Significance
Vitamin D plays an important role in regulating the immune system in health and disease and may be beneficial for patients with multiple sclerosis. It prevents CNS autoimmunity in mice by an incompletely understood mechanism. The present study is a systematic evaluation of the vitamin D effects on T lymphocytes at each step of their journey to the CNS. The data demonstrate that vitamin D does not affect generation of pathogenic cells but prevents their presence in the CNS. Unlike current long-acting drugs that impair immune cell trafficking, the effect of vitamin D is quickly reversed after treatment cessation, which could prove advantageous when immune function needs to be reestablished in the setting of infection.
Abstract
Pharmacologic targeting of T helper (TH) cell trafficking poses an attractive opportunity for amelioration of autoimmune diseases such as multiple sclerosis (MS). MS risk is associated with vitamin D deficiency, and its bioactive form, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], has been shown to prevent experimental autoimmune encephalomyelitis, a mouse model of MS, via an incompletely understood mechanism. Herein, we systematically examined 1,25(OH)2D3 effects on TH cells during their migration from the lymph nodes to the CNS. Our data demonstrate that myelin-reactive TH cells are successfully generated in the presence of 1,25(OH)2D3, secrete proinflammatory cytokines, and do not preferentially differentiate into suppressor T cells. These cells are able to leave the lymph node, enter the peripheral circulation, and migrate to the s.c. immunization sites. However, TH cells from 1,25(OH)2D3-treated mice are unable to enter the CNS parenchyma but are instead maintained in the periphery. Upon treatment cessation, mice rapidly develop experimental autoimmune encephalomyelitis, demonstrating that 1,25(OH)2D3 prevents the disease only temporarily likely by halting TH cell migration into the CNS.
Multiple sclerosis (MS) is an immune-mediated demyelinating disorder of the central nervous system (CNS). Whereas its etiology is unknown, a number of environmental and genetic factors contribute to the risk of developing MS (1). Epidemiologic studies demonstrate a strong correlation between MS risk and vitamin D deficiency (2). Higher levels of circulating 25-hydroxyvitamin D3 have been recently shown to correlate with a reduction in the number of new T2 and gadolinium-enhancing lesions (3). Moreover, the bioactive form of vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], can completely prevent and treat established experimental autoimmune encephalomyelitis (EAE) (4), a murine MS model. Nonetheless, the mechanism of 1,25(OH)2D3’s in vivo action remains incompletely understood.
The pathogeneses of MS and EAE require localization of self-reactive T cells to the CNS. Myelin-specific effector T cells are primed in the lymph nodes (LNs) and subsequently travel a complex route and arrive at the CNS barriers. There, a sequential interaction with a multitude of endothelial adhesion molecules and chemokines leads to T helper (TH) cell migration into the CNS parenchyma. Several ligand/receptor pairs have been implicated in the pathogeneses of MS and EAE (5, 6). Therefore, manipulation of molecules involved in T-cell trafficking poses an attractive opportunity for therapeutic interventions. Recent studies have highlighted a role for vitamin D in immune cell migration (7–10), but whether 1,25(OH)2D3 affects trafficking of lymphocytes in MS and/or EAE has not been investigated.
A recent study using conditional deletion of the vitamin D receptor (VDR) has demonstrated that 1,25(OH)2D3 prevents EAE by acting directly on the encephalitogenic TH cells (11). We hypothesized that 1,25(OH)2D3 could be inhibiting encephalitogenic T cells via regulation of molecules involved in their trafficking into the CNS. Herein, we provide unique evidence that 1,25(OH)2D3 prevents EAE by modulating the migratory phenotype of the pathogenic TH cells without affecting their priming or effector functions. Our findings further emphasize that 1,25(OH)2D3 is a reversible immune modulator, as treatment cessation leads to rapid disease onset. These findings may have important implications for the use of vitamin D as an adjunct treatment of immune-mediated diseases such as MS.
Results
1,25(OH)2D3 Prevents Accumulation of Inflammatory Infiltrates in the CNS.
1,25(OH)2D3 is known to inhibit EAE induction, but the mechanism of disease prevention remains unclear. To investigate its effects on TH cells, 1,25(OH)2D3 or vehicle were administered to C57BL/6 mice daily by oral gavage, beginning a day before immunization with myelin oligodendrocyte glycoprotein (MOG) 35-55. Whereas T lymphocytes infiltrated the CNS and induced EAE in vehicle-treated animals, no T cells, or CD11b+CD45hi monocytic infiltrates (Fig. S1), were recovered from the CNS of 1,25(OH)2D3-treated mice, all of which remained disease-free (Fig. 1 A and B). Moreover, both vehicle- and 1,25(OH)2D3-treated groups had significantly higher proportions of splenic CD4+ T cells compared with the naive control (Fig. 1B). Thus, EAE prevention is accompanied by TH cell expansion in the periphery and a complete absence of inflammatory infiltrates in the CNS of 1,25(OH)2D3-treated animals, leading us to hypothesize that 1,25(OH)2D3 affects the ability of encephalitogenic T cells to enter the CNS without interfering with their activation.
Fig. 1.
1,25(OH)2D3 inhibits EAE and prevents inflammatory infiltrates in the CNS. (A) Clinical scores of vehicle (Veh; n = 8) and 1,25(OH)2D3-treated [1,25(OH)2D3; n = 8] mice immunization with MOG 35-55 in CFA. **P = 0.0033 (Mann–Whitney test). Data are representative of six independent experiments. (B) Mice were killed on EAE day 16. Mononuclear cells from the CNS (Upper) and splenocytes (Lower) were isolated, and the proportion of CD4+ and CD8+ T cells were examined by FACS analysis. Representative flow plots are shown (Left). The corresponding graphs (Right) are representative of at least four independent experiments (n ≥ 5 per group per experiment). ***P < 0.0001.
