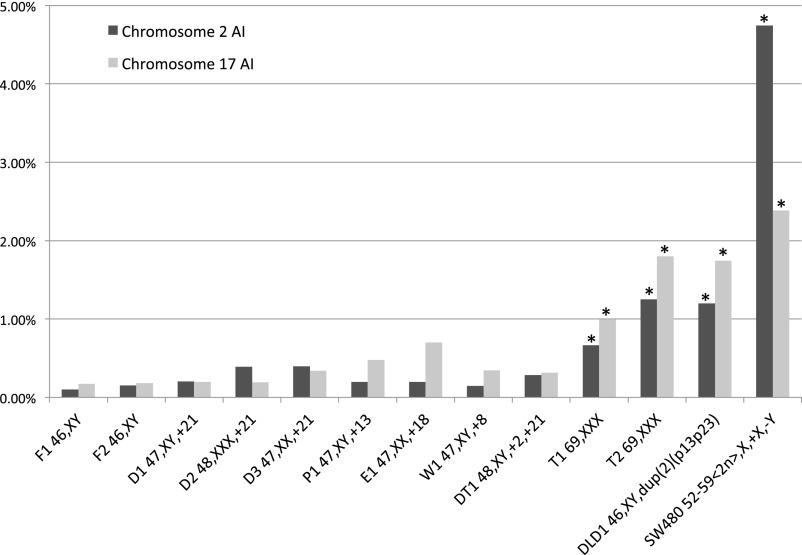
Dual-color FISH was then used to estimate AI levels in cells from constitutionally aneuploid patients (Figs. 1 and 2 A–C). A significantly higher AI than in normal fibroblasts was found in both triploid cases T1 and T2, even when their scores were adjusted for chromosome number (P < 0.01 Bonferroni adjusted after Fisher’s exact test in comparison with F1 and F2, with each chromosome tested separately; Table S3). The AI levels in triploids approached that of the well-known “chromosomally stable” colorectal cancer cell line DLD1 (14) but were far from the levels in the “chromosomally unstable” SW480 line. Using the same stringent statistical approach, none of the trisomic cases showed a significantly elevated AI compared with normal cells, although there was a trend toward elevated AI when all trisomic cases were collectively compared with AI in fibroblasts (P = 0.007; Mann–Whitney–Wilkinson test). This finding included cases with cancer-like karyotypes such as trisomy 8 (patient W1) and concurrent trisomy 2 and 21 (patient DT1). Loss of a single chromosome was the predominant pattern of aneusomy in cases of constitutional aneusomy, ranging from 0.06% to 1.67% in prevalence and making up 16–93% of all aneusomy (Table S4).
Fig. 1.
Aneusomy index for chromosomes 2 and 17 for all samples. P values are from Fisher’s exact test comparing each chromosome 2 and 17 in each aneuploid case with pooled data for the normal fibroblasts (F1 and F2). The Bonferroni correction was used to correct for multiple testing. *P < 0.001 after correction. See Table S3 for exact P values.
Fig. 2.
