Significance
Genetic alterations targeting the PI3K pathway are highly prevalent in breast cancers. Although breast cancers harboring PIK3CA mutation and HER2 amplification have enhanced sensitivity to PI3K inhibitors, the mechanism underlying this sensitivity is unknown. This study shows that PI3K inhibitors suppress MEK/ERK pathway in these cancers, and inhibition of both AKT and ERK pathways is necessary for maximal antitumoral activity. We elucidate a unique mechanistic link between PI3K and ERK via PI3K-dependent regulation of P-Rex1, which in turn regulates the Rac1/PAK/c-RAF/MEK/ERK pathway. Importantly, expression levels of the Rac-GEF, P-Rex1, correlate with sensitivity to PI3K inhibitors among these breast cancer cell lines, indicating its potential utility as a biomarker to identify cancers that will respond to PI3K inhibitors.
Abstract
The PI3K pathway is genetically altered in excess of 70% of breast cancers, largely through PIK3CA mutation and HER2 amplification. Preclinical studies have suggested that these subsets of breast cancers are particularly sensitive to PI3K inhibitors; however, the reasons for this heightened sensitivity are mainly unknown. We investigated the signaling effects of PI3K inhibition in PIK3CA mutant and HER2 amplified breast cancers using PI3K inhibitors currently in clinical trials. Unexpectedly, we found that in PIK3CA mutant and HER2 amplified breast cancers sensitive to PI3K inhibitors, PI3K inhibition led to a rapid suppression of Rac1/p21-activated kinase (PAK)/protein kinase C-RAF (C-RAF)/ protein kinase MEK (MEK)/ERK signaling that did not involve RAS. Furthermore, PI3K inhibition led to an ERK-dependent up-regulation of the proapoptotic protein, BIM, followed by induction of apoptosis. Expression of a constitutively active form of Rac1 in these breast cancer models blocked PI3Ki-induced down-regulation of ERK phosphorylation, apoptosis, and mitigated PI3K inhibitor sensitivity in vivo. In contrast, protein kinase AKT inhibitors failed to block MEK/ERK signaling, did not up-regulate BIM, and failed to induce apoptosis. Finally, we identified phosphatidylinositol 3,4,5-trisphosphate-dependent Rac exchanger 1 (P-Rex1) as the PI(3,4,5)P3-dependent guanine exchange factor for Rac1 responsible for regulation of the Rac1/C-RAF/MEK/ERK pathway in these cells. The expression level of P-Rex1 correlates with sensitivity to PI3K inhibitors in these breast cancer cell lines. Thus, PI3K inhibitors have enhanced activity in PIK3CA mutant and HER2 amplified breast cancers in which PI3K inhibition down-regulates both the AKT and Rac1/ERK pathways. In addition, P-Rex1 may serve as a biomarker to predict response to single-agent PI3K inhibitors within this subset of breast cancers.
The phosphoinositide 3-kinase (PI3K) family of lipid kinases plays a prominent role in the growth and survival of several types of cancer (1). The PI3K pathway is aberrantly activated by a number of different mechanisms in cancers. These include genetic mutation and/or amplification of key pathway components, such as amplification or mutation of the PI3K catalytic subunit p110α (encoded by PIK3CA gene), mutation or deletion of the phosphatase PTEN, amplification or mutation of the gene encoding for the PI3K effector protein kinase AKT, as well as constitutive activation of receptor tyrosine kinases (RTKs) (e.g., HER2 amplification in breast cancer) or other less frequent events (2). PI3K phosphorylates the phosphoinositide PI(4,5)P2 in the 3′OH group of the inositol ring to produce PI(3,4,5)P3. PI(3,4,5)P3 directly binds to the pleckstrin homology (PH) domains of certain proteins, such as AKT, leading to their activation, which in turn transmit growth and survival signals.
These findings have encouraged the development of several different PI3K inhibitors, many of which are either in or approaching clinical trial testing. Genotype-driven patient selection has been investigated to uncover patient populations that will be particularly susceptible to PI3K inhibitors. Cancers harboring mutations in the PIK3CA gene have emerged as among the most sensitive to single-agent PI3K inhibitors in several preclinical studies, although clinical activity to date has been mixed (3–6). These gain-of-function mutations in the PI3KCA gene are found in a broad range of cancers, and they are highly enriched in breast cancer, where they are observed in 20–25% of cases (7). In addition, breast cancers with amplified HER2, which comprise ∼20% of all breast cancers, (8) are also particularly sensitive to PI3K inhibition (9–11). However, even among patients whose cancers harbor PIK3CA mutations, a significant heterogeneity of responses has been observed to PI3K inhibitors currently being tested in clinical studies (3–5). There have been some patients with bona fide response evaluation criteria in solid tumors (RECIST) criteria responses, but the majority has not had similarly impressive outcomes. These early clinical results highlight the potential utility of a biomarker of sensitivity to single-agent PI3K inhibitors.
Interestingly, early clinical trial reports have found that inhibition of PI3K signaling may sometimes lead to suppression of protein kinase MEK (MEK)/ERK signaling (6). Although a previous laboratory study had shown that the PI3K/mammalian target of rapamycin (mTOR) inhibitors LY294002 and wortmannin can inhibit protein kinase RAF (RAF)/MEK/ERK-signaling (12), this clinical observation was initially surprising because several studies have shown that inhibitors of components of the PI3K signaling pathway (such as AKT and mTOR inhibitors) actually lead to activation of the MEK/ERK signaling in many cancer types (11, 13), and such feedback activation may impair sensitivity to PI3K pathway inhibitors (9, 11, 14). Because PIK3CA and HER2 amplified breast cancers are particularly sensitive to single-agent PI3K pathway inhibitors, we investigated how PI3K inhibitors impact MEK/ERK signaling in these genetically defined subsets of breast cancers. In our study, we found that several cell lines harboring PIK3CA mutation and/or HER2 amplification suppress MEK/ERK pathway signaling as well as the AKT pathway after treatment with PI3K inhibitors, and importantly, inhibition of both pathways is necessary for maximal antitumoral activity. Moreover we identify that the mechanistic link between PI3K and MEK/ERK is via a PI(3,4,5)P3-dependent regulation of the phosphatidylinositol 3,4,5-trisphosphate-dependent Rac exchanger 1 (P-Rex1)/ small GTPase Rac1 (Rac1)/protein kinase c-RAF (c-RAF) pathway in these cancers. Importantly, the expression levels of the Rac guanine exchange factor (Rac-GEF), P-Rex1, correlate with sensitivity to PI3K inhibitors in these breast cancer cell lines.