1,25(OH)2D3 Does Not Affect Activation of Pathogenic TH Cells.
We next examined the effect of 1,25(OH)2D3 on the peripheral inflammatory response following immunization (Fig. S2). An equivalent increase in the proportion of the CD44hiCD62Llow effector and IFNγ+ or IL-17+ TH cells was observed in the draining lymph nodes (dLNs) and spleens in both vehicle- and 1,25(OH)2D3-treated animals, compared with naive controls. Furthermore, dLN cells and splenocytes isolated 8 d after immunization secreted equivalent amounts of IFNγ and IL-17 in response to in vitro restimulation with either MOG 35-55 or anti-CD3/CD28 antibodies and exhibited equivalent MOG-specific proliferative capacity. Moreover, 1,25(OH)2D3 did not induce apoptosis of peripheral TH cells. Although it has been proposed that 1,25(OH)2D3 induces TH cell apoptosis in the CNS (9, 11, 12), absence of inflammatory infiltrates precluded us from examining potential CNS-specific apoptosis. Taken together, these findings indicate that the treatment with 1,25(OH)2D3 does not affect generation or activation of MOG-specific TH cells, suggesting an alternative mechanism for disease prevention. Similarly, 1,25(OH)2D3 ameliorated established disease and reduced the frequency of T cells in the CNS but not in the spleen, suggesting that 1,25(OH)2D3 might be blocking migration of new waves of activated MOG-specific cells into the CNS (Fig. S3).
1,25(OH)2D3 Does Not Induce Suppressor T Cells.
Induction of regulatory TH cells (Tregs) has been suggested as a potential mechanism by which 1,25(OH)2D3 prevents EAE (13, 14). No significant difference in the proportion of the CD25+Foxp3+ Tregs was detected between the treatment groups at either EAE day 15 or 36 (Fig. S4A). Furthermore, CD45.2+ 2D2 TH cells were transferred into CD45.1+ congenic mice before immunization, and examination of Foxp3+ 2D2 TH cells 8 d later revealed no difference in the proportion of antigen-specific Tregs between the treatment groups (Fig. S4B). An ex vivo suppression assay demonstrated no significant differences between the effects of TH cells from vehicle- and 1,25(OH)2D3-treated animals on proliferation of naive responder cells (Fig. S4C). Taken together, these findings demonstrate that 1,25(OH)2D3 does not increase the frequency of total or MOG-specific Tregs or augment their suppressive capacity, suggesting a direct effect of 1,25(OH)2D3 on encephalitogenic TH cells as a disease prevention mechanism.
1,25(OH)2D3 Does Not Sequester TH Cells to the Lymph Nodes but Does Sustain Leukocytosis.
To evaluate the effect of 1,25(OH)2D3 on expansion and distribution of MOG-specific TH cells, we transferred CD45.2+ 2D2 TH cells into CD45.1+ mice before immunization with either MOG 35-55 in Complete Freund's Adjuvant (CFA) or CFA only. MOG-immunized recipients were treated with vehicle, 1,25(OH)2D3, or the immunomodulatory drug FTY720 which sequesters lymphocytes in lymph nodes. Expansion of MOG-specific cells in the dLNs was equivalent in the three treatment groups compared with the CFA-only control (Fig. 2A). Whereas equivalent proportions of MOG-specific TH cells were observed in the spleen and peripheral circulation of 1,25(OH)2D3- and vehicle-treated mice, FTY720 treatment, as expected (15), led to a significant reduction of 2D2 cells in these compartments and to lymphopenia (Fig. 2 A and B). Furthermore, leuko- and lymphocytosis were observed 8 d after immunization and before disease onset in both vehicle- and 1,25(OH)2D3-treated mice compared with day 0 (Fig. 2C). After clinical symptoms had developed in the vehicle- but not in 1,25(OH)2D3-treated animals (day 16), blood counts remained elevated in the 1,25(OH)2D3 group but returned almost to baseline in the vehicle-treated animals, suggesting that, whereas encephalitogenic cells migrate into the CNS in the vehicle group, the cells remain in the circulation in the 1,25(OH)2D3-treated mice. During the chronic stage of disease (day 22), blood counts returned to baseline in both groups, illustrating the contraction phase of the immune response. In vitro restimulation of splenocytes and LN cells isolated at the peak of disease revealed that splenocytes from the 1,25(OH)2D3-treated animals secreted significantly higher levels of IFNγ and IL-17 than the cells from the vehicle group, indicating paucity of MOG-reactive TH cells in the spleens of vehicle- but not 1,25(OH)2D3-treated mice (Fig. 2D). Cells from all groups demonstrated an appropriate polyclonal response to anti-CD3/CD28 stimulation. These findings indicate that MOG-specific TH cells migrate into the CNS in vehicle-treated animals to cause EAE but remain in the periphery in the 1,25(OH)2D3-treated animals, suggesting that 1,25(OH)2D3 prevents circulating pathogenic TH cells from entering the CNS.
Fig. 2.
