Significance
Promoter recognition and specific transcription initiation depend on the binding of a sigma factor to the core RNA polymerase in bacteria. The activity of sigma factors is often regulated through sequestration by cognate anti-sigma factors. Although Rsd is known as the anti-sigma factor for the housekeeping sigma factor σ70, which is responsible for most of the gene expression in exponentially growing Escherichia coli cells, the mechanism for the sequestration of Rsd from σ70 during the exponential phase of growth has not been investigated previously. In this study, we demonstrate that regulation of Rsd activity is achieved by the binding partner switch from σ70 to the dephosphorylated histidine-containing phosphocarrier protein HPr of the bacterial phosphoenolpyruvate:sugar phosphotransferase system in Escherichia coli.
Keywords: glucose signaling, sigma factor competition, transcriptional regulation
Abstract
The bacterial phosphoenolpyruvate:sugar phosphotransferase system (PTS) is a multicomponent system that participates in a variety of physiological processes in addition to the phosphorylation-coupled transport of numerous sugars. In Escherichia coli and other enteric bacteria, enzyme IIAGlc (EIIAGlc) is known as the central processing unit of carbon metabolism and plays multiple roles, including regulation of adenylyl cyclase, the fermentation/respiration switch protein FrsA, glycerol kinase, and several non-PTS transporters, whereas the only known regulatory role of the E. coli histidine-containing phosphocarrier protein HPr is in the activation of glycogen phosphorylase. Because HPr is known to be more abundant than EIIAGlc in enteric bacteria, we assumed that there might be more regulatory mechanisms connected with HPr. The ligand fishing experiment in this study identified Rsd, an anti-sigma factor known to complex with σ70 in stationary-phase cells, as an HPr-binding protein in E. coli. Only the dephosphorylated form of HPr formed a tight complex with Rsd and thereby inhibited complex formation between Rsd and σ70. Dephosphorylated HPr, but not phosphorylated HPr, antagonized the inhibitory effect of Rsd on σ70-dependent transcriptions both in vivo and in vitro, and also influenced the competition between σ70 and σS for core RNA polymerase in the presence of Rsd. Based on these data, we propose that the anti-σ70 activity of Rsd is regulated by the phosphorylation state-dependent interaction of HPr with Rsd.
By monitoring their environment, bacteria ensure the most appropriate response for each environment. One of the sensory systems for monitoring changes in nutrient availability is the phosphoenolpyruvate (PEP):sugar phosphotransferase system (PTS). The PTS is a multicomponent system that catalyzes the concomitant phosphorylation and translocation of numerous sugar substrates across the cytoplasmic membrane. This system consists of two general components, enzyme I (EI) and the histidine-containing phosphocarrier protein HPr, which are common to all PTS sugars, along with many sugar-specific components collectively known as enzyme IIs (EIIs) (1, 2).
Each EII complex generally consists of three domains: one integral membrane domain forming the sugar translocation channel (EIIC) and two cytosolic domains (EIIA and EIIB). EI transfers a phosphoryl group from PEP to HPr, and HPr then transfers the phosphoryl group to the different EIIs. Each EII complex forms a cascade of phosphorylated intermediates, and in the presence of a PTS sugar, the EIIA and EIIB domains sequentially transfer the phosphate group from HPr to the incoming sugar. Thus, the phosphorylation states of the PTS components change depending on the availability of a PTS sugar substrate (3, 4).
In addition to sugar uptake, the PTS plays an important role as a sensory transduction system to monitor nutritional changes, and its components are involved in the regulation of various metabolic processes through protein–protein interactions in numerous bacteria. In Escherichia coli, EI regulates chemotaxis (5), and the membrane-bound glucose transporter EIICBGlc sequesters the global transcription regulator Mlc from its target promoters to induce the expression of several PTS proteins in the presence of glucose (6–8). In E. coli and other γ-proteobacteria, crr encoding EIIAGlc resides in the ptsHIcrr operon together with genes for the two general components. EIIAGlc, the central processing unit of carbon metabolism, plays multiple roles, including regulation of adenylyl cyclase, the fermentation/respiration switch protein FrsA, glycerol kinase, and non-PTS transporters for lactose, maltose, and melibiose (2, 9, 10). These regulatory functions of the PTS proteins are dependent on the phosphorylation state of the involved component. Thus, the ratio of phosphorylated to dephosphorylated PTS proteins serves as a signal input for the control of these physiological processes.
Whereas the intracellular concentration of HPr is known to be higher than that of EIIAGlc in E. coli (11), glycogen phosphorylase in E. coli is the only binding partner of HPr identified to date (12). Furthermore, in Gram-positive bacteria, HPr is known as the key regulator of carbon metabolism and performs diverse regulatory functions in response to changes in its phosphorylation state (13). Thus, we assumed that more targets might be regulated by HPr.