Results
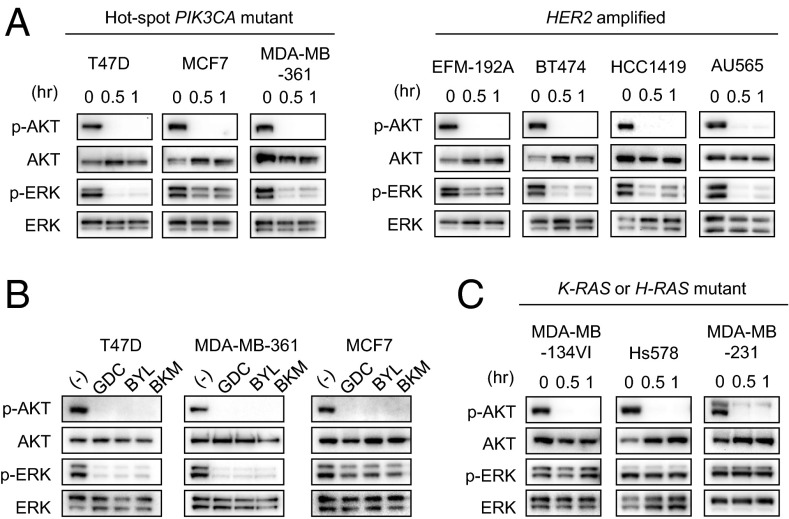
Treatment of a panel of HER2 amplified and/or PIK3CA mutant breast cancer cell lines with the pan PI3K inhibitor GDC-0941 unexpectedly revealed that both AKT and ERK signaling were suppressed (Fig. 1A). This result was recapitulated with additional PI3K inhibitors, including the p110α-specific inhibitor, BYL719, and another pan-PI3K inhibitor, BKM120 (Fig. 1B). Of note, in PIK3CA and HER2 amplified breast cancers that harbor concurrent RAS mutations, PI3K inhibitors did not suppress ERK signaling (Fig. 1C). Thus, it seems that PI3K signaling drives ERK activation specifically in HER2 amplified and/or PIK3CA mutant cells that do not have direct activation of the MEK/ERK pathway by mutant RAS.
Fig. 1.
PI3K inhibition down-regulates both AKT and ERK signaling in HER2 amplified and PIK3CA mutant breast cancer cells. (A) Cells were treated with 1 μM GDC-0941 for the indicated times, and lysates were probed with the indicated antibodies. Although BT474 has a K111N amino acid substitution, this mutation was found to have no effect on AKT phosphorylation (39). Independent experiments were performed three times, and a representative result is shown. (B) Cells were treated with 1 μM GDC-0941, 1 μM BYL719, and 1 μM BKM120 for 30 min, and lysates were probed with the indicated antibodies. Independent experiments were performed three times, and a representative result is shown. (C) Cells were treated with 1 μM GDC-0941 for the indicated times, and lysates were probed with the indicated antibodies. Independent experiments were performed three times, and a representative result is shown.
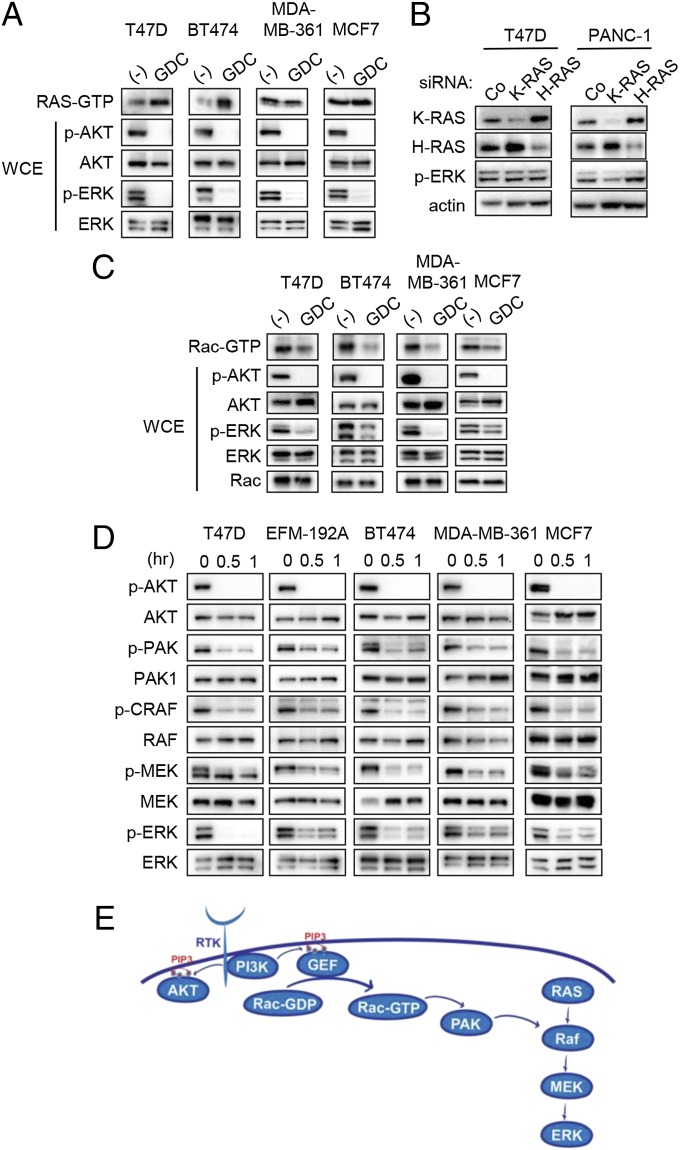
Because RAS often regulates activation of the RAF/MEK/ERK pathway, we determined whether PI3K inhibition led to concomitant suppression of RAS activity in the PIK3CA mutant and/or HER2 amplified breast cancers. However, to our surprise, we observed that RAS activity was not suppressed but rather was induced after GDC-0941 treatment in PIK3CA mutant and HER2 amplified breast cancer cells (Fig. 2A). This discordance between activation of RAS and loss of ERK signaling suggested that PI3K controls MEK/ERK signaling through a RAS-independent pathway. Indeed, we observed that knockdown of small GTPase K-RAS (K-RAS) or small GTPase H-RAS (H-RAS) failed to suppress ERK phosphorylation in T47D cells, in contrast to a K-RAS mutant pancreatic cancer cell line (Fig. 2B). Previous research has identified alternative mechanisms for activating ERK signaling, and thus we turned our attention to Rac, the small GTPase that is a key downstream effector of PI3K signaling (15). It has been shown that Rac can activate PAK (p21-activated kinase), which can directly activate the RAF/MEK/ERK pathway (16, 17). To measure Rac1 activity, cells were serum starved and then changed into media containing 10% (vol/vol) FBS with or without PI3K inhibitor. We observed that GDC-0941 treatment significantly inhibited Rac1 activity (Fig. 2C) as well as phosphorylation of PAK, C-RAF, MEK, and ERK (Fig. 2 C and D). Among the three isoforms of PAK, only siRNA against PAK1 and PAK3 suppressed ERK phosphorylation after PI3K inhibition (Fig. S1). Interestingly PAK2 down-regulation paradoxically caused increased activation of ERK for reasons unknown at this time. Taken together, these results are consistent with PI3K activating the ERK signaling pathway via Rac, independent of RAS (Fig. 2E).
