Significance
Phosphatidylinositol polyphosphates (PIPs) are transiently generated at specific membrane subdomains. Changes of PIP levels regulate the trafficking of vesicles and the activity of membrane transport proteins. To directly visualize the intracellular dynamics of phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2], a key phosphoinositide in the endosome and lysosome, we have engineered a PI(3,5)P2 probe by fusing fluorescent proteins directly to the lipid-binding domain of TRPML1, a lysosomal ion channel that is potently and specifically activated by PI(3,5)P2. This PI(3,5)P2 probe binds to PI(3,5)P2 with biochemical specificity in vitro and responds quickly to changes in the intracellular PI(3,5)P2 level in living cells. With this biosensor, rapid changes of PI(3,5)P2 on single vesicle membranes are captured prior to membrane fusion of two vesicles.
Keywords: TRP channel, PIKfyve, vesicle fusion
Abstract
Phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2] is a low-abundance phosphoinositide presumed to be localized to endosomes and lysosomes, where it recruits cytoplasmic peripheral proteins and regulates endolysosome-localized membrane channel activity. Cells lacking PI(3,5)P2 exhibit lysosomal trafficking defects, and human mutations in the PI(3,5)P2-metabolizing enzymes cause lysosome-related diseases. The spatial and temporal dynamics of PI(3,5)P2, however, remain unclear due to the lack of a reliable detection method. Of the seven known phosphoinositides, only PI(3,5)P2 binds, in the low nanomolar range, to a cytoplasmic phosphoinositide-interacting domain (ML1N) to activate late endosome and lysosome (LEL)-localized transient receptor potential Mucolipin 1 (TRPML1) channels. Here, we report the generation and characterization of a PI(3,5)P2-specific probe, generated by the fusion of fluorescence tags to the tandem repeats of ML1N. The probe was mainly localized to the membranes of Lamp1-positive compartments, and the localization pattern was dynamically altered by either mutations in the probe, or by genetically or pharmacologically manipulating the cellular levels of PI(3,5)P2. Through the use of time-lapse live-cell imaging, we found that the localization of the PI(3,5)P2 probe was regulated by serum withdrawal/addition, undergoing rapid changes immediately before membrane fusion of two LELs. Our development of a PI(3,5)P2-specific probe may facilitate studies of both intracellular signal transduction and membrane trafficking in the endosomes and lysosomes.
Phosphorylated phosphoinositide lipids are produced on the cytosolic side of cellular lipid bilayer membranes (1, 2). There are seven different known phosphoinositides lipids, which localize to distinct membrane subdomains to regulate organelle-specific membrane signaling pathways and membrane-trafficking events (1, 2). One such phosphoinositide lipid is phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], which is predominantly localized to the plasma membrane (PM). PI(4,5)P2 serves as a permissive cofactor that is required for the activity of PM channels/transporters, as a precursor for the generation of second messengers, and as a PM recruiter for cytosolic peripheral proteins (3, 4). Likewise, PI(3,4)P2 and PI(3,4,5)P3 are transiently produced at the PM to regulate various signaling effectors, such as v-akt-murine thymoma viral oncogene homolog 1 (Akt) (1, 5). Conversely, PI(3)P is primarily found on early endosomes, phagosomes, and autophagosomes to regulate the maturation of these compartments (5, 6).
Unlike the aforementioned phosphoinositides, the subcellular localization and functions of PI(3,5)P2 are poorly understood. PI(3,5)P2 is proposed to be mainly localized to late endosomes and lysosomes (LELs) (7), and also to early endosomes (8), based on the location of its synthesizing enzyme complex, which in mammalian cells consists of the phosphoinositide kinase, FYVE finger-containing (PIKfyve) the phosphatase FIG4 homolog, SAC1 lipid phosphatase domain-containing (Fig4), and the scaffolding protein VAC 14 homolog (Vac14) (9–12). Genetic disruption of any of these components in mice results in a decrease in the intracellular PI(3,5)P2 level, lysosomal trafficking defects at the cellular level, and embryonic/neonatal lethality at the animal level (9–12). Consistent with the LEL localization of PI(3,5)P2, we recently reported that PI(3,5)P2 is an endogenous agonist for mucolipin TRP (TRPML) and two-pore TPC channels, which are LEL-localized membrane channel proteins (13, 14). Despite its genetic importance, the localization and dynamics of PI(3,5)P2 remain to be established at the single-cell level, largely due to the lack of a direct method to visualize this low-abundance phosphoinositide.
The recent development of specific fluorescent probes has greatly enhanced our understanding of phosphoinositide signaling (2, 15). These probes include those for PI(3)P, PI(4)P, PI(3,4)P2, PI(4,5)P2, and PI(3,4,5,)P3, which are generated by fusing fluorescent tags with the phosphoinositide-interacting domains of their effector proteins. For example, GFP or mCherry proteins are fused directly with the FAPP1-PH domain [for PI(4)P], the phospholipase C (PLC)δ-PH domain [for PI(4,5)P2], the AKT-PH domain [for both PI(3,4)P2 and PI(3,4,5)P3], and the hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs)-FYVE and early endosome antigen 1 (EEA1)-FYVE domains [for PI(3)P] (15, 16). However, an effective, high-affinity PI(3,5)P2 probe is still lacking, possibly due to two barriers. First, most peripheral phosphoinositide-binding proteins exhibit low affinity binding and often require the simultaneous binding of additional factors to increase the affinity and specificity (2, 17). PI(3,5)P2 is a poorly characterized phosphoinositide with very few known effectors, most of which exhibit relatively low affinities (18, 19). Second, intracellular PI(3,5)P2 levels are reportedly low [around 1/10th of that of PI(3)P and less than 1/100th of that of PI(4,5)P2] (2, 10, 12).
Thus, it has proven difficult to develop an effective PI(3,5)P2 probe for in situ use by using the phosphoinositide-binding domains of peripheral proteins (18). We recently reported that TRPML1, a LEL-localized membrane channel protein, is potently activated by PI(3,5)P2 in the low nanomolar range. We further showed that PI(3,5)P2 binds to the cytosolic N-terminal polybasic domain of TRPML1 (ML1N). This specific activation/binding is abolished by mutations of basic residues within the polybasic domain (20). In this study, we report the development of a genetically encoded PI(3,5)P2 fluorescent probe based on ML1N.