Here we report that the dephosphorylated form of HPr interacts with Rsd, a negative regulator of σ70, and antagonizes its function to confer a competitive advantage on promoter selection by the σ70-RNA polymerase holoenzyme in E. coli. We propose a unique role for HPr as an anti–anti-sigma factor for σ70.
Results
Phosphorylation State-Dependent Interaction of HPr with Rsd.
To search for a new binding partner of HPr, we used an N-terminally hexahistidine-tagged version of HPr (His-HPr) as bait for the ligand fishing experiment as described previously (14–16). Crude extracts prepared from E. coli MG1655 cells grown to stationary phase were mixed with TALON metal affinity resin in the presence or absence of His-HPr and then subjected to pull-down assays. After brief washes, proteins bound to the resin were eluted with 150 mM imidazole. Analysis of the eluted proteins by SDS/PAGE followed by staining with Coomassie brilliant blue revealed dramatic enrichment of two protein bands migrating at ∼97 and 20 kDa in the fraction containing His-HPr (Fig. 1A). In-gel tryptic digestion, MALDI-TOF MS, and peptide mass fingerprinting revealed that the protein band migrating at ∼97 kDa corresponded to glycogen phosphorylase (GlgP), which is known to interact with HPr (12). Intriguingly, the protein band at ∼20 kDa was identified as Rsd, a regulator of σ70 (RpoD) (17). Rsd is known to bind to σ70 with a stoichiometry of 1:1 and to function as an anti-sigma factor (18–20). The crystal structure of Rsd bound to region 4 of σ70 indicates that Rsd binding directly interferes with both the core RNA polymerase-binding and promoter-binding functions of region 4 (21).
Fig. 1.
Specific interaction of Rsd with HPr. (A) Ligand fishing experiment to search for protein(s) interacting with His-HPr. Crude extract prepared from MG1655 grown in 400 mL of LB was mixed with buffer A (20 mM Hepes-NaOH, pH 7.6, containing 100 mM NaCl) (lane 1) or 500 μg of purified His-HPr (lane 2). Each mixture was incubated with 500 μL of TALON resin for metal affinity chromatography. (B) Ligand fishing experiment using His-Rsd as bait. Crude extract from MG1655 (lanes 1–3) or MG1655ΔptsH (lanes 4 and 5) grown in 200 mL of LB was mixed with 150 μg of His-Rsd (lanes 2, 3, and 5) or buffer A (lanes 1 and 4). Glucose (lane 2) and PEP (lane 3) were added to 2 mM to dephosphorylate and phosphorylate the PTS proteins, respectively, and these mixtures were incubated with 100 μL of TALON resin for metal affinity chromatography. The proteins bound to each column were processed as described in Materials and Methods. EzWayTM Protein Blue MW Marker (KOMABIOTECH) was used as the molecular mass markers (lane M). Protein bands bound specifically to His-tagged baits are indicated by arrows.
Interactions with target proteins and the regulatory functions of the PTS proteins typically depend on the phosphorylation state of the involved PTS component (3, 13). To confirm the interaction between Rsd and HPr, and to test whether this interaction is dependent on the phosphorylation state of HPr, we constructed Rsd with an N-terminal hexahistidine tag (His-Rsd). Pull-down assays were performed using TALON metal affinity resin charged with His-Rsd to identify Rsd-binding proteins from crude extracts of WT MG1655 and its otherwise isogenic ptsH mutant cells. These cell extracts were incubated either with glucose to dephosphorylate or with phosphoenolpyruvate (PEP) to phosphorylate the PTS components in the extracts. After a brief wash, proteins bound to the resin were eluted with 150 mM imidazole and analyzed by SDS/PAGE. As shown in Fig. 1B, a protein band migrating at ∼9 kDa was significantly enriched in the eluate from the column loaded with a mixture of WT cell extract, glucose, and His-Rsd compared with the control column without His-Rsd (compare lanes 1 and 2). Peptide mass fingerprinting after in-gel tryptic digestion identified this protein as HPr.
Interestingly, however, the HPr band was barely detectable in the eluate from the His-Rsd affinity column incubated with the WT extract and PEP (compare lanes 2 and 3), indicating that only the dephosphorylated form of HPr can interact with Rsd. As expected, the HPr band was not detectable in the eluates from the columns loaded with the ptsH mutant cell extract regardless of the presence of His-Rsd (compare lanes 4 and 5). The phosphorylation state-dependent interaction of HPr with Rsd was further confirmed by gel shift experiments using nondenaturing PAGE. Of note, the electrophoretic mobility of Rsd was increased in the presence of dephosphorylated HPr; however, phosphorylated HPr did not affect the mobility of Rsd (Fig. S1).