Fig. 2.
PI3K inhibition suppresses Rac but not RAS activation. (A) RAS activity assays were completed after 30 min of GDC-0941 treatment. Representative detection of active RAS (RAS-GTP, determined by the Raf-1 pull-down assay; Materials and Methods) is shown. The corresponding whole-cell extracts were probed with the indicated antibodies. Independent experiments were performed at least three times, and a representative result is shown. (B) Cells were transfected with control, K-RAS, or H-RAS–targeted siRNA for 72 h. Lysates were prepared and probed with the indicated antibodies. Results were confirmed by two independent experiments. (C) Cells were serum starved for 16 h, and then media containing 10% (vol/vol) FBS with or without 1 μM of GDC-0941 was added. Cells were lysed after 30 min, and Rac-GTP levels were determined with a PAK1-binding domain pull-down assay. Independent experiments were performed more than three times for T47D and twice for other cell lines, and a representative result is shown. (D) Cells were treated with 1 μM GDC-0941 for the indicated times, and lysates were probed with the indicated antibodies. Independent experiments were performed at least three times, and a representative result is shown. (E) Schematic representation of how PI3K is proposed to regulate ERK pathway.
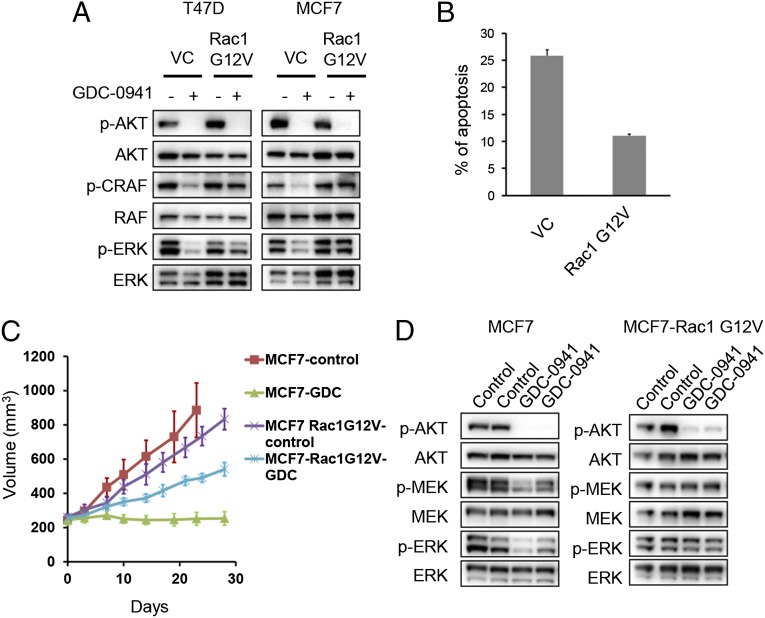
To determine whether down-regulation of Rac is essential for PI3K inhibitors to suppress ERK phosphorylation, we expressed a constitutively activated version of Rac1 in T47D and MCF7 cells. Expression of constitutively active Rac1 (G12V Rac1) abrogated PI3K-dependent down-regulation of C-RAF and ERK phosphorylation, although suppression of AKT phosphorylation was preserved (Fig. 3A and Fig. S2). Importantly, G12V Rac1 expression mitigated apoptosis induced by PI3K inhibition (Fig. 3B) even though AKT activity remained suppressed (Fig. 3A). These results suggest that the suppression of both the Rac/ERK and AKT pathways are required for maximal cell death induced by PI3K inhibition. Consistent with these results, GDC-0941 treatment blocked growth of MCF7 tumor xenografts in vivo, but efficacy of the PI3K inhibitor was substantially mitigated in the MCF7 tumors expressing constitutively active Rac1 (Fig. 3C). Western blotting of tumor lysates showed that GDC-0941 down-regulated both AKT and ERK phosphorylation in MCF7 xenografts, whereas MCF7 xenografts expressing G12V Rac1 displayed sustained MEK/ERK activity after PI3K inhibition despite suppression of AKT phosphorylation (Fig. 3D). Thus, down-regulation of Rac1 is necessary for PI3K inhibitors to suppress ERK signaling and for full efficacy in vivo.