Results
A Fluorescent PI(3,5)P2 Probe Based on the Phosphoinositide-Interacting Domain of TRPML1 Exhibits Membrane Localization That Is Dependent on PI(3,5)P2.
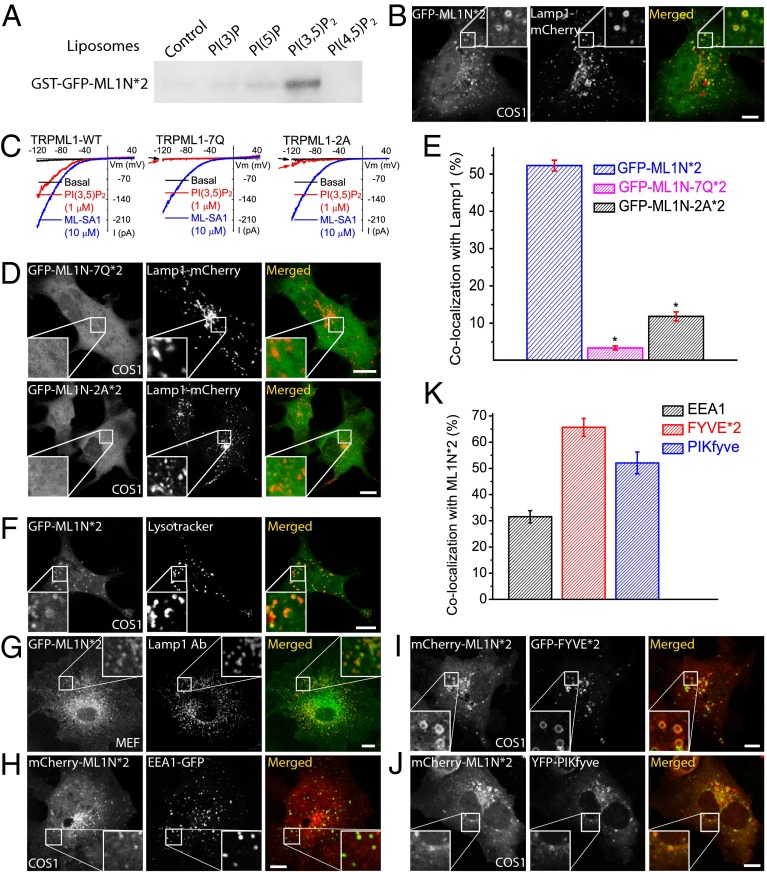
We previously showed that PI(3,5)P2 activates TRPML1 with striking specificity and a low nanomolar EC50, with no other phosphoinositides exhibiting any activation effect (20). Moreover, in vitro binding assays demonstrated that the glutathione S-transferase (GST) fusion protein of the cytosolic N-terminal segment (ML1N; residues 1–68) can bind directly to PI(3,5)P2 (20). We reasoned that fusion of fluorescence tags with ML1N would serve as a high-affinity PI(3,5)P2 sensor. To potentially increase the affinity and specificity (15), a tandem repeat of ML1N (ML1N*2) was fused with an N-terminal GFP or mCherry tag (Fig. S1). Indeed, the purified GST fusion proteins of GFP-ML1N*2 (GST-GFP-ML1N*2) exhibited a much higher specificity toward PI(3,5)P2 in in vitro protein–lipid binding assays than the single-copy GST-ML1N used in our previous study (20). In the liposome binding assay, which would best mimic the “vesicular membrane environment” in living cells, the GST-GFP-ML1N*2 proteins specifically recognized PI(3,5)P2 with a Kd of 5.6 μM (Fig. S2), but not any other phosphoinositides tested except for weak binding to PI(5)P (Fig. 1A). In the PIP strip and PIP-beads binding assays, the tandem fusion proteins also exhibited a weak binding to PI(4,5)P2, PI(3,4,5)P3, and PI(3)P (Figs. S3 and S4). Nevertheless, much stronger binding was seen for PI(3,5)P2, with no obvious binding to most other phospholipids (Figs. S3 and S4). Collectively, these results indicated that the tandem probe manifests a high in vitro specificity for PI(3,5)P2 over other phosphoinositides.
Fig. 1.
Colocalization of the ML1N-based PI(3,5)P2 probe with endolysosomal markers. (A) Purified GST-GFP-ML1N*2 proteins bound strongly to PI(3,5)P2-containing liposomes, but not to liposomes containing PI(3)P, PI(5)P, or PI(4,5)P2. Liposomes, diluted to a final concentration of 20 μM total lipids with 1 μM tested phosphoinositide, were incubated with purified GST-fusion proteins for 20 min, centrifuged, and visualized by Western blot with GST antibodies. (B) GFP-ML1N*2 exhibited a vesicular location and significant colocalization with Lamp1-mCherry. Confocal images were taken 24 h posttransfection in COS1 cells that were cotransfected with Lamp1-mCherry and GFP-ML1N*2. (C) Charge-neutralizing mutations in the phosphoinositide-interacting domain (R42Q/R43Q/R44Q/K55Q/R57Q/R61Q/K62Q, abbreviated as 7Q; R61A/K62A, abbreviated as 2A; also see ref. 21) selectively impaired or abolished the activation of TRPML1 by PI(3,5)P2 (1 µM) in the whole-endolysosome configuration; enlarged endolysosomes were isolated from COS1 cells transfected with various TRPML1 constructs. In contrast, ML-SA1 readily activated wild-type (WT) TRPML1, TRPML1-7Q, and TRPML1-2A in the same endolysosomes. (D) The localization of the ML1N*2 probe to vacuolar membranes was dependent on the basic residues in its phosphoinositide-interacting domain. Both ML1N-7Q*2 and ML1N-2A*2 displayed a diffuse, cytosolic localization pattern. (E) Quantification of the colocalization index between ML1N*2 and Lamp1 (see SI Materials and Methods for the algorithm that was used to analyze the colocalization). (F) Colocalization of GFP-ML1N*2 with LysoTracker (red) in COS1 cells. (G) Colocalization of GFP-ML1N*2 with endogenous Lamp1 (recognized by a Lamp1 antibody) in MEF cells. (H–K) Colocalization analyses between ML1N*2 and other fluorescently tagged endolysosomal proteins/markers in COS1 or MEF cells that were transfected with the indicated constructs for 24 h. (H) Colocalization of mCherry-ML1N*2 with the early endosomal marker GFP-EEA1. (I) Colocalization of ML1N*2 with the PI(3)P probe GFP-Hrs-FYVE*2. (J) Colocalization of ML1N*2 with YFP-PIKfyve. (K) Quantitative analyses revealed that ML1N*2 exhibited various degrees of colocalization with YFP-PIKfyve, GFP-FYVE*2, and GFP-EEA1. Error bars represent SEM; numbers of data points are given in the text. The asterisk indicates P < 10−5. (Scale bar: 10 μm.)
