Significance
In some organisms, respiration fluctuates cyclically, and these rhythms can be a sensitive gauge of metabolism. Constant or pulsatile exposure of yeast to visible wavelengths of light significantly alters and/or initiates these respiratory oscillations, revealing a further dimension of the challenges to yeast living in natural environments. Our results also have implications for the use of light as research tools—e.g., for excitation of fluorescence microscopically—even in organisms such as yeast that do not express specialized photoreceptive molecules. Moreover, the growth of yeast strains that are null for the yeast activator protein-1 gene that regulates oxidative stress genes is exquisitely sensitive to visible light, indicating that light can both modulate respiration and induce oxidative stress.
Abstract
Exposure of cells to visible light in nature or in fluorescence microscopy often is considered to be relatively innocuous. However, using the yeast respiratory oscillation (YRO) as a sensitive measurement of metabolism, we find that non-UV visible light has a significant impact on yeast metabolism. Blue/green wavelengths of visible light shorten the period and dampen the amplitude of the YRO, which is an ultradian rhythm of cell metabolism and transcription. The wavelengths of light that have the greatest effect coincide with the peak absorption regions of cytochromes. Moreover, treating yeast with the electron transport inhibitor sodium azide has similar effects on the YRO as visible light. Because impairment of respiration by light would change several state variables believed to play vital roles in the YRO (e.g., oxygen tension and ATP levels), we tested oxygen’s role in YRO stability and found that externally induced oxygen depletion can reset the phase of the oscillation, demonstrating that respiratory capacity plays a role in the oscillation’s period and phase. Light-induced damage to the cytochromes also produces reactive oxygen species that up-regulate the oxidative stress response gene TRX2 that is involved in pathways that enable sustained growth in bright visible light. Therefore, visible light can modulate cellular rhythmicity and metabolism through unexpectedly photosensitive pathways.
Many organisms are exposed to visible light in their environment. Full sunlight can deliver up to 10 quadrillion photons of visible light⋅cm−2⋅s−1 (i.e., 2,000 µEinsteins⋅m−2⋅s−1), and cloud cover reduces this exposure only by a factor of 10. Even though sunlight provides photosynthetic energy to plants and a medium for the vision of many animal species, its high-intensity rays damage living cells when light-absorbing molecules cannot safely disperse the energy that photons bring. Although the destructive capacity of UV light is widely appreciated, photons of visible light can be deleterious, e.g., by destroying cytochromes and thus affecting cellular respiration (1) or by producing reactive oxygen species (ROS) that cause damage to DNA, membranes, and other cellular components (2). To cope with the damaging effects of light, organisms have evolved different strategies ranging from the expression of shielding pigments, such as melanin and carotenoids (3), to active mechanisms that sense light and respond quickly to mitigate/repair damage, such as iris constriction to protect the retina (4, 5), light-avoidance movements by chloroplasts and mitochondria (6, 7), and the induction/activation of DNA photolyase (8). A third strategy to minimize damage from light is to anticipate and prepare for its effects through the use of cellular timing mechanisms such as a circadian clock (9).
The budding yeast Saccharomyces cerevisiae lacks the well-characterized photoreceptors of many other organisms [e.g., cryptochromes, phytochromes, and the photoreceptors white-collar 1 (WC-1), and rhodopsins] that sense light intensity and spectra (10). Therefore, S. cerevisiae generally is assumed to be unresponsive to visible light and moreover is not thought to exhibit light-anticipatory behavior that might be mediated by an oscillatory timekeeping mechanism. However, under certain conditions of nutrient-limited growth, yeast cultures exhibit an ultradian respiratory oscillation (yeast respiratory oscillation, or YRO), that is characterized by 1- to 6-h rhythms of oxygen consumption, metabolite production, cell division, and gene expression (11–16) which have been proposed to allow yeast to anticipate rhythmic occurrences of oxidative stress and mitigate its effect on DNA replication (13, 17). Originally, this oscillation was studied in populations of synchronized cells using slow-dilution continuous culture in bioreactors containing high cell densities. Recent evidence suggests that metabolic cycling in S. cerevisiae also occurs at the single-cell level even in unsynchronized cultures and that the conditions of continuous culture allow independently oscillating cells to synchronize (18–20). Because respiration and oxidative state play major roles in regulating the YRO (13, 15, 21–23), and because visible light is an important environmental factor that might alter respiration rates and ROS production (2, 24, 25), we sought to determine if visible light affects the YRO as a means to understand better the factors that can influence metabolism and the YRO and the strategies that yeasts in nature use to protect themselves from photodamage.
We show that visible light (especially blue light) at intensities less than that of natural full sunlight significantly modulates the period and amplitude of the YRO. On the basis of the strongest YRO modulations correlating with peaks in the cytochrome absorption spectrum and the modulations being mimicked using an electron transport inhibitor, we conclude that this effect of visible light on the YRO is likely to be mediated through light absorption by cytochromes. We further investigated the role of oxidative electron transport in propagating the YRO and found that changes in oxygen tension and/or blocking the electron transport chain can phase-shift the YRO in a manner similar to light. When light that affects the YRO is used as a potential entraining agent for a putative circadian oscillator, an ∼24-h rhythm does not persist in constant conditions. These data are consistent with the absence of a light-entrainable circadian oscillator in yeast. These results are significant both in identifying factors that influence metabolism in nature and in showing that experimental light excitation, as used in fluorescence microscopy, has the potential to impact cellular metabolism negatively.
Results and Discussion
Yeast Response to Light in Continuous Culture.