1,25(OH)2D3 treatment does not sequester TH cells to the lymph nodes but does sustain leukocytosis. (A) A total of 5 × 106 CD4+ T cells per mouse isolated from unimmunized CD45.2+ 2D2 mice were transferred i.v. into CD45.1+ congenic mice. The recipients were immunized the following day with either MOG 35-55 in CFA or CFA only. Draining LNs, spleens, and peripheral blood were isolated on day 8, and expansion of CD45.2+ 2D2 cells was analyzed by FACS. Transferred 2D2 cells as percent of total cells are graphed. (B) Representative flow plots for circulating leukocytes are shown. (C) Absolute counts of white blood cells (WBC; Left) and lymphocytes (Right) were obtained on EAE days 0, 8, 16, and 22 and are shown overlaid with the clinical scores. * denotes significant differences between the treatment groups within the same time point. @, #, and $ denote significant difference compared with day 0 (@P < 0.05, #P < 0.01, $P < 0.001). (D) Splenocytes and dLN cells isolated on EAE day 15 were restimulated with 10 µg/mL MOG 35-55 or 0.5 µg/mL soluble anti-CD3/CD28 for 72 h. Secreted IFNγ and IL-17 were quantified by ELISA. All data are representative of at least two independent experiments (n = 5 mice per group per time point).
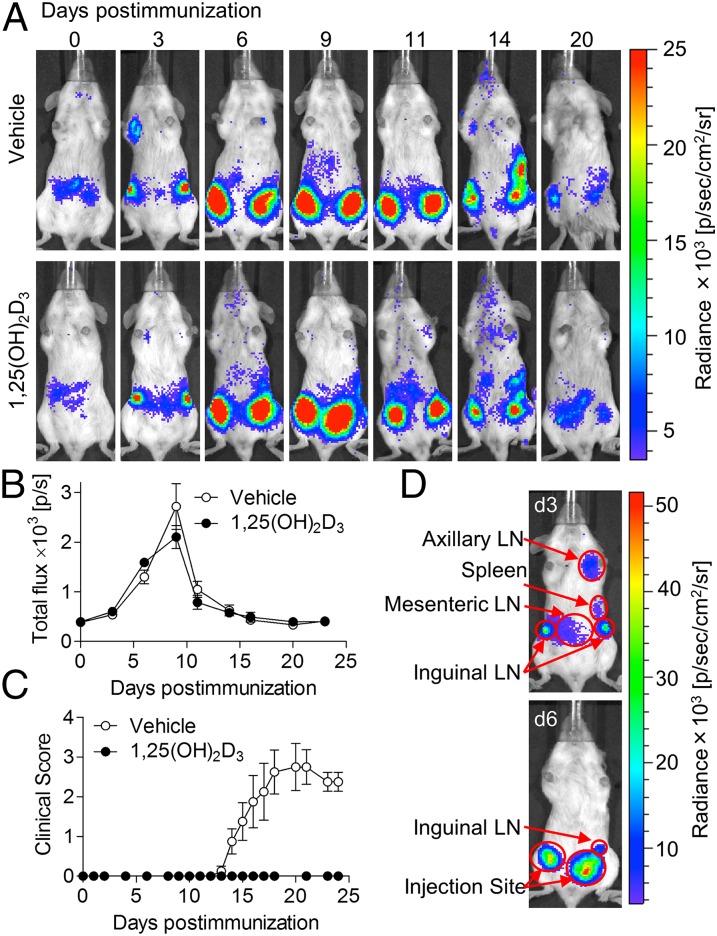
MOG-Specific TH Cells Migrate to the Immunization Sites in 1,25(OH)2D3-Treated Mice.
Next, we used in vivo bioluminescent imaging to further evaluate the effect of 1,25(OH)2D3 on location of encephalitogenic TH cells. TH cells from unimmunized 2D2-Luc mice (obtained by crossing pan-luciferase and 2D2 mice) were transferred into the B6(Cg)-Tyrc-2J/J strain of mice a day before immunization. Increased signal was observed in the dLNs 3 d postimmunization, after which a dramatic increase in the signal was detected at the immunization sites, suggesting that 2D2-Luc TH cells expand within the dLNs and then migrate to and accumulate in the nonlymphoid injection sites (Fig. 3). After signal intensity at the injection site peaked around day 9, with minimal signal observed elsewhere, the signal gradually decreased to baseline. Because significant signal was not observed outside the dLNs and sites of immunization, the decline in signal after day 10 can be best explained by cell loss during the contraction phase of the immune response rather than the redistribution of 2D2-Luc cells to other tissues such as the CNS. The signal intensity approached the preimmunization levels by the time the vehicle-treated animals developed clinically apparent disease (day 14), suggesting that the endogenous Luc-negative MOG-reactive TH cells were the cells causing the disease. Importantly, no difference in the signal intensity and distribution was observed between the vehicle- and 1,25(OH)2D3-treated animals, indicating that 1,25(OH)2D3 does not affect expansion of MOG-specific TH cells or their migration to the site of immunization. These data further confirm that 1,25(OH)2D3 does not affect generation of MOG-specific TH cells, which are not confined to the lymphoid organs but are able to enter nonlymphoid injection sites.
Fig. 3.
MOG-specific TH cells migrate to the immunization sites in 1,25(OH)2D3-treated mice; 2D2-Luc CD4+ T cells were transferred into B6(Cg)-Tyrc-2J/J mice a day before the immunization. Treatment with vehicle or 1,25(OH)2D3 was initiated on the day of transfer. The mice were subjected to bioluminescent imaging at indicated days following EAE induction. (A) Representative images at various times after immunization are shown (n = 5 per group per experiment). (B) Bioluminescent emission (p/s) was analyzed over time for the whole body. No statistically significant difference was detected between the groups at any of the examined time points. (C) Clinical scores of vehicle- and 1,25(OH)2D3-treated mice at various times after immunization (n = 5 mice per group). (D) The mice were subjected to bioluminescent imaging on EAE day 3 (d3, Upper) and day 6 (d6, Lower). Defined anatomical regions are indicated.