To determine the stoichiometry of the HPr–Rsd interaction, we compared the elution profile from a gel filtration column (Superose 12 10/300 GL; GE Healthcare) of the complex with the profiles of individual proteins (Fig. S2A). Rsd alone was eluted as a symmetrical peak at ∼13.5 mL, corresponding to the monomeric form (∼18.2 kDa), whereas HPr (∼9.1 kDa) was eluted at ∼15 mL. When the mixture of Rsd and HPr (120 and 180 μg, respectively) was subjected to gel filtration chromatography, the HPr peak was decreased, as expected, with a concomitant increase in the peak at ∼13.5 mL When the gel filtration fractions were analyzed by SDS/PAGE, those eluting at ∼13.5 mL were resolved into two bands migrating at the positions expected for HPr and Rsd (Fig. S2B).
Based on the band intensities of the two proteins in these fractions, it appears that HPr interacts with Rsd in 1:1 ratio to form a heterodimer. The biomolecular interaction studies performed using the Biacore system indicated a high-affinity interaction of HPr and Rsd with a dissociation constant (Kd) of ∼8.87 nM (Fig. S3). This low Kd value explains how the complex could survive during nondenaturing PAGE and gel filtration chromatography (Figs. S1 and S2).
Competition Between HPr and σ70 for Binding to Rsd.
The apparent Kd for the binding of Rsd to σ70 was previously measured at 32 nM using Biacore (22), representing a slightly lower affinity than that for the interaction between HPr and Rsd measured in this experiment (Fig. S3). Rsd levels reportedly increase by approximately twofold during stationary phase, with ∼6,200 molecules in an E. coli cell, compared with the levels in exponentially growing cells (∼3,300 molecules per cell), whereas the cellular level of σ70 remains relatively constant at ∼7,200 molecules per cell (18). The intracellular concentration of HPr was previously calculated as 20–100 μM (11), which corresponds to 12,000–60,000 molecules per cell when calculated assuming an E. coli cell volume of 1 × 10−15 L (18). Because the intracellular level of HPr can be 2–20 times higher than that of Rsd, it is reasonable to assume that HPr could almost completely sequester Rsd from σ70 under conditions in which HPr predominantly exists in a dephosphorylated state, such as in cells growing exponentially in the presence of glucose.
To verify this assumption, we examined the effect of HPr on the formation of the Rsd-σ70 complex by electrophoretic mobility shift assays under various conditions (Fig. 2). Each reaction mixture was divided into two aliquots. One aliquot was analyzed by native PAGE (Fig. 2B), and the other was mixed with SDS sample buffer for analysis by SDS/PAGE (Fig. 2A). As expected based on the dissociation constants, both the HPr-Rsd and Rsd-σ70 complexes could survive during PAGE under nondenaturing conditions (Fig. 2B, lanes 4 and 5). Interestingly, when HPr was added to the reaction in the absence of EI (and thus HPr was not phosphorylated), the band corresponding to the Rsd–σ70 complex was decreased, whereas the Rsd–HPr complex band increased (Fig. 2B, lanes 6 and 7). The phosphorylated form of HPr could not sequester Rsd from σ70, however (Fig. 2B, lane 9). These data indicate that only the dephosphorylated form of HPr can interact with and sequester Rsd from σ70.
Fig. 2.
Sequestration of Rsd from binding to σ70 by dephosphorylated HPr. To test the effect of HPr on the formation of the Rsd-σ70 complex, Rsd, HPr, and σ70 were incubated in different combinations in 20 mM Tris⋅HCl (pH 8.0) containing 2 mM MgCl2 and 2 mM PEP. Lane 1, 2 μg HPr; lane 2, 2 μg Rsd; lane 3, 1 μg His-σ70; lane 4, 1 μg HPr and 2 μg Rsd; lane 5, 2 μg Rsd and 1 μg His-σ70; lane 6, 1 μg HPr, 2 μg Rsd, and 1 μg His-σ70; lane 7, 2 μg HPr, 2 μg Rsd, and 1 μg His-σ70; lane 8, 1 μg EI and 1 μg HPr; lane 9, 1 μg EI, 1 μg HPr, 2 μg Rsd, and 1 μg His-σ70. Each reaction mixture was incubated at 37 °C for 10 min and then divided into two aliquots. One aliquot was analyzed by SDS/PAGE (A) to ensure the purity and the amount of each protein used in each reaction, and the other was analyzed by native PAGE (B) to measure the electrophoretic mobility shifts of Rsd complexed with HPr or σ70.
Dephospho-HPr Antagonizes the Inhibition of Eσ70-Driven Transcription by Rsd in Vitro.