Fig. 3.
Constitutive Rac1 activation abrogates the efficacy of PI3K inhibitors in vivo. (A) Cells expressing the empty vector (VC) or constitutive form of Rac1 (Rac1 G12V) were treated with 1 μM GDC-0941 for 30 min, and lysates were evaluated by Western blot with the indicated antibodies. Independent experiments were performed three times, and a representative result is shown. (B) MCF7 cells expressing the empty vector (VC) or constitutive form of Rac1 (Rac1 G12V) were treated with 1 μM GDC-0941 for 72 h. The percentage of cells undergoing apoptosis, as measured by annexin V positivity, is shown relative to untreated cells. The average ± SD is shown (n = 3). (C) MCF7 or MCF7 Rac1 G12V cells were injected into nude mice, and when tumors reached ∼250 mm3 half of the mice were treated with GDC-0941 (100 mg/kg) (n = 5) once daily for 28 d, whereas the other half (n = 5) served as untreated controls. The average tumor sizes are shown. (D) Mice harboring MCF7 or MCF7 Rac1 G12V tumors were administered with 100 mg/kg of GDC-0941 by oral gavage for 3 d, and tumors were harvested 1 h after the final treatment. Lysates were prepared and blotted with the indicated antibodies.
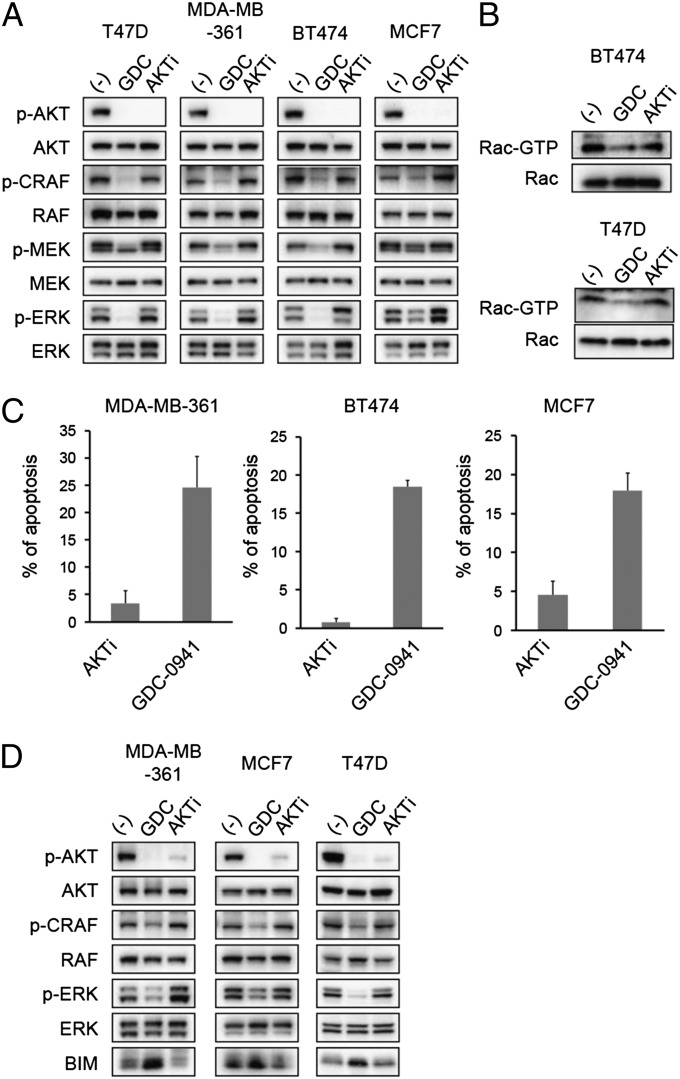
We next aimed to determine whether Rac/ERK signaling was downstream of AKT in these cancers. Therefore, we compared the effect of GDC-0941 to an AKT inhibitor (AKT1/2 inhibitor). Interestingly, AKT inhibition, unlike PI3K inhibition, did not suppress RAF/MEK/ERK signaling in either HER2 amplified or PIK3CA mutant cancer cells (Fig. 4A). Accordingly, AKT inhibition did not suppress Rac1 activity (Fig. 4B). Along this line, the AKT1/2 inhibitor also induced less apoptosis in comparison with GDC-0941 (Fig. 4C). These results were verified with a second AKT inhibitor, MK-2206 (Fig. S3). Consistent with the differential effects on ERK signaling, we observed that PI3K inhibition led to greater up-regulation of the proapoptotic protein BIM (BIM), a critical inducer of apoptosis whose protein expression is normally suppressed by ERK-mediated phosphorylation and degradation (18) (Fig. 4D). Thus, in these cells, AKT inhibitors may be less effective inducers of apoptosis than PI3K inhibitors because AKT, unlike PI3K, does not regulate the Rac/PAK/ERK/BIM pathway.
Fig. 4.