To determine the intracellular localization of the probe, COS1 cells were cotransfected with either GFP- or mCherry-tagged ML1N*1 or ML1N*2 probes. The ML1N*1 probe was mainly localized to the cytosol, with limited localization on lysosome-associated membrane protein 1 (Lamp1)-positive vesicular membranes (Fig. S5). In contrast, significant amounts of GFP-ML1N*2 or mCherry-ML1N*2 were localized to the membranes of Lamp1-positive vesicles in COS1 cells (Fig. 1B and Fig. S6).
Previous studies of other phosphoinositide probes, such as the PLCδ-PH-GFP domain for PI(4,5)P2 or the Hrs-FYVE domain for PI(3)P, have shown that mutations of key phosphoinositide-binding residues are sufficient to decrease, or even block, the localization of the probe to specific membranes (15, 16). We thus also investigated whether mutational analysis would decrease or abolish the vacuolar localization of the ML1N*2 probe. Seven positively charged amino acid residues in the phosphoinositide-interacting domain (Fig. S1B) have been shown to be crucial for PI(3,5)P2 activation/binding (20, 21). Further analyses demonstrated that two of these residues, Arg-61 and Lys-62, mediated the majority of the activation effect in the whole-endolysosome recording (Fig. 1C) or whole-cell recording of surface-expressed TRPML1 (21). We mutated all seven positively charged residues to Gln (abbreviated as 7Q) or specifically mutated the Arg-61 and Lys-62 residues to Ala (abbreviated as 2A) in the ML1N*2 background. Interestingly, the ML1N-7Q*2 probe, which exhibited a loss of its binding capability to PI(3,5)P2 in the protein–lipid interaction assays (20), showed a diffuse cytosolic pattern (Fig. 1D, Upper). Likewise, the ML1N-2A*2 mutant probe also exhibited a similar cytosolic localization, although very weak localization to Lamp1-positive vesicles was also observed (Fig. 1D, Lower), possibly due to an incomplete loss of PI(3,5)P2 affinity compared with the ML1N-7Q*2 probe (Fig. 1C). In quantitative analyses, whereas ML1N-7Q*2 was completely mislocalized (3.4 ± 0.5%, n = 18), ML1N-2A*2 still exhibited a very low level (11.8 ± 1.2%, n = 24) of colocalization with Lamp1 (Fig. 1E). These results suggest that the level of colocalization correlates with the PI(3,5)P2 binding affinity of ML1N*2.
The ML1N*2 PI(3,5)P2 Probe Is Localized to Endolysosomal Compartments in Mammalian Cells.
To further define the locations of the probe, cells were cotransfected or stained with various organellar markers of the endocytic pathway (Fig. 1 F–K). In agreement with the subcellular locations of the PI(3,5)P2-synthesizing enzyme complex (8), the ML1N*2 probe colocalized highly with the LEL marker Lamp1 (Fig. 1 B and G and Fig. S6A). Nevertheless, most ML1N*2-expressing cells also exhibited additional cytosolic, nonvesicular localization (Fig. 1B and Fig. S6A), which could be attributed to a combination of a high level of probe expression (15) and a low level of basal PI(3,5)P2. To account for this observation, positive “colocalization” was set arbitrarily as the number of pixels positive for the endocytic markers when the fluorescent intensity of the probe (membrane-bound fluorescence intensity) was more than 150% of the cytosolic, “background” intensity (see SI Materials and Methods for a detailed description of the colocalization analysis). With this algorithm, the colocalization index in the control experiments was >90% for Lamp1-mCherry and Lamp1-EGFP, but <1% for mCherry and Lamp1-EGFP, indicative of the reliability of the analysis (Fig. S7). In COS1 cells, about one-half of the Lamp1 fluorescence signal (52.3 ± 1.4%, n = 45 cells; Fig. 1E) colocalized with the ML1N*2 probe (Fig. 1B). A high degree of colocalization between ML1N*2 and Lamp1 was also observed in other cell types including mouse embryonic fibroblast (MEF), Chinese hamster ovary (CHO), and human embryonic kidney 293T (HEK293T) cells (Fig. S6A). In addition, similarly high levels of colocalization were observed between the GFP-ML1N*2 probe and LysoTracker (Fig. 1F), a probe for acidic organelles, or endogenous Lamp1 (Fig. 1G). No consistent PM localization was seen, although in some cells the probe is localized in patchy regions of PM (Fig. S8). In a proportion of certain cell types, the probe was also localized to the nucleus (Fig. 1B and Fig. S6A), a finding that was similarly observed for some other phosphoinositide probes (2, 15), and for tandem repeats of EGFP alone (22). Collectively, these results suggest that a significant pool of PI(3,5)P2 resides on Lamp1-positive compartments.