1,25(OH)2D3-Treated Mice Have Intact Blood–CNS Barrier.
The apparent inhibition of transmigration of TH cells into the CNS parenchyma could occur at the level of the blood–brain barrier (BBB). Evaluation of Evans Blue dye extravasation at the peak of EAE revealed an intact BBB of the 1,25(OH)2D3-treated mice, whereas the paralyzed vehicle-treated mice exhibited significant loss of BBB integrity (Fig. S5A). Moreover, IgG extravasated into the CNS parenchyma in the vehicle- but not 1,25(OH)2D3-treated mice (Fig. S5B). Although these findings suggest a potential direct effect of 1,25(OH)2D3 on the BBB endothelium, an intrinsic inability of encephalitogenic T cells to enter the CNS would also leave the BBB intact. Hence, these findings maintain that 1,25(OH)2D3 prevents TH cells’ migration into the CNS and argue against the possibility that the pathogenic TH cells transiently enter the CNS parenchyma and rapidly recirculate through cervical lymphatics or undergo apoptosis in the CNS, as this would undermine BBB integrity.
T Cells Do Not Cross BBB or Blood–Leptomeningeal Barrier Endothelium in 1,25(OH)2D3-Treated Mice.
To migrate into the CNS parenchyma, encephalitogenic TH cells must cross the BBB or blood–leptomeningeal barrier (BLMB) and the glia limitans (16). Cell sequestration within the perivascular or leptomeningeal space prevents clinical disease (17, 18). Before the onset of the clinical symptoms, inflammatory infiltrates were contained within the leptomeningeal space in approximately half of the vehicle-treated mice, whereas no infiltrates were detected in any of 1,25(OH)2D3-treated or naive mice (Fig. S6A). At the peak of disease, infiltrates were observed within the spinal cord parenchyma of all vehicle-treated controls, whereas no meningeal or parenchymal infiltrates were detected in the 1,25(OH)2D3-treated or naive mice (Fig. S6B). Moreover, vehicle- but not 1,25(OH)2D3-treated mice exhibited abundant perivascular cuffing within the spinal cord at the peak of disease (Fig. S6C). T-cell infiltration was further examined by CD3 staining (Fig. S7), which paralleled the histology findings. These findings indicate that the encephalitogenic T cells are not trapped in the perivascular or leptomeningeal space, suggesting that 1,25(OH)2D3 maintains TH cells in the circulation.
EAE Inhibition by 1,25(OH)2D3 Is Reversible.
Next, we sought to evaluate whether 1,25(OH)2D3 permanently alters TH cell phenotype and ability to cause EAE. Animals developed EAE within ∼7 d of treatment cessation, whereas mice maintained on 1,25(OH)2D3 remained disease-free (Fig. 4A). Equivalent proportions of splenic TH cells in vehicle-treated and withdrawal mice were CD44hiCD62Llow and produced IFNγ and IL-17 (Fig. S8 A and B). In addition, the immune response has contracted by the time withdrawal mice developed the disease, as indicated by return of leukocyte and lymphocyte counts to baseline (Fig. S8C) and undetectable IFNγ and IL-17 secretion in response to in vitro restimulation with MOG. Interestingly, whereas the proportions of TH cells in the CNS were comparable between the two groups, the proportion of CD8+ T cells was significantly lower in the withdrawal animals (Fig. 4B), suggesting an altered mode of disease induction following 1,25(OH)2D3 withdrawal. These findings demonstrate that although 1,25(OH)2D3 prevents EAE, this effect is not permanent, suggesting that pathogenic TH cells are present but unable to enter the CNS to cause disease during the treatment course. Moreover, the prompt reversal of 1,25(OH)2D3 effects upon treatment cessation illustrates that, similar to other nuclear hormones, it signals rapidly and reversibly and suggests that it affects a cellular target with a rapid turnover.
Fig. 4.

EAE inhibition by 1,25(OH)2D3 is reversible. Immunized mice were treated with 1,25(OH)2D3 for the duration of the experiment or up to day 17 postimmunization (arrow). (A) Clinical scores of mice treated with vehicle (Veh; n = 5), 1,25(OH)2D3 for the entirety of the study [1,25(OH)2D3; n = 5], or with 1,25(OH)2D3 up to day 21 (withdrawal and W/d; n = 5) are shown. Representative data from four independent experiments are shown. (B) On EAE day 29, the proportions of CD4+ and CD8+ T cells in the inflammatory infiltrates in the brain were determined by FACS analysis.
1,25(OH)2D3 Reversibly Reduces the Expression of the Chemokine Receptor CXCR3 on TH cells.
Finally, we evaluated whether 1,25(OH)2D3 can modulate the expression of molecules involved in CNS transendothelial migration of TH cells. A significant reduction in the proportion of splenic CXCR3+ TH cells in 1,25(OH)2D3-treated animals was observed, equivalent to the proportion in unimmunized naive animals (Fig. 5A). However, no change in the expression of α4-integrin (CD49d) or the chemokine receptor CCR6, receptors involved in TH cell CNS migration (19, 20), was detected on splenic TH cells of vehicle- or 1,25(OH)2D3-treated animals at the examined time point (Fig. S9). Importantly, after cessation of the 1,25(OH)2D3 treatment, the proportion of CXCR3+ TH cells reached that of the vehicle-treated animals (Fig. 5B). These findings suggest that 1,25(OH)2D3 may prevent TH cell extravasation across the BBB endothelium by regulating chemokine receptor expression, and that the effect of 1,25(OH)2D3 is easily reversible, as CXCR3 expression is rapidly turned over in the absence of treatment.