AKT inhibitors fail to down-regulate ERK signaling and induce apoptosis. (A) Cells were treated with 1 μM GDC-0941 (GDC) or 1 μM AKT1/2 kinase inhibitor (AKTi) for 30 min. Lysates were prepared and blotted with the indicated antibodies. Independent experiments were performed at least three times, and a representative result is shown. (B) Cells were serum starved for 16 h, and media containing 10% (vol/vol) FBS with or without indicated drugs was added. After 30 min cells were lysed, and Rac-GTP levels were determined with a PAK1-binding domain pull-down assay. Independent experiments were performed twice for BT474 cells and three times for T47D cells. (C) Cells were treated with 1 μM GDC-0941 (GDC) or 1 μM AKT1/2 kinase inhibitor (AKTi) for 72 h. The percentage of cells undergoing apoptosis, as measured by annexin V positivity, is shown relative to untreated cells. The average ± SD is shown (n = 3). (D) Cells were treated with 1 μM GDC-0941 (GDC) or 1 μM AKT1/2 kinase inhibitor (AKTi) for 24 h. Lysates were prepared and probed with the indicated antibodies. Independent experiments were performed three times, and a representative result is shown.
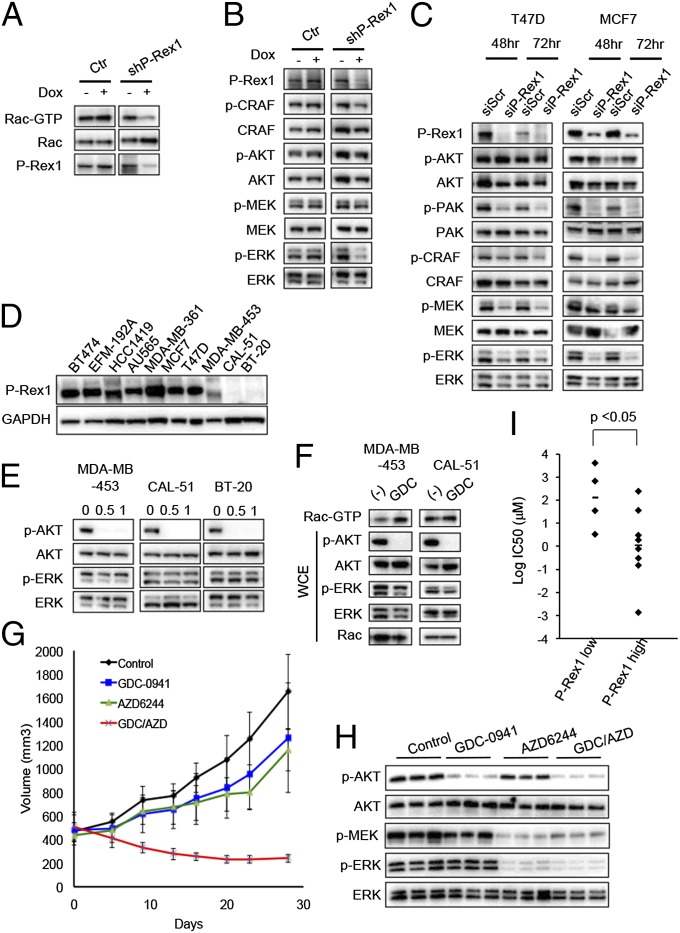
These data suggest that the Rac/PAK/ERK/BIM pathway is activated by PI3K but not AKT in this subset of breast cancers. Thus, we endeavored to identify which other PI3K effector (likely containing a PH domain) regulated Rac activation. Rac activation is directly mediated by Rac-GEFs, a class of molecules that promote the exchange of GDP for GTP (15). A recent study indicated that the PH domain-containing GEF, P-Rex1, is overexpressed in numerous breast cancers, particularly in primary tumors or cell lines of luminal origin, compared with normal mammary cells. Moreover, in these cells P-Rex1 is responsible for PI3K-dependent Rac activation, although its role in regulating ERK had not been evaluated (19). Thus, we hypothesized that P-Rex1–dependent activation of Rac may be dependent on PI(3,4,5)P3 levels on the membrane and thus would be sensitive to PI3K inhibitors (but not AKT inhibitors). To determine the role of P-Rex1 in the regulation of ERK signaling, we used RNA interference to deplete P-Rex1 levels. As expected, tetracycline-induced depletion of P-Rex1 inhibited Rac1 activity (Fig. 5A). Consistent with our results above, depletion of P-Rex1 by either siRNA or shRNA suppressed MAPK signaling, including c-RAF, MEK, and ERK (Fig. 5 B and C). Thus, these data suggest that P-Rex1 links PI3K signaling to ERK in HER2 amplified and/or PIK3CA mutant breast cancer cells.
Fig. 5.
P-Rex1 expression levels correlate with sensitivity to PI3K inhibition. (A) T47D cells expressing doxycycline-inducible control (Ctr) or P-Rex1 (shPREX) vector were treated with or without 10 ng/mL doxycycline (Dox) for 72 h. Then cells were serum starved for 16 h, and media with10% (vol/vol) FBS was added. After 30 min cells were lysed, and Rac-GTP levels were determined with a PAK1-binding domain pull-down assay. Independent experiments were performed twice, and a representative result is shown. (B) T47D cells expressing doxycycline-inducible control (Ctr) or P-Rex1 (shPREX) vector were treated with or without 10 ng/mL doxycycline (Dox) for 72 h. Lysates were prepared and probed with the indicated antibodies. Results were confirmed by three independent experiments, and a representative result is shown. (C) Cells were transfected with control or P-Rex1-targeted siRNA for 48 and 72 h. Lysates were prepared and probed with the indicated antibodies. Independent experiments were performed at least 3 times for T47D and twice for MCF7, and a representative result is shown. (D) Cells were lysed, and lysates were probed with P-Rex1 antibody; GAPDH served as loading control. Results were confirmed by independent lysates, and a representative result is shown. (E) Cells were treated with 1 μM GDC-0941 for the indicated times, and lysates were probed with the indicated antibodies. Independent experiments were performed at least three times, and a representative result is shown. (F) MDA-MB-453 and CAL-51 cells were serum starved for 16 h, and media containing 10% (vol/vol) FBS with or without 1 μM GDC-0941 (GDC) was added. Cells were lysed after 30 min, and Rac-GTP levels were determined with a PAK1-binding domain pull-down assay. (G) CAL-51 were injected into nude mice, and when tumors reached ∼450 mm3 mice were treated with GDC-0941 (100 mg/kg once daily), AZD6244 (25 mg/kg once daily), or the combination (GDC/AZD) once daily for 28 d (n = 5 mice for each group). The average tumor sizes are shown. (H) CAL-51 tumors were harvested 2 h after the last treatment (as indicated), and lysates were prepared and blotted with the indicated antibodies. (I) Correlation between IC50 of GDC-0941 and P-Rex1 RNA expression levels in a set of 12 HER2 amplified and/or PIK3CA mutant breast cancer cell lines.