We also investigated the colocalization of ML1N*2 with other endocytic markers. The early endosomal marker EEA1 showed significant colocalization with the probe (31.5 ± 2.4%, n = 25; Fig. 1 H and K), which is consistent with a previous observation that in fibroblasts, ∼40% of Vac14 proteins colocalize with EEA1-positive compartments (8). A commonly used PI(3)P probe GFP-FYVE*2 (6) exhibited a high degree of colocalization with the mCherry-ML1N*2 probe (65.7 ± 3.4%, n = 22; Fig. 1 I and K, and Fig. S9), a reflection of the fact that PI(3)P is the primary precursor and metabolite of PI(3,5)P2 (8, 11). Consistent with PIKfyve being the primary kinase responsible for the synthesis of PI(3,5)P2, a high degree of colocalization between ML1N*2 and PIKfyve-YFP was observed on vesicular structures in COS1 cells (52.1 ± 4.2%, n = 21; Fig. 1 J and K).
Reducing PI(3,5)P2 Levels Decreases or Abolishes the Endolysosomal Localization of the ML1N*2 Probe in Both Mammalian and Yeast Cells.
The results presented thus far suggest that the endolysosomal localization of the probe is likely due to its binding to PI(3,5)P2 located on endolysosomal membranes. To further test this possibility, we used several approaches to manipulate intracellular PI(3,5)P2 levels to investigate whether the localization pattern of the probe changed in response to an increase or decrease in PI(3,5)P2 levels. In mammalian cells, physiological stimuli that induce a robust elevation in PI(3,5)P2 levels have not been established, although in some cell types PI(3,5)P2 levels can be increased moderately (usually less than twofold) by certain types of extracellular stimulation, such as osmotic shock (23) or insulin application (24). Genetic and pharmacological tools to dramatically reduce PI(3,5)P2 levels are available.
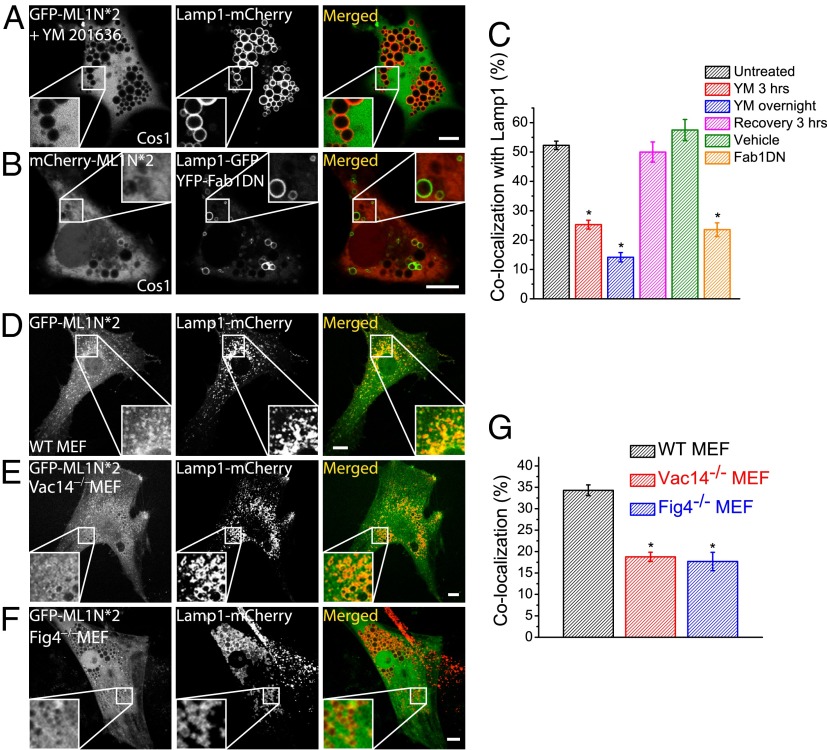
YM201636 is a widely used PIKfyve inhibitor that attenuates PI(3,5)P2 production, resulting in enlarged endolysosomes (25). In COS1 cells coexpressing the GFP-ML1N*2 probe and Lamp1-mCherry, YM201636 treatment dramatically reduced the colocalization of the probe with Lamp1 to 14.2 ± 1.6% (n = 47; Fig. 2 A and C). Similar results were seen in NIH 3T3 cells (Fig. S8). The effect of YM201636 was reversible because the colocalization index returned to the normal level 3 h after drug removal (47.7 ± 3.0%, n = 13; Fig. 2C). Moreover, the effect of YM201636 was specific because the vesicular localization of the PI(3)P probe GFP-FYVE*2 remained unaffected (Fig. S9). Note that, in the presence of YM201636, some of the ML1N-2*-GFP probe was still observed on a small number of vacuoles (Fig. S8), raising the possibility that in the absence of PI(3,5)P2, the ML1N-2*-GFP probe recognizes another epitope, either a protein or lipid. Interestingly, when purified GST-GFP-ML1N*2 proteins were exogenously applied to the fixed cells, GFP fluorescence exhibited a vesicular localization pattern and significant colocalization with Lamp1, which was abolished by YM 201636 treatment for 30 min (Fig. S10). These results suggested that the probe binds directly to the Lamp1-positive membranes in a YM201636-sensitive manner.
Fig. 2.
Genetically or pharmacologically induced decreases in PI(3,5)P2 levels diminish or abolish the vesicular localization of the ML1N*2 probe. (A) GFP-ML1N*2– and Lamp1-mCherry–transfected COS1 cells were treated with YM201636 (800 nM) overnight before confocal imaging analysis. Although Lamp1-positive compartments were dramatically enlarged, the GFP-ML1N*2 probe was primarily localized to the cytosol and was not found on enlarged vacuolar membranes. (B) The vesicular localization of the mCherry-ML1N*2 probe was significantly reduced in cells cotransfected with the 1-phosphatidylinositol-3-phosphate 5-kinase (Fab1)-DN construct. Images were taken 24 h posttransfection. (C) YM201636 treatment resulted in a progressive decrease in ML1N*2’s colocalization with Lamp1. Washout of the drug in the culture medium led to a complete recovery of the colocalization. (D–F) The ML1N*2 probe exhibited an apparent vesicular localization pattern in WT (D), but not in Vac14−/− (E) or Fig4−/− (F) MEF cells. (G) The colocalization index between ML1N*2 and Lamp1 was significantly reduced in Vac14−/− and Fig4−/− MEFs. Error bars represent SEM; numbers of data points are given in the text. The asterisk indicates P < 10−5. (Scale bar: 10 μm.)
