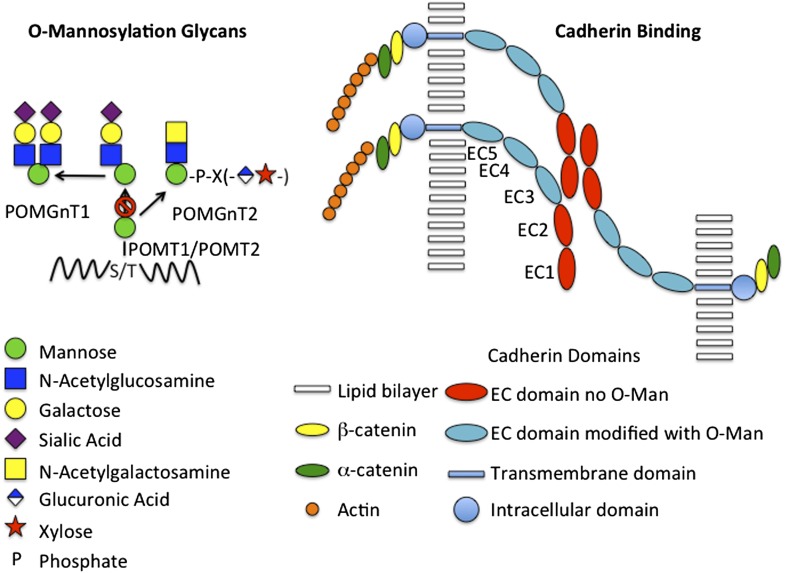
Two reports in PNAS by Vester-Christensen et al. (1) and Lommel et al. (2) provide critical new insight into how a unique family of glycans contributes to early embryonic development, brain development, and malignant transformation. Following transfer of mannose (Man) to Ser or Thr by protein O-mannosyltransferase (POMT), either POMT1 or POMT2, the O-Man can be further modified with N-acetylglucosamine, galactose, sialic acid, PO4, N-acetylgalactosamine, glucuronic acid, and xylose to generate structures, such as those illustrated in Fig. 1. Glycans linked to proteins via O-Man represent a major fraction of the O-glycans in the brain, where they were first described (3). However, just which glycoproteins are modified with O-Man in the brain, as well as in other tissues, has yet to be determined.
Fig. 1.
O-mannosylation glycans and cadherin binding. (Left) The structures of O-mannosylated glycans found on glycoproteins such as αDG are illustrated schematically. Man is transferred to Ser or Thr by either POMT1 or POMT2. Most often (Far Left) N-acetylglucosamine is added in β1,2-linkage by POMGnT1, yielding glycans with either one or two branches that terminate with sialic acid. Alternatively POMGnT2 can add N-acetylglucosamine in β1,4-linkage (Near Left) leading to the synthesis of a complex structure that is still under investigation. Elimination of POMGnT1 results in the presence of O-Man at all sites except those that undergo N-acetylglucosamine addition by POMGnT2 (Right). The classic cadherins form trans homodimers across apposing cells via interactions involving EC1 and EC2, which are not O-mannosylated. The EC domains more proximal to the membrane, EC3-5, are O-mannosylated and present the more distal EC domains to each other. O-mannosylation and calcium binding between the proximal EC domains may be critical for presenting the EC1 and EC2 domains in a manner that permits trans homodimer formation and adhesion. The intracellular domain interacts with actin via α- and β-catenin and is also able to modulate activation of signaling pathways. Loss of adhesion is seen in transformed cells and may increase the chances of these cells metastasizing.
Abnormalities in the posttranslational modification of α-Dystroglycan (αDG) are the basis of congenital muscular dystrophies (CMD). In prior research driven by the need to understand the genetic and biochemical basis for CMDs, αDG has become the most extensively characterized glycoprotein that is modified with O-Man (4–6). αDG, a basement membrane glycoprotein, is a key component of the dystrophin–glycoprotein complex that links the cytoskeleton with the extracellular matrix. Congenital deficiencies of the glycosyltransferases responsible for O-Man transfer (POMT1 and POMT2) and each of the further modifications generating the structures shown in Fig. 1 are responsible for CMDs, referred to as dystroglycanopathies. A particularly severe form CMD, Walker–Warburg syndrome, is associated with brain malformations, ocular abnormalities, and death in the first year of life (5). The presence of brain malformations raised the possibility that O-Man structures on either αDG or other glycoproteins might be responsible for these developmental abnormalities. αDG is widely expressed in tissues; however, ablation of αDG in the brain does not change the levels of O-Man glycans in the brain, indicating that other glycoproteins must also bear these structures.