As previously reported (19), there is a spectrum of expression of levels of P-Rex1 across breast cancer cell lines (Fig. 5D). Interestingly, P-Rex1 was expressed in all of the cell lines in which PI3K inhibition led to suppression of the ERK pathway. Conversely, in three breast cancer cell lines—MDA-MB-453 (HER2 amplified and PIK3CA mutant), BT-20 (PIK3CA mutant), and CAL-51 (PIK3CA mutant)—that had undetectable levels of P-Rex1 (Fig. 5D), PI3K inhibition did not suppress Rac1 activation or ERK phosphorylation (Fig. 5 E and F). Indeed, there was no correlation between Rac-GTP (Fig. S4A) and P-Rex1 levels (Fig. 5D), underscoring the finding that PI3K-dependent activation of Rac, not absolute Rac-GTP levels, correlate with PI3K-dependent regulation of ERK signaling. Unlike the MCF7 xenograft tumors, the growth of CAL-51 tumors was minimally impacted by PI3K inhibition (Fig. 5G), and as expected, the PI3K inhibitor did not impair ERK signaling in these low P-Rex1 tumors in vivo (Fig. 5H). However, the combination of PI3K inhibitor and MEK inhibitor induces down-regulation of both AKT and ERK pathways (Fig. 5H) and tumor regression (Fig. 5G). Notably, examination of P-Rex1 expression levels among a panel of HER2 amplified and PIK3CA mutant breast cancer cells revealed that those cell lines that express low levels of P-Rex1 were less sensitive to the antiproliferative effects of GDC-0941 than high P-Rex1–expressing cells (Fig. 5I). Taken together, these data demonstrate that PI3K-dependent regulation of ERK signaling is mediated through P-Rex1. Consequently, P-Rex1 expression levels may serve as a biomarker to predict which HER2 amplified and PIK3CA mutant breast cancers have ERK signaling under the regulation of PI3K and accordingly may help identify those cancers that will be most susceptible to single-agent PI3K inhibitors in the clinic.
Discussion
In this study we observe that in HER2 amplified and/or PIK3CA mutant breast cancers PI3K inhibition leads to suppression of not only AKT but ERK as well. Interestingly, ERK down-regulation had been observed also in patients treated with XL147, a potent inhibitor of the class I PI3K family members (6), supporting the observation that in some cancers PI3K signaling controls ERK signaling. All together our data support a model in which PI3K regulates P-Rex1–dependent activation of Rac1, which in turn activates the PAK/RAF/ERK/ERK pathway (Fig. S5).
In accordance with these results, previous studies indicate that Rac activates the RAF/MEK/ERK pathway (16, 17) and that Rac/PAK regulation of ERK is essential for HER2-induced transformation of human breast epithelial cancer cells (20). However, the regulation of ERK signaling in a PI3K-dependent manner via P-Rex1 has not been previously described. Although our data suggest that ERK is one key downstream effector of PI3K-dependent Rac1 activity in these tumors, we cannot exclude the possibility that Rac1 may also regulate signaling pathways in addition to ERK that contribute to the survival of these cells. In this study we identified P-Rex1 as the Rac-GEF that regulates PI3K-mediated ERK pathway activation. We think it is unlikely that other Rac-GEFs with a PH domain, such as P-Rex2a, exert a redundant role in activating the ERK pathway in these cells for several reasons: (i) P-Rex1 is the only Rac-GEF controlling Rac activity when overexpressed (19); (ii) P-Rex2a is almost undetectable in cells overexpressing P-Rex1 (19); and (iii) P-Rex2a regulates PI3K pathway through inhibition of PTEN independently of its Rac-GEF activity (21). Our finding that expression levels of P-Rex1 correlate with sensitivity to GDC-0941 suggests that P-Rex1 expression may serve as a clinical biomarker predicting clinical benefit from PI3K inhibitors. Analysis of patient specimens from ongoing clinical trials of PI3K inhibitors will be needed to assess this hypothesis. Although these data demonstrate that ERK activation is controlled by PI3K in breast cancers that express high levels of P-Rex1, we do not have data to determine whether ERK activation is under control of Rac in breast cancers that express low levels of P-Rex1. It is quite possible that low P-Rex1 breast cancers use Rac-independent pathways to control ERK activation.
To our initial surprise, PI3K inhibitor-induced suppression of the ERK pathway seems to be largely independent of RAS. In fact, whereas GDC-0941 treatment results in inhibition of both ERK and AKT activation, RAS activity was modestly increased. Although more studies are needed to elucidate the feedback mechanism leading from PI3K inhibition to RAS activation, it is possible that PI3K/AKT inhibition releases a negative feedback on receptor tyrosine kinases (RTKs) that, in turn, stimulate RAS (11, 14, 22, 23). Additionally, other regulators of RAS activation may be regulated by PI3K, such as a RAS-GAP that contains a PH domain (24).