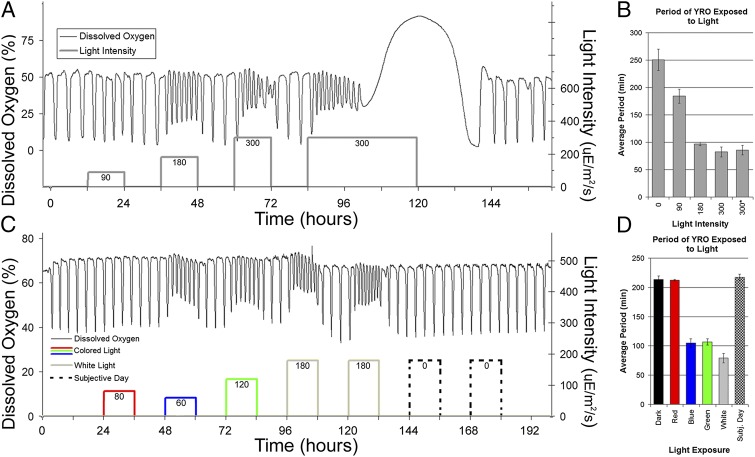
We established a stable YRO in the CEN.PK yeast strain and administered light of increasing intensities from cool white fluorescence (CWF) bulbs for 12-h intervals that alternated with 12 h of darkness (Fig. 1). The YRO had a period of about 250 min in total darkness, but white light caused the period of the oscillation to shorten and the amplitude to decrease (Fig. 1 A and B). These changes became more apparent with greater intensities of light (90–300 µE⋅m−2⋅s−1). The lowest intensity of light applied (90 µE⋅m−2⋅s−1) had only minor effects on period and amplitude; however, the brightest light applied (300 µE⋅m−2⋅s−1) caused higher frequency (∼85 min) and unstable oscillations. Treating the culture with 300 µE⋅m−2⋅s−1 of light for longer than 16 h destroyed the oscillation (Fig. 1A), but returning the culture to total darkness allowed the oscillation to recover spontaneously about 20 h later. Typical light intensities that are found outdoors on a sunny day can exceed 2,000 µE⋅m−2⋅s−1, whereas indoor light often is around 5–15 µE⋅m−2⋅s−1. In fact, direct natural sunlight through a window affects the YRO similarly to artificial light (Fig. S1). In our experiments, the application of light did not change the temperature of the culture, because its temperature was maintained at 30 ± 0.2 °C by a temperature-controlled water jacket that surrounded the entire culture and was between the culture and the light source (Fig. S2).
Fig. 1.
The effects of visible light on the YRO. (A) Different light intensities affect the YRO differentially. Oscillations were initiated in a culture grown in the dark until stable DO oscillations formed (black line, left y-axis). Then 12-h treatments of white light were administered at intensities of 90, 180, and 300 µE⋅m−2⋅s−1 (gray line, right y-axis) with 12 h of darkness between light treatments. The final 300 µE⋅m−2⋅s−1 light treatment was maintained for 35 h. Culture temperature was 30 °C in light and dark. (B) The average periods for oscillations during the initial dark phase or during each of the different light treatments for the YRO shown in A (± SD). The x-axis is arranged by light intensity in µE⋅m−2⋅s−1, and the bar labeled “300*” represents the average period for the second and longer 300 µE⋅m−2⋅s−1 light treatment. (C) The effects of different spectra of light on the YRO. Oscillations were initiated in the dark until stable DO oscillations formed (black line, left y-axis). Then 12-h treatments of red, blue, or green light were administered (colored lines matching color of light, right y-axis) with 12 h of darkness between treatments. After the application of colored light, two 12-h white light treatments were given. Light intensities of each treatment are shown on the right y-axis and are indicated by numbers under each of the colored or gray lines showing light treatment. (D) The average periods for oscillations occurring during the initial dark phase or during each of the different colored light treatments for the YRO shown in C (± SD). The subjective day bar pertains to the portion of the YRO indicated by dashed black lines of C and represents the times when light would have recurred in accordance with the 12-h light/dark cycle maintained for the previous 5 d.
Determining the wavelength(s) of light that mediate biological responses has proved valuable for identifying the underlying light-absorbing pigments (26, 27), so we tested the effect that red, green, and blue light had on the YRO by placing colored filters in the light path. An oscillating culture with a period of about 210 min was established, and light of different colors was administered in a light/dark cycle with 12 h of light (of different spectra) alternating with 12 h of darkness (see Fig. S3 for the spectra and transmittance of the filters). Red light (80 µE⋅m−2⋅s−1) had no effect on the YRO’s period and only very minor effects on amplitude, whereas stronger green light (120 µE⋅m−2⋅s−1) and dimmer blue light (60 µE⋅m−2⋅s−1) significantly affected the YRO in a manner similar to that of moderately bright white light (180 µE⋅m−2⋅s−1) (Fig. 1 C and D). Dim green light (80 µE⋅m−2⋅s−1) similar in intensity to blue and red light caused only minor effects (Fig. S3C).
To test if the light/dark cycle might entrain/synchronize a putative circadian oscillation in the yeast (28), the three 12-h treatments of colored light (red, green, blue) were followed by two 12-h treatments of unfiltered white light of sufficient intensity to cause noticeable effects to the YRO (for total of five 24-h light/dark cycles) followed by constant darkness to see if any persisting circadian patterns of period or amplitude changes were evident in the YRO. As shown in Fig. 1 C and D, the YRO showed stable periods and amplitudes during this free run similar to those seen before light treatment, thus providing no evidence that a circadian clock influences this phenomenon. Moreover, Fig. 1C shows that the light-induced changes in the period and amplitude of the YRO are rapidly reversible.