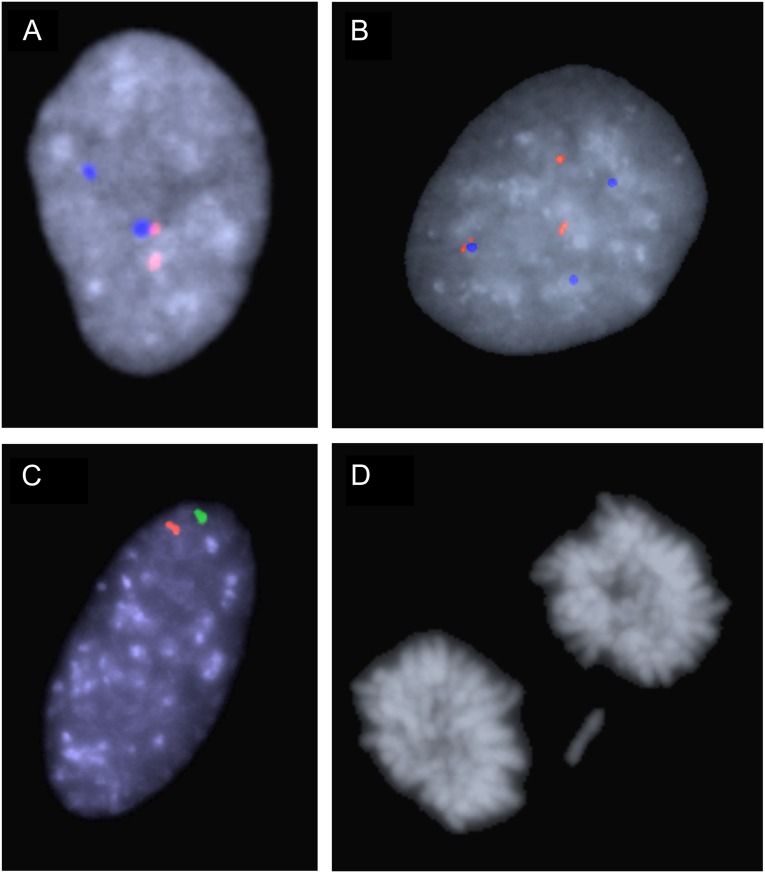
Examples of aneusomy and chromosomal mis-segregation. (A) Nucleus from case W1 with two signals from both chromosome 17 probes, corresponding to disomy. (B) Trisomy 17 in a cell from case D3, using the same probe combination as in A. (C) Monosomy 2 in a cell from case P1, using the chromosome 2 probe pair. (D) Lagging chromosome at telophase in triploidy case T1.
In the triploid cases, the majority (60–93%) of aneusomies consisted of reversion from trisomy to disomy. To assess whether the high frequency of disomics in the triploid cases could be explained by increased frequency of chromosomal mis-segregation at mitosis, the frequency of lagging chromosomes at ana-telophase cells was scored in T1 and T2 (Fig. 2D). In both these cases, lagging of whole chromosomes was observed at a higher rate than in diploid control cells (5 of 212 in T1 and 5 of 208 in T2 compared with 1 of 200 in F2). However, after normalization for the 1.5× increased chromosomal content in triploid compared with diploid cells, there was no significant difference in mis-segregation rate between T1 or T2 compared with fibroblasts (P = 0.62; Fisher’s exact test). Furthermore, the increase in mis-segregation was not proportional to the approximately tenfold increase in frequency of cells carrying single-chromosome losses in the triploid cases compared with diploid fibroblasts. Taken together with the finding that the majority of cells with a chromosome number deviating from three in the triploids were disomic, this indicated that the increased somatic mosaicism in T1 and T2 was predominantly caused by a specific accumulation of cells with loss of whole chromosomes over time and was not primarily an effect of an elevated chromosomal mis-segregation rate.
Discussion
Whether alteration in chromosome number of a diploid human cell by itself causes further aneuploidy through the induction of chromosomal instability has been much debated, in part because of conflicting experimental data (14, 20, 21). The ambiguities from previous experiments could to some extent be explained by the fact that these were almost exclusively performed on neoplastic cells, which are expected to be highly diverse in genotype and phenotype, making it difficult to form groups for comparison. In addition, the multiple epigenetic and DNA sequence changes contributing to the cancer phenotype may further confound the situation by having a direct effect on chromosome segregation (7, 14, 22). Furthermore, a positive correlation between the degree of aneuploidy on the one hand, and the level of chromosomal instability on the other hand, that has been found in some studies of cancer cells (21) is not sufficient to discriminate cause from effect, even though such data have been interpreted as a sign that aneuploidy per se triggers more aneuploidy in an autocatalytic fashion (8, 10, 23). In addition, the few previous studies in which the issue has been addressed in nonneoplastic aneuploid cells, typically constitutional trisomy syndromes, have been confusing, even though constitutional aneuploidy ought to provide a cleaner system for studying the connection between chromosomal instability and aneuploidy than cancer cells. These studies also have shown very high frequencies (in excess of 20% when extrapolated to all chromosomes) of somatic aneuploidy in normal (control) blood lymphocytes, comparable with levels in cancerous cells (16, 17).