The kinase activity of PIKfyve can also be inhibited by overexpression of dominant-negative (DN) PIKfyve (26). In COS1 cells cotransfected with YFP-PIKfyve-DN, mCherry-ML1N*2, and Lamp1-GFP, enlarged vacuoles were seen (Fig. 2B) with an accompanying, profound decrease in the colocalization between Lamp1 and the probe (23.6 ± 2.4%, n = 25; Fig. 2C).
Although PIKfyve knockout (KO) mice are embryonic lethal (11), Vac14 and Fig4 KO mice can survive postnatally with reduced intracellular PI(3,5)P2 levels (∼50% of WT control) (9, 10). In both Vac14 KO and Fig4 KO MEF cells, the colocalization between GFP-ML1N*2 and Lamp1 was significantly reduced compared with WT MEF cells (18.8 ± 1.3%, n = 38, and 17.7 ± 2.2%, n = 29, for Vac14 and Fig4 KO MEFs, respectively, vs. 34.3 ± 1.3%, n = 41, for WT cells; Fig. 2 D–G). Taken together, these results suggest that the endolysosomal localization of the probe was dependent on the activity of PIKfyve/Fab1 and that the probe was capable of detecting changes in intracellular PI(3,5)P2 levels.
Because genetic means of altering PI(3,5)P2 levels are well established in the yeast system (7, 20, 27), we also performed the assay in yeast cells. In WT yeast cells, the GFP-ML1N*2 probe showed a very clear vacuolar localization, as shown by its colocalization with the FM4-64 dye, a fluorescent marker of vacuolar membranes (Fig. S11). Hypertonic shock activation of Fab1-mediated PI(3,5)P2 production (20) further increased the vacuolar localization of the probe (Fig. S11). Yeast cells lacking fab1 (yeast ortholog of PIKfyve; fab1Δ) contain an undetectable level of PI(3,5)P2 with a characteristic enlarged vacuole phenotype (7, 20, 27). In fab1Δ yeast cells, the vacuolar localization was dramatically diminished (Fig. S11). These results suggest that the vacuolar localization pattern of the probe in yeast cells is most likely mediated by PI(3,5)P2. Unlike mammalian cells (Fig. 1), the GFP-ML1N*2 probe also showed consistent, evenly distributed PM localization in both WT and fab1Δ yeast strains (Fig. S11). Prominent PM localization was seen (Fig. S11) even with the GFP-ML1N*1 probe in both WT and PI(4,5)P2-deficient Mss4 mutant (28) yeast cells (Fig. S11). Thus, the PM localization of the probe in yeast is likely unrelated to PI(3,5)P2 or PI(4,5)P2. This may compromise the use of the probe for yeast studies.
Endolysosomal PI(3,5)P2 Levels Are Regulated by Serum-Derived Factors.
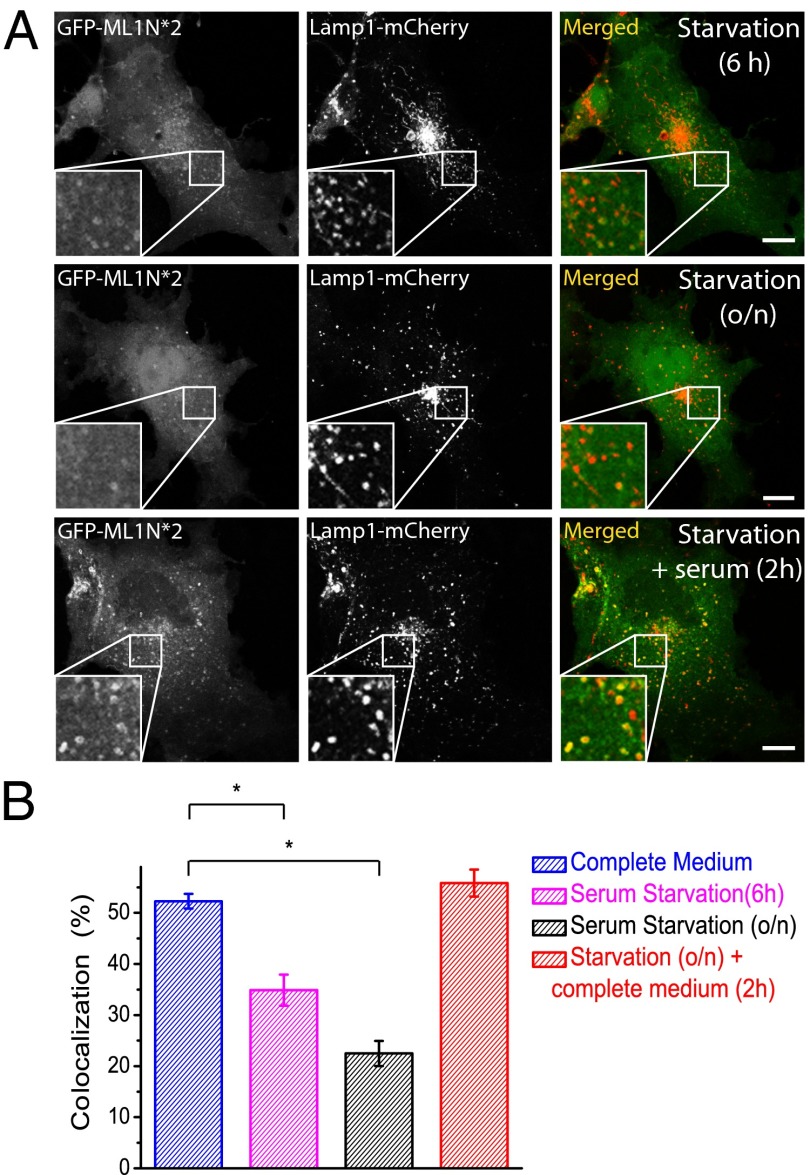
We used the probe to track the dynamic and regulatory changes of PI(3,5)P2 in living cells. In 3T3L1 adipocytes, insulin has been shown to induce a modest increase in PI(3,5)P2 levels in serum-starved cells (19, 26). Likewise, serum-derived factors such as hormones and growth factors would regulate the levels of PI(3,5)P2 and other phosphoinositides in other cell types (12). Consistently, serum starvation elicited a striking change in the pattern of the probe localization in COS1 cells. Under normal conditions, the GFP-ML1N*2 probe was seen on Lamp1-positive vacuole membranes as intensely bright circles or dots (depending on the vacuole size) (Fig. 1B). However, in COS1 cells serum-starved for 6 h (Fig. 3A, Top) or overnight for 18 h (Fig. 3A, Middle), the percentage of the colocalization between Lamp1 and the ML1N*2 probe decreased significantly (34.9 ± 3.0%, n = 23, for 6 h; 22.9 ± 2.5%, n = 24, for 18 h; Fig. 3B). Furthermore, the relative intensity of the probe on the positive vacuoles was greatly reduced compared with serum-replete or normal conditions. When cells were replenished with serum for 2 h, both colocalization (55.8 ± 2.6%, n = 25) and signal intensity returned to normal levels (Fig. 3A, Bottom). Thus, intracellular PI(3,5)P2 levels are regulated by unknown hormones or growth factors in the serum, the identification of which can be facilitated by the use of the ML1N*2 probe.