Steentoft et al. (7, and see ref. 8) recently developed a powerful strategy to identify glycoproteins bearing O-GalNAc–linked structures and to map the location of O-GalNAc–modified Ser/Thr residues. The authors have now extended this approach to map the O-Man glycoproteome by generating “SimpleCells” that have a simplified pattern of O-Man glycosylation. Zinc-finger nuclease gene targeting was used to eliminate UDP-GlcNAc: Manα1-O-Ser/Thr β1,2GlcNAc-transferase (POMGnT1) that adds GlcNAc to the O-Man, leaving Ser/Thr residues modified with only O-Man. Following protease digestion, peptides bearing O-Man are fractionated by lectin weak-affinity chromatography and O-Man–containing peptides identified by mass spectrometry. All of the previously described sites bearing O-Man, as well as those bearing O-GalNAc, on αDG and two additional sites were identified, demonstrating the robustness of this strategy.
Even though αDG was one of the glycoproteins identified as having O-Man, it was not the major component. Remarkably, 37 members of the cadherin superfamily of cell-membrane receptors were identified as the major carriers of O-Man glycans. The cadherins are cell-surface membrane glycoproteins that have multiple repeats of an extracellular cadherin (EC) domain (9–11) (Fig. 1). The EC domains have an Ig-like fold. Cadherins mediate cell–cell adhesion by trans homodimerization between the most distal EC1 and EC1–2 domains on apposed cells. The other EC domains play a critical role in presenting the EC1 and EC2 domains so they can form homodimers. The O-Man–modified sites identified are confined to the EC domains EC2–5 of both classic type 1 and 2 cadherins and appear to have been evolutionarily conserved. Clustered protocadherins, which are predominantly expressed in the brain, contained O-Man sites predominantly in EC2–3. The expression of the clustered protocadherins is highly regulated during brain development and they appear to form oligomers that serve to increase the molecular diversity at the cell surface. Plexins and a mucin-like membrane glycoprotein KIAA1549 were also found to have O-Man glycans and are expressed in the brain.
Lommel et al. (2) provide direct evidence that O-mannosylation is essential for E-cadherin–mediated cell adhesion. E-cadherin is known to play a central role in cell–cell adhesion during embryo development before implantation. If O-mannosylation is prevented either genetically by ablating Pomt2 or biochemically using a specific inhibitor of POMT (R3A-5a), embryo development is arrested at the morula-to-blastocyst transition stage. O-Man–bearing glycoproteins were detected at the surface of preimplantation four-cell morulas and blastocysts using a polyclonal antibody (T[α-Man]) raised to a Threonine O-mannosyl–conjugated peptide. Pomt2−/− and inhibitor-treated embryos no longer stained for O-Man with T[α-Man]. Furthermore, although E-cadherin staining was retained basolaterally, it was reduced at sites that also displayed reduced cellular adhesion. In other words, the localization of the E-cadherin was disrupted in the absence of O-Man precisely at the sites where adhesions should form. The importance of O-Man for E-cadherin–mediated adhesion was further demonstrated using Madin–Darby canine kidney cells in an adhesion assay. Both T[α-Man] and R3A-5a selectively inhibited the aggregation of Madin–Darby canine kidney cells expressing E-cadherin. Thus, it is highly like that O-mannosylation of E-cadherin is required for the morula to blastocyst transition before implantation.