Fig. 5.
1,25(OH)2D3 reversibly reduces CD4+ T-cell CXCR3 expression. (A) On EAE day 15, difference in the proportion of splenic CXCR3+ TH cells from 1,25(OH)2D3- (D3) and vehicle-tread (Veh) mice was evaluated by flow cytometric analysis (**P = 0.0068). (B) Treatment with 1,25(OH)2D3 was stopped on EAE day 17, and the animals were killed on day 29, when the withdrawal animals reached peak disease severity. The difference in the proportion of splenic CXCR3+ TH cells from vehicle-treated (Veh) and the withdrawal (W/d) groups was evaluated. The representative flow plots are shown (Left). The corresponding graphs (Right) are representative of at least two independent experiments (n = 5 per group per experiment).
Discussion
To induce an organ-specific autoimmune response, self-reactive T cells must migrate to their target tissues, a process that is regulated by chemokines and their cognate receptors, selectins, integrins, and metalloproteinases. Pharmacologic targeting of TH cell migration poses an attractive opportunity for amelioration of immune-mediated diseases such as MS. The data presented herein provide unique evidence that 1,25(OH)2D3 reversibly prevents EAE by modulating encephalitogenic TH-cell CNS localization. Specifically, we demonstrate that TH cells from 1,25(OH)2D3-treated EAE mice become activated in response to immunization, secrete proinflammatory cytokines, and do not differentiate into suppressor T cells. These cells are able to leave the lymph nodes, enter the peripheral circulation, and arrive at the s.c. immunization sites. However, TH cells from 1,25(OH)2D3-treated mice do not migrate into the CNS parenchyma, but are instead maintained in the periphery and display down-regulation of the homing receptor CXCR3. Moreover, treatment cessation leads to rapid disease onset, demonstrating that 1,25(OH)2D3 halts TH-cell CNS invasion only temporarily. Our findings emphasize the direct and reversible effects of 1,25(OH)2D3 on autoreactive TH cells in prevention of autoimmune conditions.
1,25(OH)2D3 has been shown to prevent and reverse EAE (4, 21); however, the mechanism of its in vivo action has not been fully elucidated and may differ for prevention and treatment paradigms. A recent study using conditional deletion of VDR demonstrated that 1,25(OH)2D3 prevents EAE by acting directly on the encephalitogenic TH cells (11). 1,25(OH)2D3 has been shown to inhibit T-cell proliferation and IL-17 and IFNγ production in in vitro studies (7, 22), and it has been suggested that 1,25(OH)2D3 prevents EAE by inhibiting the differentiation and function of the pathogenic TH1 and TH17 cells (7, 14). However, in our prevention paradigm, 1,25(OH)2D3 did not lower the proportion of CD44hiCD62Llow effector or IL-17– and IFNγ-producing TH cells in the peripheral lymphoid organs or reduce the profound leukocytosis elicited by immunization, uniquely demonstrating, to our knowledge, that the dramatic systemic immune reaction in EAE is unaffected by 1,25(OH)2D3. Further examination of MOG-reactive TH cells confirmed that 1,25(OH)2D3 does not inhibit generation of a myelin-specific response. The discrepancies between our and previous findings could be related to dosing, time, or mode of 1,25(OH)2D3 administration. Importantly, our findings indicate that 1,25(OH)2D3 acts as a selective immunomodulator, thus highlighting its potential advantages over broadly immunosuppressive agents that heighten infection susceptibility.
EAE in IL-10/IL-10R–deficient mice has been shown to be unresponsive to 1,25(OH)2D3, indicating potential immunoregulatory mechanisms of its action (13). However, more recently, Hayes and coworkers (11) have demonstrated that TH cells expressing the transcription factor Foxp3 have virtually no VDR transcripts, whereas Foxp3– effector cells exhibit abundant expression. In agreement with these observations, we detected no changes in the proportion of total or MOG-specific Tregs or in the suppressive capacity of TH cells from 1,25(OH)2D3-treated animals. Although it is possible that 1,25(OH)2D3 exerted undetected immunoregulatory effects, one would expect this to be long lasting. Tregs have been shown to lose regulatory activity and acquire a proinflammatory phenotype under certain conditions (23); however, the majority of Tregs are considered to be phenotypically stable (24). Thus, the rapid EAE onset observed upon 1,25(OH)2D3 withdrawal suggests a lack of Treg induction and instead supports the direct effect of 1,25(OH)2D3 on the pathogenic TH cells. This intriguing phenomenon, which has not been reported in the literature, raises a question regarding the fate of encephalitogenic T cells during the treatment. For disease to develop within a week of treatment cessation, activated pathogenic T cells must already be present in the periphery, awaiting a chance to reach the CNS. The onset of clinical symptoms coincided with a reduction in circulating leukocytes and MOG-specific TH cells in vehicle-treated mice, whereas in the 1,25(OH)2D3-treated animals, leukocytes remained in the periphery and responded strongly to in vitro restimulation with MOG 35-55. These findings indicate that the pathogenic TH cells are present in the peripheral circulation but are unable to enter the CNS while exposed to 1,25(OH)2D3. The nature and location of antigen presentation that would be required for TH cell reactivation upon 1,25(OH)2D3 withdrawal remains to be elucidated. Of note, rebound effect has been previously shown only for treatment of established disease (21), where TH cells have already caused damage in the CNS. In this scenario, 1,25(OH)2D3 withdrawal would allow TH cells in the CNS to reinitiate their effector functions, which is different from the prevention paradigm, where TH cells do not enter the CNS until the drug withdrawal.