The photoreceptors WC-1 and WC-2 mediate blue-light responses in some fungi (27), but the S. cerevisiae genome lacks the WC-1 gene, the WC-2 gene, and any homologs (10). However, cytochromes are pigmented cellular components that are known to absorb blue-green photons (29, 30) and are an integral part of the electron transport chain in the mitochondria of practically all eukaryotes, including yeast. Observations from 1969 to 1979 indicated that visible light can destroy or deactivate cytochromes in mammalian cells, algae, and yeast (1, 25), and this action was suggested to be responsible for impaired yeast growth at low temperatures (31). On the basis of those reports implicating cytochromes as candidate absorbers of light that can inhibit respiratory electron transport, we compared the wavelengths of our light treatments with the absorbance spectra of cytochrome oxidase (Fig. S3B). Our blue-light treatment has modest emission at wavelengths for which reduced cytochrome oxidase has the greatest absorption. This comparison, however, does not exclude the possibility that other pigments (including other cytochromes) may contribute to these effects of light on yeast.
Visible Light Induces the ROS Stress Response, but ROS Does Not Modulate the YRO Period.
We hypothesize that there are two likely mechanisms by which light might impinge upon cytochromes to affect the YRO. One is that photoinhibition of electron transport produces ROS that may affect protein/enzyme integrity and cellular oxidative balance. The other is that the absorption of blue light by cytochromes stalls respiratory electron transport and ATP production.
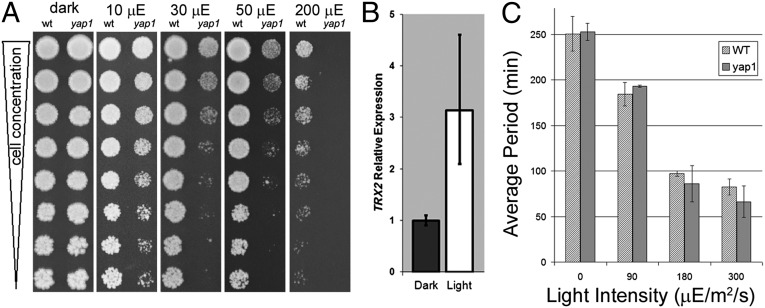
To distinguish between those alternatives, we studied a strain of yeast lacking the gene yeast activator protein-1 (yap1). YAP1 encodes a basic leucine zipper transcription factor that localizes to the yeast nucleus in the presence of H2O2 and up-regulates transcription of oxidative stress genes such as thioredoxin reductase (TTR1), cytosolic thioredoxin (TRX2), and cytochrome-c peroxidase (CCP1) (32). If visible light impairs electron transport, high-energy electrons react prematurely with O2 and form superoxide radicals (O2⋅−), H2O2, and hydroxyl radicals (⋅OH) (32, 33). Therefore, yeast with an impaired ROS defense might be more susceptible to the deleterious effects of visible light. We predicted that a yeast strain that is deficient for Yap1p would show inhibited growth compared with WT when exposed to visible light, so we grew both a yap1-knockout strain (34) and its WT strain at various dilutions side-by-side on plates at different light intensities. Both strains grew equally well when grown in darkness; however, as little as 10 µE⋅m−2⋅s−1 of white light had an obvious inhibitory effect on the growth of the yap1-knockout strain (yap1) compared with the WT (Fig. 2A). Similar growth inhibition was not seen in WT cells until light intensities reached 200 µE⋅m−2⋅s−1 (Fig. 2A).
Fig. 2.
The Yap1p oxidative stress-responsive transcription factor plays a role in yeast’s ability to tolerate harmful effects of visible light. (A) A yap1-knockout strain (yap1) and a WT strain of the same background were exposed to varying intensities of visible white light (0–200 µE⋅m−2⋅s−1) at room temperature and were imaged after 4 d. Both strains grew equally well in the dark, but the yap1-deletion strain showed impaired growth even at 10 µE⋅m−2⋅s−1. (B) Quantitative RT-PCR shows that transcription of the gene TRX2 (for the peroxide scavenger thioredoxin enzyme) is up-regulated when yeast cells are exposed to 280 µE⋅m−2⋅s−1 visible light compared with dark (n = 3 ± SD). (C) YRO modulation in a yap1-knockout strain (yap1) oscillating in continuous culture does not differ from that in WT when exposed to the same intensities of visible light. Each bar represents the average period (± SD) in minutes of complete oscillations occurring during the treatments shown in Fig. S4 A and B.
Because the loss of the ROS-responsive transcription factor Yap1p had such a noticeable effect on yeast survival in the presence of visible light, we reasoned that WT yeast grown in continuous culture under strong white light should express an elevated level of TRX2 (the cytosolic thioredoxin gene that is up-regulated by Yap1p in the presence of ROS) compared with cells grown in the dark. To test that prediction, we grew a nonoscillating strain of CEN.PK (yBR-ura3ΔCEN.PK113-7D, to exclude the influence of an oscillation in Yap1p expression) in continuous culture, taking yeast samples from the culture after 24 h of darkness and then again after 12 h of 280 µE⋅m−2⋅s−1 light. RNA levels (measured by quantitative RT-PCR) collected from these samples showed a roughly threefold difference in TRX2 expression in light-treated yeast and yeast from the same culture in darkness (Fig. 2B).
Therefore, the yap1 strain of yeast is more sensitive to ROS than is WT. If ROS production plays a significant role in light-induced YRO modulation, then the YRO period of yap1 yeast should respond to light differently from WT yeast. However, the YRO period in the yap1-knockout strain of CEN.PK was not significantly different from that in WT when exposed to similar light intensities (Fig. 2C and Fig. S4 A and B). Unexpectedly, however, oscillations initially were absent or at very low amplitude until the exposure to light, whereupon large-amplitude rhythms quickly initiated and remained strong, even with return to darkness (Fig S4). Similarly, bright light abolished oscillations, and this nonoscillatory status persisted in darkness until rhythmicity was reinstated by light of an intermediate intensity (Fig. S4C).