Fig. 3.
Endolysosomal PI(3,5)P2 levels are regulated by serum-derived factors. (A) In COS1 cells dually transfected with GFP-ML1N*2 and Lamp1-mCherry, serum starvation induced a progressive reduction in the colocalization of ML1N*2 and Lamp1. Readdition of complete medium rapidly (<2 h) restored the high degree of colocalization. (B) Serum-dependent colocalization of the ML1N*2 probe and Lamp1. Error bars represent SEM; numbers of data points are given in the text. The asterisk indicates P < 10−5. (Scale bar: 10 μm.)
PI(3,5)P2 Dynamics in Relation to Endolysosomal Membrane Fusion in Mammalian Cells.
PI(3,5)P2 is a key regulator of membrane trafficking (membrane fusion and fission) in LELs, and PI(3,5)P2 deficiency causes endolysosomal enlargement due to trafficking defects (7). Because PI(3,5)P2 directly activates lysosomal TRPML1 channels, it was hypothesized that PI(3,5)P2 elevation may induce lysosomal Ca2+ release to trigger endolysosomal membrane fusion/fission (13, 20, 29). To visualize PI(3,5)P2 dynamics in relation to membrane fusion/fission in live cells, we performed time-lapse confocal imaging in COS1 cells that were dually transfected with GFP-ML1N*2 and Lamp1-mCherry, with Lamp1-mCherry revealing the fusion events and GFP-ML1N*2 tracking PI(3,5)P2 dynamics. Due to the small size (<0.5 μm) of most Lamp1-positive compartments, we were not able to clearly resolve membrane fusion/fission events for these vesicles due to the limitation imposed by the image resolution. For larger vesicles (>0.5–1 μm for both vacuoles undergoing fusion), however, the naturally occurring fusion rate appeared to be very low (∼0.01 events per cell per min). Nevertheless, in the few observations we made, a rapid and robust increase in the vacuolar GFP fluorescence was detected immediately before fusion of the two Lamp1-positive vacuoles occurred (Fig. 4 A and C, and Movie S1). The intensity of the probe peaked at the time of fusion, and then gradually decreased over time (Fig. 4B). In contrast, mCherry-Lamp1 intensity and cytoplasmic GFP-ML1N*2 fluorescence intensity remained relatively constant, suggesting that the increase in the vacuolar GFP-ML1N*2 fluorescence [hence PI(3,5)P2 level] was the cause, and not the result, of membrane fusion. These results suggest that PI(3,5)P2 is rapidly produced to reach a high level before fusion events.
Fig. 4.
PI(3,5)P2 is transiently increased immediately before membrane fusion in LELs. (A–C) Time-lapse live imaging of COS1 cells that were dually transfected with GFP-ML1N*2 and Lamp1-mCherry. (A) Image of a COS1 cell immediately before membrane fusion between two Lamp1-positive vacuoles occurred (highlighted in the white box). (B) Time-dependent changes in the fluorescence intensities of GFP and mCherry for the selected region (white box, A). (C) Series of images of the selected region at corresponding time points as in B. For a 4-min track period, one fusion event between two vacuoles occurred between every 1 min and 1 min 15 s (D and E) GFP-ML1N*2 and Lamp1-mCherry dually transfected COS1 cells were treated with acetate Ringer’s solution for 30 min and then subjected to live imaging for 5–20 min after being returned to the normal Ringer’s solution. Approximately one-half of the vacuoles (45% of the 22 monitored events; D) exhibited a significant increase (>20% of the baseline membrane intensity) in their membranous PI(3,5)P2 levels immediately before fusion, whereas the other half (55%; E) had little or no changes. The white and blue arrows mark vacuoles prefusion and postfusion, respectively. (F) Changes in vacuolar PI(3,5)P2 levels in relation to fusion events. The fluorescent intensity of GFP-ML1N*2 was normalized to the cytosolic intensity at time 0, and two representative traces are shown; the vertical dotted line indicates the time when the fusion took place. The cytosolic intensity of the probe remained relatively constant over the time period of the live-imaging experiment. [Scale bar: 10 μm (for A) and 2 μm (for C and D).]