The complex route of encephalitogenic TH cells from the LNs to the CNS is only partially defined. With the use of in vivo bioluminescent imaging, we demonstrate that migration to the s.c. immunization sites is an important step in TH cell journey to the CNS and is unaffected by 1,25(OH)2D3. However, the functional significance of this localization for EAE induction remains to be elucidated. Interestingly, no bioluminescent signal was detected in the CNS in either treatment group, and the total body signal intensity approached the preimmunization levels by the time clinical symptoms were apparent, indicating that the 2D2-Luc cell population contracted by the onset of disease. This finding suggests that the endogenous TH cells were the EAE-inducing cells. Low avidity for the MHC–MOG complex could allow 2D2 cells to be outcompeted by the endogenous MOG-specific TH cells over time, leading to their contracture before the onset of disease (25). Alternatively, this imaging modality might not be capable of detecting the few 2D2-Luc cells that do arrive in the CNS. Overall, the imaging data established that 1,25(OH)2D3 does not affect the ability of the MOG-specific TH cells to migrate to the immunization sites, whereas FACS and immunohistochemical approaches demonstrate that TH cells are not sequestered in the perivascular/leptomeningeal space, suggesting that 1,25(OH)2D3 prevents T cells from crossing the CNS endothelium and thus maintains them in the periphery. Taken together, these findings emphasize a potential difference between migration of TH cells to the s.c. injection sites versus the CNS, indicating that 1,25(OH)2D3 might be selectively modulating the CNS-tropic TH cell migration.
Although much progress has been made in understanding T-cell trafficking to the CNS (6, 16), the molecular signature that permits myelin-reactive TH cells to breach the CNS barriers and infiltrate the parenchyma remains to be fully defined. Chemokine receptor expression profiles differ by TH cell subtype and, in combination with selectins and integrins, define their final destination (26, 27). Chemokine receptors are dynamic G protein-coupled receptors, and their expression on TH cells is regulated by T cell receptor (TCR) stimulation, cytokines, vitamins, and other mediators present at the time of priming (28). 1,25(OH)2D3 has been previously shown to modulate the expression of several chemokine receptors on both mouse and human TH cells in vitro (7, 10). However, the effects of 1,25(OH)2D3 on in vivo expression of chemokine receptors and adhesion molecules known to play a role in EAE pathogenesis have not been examined. A significant and reversible reduction in CXCR3 expression was detected on peripheral TH cells in 1,25(OH)2D3-treated mice. Although the role of CXCR3 and its ligands in migration of TH cells into the CNS remains controversial (29), the majority of TH cells found in MS active demyelinating plaques are CXCR3+ (30, 31), and blockade of CXCR3 with antibodies or other antagonists prevents TH1 cells from entering the CNS and subsequently ameliorates EAE (32–34). Moreover, a recent study by Hayes and coworkers (35) demonstrated a requirement for a functional IFNγ gene for 1,25(OH)2D3 to prevent the accumulation of pathogenic TH cells in the CNS, suggesting that IFNγ-producing CXCR3+ cells may be a major target of vitamin D therapy. Because naturally occurring IFNγ-producing TH1 cells are almost exclusively CXCR3 positive, and CXCR3 has been demonstrated to be essential for the establishment of the TH1 amplification loop, it is possible that 1,25(OH)2D3-mediated down-regulation of CXCR3 could at least partially explain its ability to prevent EAE.
Whereas no changes in α4-integrin expression were detected in 1,25(OH)2D3-treated mice, the integrin conformation was not examined. G protein-coupled chemokine receptor signaling leads to integrin activation; hence, changes in chemokine receptor expression could potentially impair interactions between T-cell integrins and their endothelial ligands, inhibiting stable adhesion of T cells to the CNS endothelium. Furthermore, although 1,25(OH)2D3 has been shown to reduce expression of CCR6 and to inhibit trafficking of TH17 cells toward the chemokine CCL20 in vitro (7), the role of CCR6 in EAE induction is controversial. CCR6 deletion has been shown to prevent TH17 cell CNS entry through the choroid plexus, thus inhibiting EAE (19), but contradictory studies have undermined this observation (36, 37). Whereas the molecular signature that defines encephalitogenic TH cells is not fully characterized, our findings suggest that the presence of 1,25(OH)2D3 during the priming of MOG-specific TH cells can alter their expression of chemokine receptors without affecting differentiation, thus potentially reversibly modulating TH cell trafficking phenotype and ameliorating EAE. The reversal of 1,25(OH)2D3 effects could prove advantageous when immune function needs to be reestablished during an infection. However, this rapid rebound effect raises concern about the use of vitamin D for MS treatment. If vitamin D supplementation sequesters the pathogenic T cells in the periphery, treatment cessation could potentially release these cells into the CNS and lead to a clinical relapse. Overall, the present study expands the understanding of the mechanism by which 1,25(OH)2D3 prevents EAE and emphasizes the importance of understanding the molecular mechanisms underlying the extravasation of encephalitogenic T cells into the CNS, as well as its prevention, in advancing the treatment for diseases caused by CNS inflammation such as MS.
Methods
Additional methods can be found in SI Methods.
Mice.