Our data support the notion that inhibition of PI3K is qualitatively different from AKT inhibition in some cancers. Our finding that AKT inhibitors do not suppress ERK and are inferior to PI3K inhibitors in terms of induction of the proapoptotic molecule BIM and apoptosis suggests that PI3K inhibitors may have superior antitumor activity compared with AKT inhibitors for certain cancers. Previous studies have demonstrated that combined inhibition of both PI3K/AKT and ERK pathways are substantially more effective in promoting durable tumor regression in other cancer models (25–30). However, the functional differences between PI3K and AKT inhibitors may extend beyond the regulation of P-Rex1 and ERK signaling. Previously, others reported that the PI(3,4,5)P3 produced by PI3K can activate AKT-independent signaling pathways that are critical for cancer growth. For example, it has been shown that the PDK1 substrate SGK3 can play a role in promoting PI3K-dependent viability in some breast cancers harboring PIK3CA mutations (31). Additionally, Bruton’s tyrosine kinase (BTK), a PH domain-containing mediator of B-cell receptor signaling implicated in the pathogenesis of B-cell malignancies (32), can be recruited by PI(3,4,5)P3 to the plasma membrane, where it becomes activated (33). In early clinical trials, BTK inhibitors are yielding promising activity in lymphoid malignancies (34, 35). Thus, in several cancers, PI3K seems to control oncogenic pathways other than just AKT.
Interestingly, in contrast to our findings, previous studies revealed activation of ERK signaling after more prolonged treatment with PI3K inhibitors in HER2 amplified breast cancers (9, 11). In the PIK3CA mutant T47D and MCF7 breast cancer cells, both Rac and ERK signaling remained suppressed for up to 24 h (Fig. S4 B and C). In contrast, both Rac and ERK signaling recovered after 24 h of treatment with GDC-0941 in BT474 cells (Fig. S4 B and C). Although these findings do not explain why BT474 cells recover Rac activation, these results continue to support a tight relationship between Rac and ERK activation among all of these breast cancer cell lines. It is also notable that, in HER2 amplified breast cancers, compensatory activation of ERK was stronger with dual PI3K/mTOR inhibitors in comparison with pure PI3K inhibitors and was mediated by activated ErbB receptor signaling (11). Subsequent studies using selective mTORC1/2 inhibitors, designed to inhibit TORC1/2 while sparing PI3K, revealed a similar activation of ErbB receptors (36). Thus, it seems that potent inhibition of mTOR may have a greater capacity than PI3K inhibitors to activate ERK in HER2 amplified breast cancers. However, the clinical significance of this distinction remains to be determined.
Our findings raise the question of why ERK signaling is regulated by a PI3K-dependent mechanism in many breast cancer cell lines, particularly those without RAS mutations. We hypothesize that many of these cancers, such as those with PIK3CA mutations and/or HER2 amplification, may initially develop genetic mutations that strongly activate PI3K but are not potent inducers of ERK signaling. In these cancers, there may be less input into ERK by RTKs, especially because PI3K activation normally suppresses RTK activation. In this scenario, those clones that effectively used PI(3,4,5)P3 to also activate the ERK signaling pathway (i.e., those with high P-Rex1) would grow out via Darwinian selection. This may explain the relatively high expression levels of P-Rex1 observed in many luminal breast cancers (19).
Altogether, our studies reveal a unique mechanism of PI3K-dependent regulation of ERK activation and provide additional insight as why breast cancers harboring PIK3CA mutations and/or HER2 amplification may have enhanced sensitivity to single-agent PI3K inhibitors. Furthermore, these results provide a rationale for assessing P-Rex1 as a biomarker in clinical trials of breast cancers treated with PI3K inhibitors.
Materials and Methods
Immunoblotting.
Lysates were prepared as previously described (37). Antibodies against phospho-AKT (Ser-473), phospho-CRAF (Ser-338), phospho-MEK1/2 (Ser-217/221), MEK1/2, phospho-p42/44 MAP kinase (Thr-202/Tyr-204), p42/44 MAP kinase, phospho-PAK1 (Ser199/204)/PAK2 (Ser192/197), PAK1, PAK2, and BIM were from Cell Signaling Technology. Antibodies against AKT and RAF-1 were purchased from Santa Cruz Biotechnology. Total RAS and Rac were from Millipore. P-Rex1 antibody was obtained from Medical & Biological Laboratories.
RAS and Rac Activity Assay.
RAS and Rac1 activation assays were performed using RAS and Rac Activity Assay kit (Millipore). Briefly, cell lysates were immunoprecipitated with a GST fusion protein corresponding to the RAS-binding domain of Raf-1 bound to glutathione-agarose to identify RAS-GTP or the p21-binding domain of human PAK1 bound to glutathione-agarose to identify Rac1-GTP. GTPγS and GDP protein loading were used for positive and negative controls, respectively.
Xenograft Mouse Studies.
For xenograft experiments, a suspension of 5–10 × 106 cells was inoculated s.c. into the left flank of 6- to 8-wk-old female athymic nude mice (for MCF7 experiment mice were implanted with estrogen pellet). The mice were maintained in laminar airflow units under aseptic conditions, and the care and treatment of experimental animals were in accordance with institutional guidelines. GDC-0941 was dissolved in 0.5% methylcellulose and administered at 100 mg/kg once per day by oral gavage. AZD6244 was dissolved in 0.5% methylcellulose and 0.4% polysorbate and administered at 25 mg/kg once per day by oral gavage.
Database Analyses.
PIK3CA mutation and HER2 amplification status for cell lines was obtained from the Sanger Institute COSMIC database, drug sensitivity data, represented as IC50, were obtained from Supplementary Data 1 of Garnett et al. (38), and transcript levels for P-Rex1 were obtained from the CCLE database (www.broadinstitute.org/ccle/home). A total of 12 HER2 amplified and/or PIK3CA mutant cell lines with corresponding drug sensitivity data to GDC-0941 were identified. Cell lines were classified into two groups—high and low—depending on the levels of P-Rex1 they expressed, and a two-tailed Student t test was performed on the IC50 (Fig. 5I). Differences of P < 0.05 were considered statistically different.