Taken together, these data have several implications. First and most importantly, light-induced ROS production alone cannot account for the changes in YRO period or amplitude that are seen during light exposure, because the YRO light sensitivities are equivalent in WT yeast and the yap1 strain (Fig. 2C). Second, the Yap1p transcription factor that responds to oxidative stress plays a role in yeast’s ability to tolerate the harmful effects of visible light (Fig. 2 A and B). Third, an impaired ROS-scavenging pathway interferes with the continuous culture’s ability to synchronize subpopulations of oscillating cells spontaneously (Fig. S4). Finally, sudden ROS production by light may provide a signal for individual cells that are oscillating out of phase to synchronize their YROs (Fig. S4).
Inhibition of Respiration Is the Mechanism of YRO Modulation.
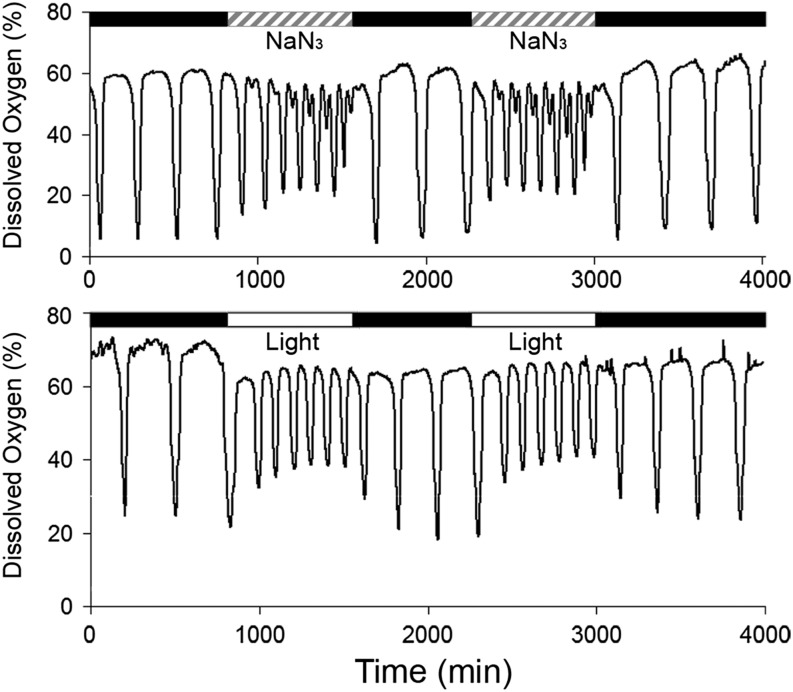
Because light-induced ROS production alone cannot account for the changes in YRO period or amplitude (Fig. 2C), we considered the alternative hypothesis of photoinhibition of electron transport. If the shortened period and reduced amplitude of the YRO are caused by light inhibiting or destroying mitochondrial cytochromes, then it is likely that another method of inhibiting or destroying cytochromes (or their effectiveness as transporters of electrons) would have an effect similar to that of light. Sodium azide is a chemical that inhibits respiration by binding and inhibiting cytochrome c (35) and cytochrome oxidase (36) of the electron transport chain. We introduced sodium azide into the oscillating culture at a steady drip (3.4 µmol/h). Perfusion of azide in 12-h treatments separated by 12 h with no azide showed effects on the YRO similar to those of 12-h light/dark treatments (Fig. 3). That is, sodium azide shortened the period and reduced the amplitude of the dissolved oxygen (DO) oscillation during treatment, and the oscillation returned to its longer period and amplitude once the delivery of the chemical was stopped. Treating the culture with a higher concentration of sodium azide (10 µmol/h) had an effect similar to treatment with very bright light, in that it destroyed the YRO and caused the DO levels to rise during treatment and to fall after the end of treatment (Fig. S5). These data support the conclusion that light affects the YRO by inhibiting the activity of light-absorbing cytochromes, thereby inhibiting electron transport and oxidative phosphorylation.
Fig. 3.
Sodium azide and white light have similar effects on the YRO. Separate oscillating 850-mL cultures were given two 12-h treatments of either sodium azide at 3.4 µmol/h (Upper) or white light at 180 µE⋅m−2⋅s−1 (Lower), with 12 h between treatments (30 °C). Both light and azide shorten the period and reduce the amplitude of the YRO, and both have a transient effect on period after the initial administration of each treatment.
Oxygen Shortage to the Respiration Pathway Can Reset the YRO.
The redox state of the culture is believed to play a vital role in the YRO as it alternates between a high oxygen consumption (HOC) phase and a low oxygen consumption (LOC) phase (13, 21, 22). As shown above, damage to or impairment of the respiratory cytochromes of the cell resulted in a shortened HOC phase of the YRO when DO is low (or dropping) and reduced the time before the culture returned to an LOC mode of energy metabolism when DO is high or rising. In addition, the introduction of chemicals (metabolites) such as ethanol and acetaldehyde has been shown previously to result in differing degrees of YRO phase resetting, depending upon the phase of the oscillation at which those substances were introduced (14). This phase resetting in response to the artificial introduction of metabolites prematurely switches the YRO from an LOC phase to an HOC phase characterized by an immediate drop in DO in the culture. Moreover, our results suggest that light and azide may affect the YRO by inhibiting cytochromes. Because azide is known to bind cytochrome oxidase, thereby preventing oxygen from terminally accepting electrons from the electron transport chain, we questioned whether rapidly depriving the culture of oxygen could have a similar phase-resetting effect without the addition of metabolites. We conjectured that the oscillating oxygen level in the culture might be an environmental signal that allows the YRO to remain synchronized among the cells in the population.