C57BL/6 and CD45.1+ congenic mice were purchased from the National Cancer Institute. B6(Cg)-Tyrc-2J/J mice and 2D2 mice (TCR-transgenic specific for MOG 35-55) were purchased from The Jackson Laboratory. Luc mice were a kind gift from Robert Negrin (Stanford University, Stanford, CA). Luc mice expressing MOG-specific TCR (2D2-Luc) were generated and bred in our facility. All mice were maintained in a federally approved animal facility at Johns Hopkins University in accordance with the Institutional Animal Care and Use Committee. Female mice of 8–12 wk of age were used in all of the experiments.
Induction of EAE and Treatment.
MOG EAE was induced and monitored as previously described (38). For adoptive transfer of 2D2 TH cells, 5 × 106 splenic TH cells isolated from unimmunized CD45.2+ 2D2 mice were transferred i.v. into CD45.1+ congenic recipients a day before immunization. 1,25(OH)2D3 (Tocris Bioscience) was dissolved in 100% (vol/vol) ethanol and stored at −80 °C in light-proof containers. FTY720 (Cayman Chemical) was dissolved in 100% (vol/vol) ethanol and stored in −80 °C. 1,25(OH)2D3 (1.5 µg/kg mouse weight/d), FTY720 (1.5 mg/kg mouse weight/d), and vehicle control (1% ethanol in water) were administered daily by oral gavage in 100 μL H2O.
Cell Isolation and Culture.
Single-cell suspensions were made by passing spleens and lymph nodes through a 70-μm nylon cell strainer (BD Biosciences). Splenic TH cells were isolated using EasySep Mouse CD4+ T-cell Enrichment kit (Stemcell Technologies), following the manufacturer's protocol. Mononuclear cells were isolated from the CNS using a 70–30% Percoll step gradient as previously described (39). Cells were cultured in RPMI 1640 supplemented with 10% vol/vol FBS, 100 μg/mL penicillin and streptomycin, 0.5 μM 2-mercaptoethanol, 10 mM Hepes buffer, 1 mM sodium pyruvate, and MEM Nonessential amino acids.
ELISA.
Spleen and dLN cells were cultured in complete RPMI medium 1640 (5 × 106 cells per well in 24-well plates) in the presence of 0 or 10 μg/mL MOG 35-55, or 0.5 μg/mL anti-CD3 and anti-CD28 antibodies (BD Biosciences). Supernatants were collected after 72 h, and the levels of IFNγ and IL-17 were quantified using ELISA kits according to manufacturer’s instructions (BioLegend).
Flow Cytometry.
Surface antigens were stained with anti-CD4 (BD Biosciences; RM4-5), anti-CD8 (BD Biosciences; 53-6.7), anti-CD45.2 (BD Biosciences; 104), and anti-CXCR3 (eBioscience; CXCR3-173). Flow cytometric analyses were performed on a FACSCalibur instrument (BD Biosciences) and the results were analyzed using FlowJo software (TreeStar).
Complete Blood Counts.
Absolute counts of white blood cells and lymphocytes in peripheral blood were obtained using Drew Scientific Hemavet 950FS (Drew Scientific).
In Vivo Bioluminescent Imaging.
B6(Cg)-Tyrc-2J/J mice were used as recipients because white coats minimize light absorption. Treatment with vehicle or 1,25(OH)2D3 was started on the day of transfer of 2D2-Luc TH cells, and EAE was induced the following day. Mice were anesthetized with isoflurane, and images were obtained 8 min after i.p. injections of d-luciferin (150 mg/kg body weight; Regis Technologies) with a CCD camera cooled to −180 °C, using the IVIS Spectrum Imaging system (Xenogen) with the field of view set at subject height (1.2 cm). The photographic images were obtained using a 0.2-s exposure, 8f/stop, and small binning. The bioluminescent images were obtained using 300-s exposures, 1f/stop, medium binning, and an open filter. The bioluminescent and gray-scale images were overlaid using Living Image software (Xenogen). Regions of interest were drawn around entire animals, and the total flux (p/s) per region of interest was obtained.
Statistical Analysis.
Statistical analysis was conducted using GraphPad Prism software (GraphPad). Mann–Whitney test was used to compare data from noninterval scales. Two-tailed Student t test was used to analyze normally distributed data. Results were considered significant if the P value was <0.05: *P < 0.05, **P < 0.01, ***P < 0.001; NS, no significant difference. All error bars represent SEM.
Supplementary Material
Acknowledgments
We thank Drs. J. Bream, J. Powell, and K. Whartenby for helpful discussion. This work was supported by National Institutes of Health Grant R01 NS041435 (to P.A.C.), the Kenneth and Claudia Silverman Family Foundation, and an American Medical Association Foundation Seed Grant (to I.V.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.A.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306072110/-/DCSupplemental.