It was previously reported that Rsd negatively regulates the transcription of σ70-dependent genes in vitro, and thus is proposed to function as an anti-σ factor for σ70 (17–20). Because dephosphorylated HPr interacts with Rsd to prevent formation of the Rsd–σ70 complex, we tested whether HPr can antagonize the inhibitory effect of Rsd on σ70-dependent transcription in vitro, using a supercoiled plasmid containing the σ70-dependent alaS promoter as template (Fig. 3A). As reported previously (17), transcription from the alaS promoter was almost completely inhibited in the presence of Rsd (Fig. 3A, lanes 2 and 3). HPr alone did not show any effect on alaS transcription (lane 4).
Fig. 3.
Effects of the HPr–Rsd interaction on the transcription from σ70- and σS-dependent promoters in vitro. (A) pIVT-alaS was used as a template for σ70-dependent alaS transcription. Transcription reactions were performed as described in Materials and Methods. Each reaction contained 37 nM (0.4 U) of E. coli core RNAP (Epicentre) and 500 nM σ70. The amounts of other proteins added to the reactions are shown below each lane except for lane 8, where 1 μM EI and 1 mM PEP were added together with the indicated amounts of Rsd and HPr to phosphorylate HPr. (B) A linear template containing the σS-dependent promoter gadA P was used. Each reaction contained 0.4 U of core RNAP and σ70 and σS were added to 200 nM, where indicated. Amounts of other proteins added to the reactions are shown below each lane. The synthesized transcripts were analyzed by electrophoresis on a 5% polyacrylamide gel containing 8 M urea, and transcript levels were measured by a phosphoimager and are presented as values relative to lane 1 at the bottom of each lane.
Intriguingly, when Rsd was added to the reaction in the presence of HPr, the inhibitory effect of Rsd on alaS transcription was decreased, and this Rsd-antagonizing effect was dependent on the amount of added HPr (Fig. 3A, lanes 5–7). However, when HPr became phosphorylated by incubation with PEP and EI, it did not affect the function of Rsd as an anti-σ70 factor (Fig. 3A, lane 8). The anti-Rsd activity of dephospho-HPr also could be observed in in vitro transcription assays using a supercoiled plasmid containing the icdA P1 and P2 promoters (Fig. S4). Rsd inhibited the σ70-dependent transcription from both promoters, and only the dephosphorylated form of HPr decreased the inhibitory effect of Rsd on the icdA P1 and P2 transcripts.
In bacterial cells, various sigma factors compete for a limited amount of core RNA polymerase (RNAP). It was previously demonstrated that the level of one σ factor can affect the expression of genes dependent on other σ factors owing to the competition between σ factors for a common RNAP (23). Furthermore, the RNAP holoenzymes Eσ70 and EσS recognize the same consensus hexamer sequences, and many stationary phase-specific genes are transcribed in vitro by both holoenzymes (24). In the presence of both Eσ70 and EσS, Rsd has been shown to increase the transcription from a σS-dependent promoter (20).
To test the anti-Rsd effect of HPr on σS-dependent transcription under conditions in which σ70 and σS are competing for RNAP, we performed in vitro transcription experiments in the presence of both holoenzymes and a linear gadA promoter template. A gadA transcript was produced in the reaction containing Eσ70 alone, consistent with a previous finding that gadA can be transcribed in an rpoS mutant (25). The gadA transcript was significantly increased in the reaction containing EσS alone compared with when the same amount of Eσ70 was used (Fig. 3B, lanes 1 and 2). As expected from the much higher affinity of σ70 for core RNA polymerase compared with σS (26), the transcript level was remarkably decreased in the reaction containing both σ70 and σS compared with that generated in the presence of EσS alone, but was still slightly higher than that produced in the presence of Eσ70 alone (Fig. 3B, lane 3); however, the addition of Rsd to the reaction mixture containing both σ70 and σS increased gadA transcription in a concentration-dependent manner, suggesting that the inhibition of EσS-driven transcription by σ70 was neutralized by Rsd (Fig. 3B, lanes 4–6).
This result is in agreement with earlier studies demonstrating that Rsd facilitates switching of the σ subunit on RNA polymerase from σ70 to σS (20, 27, 28). Intriguingly, dephosphorylated HPr antagonized the Rsd effect and decreased the transcript level in a concentration-dependent manner (Fig. 3B, lanes 7–9). Thus, these results support the idea that HPr functions as an anti-Rsd factor and confers a competitive advantage for promoter selection by Eσ70 holoenzyme when a favorable carbon source, such as glucose, is available.
In Vivo Effect of the HPr–Rsd Interaction on Gene Expression from Different Promoters.