To facilitate the detection of fusion events between large vacuoles, we treated the cells with an acetate Ringer’s solution for 20–30 min. Treatment with acetate Ringer’s has long been known to cause cytoplasmic acidification that induces vacuolar fragmentation and the subsequent redistribution of vacuoles to the periphery of the cells, the mechanism of which is poorly understood (30, 31). Upon removal of acetate Ringer’s solution, the lysosomes quickly regain their size through increased fusion frequency, typically in 10–30 min (30, 31). In COS1 cells that were dually transfected with GFP-ML1N*2 and Lamp1-mCherry, withdrawal of the acetate Ringer’s solution resulted in an increased (∼20-fold) rate of fusion between large vacuoles (∼0.17 events per cell per min). All vacuoles undergoing fusion had a much higher (164.4 ± 1.5%, n = 22) probe intensity on the vacuolar membranes than the cytosolic background at the time of fusion. Based on the PI(3,5)P2 responses, the fusogenic vacuoles could be classified into two groups. The first group (∼45% of all events) had significant increases (>20%) in the membrane intensity within 1 min of prefusion (Fig. 4 D and E, upper panels, and Movie S2), whereas the second group (∼55% of all events) exhibited little or no increase (Fig. 4E and Movie S3). Interestingly, the basal fluorescence intensity of the probe in the second group was significantly higher than that of the first group (Fig. 4 D–F). These results suggest that recovery from the acetate Ringer’s treatment uses PI(3,5)P2-dependent mechanisms to facilitate membrane fusion between LELs.
Discussion
Although the importance of PI(3,5)P2 in endolysosomal membrane trafficking is well accepted, the dynamics and localization of PI(3,5)P2 are unclear, largely due to the lack of a method to directly monitor PI(3,5)P2 levels in intact cells. In the present study, we report a fully characterized PI(3,5)P2 fluorescent probe that can be used to track in situ PI(3,5)P2 dynamics. Our PI(3,5)P2 fluorescent probe, consisting of two repeats of the cytosolic N terminus of the lysosomal channel TRPML1, responds specifically to changes in the intracellular PI(3,5)P2 level and localization, as indicated by the following key findings. First, the probe is colocalized with the PI(3,5)P2-synthesizing enzyme complex. Second, charge-neutralizing mutations in the putative PI(3,5)P2 binding domain abolish the vacuolar localization of the probe. Third, increasing or decreasing PI(3,5)P2 levels in both yeast and mammalian cells cause corresponding changes in the vacuolar localization of the probe. Thus, the probe can reliably detect PI(3,5)P2 in living cells.
The predominantly LEL localization of the probe is somewhat unexpected. Although PI(3,5)P2, unlike other phosphoinositides, activates TRPML1 in the nanomolar range, PI(4,5)P2 and PI(3,4,5)P3, two PM-localized phosphoinositides, inhibit TRPML1 (21). Although very weakly, the in vitro binding assays demonstrated that ML1N (20) and also its tandem dimer bound to PI(4,5)P2 and PI(3,4,5)P3. However, no consistent PM localization was detected in the mammalian cell types that we tested, although in some cell types patchy PM localization was observed. One plausible explanation is that the affinity of TRPML1 for PI(3,5)P2 is 5- to 10-fold higher than that of PI(4,5)P2 and PI(3,4,5)P3 (21). Alternatively, the probe overexpression might have resulted in PI(3,5)P2 enrichment in LELs by protecting them from phosphatase-mediated degradation, as was observed for the PI(4,5)P2 probe, where the probe expression attenuated the PLC hydrolysis of PI(4,5)P2 (32). It is also likely that ML1N contains additional PI(3,5)P2-dependent or -independent motifs that specifically strengthen the LEL localization of the probe. Similar observations have been reported for other phosphoinositide probes, including the PI(4)P probe (2, 15, 17). Although not identified yet, an additional recruiting mechanism may play a crucial and permissive role in the LEL localization of the PI(3,5)P2 probe. Nevertheless, such putative dual recruiting mechanism would not prevent the use of the current probe to detect PI(3,5)P2 dynamics in LELs. Probe localization in other compartments, however, needs to be cautiously interpreted, as other phosphoinositides may be much more abundant than PI(3,5)P2 despite their very weak binding affinities. In addition, the weak binding of the tandem probe to PI(5)P could be potentially problematic, as this lipid is also known to be present in the late endocytic compartments of mammalian cells, and is produced from the PI(3,5)P2 generated by PIKfyve (12). Because the production of these two phosphoinositides are linked, and their biological roles have not been clearly separated, the probe may also detect the changes of the PI(5)P, similar to the case of detection of PI(3,4)P2 and PI(3,4,5)P3 with the AKT-PH probe (15).
Due to the lack of an effective PI(3,5)P2 fluorescent probe, the measurement of intracellular PI(3,5)P2 levels has thus far been restricted to indirect approaches such as radiolabeled HPLC, a technique that requires lengthy protocols, and provides no information about subcellular localization and real-time dynamics. By using the PI(3,5)P2 probe that we have developed and characterized, we have shown that PI(3,5)P2 elevation occurs immediately before endolysosomal fusion, highlighting the role of PI(3,5)P2 in regulating endolysosomal fusion. TRPML1 is a lysosomal channel that mediates Ca2+ release from the lysosomal lumen (13) to trigger membrane fusion in LELs (29). Because PI(3,5)P2 elevation may activate TRPML1-mediated Ca2+ release (13, 20), it is likely that PI(3,5)P2 is the trigger for fusion between lysosomes and various other compartments (e.g., late endosomes, autophagosomes, phagosomes, and plasma membrane). We have recently shown that PI(3,5)P2 elevation occurs during lysosomal exocytosis and phagosome formation (33). With the aid of high-resolution live imaging in combination with genetic and pharmacological approaches, the PI(3,5)P2 probe will prove invaluable in studying the regulation of PI(3,5)P2 dynamics in signal transduction and membrane trafficking.
Materials and Methods
For fixed samples, cells were fixed and immunostained following a standard protocol, and then imaged with a Leica confocal microscope. Live imaging was performed with an Olympus Spindisk confocal microscope. Images were analyzed with ImageJ software. Colocalization was performed with the Colocalization Plug-in of ImageJ. See SI Materials and Methods for details of experimental procedures.