References
- 1.Zuvich RL, McCauley JL, Pericak-Vance MA, Haines JL. Genetics and pathogenesis of multiple sclerosis. Semin Immunol. 2009;21(6):328–333. doi: 10.1016/j.smim.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 3.Mowry EM, et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol. 2012;72(2):234–240. doi: 10.1002/ana.23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87(3):1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: Anatomical sites and molecular mechanisms. Trends Immunol. 2005;26(9):485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Engelhardt B. Immune cell entry into the central nervous system: Involvement of adhesion molecules and chemokines. J Neurol Sci. 2008;274(1–2):23–26. doi: 10.1016/j.jns.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Chang JH, Cha HR, Lee DS, Seo KY, Kweon MN. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLoS ONE. 2010;5(9):e12925. doi: 10.1371/journal.pone.0012925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gysemans CA, et al. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: Implications for prevention of diabetes in nonobese diabetic mice. Endocrinology. 2005;146(4):1956–1964. doi: 10.1210/en.2004-1322. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen LB, Nashold FE, Spach KM, Hayes CE. 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J Neurosci Res. 2007;85(11):2480–2490. doi: 10.1002/jnr.21382. [DOI] [PubMed] [Google Scholar]
- 10.Sigmundsdottir H, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8(3):285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 11.Mayne CG, Spanier JA, Relland LM, Williams CB, Hayes CE. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur J Immunol. 2011;41(3):822–832. doi: 10.1002/eji.201040632. [DOI] [PubMed] [Google Scholar]
- 12.Spach KM, et al. Gene expression analysis suggests that 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by stimulating inflammatory cell apoptosis. Physiol Genomics. 2004;18(2):141–151. doi: 10.1152/physiolgenomics.00003.2004. [DOI] [PubMed] [Google Scholar]
- 13.Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2006;177(9):6030–6037. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- 14.Joshi S, et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol. 2011;31(17):3653–3669. doi: 10.1128/MCB.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brinkmann V, Pinschewer D, Chiba K, Feng L. FTY720: A novel transplantation drug that modulates lymphocyte traffic rather than activation. Trends Pharmacol Sci. 2000;21(2):49–52. doi: 10.1016/s0165-6147(99)01419-4. [DOI] [PubMed] [Google Scholar]
- 16.Engelhardt B, Ransohoff RM. Capture, crawl, cross: The T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33(12):579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Agrawal S, et al. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203(4):1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toft-Hansen H, et al. Metalloproteinases control brain inflammation induced by pertussis toxin in mice overexpressing the chemokine CCL2 in the central nervous system. J Immunol. 2006;177(10):7242–7249. doi: 10.4049/jimmunol.177.10.7242. [DOI] [PubMed] [Google Scholar]
- 19.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10(5):514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 20.Yednock TA, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356(6364):63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 21.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA. 1996;93(15):7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cippitelli M, Santoni A. Vitamin D3: A transcriptional modulator of the interferon-gamma gene. Eur J Immunol. 1998;28(10):3017–3030. doi: 10.1002/(SICI)1521-4141(199810)28:10<3017::AID-IMMU3017>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21(3):281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey-Bucktrout SL, Bluestone JA. Regulatory T cells: Stability revisited. Trends Immunol. 2011;32(7):301–306. doi: 10.1016/j.it.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chewning JH, Dugger KJ, Chaudhuri TR, Zinn KR, Weaver CT. Bioluminescence-based visualization of CD4 T cell dynamics using a T lineage-specific luciferase transgenic model. BMC Immunol. 2009;10:44. doi: 10.1186/1471-2172-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foxman EF, Campbell JJ, Butcher EC. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol. 1997;139(5):1349–1360. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ransohoff RM. Chemokines and chemokine receptors: Standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31(5):711–721. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9(9):981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller M, Carter S, Hofer MJ, Campbell IL. Review: The chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity—a tale of conflict and conundrum. Neuropathol Appl Neurobiol. 2010;36(5):368–387. doi: 10.1111/j.1365-2990.2010.01089.x. [DOI] [PubMed] [Google Scholar]
- 30.Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96(12):6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN. Expression of the interferon-gamma-inducible chemokines IP-10 and Mig and their receptor, CXCR3, in multiple sclerosis lesions. Neuropathol Appl Neurobiol. 2000;26(2):133–142. doi: 10.1046/j.1365-2990.2000.026002133.x. [DOI] [PubMed] [Google Scholar]
- 32.Kohler RE, et al. Antagonism of the chemokine receptors CXCR3 and CXCR4 reduces the pathology of experimental autoimmune encephalomyelitis. Brain Pathol. 2008;18(4):504–516. doi: 10.1111/j.1750-3639.2008.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sporici R, Issekutz TB. CXCR3 blockade inhibits T-cell migration into the CNS during EAE and prevents development of adoptively transferred, but not actively induced, disease. Eur J Immunol. 2010;40(10):2751–2761. doi: 10.1002/eji.200939975. [DOI] [PubMed] [Google Scholar]
- 34.Ni J, et al. The chemokine receptor antagonist, TAK-779, decreased experimental autoimmune encephalomyelitis by reducing inflammatory cell migration into the central nervous system, without affecting T cell function. Br J Pharmacol. 2009;158(8):2046–2056. doi: 10.1111/j.1476-5381.2009.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spanier JA, Nashold FE, Olson JK, Hayes CE. The Ifng gene is essential for Vdr gene expression and vitamin D₃-mediated reduction of the pathogenic T cell burden in the central nervous system in experimental autoimmune encephalomyelitis, a multiple sclerosis model. J Immunol. 2012;189(6):3188–3197. doi: 10.4049/jimmunol.1102925. [DOI] [PubMed] [Google Scholar]
- 36.Elhofy A, Depaolo RW, Lira SA, Lukacs NW, Karpus WJ. Mice deficient for CCR6 fail to control chronic experimental autoimmune encephalomyelitis. J Neuroimmunol. 2009;213(1–2):91–99. doi: 10.1016/j.jneuroim.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamazaki T, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181(12):8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gocke AR, et al. Kv1.3 deletion biases T cells toward an immunoregulatory phenotype and renders mice resistant to autoimmune encephalomyelitis. J Immunol. 2012;188(12):5877–5886. doi: 10.4049/jimmunol.1103095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lebson L, et al. Cutting edge: The transcription factor Kruppel-like factor 4 regulates the differentiation of Th17 cells independently of RORγt. J Immunol. 2010;185(12):7161–7164. doi: 10.4049/jimmunol.1002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.