Despite the fact that Rsd is an anti-σ70 factor, to date no specific phenotype for rsd mutant strains has been reported (17, 18, 27). A recent transcriptome analysis demonstrated that Rsd overexpression increased the expression of some σS-dependent promoters, including hdeA and gadA (28), whereas rsd deletion mutants did not display measurable differences in gene expression compared with WT cells (27, 28). For this reason, it has been postulated that the function of Rsd is to sequester σ70 and to displace RNAP core enzyme, which in turn becomes accessible to σS and possibly other σ factors during stationary phase.
To confirm the effect of HPr on Rsd activity in vivo, we determined the expression levels of these genes by measuring the β-galactosidase activities of promoter-lacZ fusion constructs introduced into rsd deletion mutants carrying pACYC184 or pACYC-Rsd. When the cells were grown to stationary phase, Rsd increased the expression levels of hdeA and gadA (Fig. 4 A and B). To test whether dephospho-HPr affects gene expression through an interaction with Rsd, we constructed pACYC-Rsd-H15A, a vector coexpressing Rsd and an unphosphorylatable form of HPr (H15A). As shown in Fig. 4 A and B, the stimulatory effect of Rsd on hdeA and gadA expression was completely antagonized when Rsd was coexpressed with HPr(H15A). This same tendency was also observed for other stress-responsive genes, such as gadB, katE, and ybaS (Fig. S5).
Fig. 4.
Effects of Rsd and dephosphorylated HPr on transcription from σ70- and σS-dependent promoters in vivo. Expression levels of hdeA (A), gadA (B), and ilvB (C) were determined by measuring the β-galactosidase activities of promoter-lacZ fusion constructs introduced into rsd deletion mutants harboring the indicated plasmids. The hdeA-lacZ and gadA-lacZ fusion strains were grown in LB medium, whereas the ilvB-lacZ strain was grown in M9 minimal medium containing 0.1% casamino acids and 0.5% glycerol. β-Galactosidase activity was measured using overnight-cultured cells. Data are mean ± SD (n = 3).
A previous study demonstrated that expression of the ilvBN operon is dependent on Eσ70 (29). To investigate the effect of the HPr–Rsd interaction on ilvB expression in vivo, we compared the β-galactosidase activity of the ilvB–lacZ fusion in rsd-deficient cells carrying pACYC184, pACYC-Rsd, and pACYC-Rsd-H15A. As expected, the expression level of ilvB was decreased in the presence of Rsd, and this inhibitory effect was completely neutralized when Rsd was coexpressed with HPr(H15A) (Fig. 4C). All of the foregoing results are consistent with the prediction that the dephosphorylated form of HPr forms a complex with Rsd and interferes with the interaction between Rsd and σ70, thereby increasing the concentration of Eσ70 and leading to an increase in σ70-dependent transcription and a decrease in σS-dependent transcription in vivo.
In Vivo Phosphorylation State of HPr and Its Effect on Rsd Activity Are Dependent on the Presence of Glucose.
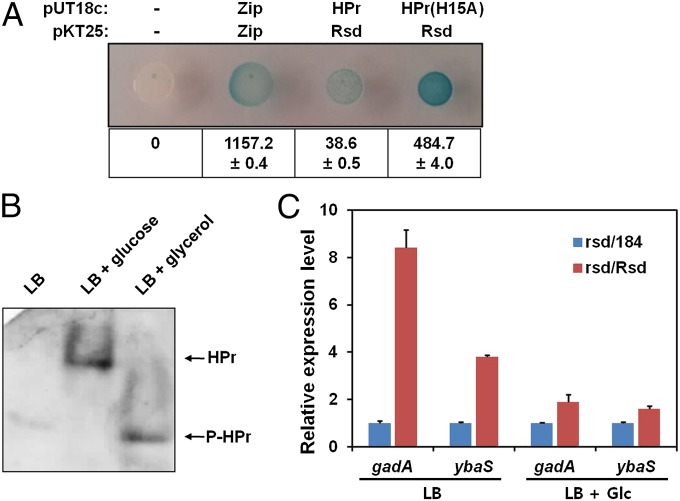
To confirm that the tight interaction between HPr and Rsd occurs in vivo, we used bacterial two-hybrid (BACTH) assays, which rely on the reconstitution of the activity of split Bordetella pertussis adenylyl cyclase CyaA in cya mutant E. coli (30). When HPr and Rsd were fused to the C-termini of the T18 and T25 fragments of CyaA, respectively, and coexpressed, the cells developed a blue color; however, the interaction between the T25 and T18 fragments themselves (negative control) was not detected by the BACTH system (Fig. 5A).
Fig. 5.