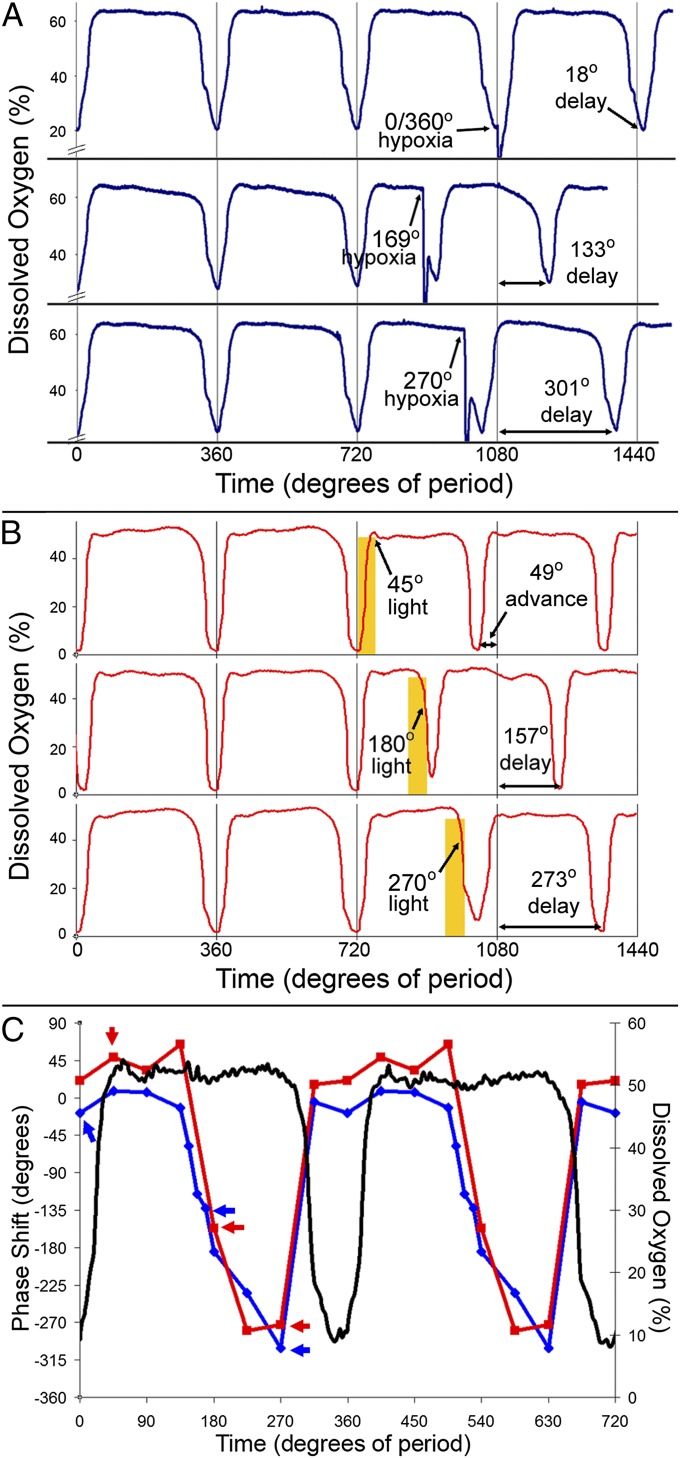
To test this prediction, the air supply was replaced with a blend of air and nitrogen to replicate artificially the characteristic DO troughs of the YRO at various phases of the YRO. Simply reproducing these troughs in amplitude and duration had no phase-resetting effect on the oscillation (Fig. S6). However, extreme deprivation of oxygen in the culture by bubbling 100% nitrogen (in place of air) into the culture at 0.9 L/min for 3 min did result in phase resetting at particular phases during the YRO. Fig. 4A shows three representative examples of this phase resetting.
Fig. 4.
Phase responses of the YRO to treatment with hypoxia (by nitrogen gas) or visible light (600 µE⋅m−2⋅s−1). (A) Representative N2 treatments used to generate the hypoxia PRC in C. Single-headed arrows show phase at the end of the 3-min hypoxia treatment. Double-headed arrows show phase response. (B) Representative light treatments used to generate the light PRC in C. Yellow bars show time of treatment with 600 µE⋅m−2⋅s−1of CWF light. Single-headed arrows show phase at the end of the 30-min treatment. Double-headed arrows show phase response. (C) A double-plotted PRC to the endpoint of hypoxia (blue trace) or the endpoint of light (red trace) treatments. Phase shifts are plotted in degrees of period with advances plotted as positive values and delays as negative values. For coherence and comparison across the −180° phase mark, treatments between phases 135° and 315° were plotted as delays. A representative DO trace (black trace) shows the YRO for reference. Blue arrows show phase points from A. Red arrows show phase points from B. 0° was defined as the time when DO started to rise from the YRO trough. 360° = 3.3 h for the N2 PRC and 4 h for the light PRC.
By testing hypoxia-induced phase resetting across different phases of the YRO, we were able to determine which portions of the YRO are most susceptible to the disruption of respiration. Given that light interferes with electron transport (1, 25) and that oxygen shortage resets the YRO at certain phases, we expected the phases of YRO sensitivity to light to be similar to those seen during oxygen deprivation. We tested this prediction by delivering bright light pulses for 30 min at different phases of the YRO. Fig. 4B shows three representative examples of phase resetting caused by light.
Fig. 4C compares the phase-resetting effects for both light (red trace) and hypoxia (blue trace), highlighting that light and hypoxia treatments have similar phases for phase-shifting (between 135° and 315°) and similar phases of relative unresponsiveness (between 315° and 450°). These phase–response curves (PRCs) show that light and oxygen deprivation affect the YRO in similar ways. In both hypoxia and light treatment the cells are forced to alter their metabolic strategy (either immediately or gradually), resulting in phase resetting at certain phases of the YRO. Together, these results imply that absolute DO levels are not the environmental synchronizing signal that maintains the YRO’s population synchrony; rather, the relative change from one metabolic state to another (as a consequence of the inhibition of respiration) is likely to be responsible for resetting the phase of the YRO.