We hypothesized that these high estimates were at least partly caused by the high risk of finding cells falsely positive for aneuploidy when assessing minority cell populations in single-probe FISH experiments. The more stringent dual-color approach used here indeed found that the average prevalence of aneusomic cells in a diploid fibroblast population was as low as 1.5 × 10−3 per chromosome pair, corresponding to a prevalence of aneuploid cells of about 2–3% when extrapolated to all chromosomes (=23 chromosome pairs). It is also noteworthy that the AI levels measured in the control cases in the present study (DLD1 and SW480) were significantly lower than earlier reports (14), further reinforcing that single-color FISH can give different results than the stricter dual-color approach. The difference in AI for chromosomes 2 and 16 in SW480 found in the present study was, however, consistent with a previous study on colorectal cancer cell lines using single-color FISH, where it was shown that different degrees of telomere-dependent chromosomal instability among chromosomes may cause widely different rates of aneusomy (19). This illustrates that single-color FISH is probably a sufficient method for comparing degrees of aneusomy between chromosomes in the same cell population (i.e., relative quantifications), as well as for showing whether aneusomy is present at a high level. However, it is a less suitable method for quantifying low AI levels. On this basis, we believe our study could be unique in showing that a specific type of constitutional chromosome number alteration (i.e., triploidy) is coupled to significant secondary somatic aneuploidization, using solid methodology on aneuploid nonneoplastic cells, even though care is warranted when translating our in vitro results to an in vivo context.
On the basis of a correlation between degrees of chromosomal instability and aneuploidy in transformed cells, it has been claimed that “maximal instability is observed with triploidy and decreases towards tetraploidy” (24). However, most of the somatic aneuploidy in constitutionally triploid cells in the current study was a result of reversion from trisomy to disomy, and the increase in somatic aneuploidy in triploids was larger than their increase in chromosomal mis-segregation compared with normal cells, indicating that the somatic aneuploidy observed was a result of accumulation of disomic cells over time, rather than increased chromosomal instability. Compared with the range of whole chromosome lagging (1.8–66%) in colon cancer cell lines (25, 26), the rate of lagging chromosomes found in triploid cells indeed overlapped with the lower, “chromosomally stable” end of the spectrum. This agrees well with our finding that the somatic mosaicism observed in the triploids was comparable to, but not higher than, that found in the “chromosomally stable” DLD1 cancer line, and far lower than in the “chromosomally unstable” SW480 line. It is tempting to frame the very high percentage of disomy seen in the triploid cases in the context of the diploid/triploid mixoploidy syndrome, a very rare variant of triploidy syndrome that has far better life expectancy than pure triploidy (27). This type of mixoploidy is very rare, but case reports of in vitro culture of amniocytes from such patients have described a reduction in frequency of triploid cells compared with uncultured samples (28), which is indicative of a growth advantage of diploid cells versus triploid cells. In line with this, the most likely explanation for the relatively high prevalence of somatic mosaicism in T1 and T2 is a growth advantage of cells having undergone reversion to disomy for one or several chromosomes, either during short-term in vitro culture or already in vivo. We found no evidence for mixoploidy in either of T1 or T2 by cytogenetic analyses.