Dependence of the phosphorylation state of HPr and its interaction with Rsd on the presence of glucose in vivo. (A) BACTH assays to measure the phosphorylation state-dependent interaction of HPr with Rsd in vivo. Zip, the leucine zipper of Saccharomyces cerevisiae GCN4, served as a positive control. β-Galactosidase activities were monitored by both color development on an X-gal plate and by direct enzyme assay (Lower, numbers in Miller unit). (B) The phosphorylation states of HPr were determined from late-exponentially growing cultures of the MG1655/pACYC-HPr in LB and LB containing the indicated sugars by native PAGE, as described in Materials and Methods. (C) Total RNA was isolated from the rsd deletion mutant harboring the control vector pACYC184 (blue bars) or the Rsd expression vector pACYC-Rsd (red bars) grown to late exponential phase in LB or LB containing 0.2% glucose, and gadA and ybaS mRNA levels were then measured by qRT-PCR. Representative data (mean ± SD) from two independent experiments (n = 3 each) are shown.
Interestingly, much higher β-galactosidase activity was observed with coexpression of T25-Rsd and T18-HPr(H15A) compared with coexpression of T25-Rsd and T18-HPr, suggesting that T18-fused HPr may exist predominantly in the phosphorylated state in the medium tested in this study. Thus, we measured the phosphorylation level of HPr in cells grown in LB medium supplemented with either glycerol or glucose. As shown in Fig. 5B, HPr was almost completely dephosphorylated in the LB medium supplemented with glucose, whereas it was predominantly in the phosphorylated state in the LB medium and LB medium supplemented with glycerol.
Based on these data, it can be assumed that Rsd regulates σ70 activity in response to the availability of a preferred sugar, which determines the phosphorylation state of HPr. Thus, we measured the expression level of two rsd-dependent genes in the presence or absence of glucose by quantitative RT-PCR (qRT-PCR) (Fig. 5C). The relative expression levels of gadA and ybaS increased by 8.4- and 3.9-fold, respectively, in the Rsd-overproducing cells in the absence of glucose, consistent with a previous report (28). However, the increases in expression levels of both genes were significantly diminished in the presence of glucose (by 1.9- and 1.6-fold, respectively). These data imply that the Rsd activity is dependent on the phosphorylation state of HPr, which is determined by the availability of preferred PTS sugars, such as glucose.
Discussion
In this study, we have identified Rsd as an interaction partner of HPr and have demonstrated that only the dephosphorylated form of HPr can interact with Rsd, thereby antagonizing its function both in vitro and in vivo. Rsd was first identified as a regulator of σ70 (17) and later shown to function as an anti-sigma factor for the housekeeping σ factor σ70 (18, 19). Several lines of evidence led us to believe that the interaction between Rsd and σ70 must somehow be regulated in response to physiological needs, and that there might be as-yet unknown effectors that can bind to and regulate the activity of Rsd. It was first shown that Rsd levels are only twofold lower in exponentially growing cells, with ∼3,300 molecules per cell, compared with levels in stationary phase cells (18).
Considering the binding affinity of Rsd to σ70 (22), the concentration of Rsd should be sufficiently high in exponentially growing cells to allow binding to σ70; however, the complex between Rsd and σ70 was detected predominantly in stationary phase extracts (18). Furthermore, based on the structure of Rsd bound to domain 4 of σ70, functional coupling was suggested between the σ70 binding surface and other binding surfaces of Rsd, either for other proteins or for as-yet unknown small molecule effectors. The present study provides direct evidence that HPr is such a regulator, and that it can sequester Rsd from σ70 depending on its phosphorylation state.
It has been proposed that Rsd plays a role in alternative σ factor-dependent transcription by biasing the competition between σ70 and σS and/or possibly other σ factors for the available core RNAP during stationary phase (17, 28). Consistent with this assumption, it was previously demonstrated that Rsd increased the expression from σS-dependent promoters and decreased the expression from some σ70-dependent promoters (27, 28). Our present findings demonstrate that the dephosphorylated form, but not the phosphorylated form, of HPr antagonizes Rsd activity, so that HPr stimulates transcription from σ70-dependent promoters and reduces transcription from σS-dependent promoters in the presence of Rsd (Figs. 3, 4, and 5).
In the presence of glucose, HPr expression is maximized (31), and the intracellular [HPr]/[σ70] ratio will exceed 8 (see above). However, the expression of Rsd is minimized (18, 27), and the [HPr]/[Rsd] ratio will reach ∼20. Therefore, although HPr has only a slightly higher binding affinity for Rsd compared with σ70 (Fig. S3) (22), HPr would completely sequester Rsd, and most σ70 would be accessible for core RNAP to express housekeeping genes in the presence of a preferred sugar such as glucose, as exemplified in Fig. 2.