Concluding Remarks
Using the YRO as a sensitive indicator of the status of intracellular metabolism, we have shown that S. cerevisiae can respond to light with physiological and metabolic consequences that are obvious almost immediately after irradiation. Because of the similar observations with azide treatment, this effect of light probably is mediated by inhibiting heme-containing cytochromes that participate in electron transport for oxidative phosphorylation. Ultimately this effect on respiration has consequences for the YRO and ROS-responding genes.
Our results and conclusions are important for two major reasons. First, these results have important implications for the use of light as a research tool when investigating yeast (and almost certainly all cells, including those without specialized photoreceptive molecules). Exposing cells to visible light for experiments (e.g., using light to excite fluorophores) should not be assumed to be innocuous. At the cellular level, we have shown that blue and green light especially affect respiration, and strong white light alters gene expression. In many cases these temporary consequences may be experimentally acceptable; however researchers should not assume that the light has no effect on the cells, especially when performing live-cell time-lapse fluorescent microscopy or investigating ROS production.
Second, light-driven modulations of respiratory rhythms give a further dimension to the challenges of yeast living in a natural environment. Light is more toxic to actively respiring yeast cells than is light in the absence of oxygen or light in the absence of respiration (31, 37, 38). Cells that are deprived of oxygen or are respiration-deficient show fewer damaging effects from light when fermenting (rather than respiring). Therefore, an adaptive strategy for yeast in natural sunlight could be to have shortened bouts of respiration when light is too strong as a way to minimize long stretches of time when cells would be subjected to this dangerous combination of light and respiration. Higher-frequency oscillations under light treatment do not mean the culture respires less but rather that the bouts of HOC phases are broken up into shorter bouts, allowing the culture more frequent opportunities to recover from accumulated damage.
Our observations that visible light can impact metabolic oscillations even in cells without dedicated photoreceptive pigments are consistent with the hypothesis that adaptive timekeeping mechanisms such as circadian clocks evolved as a result of a selective pressure generated by light that was deleterious to the optimal growth of the organism. The so-called “Escape from Light” hypothesis for the origin of circadian clocks postulates that organisms might respond to the daily bombardment of photons of both UV and visible light by evolving a timing system to segregate light-sensitive reactions temporally to the nighttime, when they will not be inhibited (39, 40). The results summarized here demonstrate that even visible light can negatively affect metabolism and could serve as a selective pressure for the evolution of a timekeeping mechanism to anticipate the daily onslaught of photons. Finally, metabolic oscillations are not limited to artificial conditions of continuous culture but rather are inherent in yeast and other types of cells under slow-growth conditions, affording a method for strengthening transcriptional regulation and minimizing signal noise when concentrations of transcription factors, cellular transporters, and other protein regulators are in low abundance (18–20, 41–43). Light-modulated respiratory status and ROS production seem to play a role in these cellular oscillations. Although our methods limited us to investigating population rhythms of respiratory oscillations, our results show that light dampens this rhythm within the population and that ROS weaken the signals that tie synchronous populations together.
Materials and Methods
See SI Materials and Methods for additional methods.
Illumination During Continuous Culture.
The YRO was established with the CEN.PK113-7D strain of S. cerevisiae in continuous culture in a Bioflo 115 reactor as described previously (14). White light was applied to the culture vessel by placing one, two, or three 65-W compact CWF floodlights (Lithonia Lighting) around the vessel’s water jacket (Fig. S2). For colored light treatment, a single layer of a Roscolux color filter (#74 for blue, #89 for green, #19 for red; Rosco Laboratories) was wrapped around the water jacket of the Bioflo 115 reactor, and three CWF lamps were used as described above. For the red-light treatment, in addition to the three CWF lamps, a 60-W incandescent lamp was positioned 15 cm from the red-filtered vessel. Light intensity from these arrangements was measured in the empty vessel using a LI-COR quantum radiometer/photometer (LI-250A) and is shown as the average of eight measurements taken at 45° increments around the vessel’s interior (Table S1).
PRCs.
An oscillating culture of CEN.PK113-7D with a stable period was initiated with 0.9 L/min air as described (14). The gas supply to the culture was switched from air to 100% N2 at 0.9 L/min for 3 min at each phase point. After treatment, the air supply was returned to normal, 0.9 L/min air. Phase 0° was defined as the time when DO started to rise from the trough. Phase shifts were determined by measuring the difference between the time of the DO trough in the cycle after treatment and the time that DO was predicted to reach the trough without treatment as extrapolated from the previous oscillation’s period. PRCs were normalized for 360° of period and were double-plotted for the end of treatment. Thirty-minute light pulses of 600 µE⋅m−2⋅s−1 were delivered by four CWF 65-W flood lamps (Lithonia Lighting), and phase points were plotted for the end of treatment. Phase points tested with hypoxia were 0°, 45°, 90°, 135°, 146°, 157°, 169°, 180°, 225°, 270°, and 315°. Phase points tested with light were 0°, 45°, 90°, 135°, 180°, 225°, 270°, and 315°.
Light Sensitivity Assay of the yap1-Knockout Strain.