In contrast to triploids, we did not find a significantly increased level of somatic chromosome number variation in any of the trisomic cases, even though there was a trend for this group as a whole to have higher AI than normal cells. It is possible that trisomy is nevertheless associated with somatic mosaicism, but the level of variation is too small for detection by our approach. However, few, if any, methods exist for application on primary human cells that could provide higher sensitivity for small cell populations than interphase FISH. Also, it is possible that individual differences in karyotype stability may confound data on a low, but still elevated, level of chromosome number variation present in viable trisomy syndromes. It should be noted that our data pertain only to whole chromosomes and do not exclude that aneuploidy induces instability in chromosome structure, as indeed indicated by some studies of cancer cell lines (15). Furthermore, it does not exclude that certain specific combinations of aneusomy induce chromosomal instability. Still, not even the cases with double trisomies in the current study showed AI levels approaching those of even the “chromosomally stable” DLD1 cancer cell line, strongly arguing against a cancer-like level of chromosomal instability. In line with this, triploidy, which includes an extra whole haploid set, was not sufficient to generate chromosomal instability at a level comparable to cancer cells with a similar chromosome number.
To summarize, we present evidence using a stringent method that constitutional aneuploidy is in some cases associated with increased somatic mosaicism with regard to chromosome copy number, but at a very low level at most comparable to “chromosomally stable” cancer cells, and that in its most pronounced form, constitutional aneuploidy primarily results from accumulation of cells with reversion to disomy, and not from a significantly increased level of chromosomal instability. Our data strongly argue against the hypothesis that aneuploidy per se is bound to cause chromosomal instability.
Materials and Methods
The study was approved by the Lund University Ethics Committee. Low-passage fibroblast cultures from patients with constitutional aneuploidy (D1, D2, D3 from Down syndrome; P1 from Patau syndrome; E1 from Edwards syndrome; W1 from complete trisomy 8/Warkany syndrome; and DT1 from double trisomy 2 and 21) and from similar-age diploid controls (F1 and F2) were obtained from National Institute of General Medical Sciences (NIGMS) Human Genetic Cell Repository/Corriell Institute for Medical Research and American Type Culture Collection, through LGC Standards. P1, E1, F1, F2, W1, and DT1 were from NIGMS, corresponding to GM00526, GM00143, GM05399, GM00500B, GM00425, and GM03576, respectively. D1, D2, and D3 were from the ATCC, corresponding to CCL-54, CCL-66, and CCL-84, respectively. The triploid cases T1 (fetal fibroblasts) and T2 (placental fibroblasts), as well as an additional 14 samples of karyotypically normal fibroblasts (Tables S1 and S2), were obtained from the biobank at the Department of Clinical Genetics, Lund University. To assess potential maternal contamination in T2, we performed quantitative flow cytometry, which gave no indications of a diploid population, showing only peaks corresponding to a triploid clone’s G1/0 and G2/M populations. Flow cytometry was carried out as described (29). For comparison with neoplastic cells, we used the colon cancer cell lines DLD1 and SW480 (ATCC). DLD1 is a chromosomally stable, microsatellite unstable cell line that has a pseudodiploid karyotype, whereas SW480 is a chromosomally unstable, microsatellite stable cell line with a complex karyotype of 52–55 chromosomes. All cell cultures obtained from an external biobank were rekaryotyped using standard G-banding obtained by Wright’s stain. For all samples, our in-house karyotype was identical to the one provided by the biobank.
Cell culture, harvest, chromosome preparation, and FISH were performed according to standard methods (30). In brief, cells were cultured through one to two subcultures in DMEM F-12 with antibiotics and 10% FBS and harvested and fixed in methanol:acetic acid (3:1), after which slides were pepsinized, dehydrated, formalin-fixed, and used for FISH. For the diploid control samples, at least 3,000 nuclei per chromosome per sample were scored. For samples from cases with constitutional aneuploidy, at least 1,000 nuclei per chromosome per sample were scored, and in DLD1 and SW480, 500 nuclei were scored. Scoring was done through manual epifluorescence microscopy. Images were acquired using a video camera coupled to an Axioplan 2 fluorescence microscope (Carl Zeiss). Image analysis was done using the CytoVision system (Applied Imaging). For the assessment of ana-telophase lagging, cells were grown on chamber slides in standard medium, fixed with methanol:acetic acid (3:1), and then harvested and stained with DAPI.