Supplementary Material
Acknowledgments
We thank Matthew Niederst for careful reading of the manuscript, and the Targeting PI3K in Women’s Cancers Stand Up to Cancer Dream Team for supplying GDC-0941. This study is supported by National Institutes of Health Grants R01CA137008 and R01CA140594 (to J.A.E.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314124110/-/DCSupplemental.
References
- 1.Engelman JA. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 2.Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16(Suppl 1):12–19. doi: 10.1634/theoncologist.2011-S1-12. [DOI] [PubMed] [Google Scholar]
- 3.Juric D, Baselga J. Tumor genetic testing for patient selection in phase I clinical trials: The case of PI3K inhibitors. J Clin Oncol. 2012;30(8):765–766. doi: 10.1200/JCO.2011.39.6390. [DOI] [PubMed] [Google Scholar]
- 4.Juric D, et al. BYL719, a next generation PI3K alpha specific inhibitor: Preliminary safety, PK, and efficacy results from the first-in-human study. Cancer Res. 2012;72(8 Suppl 1):CT-01. [Google Scholar]
- 5.Von Hoff DD, et al. A phase I dose-escalation study to evaluate GDC-0941, a pan-PI3K inhibitor, administered QD or BID in patients with advanced or metastatic solid tumors. J Clin Oncol. 2011;29(15 Suppl):3052. [Google Scholar]
- 6.Edelman G, et al. A phase I dose-escalation study of XL147 (SAR245408), a PI3K inhibitor administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2010;28(15 Suppl):3004. [Google Scholar]
- 7.Markman B, Atzori F, Pérez-García J, Tabernero J, Baselga J. Status of PI3K inhibition and biomarker development in cancer therapeutics. Ann Oncol. 2010;21(4):683–691. doi: 10.1093/annonc/mdp347. [DOI] [PubMed] [Google Scholar]
- 8.Slamon DJ, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 9.Faber AC, et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci USA. 2009;106(46):19503–19508. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiser LM, et al. Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc Natl Acad Sci USA. 2012;109(8):2724–2729. doi: 10.1073/pnas.1018854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serra V, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30(22):2547–2557. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17(8):4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carracedo A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118(9):3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandarlapaty S, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19(1):58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wertheimer E, et al. Rac signaling in breast cancer: A tale of GEFs and GAPs. Cell Signal. 2012;24(2):353–362. doi: 10.1016/j.cellsig.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang K, et al. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol. 2000;1(5):419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- 17.Arai A, et al. SDF-1 synergistically enhances IL-3-induced activation of the Raf-1/MEK/Erk signaling pathway through activation of Rac and its effector Pak kinases to promote hematopoiesis and chemotaxis. Cell Signal. 2005;17(4):497–506. doi: 10.1016/j.cellsig.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Faber AC, Ebi H, Costa C, Engelman JA. Apoptosis in targeted therapy responses: The role of BIM. Adv Pharmacol. 2012;65:519–542. doi: 10.1016/B978-0-12-397927-8.00016-6. [DOI] [PubMed] [Google Scholar]
- 19.Sosa MS, et al. Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol Cell. 2010;40(6):877–892. doi: 10.1016/j.molcel.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arias-Romero LE, et al. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene. 2010;29(43):5839–5849. doi: 10.1038/onc.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine B, et al. Activation of the PI3K pathway in cancer through inhibition of PTEN by exchange factor P-REX2a. Science. 2009;325(5945):1261–1265. doi: 10.1126/science.1173569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakrabarty A, Sánchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci USA. 2012;109(8):2718–2723. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muranen T, et al. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell. 2012;21(2):227–239. doi: 10.1016/j.ccr.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grewal T, Koese M, Tebar F, Enrich C. Differential regulation of RasGAPs in cancer. Genes Cancer. 2011;2(3):288–297. doi: 10.1177/1947601911407330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelman JA, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14(12):1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sos ML, et al. Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc Natl Acad Sci USA. 2009;106(43):18351–18356. doi: 10.1073/pnas.0907325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoeflich KP, et al. Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res. 2012;72(1):210–219. doi: 10.1158/0008-5472.CAN-11-1515. [DOI] [PubMed] [Google Scholar]
- 28.Posch C, et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proc Natl Acad Sci USA. 2013;110(10):4015–4020. doi: 10.1073/pnas.1216013110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinross KM, et al. In vivo activity of combined PI3K/mTOR and MEK inhibition in a Kras(G12D);Pten deletion mouse model of ovarian cancer. Mol Cancer Ther. 2011;10(8):1440–1449. doi: 10.1158/1535-7163.MCT-11-0240. [DOI] [PubMed] [Google Scholar]
- 30.Steinbrunn T, et al. Combined targeting of MEK/MAPK and PI3K/Akt signalling in multiple myeloma. Br J Haematol. 2012;159(4):430–440. doi: 10.1111/bjh.12039. [DOI] [PubMed] [Google Scholar]
- 31.Vasudevan KM, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16(1):21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol. 2012;31(2):119–132. doi: 10.3109/08830185.2012.664797. [DOI] [PubMed] [Google Scholar]
- 33.Miller AT, Berg LJ. New insights into the regulation and functions of Tec family tyrosine kinases in the immune system. Curr Opin Immunol. 2002;14(3):331–340. doi: 10.1016/s0952-7915(02)00345-x. [DOI] [PubMed] [Google Scholar]
- 34.Byrd JC, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Advani RH, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31(1):88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrik-Outmezguine VS, et al. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011;1(3):248–259. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebi H, et al. Receptor tyrosine kinases exert dominant control over PI3K signaling in human KRAS mutant colorectal cancers. J Clin Invest. 2011;121(11):4311–4321. doi: 10.1172/JCI57909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garnett MJ, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483(7391):570–575. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gymnopoulos M, Elsliger MA, Vogt PK. Rare cancer-specific mutations in PIK3CA show gain of function. Proc Natl Acad Sci USA. 2007;104(13):5569–5574. doi: 10.1073/pnas.0701005104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.