YPD [1% (wt/vol) yeast extract, 2% (wt/vol) peptone, 2% (wt/vol) dextrose] cultures of WT strain BY4741 and the yap1 deletion strain from the MAT alpha yeast knockout collection (34) (Thermo Scientific) were diluted to OD600 = 0.5 in YPD and placed in alternating wells across the top of a 96-well plate. An array of eight twofold serial dilutions was constructed in the 96-well plate and spotted onto YPD 2% (wt/vol) agar plates. Plates were grown at room temperature over a 65-W CWF lamp (Lithonia Lighting) that had been covered with a glass tray of water which acted as a heat sink and infrared filter. Dark-treated controls were wrapped in aluminum foil and placed on the light apparatus next to light-treated plates. Different light intensities were achieved by adjusting the distance between the yeast and the light source. Light intensities were measured using a LI-COR light meter. Images of the plates were taken with a Bio-Rad MP ChemiDoc system after 4 d of growth.
Supplementary Material
Acknowledgments
We thank Peter Kötter (University of Frankfurt) for his gift of yeast strain CEN.PK113-7D and Matt Elrod-Erickson (Middle Tennessee State University) for providing equipment and advice. This research was supported by National Institutes of Health/National Institute of General Medical Sciences Grant R01GM088595 (to C.H.J.) and by seed money from the Middle Tennessee State University Biology Department.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1313369110/-/DCSupplemental.
References
- 1.Epel B, Butler WL. Cytochrome a3: Destruction by light. Science. 1969;166(3905):621–622. doi: 10.1126/science.166.3905.621. [DOI] [PubMed] [Google Scholar]
- 2.King A, Gottlieb E, Brooks DG, Murphy MP, Dunaief JL. Mitochondria-derived reactive oxygen species mediate blue light-induced death of retinal pigment epithelial cells. Photochem Photobiol. 2004;79(5):470–475. doi: 10.1562/le-03-17.1. [DOI] [PubMed] [Google Scholar]
- 3.Moore MM, Breedveld MW, Autor AP. The role of carotenoids in preventing oxidative damage in the pigmented yeast, Rhodotorula mucilaginosa. Arch Biochem Biophys. 1989;270(2):419–431. doi: 10.1016/0003-9861(89)90524-9. [DOI] [PubMed] [Google Scholar]
- 4.Ellis CJ. The pupillary light reflex in normal subjects. Br J Ophthalmol. 1981;65(11):754–759. doi: 10.1136/bjo.65.11.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaidi FH, et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17(24):2122–2128. doi: 10.1016/j.cub.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasahara M, et al. Chloroplast avoidance movement reduces photodamage in plants. Nature. 2002;420(6917):829–832. doi: 10.1038/nature01213. [DOI] [PubMed] [Google Scholar]
- 7.Islam MS, Niwa Y, Takagi S. Light-dependent intracellular positioning of mitochondria in Arabidopsis thaliana mesophyll cells. Plant Cell Physiol. 2009;50(6):1032–1040. doi: 10.1093/pcp/pcp054. [DOI] [PubMed] [Google Scholar]
- 8.Berrocal-Tito G, Sametz-Baron L, Eichenberg K, Horwitz BA, Herrera-Estrella A. Rapid blue light regulation of a Trichoderma harzianum photolyase gene. J Biol Chem. 1999;274(20):14288–14294. doi: 10.1074/jbc.274.20.14288. [DOI] [PubMed] [Google Scholar]
- 9.Nikaido SS, Johnson CH. Daily and circadian variation in survival from ultraviolet radiation in Chlamydomonas reinhardtii. Photochem Photobiol. 2000;71(6):758–765. doi: 10.1562/0031-8655(2000)071<0758:dacvis>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Salichos L, Rokas A. The diversity and evolution of circadian clock proteins in fungi. Mycologia. 2010;102(2):269–278. doi: 10.3852/09-073. [DOI] [PubMed] [Google Scholar]
- 11.Satroutdinov AD, Kuriyama H, Kobayashi H. Oscillatory metabolism of Saccharomyces cerevisiae in continuous culture. FEMS Microbiol Lett. 1992;77(1-3):261–267. doi: 10.1016/0378-1097(92)90167-m. [DOI] [PubMed] [Google Scholar]
- 12.Klevecz RR, Bolen J, Forrest G, Murray DB. A genomewide oscillation in transcription gates DNA replication and cell cycle. Proc Natl Acad Sci USA. 2004;101(5):1200–1205. doi: 10.1073/pnas.0306490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu BP, Kudlicki A, Rowicka M, McKnight SL. Logic of the yeast metabolic cycle: Temporal compartmentalization of cellular processes. Science. 2005;310(5751):1152–1158. doi: 10.1126/science.1120499. [DOI] [PubMed] [Google Scholar]
- 14.Robertson JB, Stowers CC, Boczko E, Johnson CH. Real-time luminescence monitoring of cell-cycle and respiratory oscillations in yeast. Proc Natl Acad Sci USA. 2008;105(46):17988–17993. doi: 10.1073/pnas.0809482105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd D, Lemar KM, Salgado LE, Gould TM, Murray DB. Respiratory oscillations in yeast: Mitochondrial reactive oxygen species, apoptosis and time; a hypothesis. FEMS Yeast Res. 2003;3(4):333–339. doi: 10.1016/S1567-1356(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 16.Murray DB, Roller S, Kuriyama H, Lloyd D. Clock control of ultradian respiratory oscillation found during yeast continuous culture. J Bacteriol. 2001;183(24):7253–7259. doi: 10.1128/JB.183.24.7253-7259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Odstrcil EA, Tu BP, McKnight SL. Restriction of DNA replication to the reductive phase of the metabolic cycle protects genome integrity. Science. 2007;316(5833):1916–1919. doi: 10.1126/science.1140958. [DOI] [PubMed] [Google Scholar]