To increase the precision of the copy number analysis, each chromosome was detected with two probes with different spectral signatures: one targeting centromeric repeats and one targeting a single-copy region located to a chromosome arm. Chromosome 2 was detected by the probes LSI NMYC SG/CEP 2 SO and chromosome 17 by the probes LSI 17q SO/CEP 17 SA (Abbott Molecular Inc). In SW480, showing a structurally aberrant chromosome 17, we instead analyzed the copy number of chromosome 16 using CEP 16 SB/IGH-MAF SG SO. (Abbot Molecular Inc). We defined true aneusomy as a nondisomic signal pattern consisting of the same number of signals for both probes. For instance, one green and one red signal (1G1R) corresponded to true monosomy 2, whereas patterns consisting of an uneven number of signals (e.g., 1G2R) were eliminated from calculation of AI. Statistical analyses were performed using the R statistical software package (31).
Supplementary Material
Acknowledgments
We are grateful to Aseel Albayati for technical assistance. The study was supported by grants from the Swedish Research Council, the Swedish Cancer Society, the Swedish Childhood Cancer Society, and the Hains Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311163110/-/DCSupplemental.
References
- 1.Holland AJ, Cleveland DW. Losing balance: The origin and impact of aneuploidy in cancer. EMBO Rep. 2012;13(6):501–514. doi: 10.1038/embor.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan AW, et al. Frequent aneuploidy among normal human hepatocytes. Gastroenterology. 2012;142(1):25–28. doi: 10.1053/j.gastro.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yurov YB, et al. Aneuploidy and confined chromosomal mosaicism in the developing human brain. PLoS ONE. 2007;2(6):e558. doi: 10.1371/journal.pone.0000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregan J, Polakova S, Zhang L, Tolić-Nørrelykke IM, Cimini D. Merotelic kinetochore attachment: Causes and effects. Trends Cell Biol. 2011;21(6):374–381. doi: 10.1016/j.tcb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460(7252):278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silkworth WT, Nardi IK, Scholl LM, Cimini D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS ONE. 2009;4(8):e6564. doi: 10.1371/journal.pone.0006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber TD, et al. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci USA. 2008;105(9):3443–3448. doi: 10.1073/pnas.0712384105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li R, Sonik A, Stindl R, Rasnick D, Duesberg P. Aneuploidy vs. gene mutation hypothesis of cancer: Recent study claims mutation but is found to support aneuploidy. Proc Natl Acad Sci USA. 2000;97(7):3236–3241. doi: 10.1073/pnas.040529797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duesberg P, Mandrioli D, McCormack A, Nicholson JM. Is carcinogenesis a form of speciation? Cell Cycle. 2011;10(13):2100–2114. doi: 10.4161/cc.10.13.16352. [DOI] [PubMed] [Google Scholar]
- 10.Duesberg P. Does aneuploidy or mutation start cancer? Science. 2005;307(5706):41–42. doi: 10.1126/science.307.5706.41d. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, Pavelka N, Bradford WD, Rancati G, Li R. Karyotypic determinants of chromosome instability in aneuploid budding yeast. PLoS Genet. 2012;8(5):e1002719. doi: 10.1371/journal.pgen.1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sengupta K, et al. Artificially introduced aneuploid chromosomes assume a conserved position in colon cancer cells. PLoS ONE. 2007;2(2):e199. doi: 10.1371/journal.pone.0000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Upender MB, et al. Chromosome transfer induced aneuploidy results in complex dysregulation of the cellular transcriptome in immortalized and cancer cells. Cancer Res. 2004;64(19):6941–6949. doi: 10.1158/0008-5472.CAN-04-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386(6625):623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 15.Kost-Alimova M, Fedorova L, Yang Y, Klein G, Imreh S. Microcell-mediated chromosome transfer provides evidence that polysomy promotes structural instability in tumor cell chromosomes through asynchronous replication and breakage within late-replicating regions. Genes Chromosomes Cancer. 2004;40(4):316–324. doi: 10.1002/gcc.20054. [DOI] [PubMed] [Google Scholar]