In exponentially growing cells, a residual carbon source can dephosphorylate the PTS components, including HPr, although the extent of the phosphorylation of the PTS components is determined by the carbon source remaining in the medium (32). PTS sugars, such as glucose, usually lead to a greater dephosphorylation compared with non-PTS carbon sources. Dephosphorylated HPr can then sequester Rsd, and σ70 can bind to core RNAP and express housekeeping genes. During stationary phase, however, essential nutrients, such as carbon sources, are depleted, and organic acids are accumulated. Under these conditions, the phosphorylation of PTS components increases. Because the phosphorylated form of HPr cannot function as an anti-Rsd factor, Rsd could form a complex with σ70. Thus, σS would outcompete σ70 for binding to core RNAP and express stationary phase genes (Fig. S6).
Materials and Methods
Bacterial Strains, Plasmids, and Culture Conditions.
The bacterial strains and plasmids used in this study are listed in Table S1. The rsd mutant was constructed using E. coli DY330 as described previously (33). The rsd gene was replaced with a tetracycline-resistant cassette. Bacterial cells were grown as described previously (34).
Purification of Overexpressed Proteins.
Purification of EI and HPr was accomplished as described previously (9, 12), and Rsd and σ70 were purified in a similar manner. His-tagged proteins were purified using BD TALON metal affinity resin (BD Biosciences) according to the manufacturer’s instructions, and His-tagged proteins were eluted with binding buffer containing 150 mM imidazole. The fractions containing His-HPr or His-Rsd were concentrated using Amicon Ultra Ultracel-3K centrifugal filters (Millipore Ireland). To obtain homogeneous His-tagged proteins (>98% pure) and to remove imidazole, the concentrated pool was chromatographed on a HiLoad 16/60 Superdex 75 prepgrade column (GE Healthcare Life Sciences) equilibrated with buffer A (20 mM Hepes-NaOH, pH 7.6, containing 100 mM NaCl). For purification of sigma factors, 10% glycerol was added to buffer A.
Ligand Fishing Experiments Using Metal Affinity Chromatography.
E. coli MG1655 or its isogenic ptsH mutant cells grown overnight in LB medium were harvested and then washed with and resuspended in buffer A. Cells were disrupted by two passages through a French pressure cell at 10,000 psi. After centrifugation at 100,000 × g for 60 min at 4 °C, the supernatant was divided into aliquots and mixed either with a His-tagged protein as bait or with buffer A as a control. Each mixture was then incubated with BD TALON metal affinity resin in a Poly-Prep chromatography column (8 × 40 mm) for 30 min. After two brief washes with buffer A containing 10 mM imidazole, the proteins bound to the column were eluted with buffer A containing 150 mM imidazole. Aliquots of the eluted protein sample (10 μL each) were analyzed by SDS/PAGE, followed by staining with Coomassie brilliant blue R. Protein bands specifically bound to the His-tagged bait protein were excised from the gel, and in-gel digestion and peptide mapping of the tryptic digests were performed as described previously (16).
Native Polyacrylamide Gel Electrophoresis.
Mobility shifts of Rsd owing to its interaction with HPr or σ70 were demonstrated in a nondenaturing 4–20% gradient gel (KOMABIOTECH). Tris-glycine (pH 8.0) was used as the running buffer. A binding mixture (20 μL) of 20 mM Tris⋅HCl (pH 8.0), 2 mM MgCl2, 2 mM PEP, 2 μg Rsd, 1 μg His-σ70, and 1 or 2 μg HPr was incubated for 10 min at 37 °C. To phosphorylate HPr, 1 μg of EI was added to the indicated reactions. Each reaction mixture was combined with 5 μL of 6× loading buffer [0.01% bromophenol blue, 0.5 M Tris⋅HCl (pH 6.8), 50% glycerol] and electrophoresed in the gel. After electrophoresis, the gel was stained with Coomassie brilliant blue R.
In Vitro Transcription Assay.
Supercoiled plasmids or linear DNA incorporated with promoters of various genes were used as templates. Reactions were performed in a 20-µL total volume containing 20 mM Tris⋅HCl (pH 8.0), 200 mM potassium glutamate, 1 mM DTT, 3 mM MgSO4, 50 ng of supercoiled DNA or 10 ng of linear DNA template, 20 μg/mL BSA, 1 mM ATP, 100 μM each GTP and UTP, 10 μM CTP, 5 μCi of [α-32P]CTP (800 Ci/mmol), and 37 nM E. coli RNA polymerase. σ70, σS, Rsd, and HPr were added to the reaction as described in Fig. 3 and Fig. S4. The transcription reactions were started by addition of the nucleotides, and the reaction mixtures were incubated for 30 min at 37 °C. After the reactions were stopped by mixing with 20 μL of formamide loading buffer containing 5 mM EDTA, the synthesized transcripts were analyzed by electrophoresis on a 5% polyacrylamide gel containing 8 M urea.