Supplementary Material
Acknowledgments
We are grateful to Dongbiao Shen, Titus Franzmann, and Stefan Walter for assistance; Drs. William Brown and Jennifer Meagher from the Center for Structural Biology, Life Sciences Institute, for the optimization of the purification of GST-fusion protein; Drs. Harald Stenmark for the FYVE*2-GFP construct; and Abigail Garrity and Richard Hume for comments on an earlier version of the manuscript. We appreciate the encouragement and helpful comments of other members of the H.X. laboratory. This work was supported by National Institutes of Heath Grants R01 NS062792 (to H.X.) and R01 GM50403 (to L.S.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. T.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311864110/-/DCSupplemental.
References
- 1.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443(7112):651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 2.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9(2):99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 3.Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol. 1998;8(6):343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 4.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: How and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee WL, Mason D, Schreiber AD, Grinstein S. Quantitative analysis of membrane remodeling at the phagocytic cup. Mol Biol Cell. 2007;18(8):2883–2892. doi: 10.1091/mbc.E06-05-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillooly DJ, et al. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19(17):4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dove SK, Dong K, Kobayashi T, Williams FK, Michell RH. Phosphatidylinositol 3,5-bisphosphate and Fab1p/PIKfyve underPPIn endo-lysosome function. Biochem J. 2009;419(1):1–13. doi: 10.1042/BJ20081950. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, et al. Modulation of synaptic function by VAC14, a protein that regulates the phosphoinositides PI(3,5)P(2) and PI(5)P. EMBO J. 2012;31(16):3442–3456. doi: 10.1038/emboj.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow CY, et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448(7149):68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proc Natl Acad Sci USA. 2007;104(44):17518–17523. doi: 10.1073/pnas.0702275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikonomov OC, et al. The phosphoinositide kinase PIKfyve is vital in early embryonic development: Preimplantation lethality of PIKfyve−/− embryos but normality of PIKfyve+/− mice. J Biol Chem. 2011;286(15):13404–13413. doi: 10.1074/jbc.M111.222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zolov SN, et al. In vivo, Pikfyve generates PI(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proc Natl Acad Sci USA. 2012;109(43):17472–17477. doi: 10.1073/pnas.1203106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen D, et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun. 2012;3:731. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, et al. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151(2):372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balla T. Imaging and manipulating phosphoinositides in living cells. J Physiol. 2007;582(Pt 3):927–937. doi: 10.1113/jphysiol.2007.132795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halet G. Imaging phosphoinositide dynamics using GFP-tagged protein domains. Biol Cell. 2005;97(7):501–518. doi: 10.1042/BC20040080. [DOI] [PubMed] [Google Scholar]
- 17.Carlton JG, Cullen PJ. Coincidence detection in phosphoinositide signaling. Trends Cell Biol. 2005;15(10):540–547. doi: 10.1016/j.tcb.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuruta F, Green EM, Rousset M, Dolmetsch RE. PIKfyve regulates CaV1.2 degradation and prevents excitotoxic cell death. J Cell Biol. 2009;187(2):279–294. doi: 10.1083/jcb.200903028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridges D, et al. Phosphatidylinositol 3,5-bisphosphate plays a role in the activation and subcellular localization of mechanistic target of rapamycin 1. Mol Biol Cell. 2012;23(15):2955–2962. doi: 10.1091/mbc.E11-12-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong XP, et al. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Li X, Xu H. Phosphoinositide isoforms determine compartment-specific ion channel activity. Proc Natl Acad Sci USA. 2012;109(28):11384–11389. doi: 10.1073/pnas.1202194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seibel NM, Eljouni J, Nalaskowski MM, Hampe W. Nuclear localization of enhanced green fluorescent protein homomultimers. Anal Biochem. 2007;368(1):95–99. doi: 10.1016/j.ab.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Hill EV, Hudson CA, Vertommen D, Rider MH, Tavaré JM. Regulation of PIKfyve phosphorylation by insulin and osmotic stress. Biochem Biophys Res Commun. 2010;397(4):650–655. doi: 10.1016/j.bbrc.2010.05.134. [DOI] [PubMed] [Google Scholar]
- 24.Shisheva A, Rusin B, Ikonomov OC, DeMarco C, Sbrissa D. Localization and insulin-regulated relocation of phosphoinositide 5-kinase PIKfyve in 3T3-L1 adipocytes. J Biol Chem. 2001;276(15):11859–11869. doi: 10.1074/jbc.M008437200. [DOI] [PubMed] [Google Scholar]
- 25.Jefferies HB, et al. A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 2008;9(2):164–170. doi: 10.1038/sj.embor.7401155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikonomov OC, Sbrissa D, Shisheva A. YM201636, an inhibitor of retroviral budding and PIKfyve-catalyzed PtdIns(3,5)P2 synthesis, halts glucose entry by insulin in adipocytes. Biochem Biophys Res Commun. 2009;382(3):566–570. doi: 10.1016/j.bbrc.2009.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duex JE, Nau JJ, Kauffman EJ, Weisman LS. Phosphoinositide 5-phosphatase Fig 4p is required for both acute rise and subsequent fall in stress-induced phosphatidylinositol 3,5-bisphosphate levels. Eukaryot Cell. 2006;5(4):723–731. doi: 10.1128/EC.5.4.723-731.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desrivières S, Cooke FT, Parker PJ, Hall MN. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J Biol Chem. 1998;273(25):15787–15793. doi: 10.1074/jbc.273.25.15787. [DOI] [PubMed] [Google Scholar]
- 29.Shen D, Wang X, Xu H. Pairing phosphoinositides with calcium ions in endolysosomal dynamics: Phosphoinositides control the direction and specificity of membrane trafficking by regulating the activity of calcium channels in the endolysosomes. Bioessays. 2011;33(6):448–457. doi: 10.1002/bies.201000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heuser J. Changes in lysosome shape and distribution correlated with changes in cytoplasmic pH. J Cell Biol. 1989;108(3):855–864. doi: 10.1083/jcb.108.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durchfort N, et al. The enlarged lysosomes in beige j cells result from decreased lysosome fission and not increased lysosome fusion. Traffic. 2012;13(1):108–119. doi: 10.1111/j.1600-0854.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Várnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: Calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143(2):501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samie M, et al. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev Cell. 2013;26(5):511–524. doi: 10.1016/j.devcel.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.