- 16.Reish O, et al. Sporadic aneuploidy in PHA-stimulated lymphocytes of Turner’s syndrome patients. Chromosome Res. 2006;14(5):527–534. doi: 10.1007/s10577-006-1050-9. [DOI] [PubMed] [Google Scholar]
- 17.Reish O, Regev M, Kanesky A, Girafi S, Mashevich M. Sporadic aneuploidy in PHA-stimulated lymphocytes of trisomies 21, 18, and 13. Cytogenet Genome Res. 2011;133(2-4):184–189. doi: 10.1159/000323504. [DOI] [PubMed] [Google Scholar]
- 18.Petersson H, Mitelman F. Nonrandom de novo chromosome aberrations in human lymphocytes and amniotic cells. Hereditas. 1985;102(1):33–38. doi: 10.1111/j.1601-5223.1985.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 19.Stewénius Y, et al. Structural and numerical chromosome changes in colon cancer develop through telomere-mediated anaphase bridges, not through mitotic multipolarity. Proc Natl Acad Sci USA. 2005;102(15):5541–5546. doi: 10.1073/pnas.0408454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gisselsson D. Advances in cancer research. San Diego: Elsevier Inc; 2011. pp. 1–9. [Google Scholar]
- 21.Duesberg P, Rausch C, Rasnick D, Hehlmann R. Genetic instability of cancer cells is proportional to their degree of aneuploidy. Proc Natl Acad Sci USA. 1998;95(23):13692–13697. doi: 10.1073/pnas.95.23.13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajagopalan H, et al. Inactivation of hCDC4 can cause chromosomal instability. Nature. 2004;428(6978):77–81. doi: 10.1038/nature02313. [DOI] [PubMed] [Google Scholar]
- 23.Duesberg P, Li R. Multistep carcinogenesis: A chain reaction of aneuploidizations. Cell Cycle. 2003;2(3):202–210. [PubMed] [Google Scholar]
- 24.Fabarius A, et al. Genomic instability in context of the chromosomal theory. Cell Oncol. 2008;30(6):503–504. doi: 10.3233/CLO-2008-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burrell RA, et al. Replication stress links structural and numerical cancer chromosomal instability. Nature. 2013;494(7438):492–496. doi: 10.1038/nature11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson SL, Compton DA. Examining the link between chromosomal instability and aneuploidy in human cells. J Cell Biol. 2008;180(4):665–672. doi: 10.1083/jcb.200712029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jadhav AR, Dildy GA, Belfort MA, Lacassie Y, Carey JC. Mixoploidy: Perinatal diagnosis and pregnancy outcome. Am J Perinatol. 2010;27(8):599–602. doi: 10.1055/s-0030-1249361. [DOI] [PubMed] [Google Scholar]
- 28.Wegner RD, et al. Fetal 46,XX/69,XXY mixoploidy: Origin and confirmation by analysis of fetal urine cells. Prenat Diagn. 2009;29(3):287–289. doi: 10.1002/pd.2213. [DOI] [PubMed] [Google Scholar]
- 29.Holm A, Baldetorp B, Olde B, Leeb-Lundberg LMF, Nilsson B-O. The GPER1 agonist G-1 attenuates endothelial cell proliferation by inhibiting DNA synthesis and accumulating cells in the S and G2 phases of the cell cycle. J Vasc Res. 2011;48(4):327–335. doi: 10.1159/000322578. [DOI] [PubMed] [Google Scholar]
- 30.Gisselsson D. Refined characterisation of chromosome aberrations in tumours by multicolour banding and electronic mapping resources. Methods Cell Sci. 2001;23(1-3):23–28. [PubMed] [Google Scholar]
- 31.R Core Team 2013. R: A Language and Environment for Statistical Computing. Available at www.r-project.org. Accessed June 1, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.