- 18.Silverman SJ, et al. Metabolic cycling in single yeast cells from unsynchronized steady-state populations limited on glucose or phosphate. Proc Natl Acad Sci USA. 2010;107(15):6946–6951. doi: 10.1073/pnas.1002422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laxman S, Sutter BM, Tu BP. Behavior of a metabolic cycling population at the single cell level as visualized by fluorescent gene expression reporters. PLoS ONE. 2010;5(9):e12595. doi: 10.1371/journal.pone.0012595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aon MA, Cortassa S, Lemar KM, Hayes AJ, Lloyd D. Single and cell population respiratory oscillations in yeast: A 2-photon scanning laser microscopy study. FEBS Lett. 2007;581(1):8–14. doi: 10.1016/j.febslet.2006.11.068. [DOI] [PubMed] [Google Scholar]
- 21.Xu Z, Tsurugi K. A potential mechanism of energy-metabolism oscillation in an aerobic chemostat culture of the yeast Saccharomyces cerevisiae. FEBS J. 2006;273(8):1696–1709. doi: 10.1111/j.1742-4658.2006.05201.x. [DOI] [PubMed] [Google Scholar]
- 22.Murray DB, Engelen FA, Keulers M, Kuriyama H, Lloyd D. NO+, but not NO., inhibits respiratory oscillations in ethanol-grown chemostat cultures of Saccharomyces cerevisiae. FEBS Lett. 1998;431(2):297–299. doi: 10.1016/s0014-5793(98)00777-7. [DOI] [PubMed] [Google Scholar]
- 23.Murray DB, Engelen F, Lloyd D, Kuriyama H. Involvement of glutathione in the regulation of respiratory oscillation during a continuous culture of Saccharomyces cerevisiae. Microbiology. 1999;145(Pt 10):2739–2745. doi: 10.1099/00221287-145-10-2739. [DOI] [PubMed] [Google Scholar]
- 24.D’Aoust JY, Giroux J, Baraan LR, Schneider H, Martin WG. Some effects of visible light on Escherichia coli. J Bacteriol. 1974;120(2):799–804. doi: 10.1128/jb.120.2.799-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ninnemann H. Photoinhibition of isolated complexes I, II, and 3 of beef heart mitochondria. FEBS Lett. 1974;39(3):353–358. doi: 10.1016/0014-5793(74)80148-1. [DOI] [PubMed] [Google Scholar]
- 26.Linden H, Ballario P, Macino G. Blue light regulation in Neurospora crassa. Fungal Genet Biol. 1997;22(3):141–150. doi: 10.1006/fgbi.1997.1013. [DOI] [PubMed] [Google Scholar]
- 27.Bahn YS, et al. Sensing the environment: Lessons from fungi. Nat Rev Microbiol. 2007;5(1):57–69. doi: 10.1038/nrmicro1578. [DOI] [PubMed] [Google Scholar]
- 28.Eelderink-Chen Z, et al. A circadian clock in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2010;107(5):2043–2047. doi: 10.1073/pnas.0907902107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butt WD, Keilin D. Absorption spectra and some other properties of cytochrome c and of its compounds with ligands. Proc R Soc Lond B Biol Sci. 1962;156:429–458. doi: 10.1098/rspb.1962.0049. [DOI] [PubMed] [Google Scholar]
- 30.Horie S. On the Absorption Spectrum of Cytochrome a-3. J Biochem. 1964;56:57–66. doi: 10.1093/oxfordjournals.jbchem.a127958. [DOI] [PubMed] [Google Scholar]
- 31.Ułaszewski S, et al. Light effects in yeast: Evidence for participation of cytochromes in photoinhibition of growth and transport in Saccharomyces cerevisiae cultured at low temperatures. J Bacteriol. 1979;138(2):523–529. doi: 10.1128/jb.138.2.523-529.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Temple MD, Perrone GG, Dawes IW. Complex cellular responses to reactive oxygen species. Trends Cell Biol. 2005;15(6):319–326. doi: 10.1016/j.tcb.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Nohl H, Gille L, Kozlov A, Staniek K. Are mitochondria a spontaneous and permanent source of reactive oxygen species? Redox Rep. 2003;8(3):135–141. doi: 10.1179/135100003225001502. [DOI] [PubMed] [Google Scholar]
- 34.Winzeler EA, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285(5429):901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 35.Horecker BL, Stannard JN. The cytochrome c-azide complex. J Biol Chem. 1948;172(2):589–597. [PubMed] [Google Scholar]
- 36.Stannard JN, Horecker BL. The in vitro inhibition of cytochrome oxidase by azide and cyanide. J Biol Chem. 1948;172(2):599–608. [PubMed] [Google Scholar]
- 37.Ninnemann H, Butler WL, Epel BL. Inhibition of respiration in yeast by light. Biochim Biophys Acta. 1970;205(3):499–506. doi: 10.1016/0005-2728(70)90115-5. [DOI] [PubMed] [Google Scholar]
- 38.Ułaszewski S, Kołodyński J, Kotylak Z. Light effects in yeast: Relation between the respiratory deficiency and light sensitivity in yeast. Acta Microbiol Pol. 1982;31(3-4):227–237. [PubMed] [Google Scholar]
- 39. Pittendrigh CS (1965) Biological clocks: The functions, ancient and modern, of circadian oscillations. Science and the Sixties: Proceedings of the Air Force Office of Scientific Research Cloudcraft Symposium, ed Arm DL (Univ of New Mexico, Albuquerque, NM), pp 95–111.
- 40.Pittendrigh CS. Temporal organization: Reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 41.Nelson DE, et al. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science. 2004;306(5696):704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- 42.Slavov N, Macinskas J, Caudy A, Botstein D. Metabolic cycling without cell division cycling in respiring yeast. Proc Natl Acad Sci USA. 2011;108(47):19090–19095. doi: 10.1073/pnas.1116998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slavov N, Airoldi EM, van Oudenaarden A, Botstein D. A conserved cell growth cycle can account for the environmental stress responses of divergent eukaryotes. Mol Biol Cell. 2012;23(10):1986–1997. doi: 10.1091/mbc.E11-11-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.