The identification of cadherins, particularly clustered protocadherins, as the major targets for O-Man in the brain provides an attractive potential explanation for why individuals with severe forms of CMD also manifest
Lommel et al. provide direct evidence that O-mannosylation is essential for E-cadherin–mediated cell adhesion.
the brain malformations and ocular abnormalities seen in Walker–Warburg syndrome. Following mannosylation of Ser/Thr residues, a range of unique structures can be synthesized upon the O-Man, depending on the repertoire of glycosyltransferases that are expressed and other factors that remain to be defined. Genetic evidence indicates that congenital deficiencies of each of these enzymes can result in dystroglycanopathies thought to reflect a disruption of the interaction of αDG with the extracellular matrix. The molecular basis for these disruptions of αDG function is not yet understood.
Cadherins mediate calcium-dependent cell–cell interactions and interact with the actin cytoskeleton via α-, β-, and γ-catenins. In addition, the cadherins are able to modulate the activation of a number of signaling pathways, reflecting events occurring at the cell surface. The cadherins are complex molecules and much remains to be learned about their interactions. The majority of O-Man sites are located in EC repeats that are not directly involved in trans homodimerization of classic cadherins but are critical for the presentation of EC1 and EC2 across apposed cells. The clustered protocadherins are particularly complex because they can form cis heterodimers that have the capacity to interact with integrins via an RGD motif (10). The highly regulated expression of members of the cadherin family at specific times during development of the embryo and the brain is indicative of the important role that they play in directing cell–cell and cell–matrix interactions. Reduced cadherin-mediated cell–cell interactions is one of the hallmarks of malignant transformation that may contribute to the ability of transformed cells to metastasize as well as to grow.
The presence of O-Man glycans on the cadherins provides yet another mechanism to modulate the properties of these complex molecules. The presence or absence of these glycans or alterations in their structure could have an impact on the presentation of EC1 and EC2 domains during trans homodimerization or alter homo or hetero dimerization during cis interactions in the membrane. Altered patterns of O-Man glycosylation could be associated with malignant transformation and account for reduced cadherin-mediated adhesion. Although there is still much to be learned about the role of O-Man glycans in vivo, understanding how this modification contributes to cadherin function has the potential to provide critical new insights into embryonic development, brain development, and malignant transformation. In addition, the O-Man glycans may prove to be reasonable targets for therapeutic intervention.
Footnotes
References
- 1.Vester-Christensen MB, et al. Mining the O-mannose glycoproteome reveals cadherins as major O-mannosylated glycoproteins. Proc Natl Acad Sci USA. 2013;110:21018–21023. doi: 10.1073/pnas.1313446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lommel M, et al. Protein O-mannosylation is crucial for E-cadherin–mediated cell adhesion. Proc Natl Acad Sci USA. 2013;110:21024–21029. doi: 10.1073/pnas.1316753110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finne J, Krusius T, Margolis RK, Margolis RU. Novel mannitol-containing oligosaccharides obtained by mild alkaline borohydride treatment of a chondroitin sulfate proteoglycan from brain. J Biol Chem. 1979;254(20):10295–10300. [PubMed] [Google Scholar]
- 4.Barresi R, Campbell KP. Dystroglycan: From biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119(Pt 2):199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 5.Godfrey C, Foley AR, Clement E, Muntoni F. Dystroglycanopathies: Coming into focus. Curr Opin Genet Dev. 2011;21(3):278–285. doi: 10.1016/j.gde.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Inamori K, et al. Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science. 2012;335(6064):93–96. doi: 10.1126/science.1214115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steentoft C, et al. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat Methods. 2011;8(11):977–982. doi: 10.1038/nmeth.1731. [DOI] [PubMed] [Google Scholar]
- 8.Baenziger JU. Moving the O-glycoproteome from form to function. Proc Natl Acad Sci USA. 2012;109(25):9672–9673. doi: 10.1073/pnas.1206735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasch J, Harrison OJ, Honig B, Shapiro L. Thinking outside the cell: How cadherins drive adhesion. Trends Cell Biol. 2012;22(6):299–310. doi: 10.1016/j.tcb.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yagi T. Clustered protocadherin family. Dev Growth Differ. 2008;50(Suppl 1):S131–S140. doi: 10.1111/j.1440-169X.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- 11.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4(2):118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]