Determination of the in Vivo Phosphorylation State of HPr.
Both HPr and EIIAGlc are phosphorylated at histidine residues: the N-1 position of His-15 and the N-3 position of His-90, respectively (2). Phosphohistidine residues are known to be very unstable at neutral and acidic pH, and 1-phosphohistidine residues, such as in phospho-HPr, are even less stable at pH <9.0 compared with the 3-phosphohistidine residues, such as in EIIAGlc (35); thus, exposure of samples to pH <9.0 was minimized. Furthermore, whereas EIIAGlc shows a phosphorylation-dependent mobility shift (PDMS) on SDS/PAGE, HPr shows a PDMS only on native PAGE (36); therefore, to determine the in vivo phosphorylation state of HPr, the procedure developed for EIIAGlc was modified significantly (37).
Cell cultures (0.2 mL at A600 = 1.0) were quenched, the phosphorylation states of HPr were fixed, and the cells were disrupted at the same time by mixing with 20 μL of 5 M NaOH, followed by vortexing for 10 s and the addition of 80 μL of 3 M sodium acetate (pH 5.2) and 0.9 mL of ethanol. The samples were centrifuged at 4 °C for at least 10 min. The pellet was suspended in 40 μL of native PAGE sample buffer containing 1 M urea, and 30 µL of this solution was immediately analyzed by 14% native PAGE. Proteins were then electrotransferred onto Immobilon-P (Millipore) following the manufacturer's protocol and were detected by immunoblotting using antiserum against HPr raised in rabbits. The protein bands were visualized using Immobilon Western chemiluminescent HRP substrate (Millipore) following the manufacturer's instructions.
RNA Isolation and qRT-PCR.
Total RNA was prepared using an RNeasy Mini Kit (Qiagen) from cells grown to late logarithmic phase in LB medium containing 20 μg/mL chloramphenicol, and DNA was removed using an RNase-free DNase (Promega). The same amount of RNA from each culture was converted into cDNA using cDNA EcoDry Premix (Clontech). cDNAs were diluted 20-fold and subjected to qRT-PCR analyses using gene-specific primers and SYBR Premix Ex Taq II (Takara). Amplification and detection of specific products were performed using the CFX96 Real-Time System (Bio-Rad). For normalization of the transcript level, the rrsG or glnD gene was used as a reference. The relative expression level was calculated as the difference between the threshold cycle (Ct) of the target gene and the Ct of the reference gene for each template.
BACTH Assays.
To detect protein–protein interactions in living E. coli, we performed a BACTH assay as described previously (30). The pUT18c derivatives contain the fusion protein T18HPr or T18HPr(H15A), and the pKT25 derivative contains the fusion protein, K25Rsd. The cya-deficient E. coli K-12 strain, BTH101, was cotransformed with derivatives of pUT18c and pKT25 encoding T18 and K25 fragments of B. pertussis adenylyl cyclase, respectively. The transformants were spotted on LB plates containing 100 μg/mL streptomycin, 100 μg/mL ampicillin, and 50 μg/mL kanamycin with 40 μg/mL 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) as the color indicator for β-galactosidase activity. The plates were incubated at 30 °C. To determine the level of β-galactosidase activity, overnight-cultured transformants were diluted into 4 mL of fresh LB medium to an OD600 = 0.4. The cultures were grown at 30 °C to stationary growth phase, and the β-galactosidase activities were measured.
Supplementary Material
Acknowledgments
This work was supported by National Research Foundation Grant NRF 2010-0017384 funded by the Ministry of Science, ICT, and Future Planning and Grant NRF 2013-0076234 funded by the Ministry of Education, and by the Marine and Extreme Genome Research Center Program of the Ministry of Oceans and Fisheries, Republic of Korea.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316629111/-/DCSupplemental.
References
- 1.Barabote RD, Saier MH., Jr Comparative genomic analyses of the bacterial phosphotransferase system. Microbiol Mol Biol Rev. 2005;69(4):608–634. doi: 10.1128/MMBR.69.4.608-634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postma PW, Lengeler JW, Jacobson GR. Phosphoenolpyruvate: carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57(3):543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev. 2006;70(4):939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettenbrock K, et al. Correlation between growth rates, EIIACrr phosphorylation, and intracellular cyclic AMP levels in Escherichia coli K-12. J Bacteriol. 2007;189(19):6891–6900. doi: 10.1128/JB.00819-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lux R, Jahreis K, Bettenbrock K, Parkinson JS, Lengeler JW. Coupling the phosphotransferase system and the methyl-accepting chemotaxis protein-dependent chemotaxis signaling pathways of Escherichia coli. Proc Natl Acad Sci USA. 1995;92(25):11583–11587. doi: 10.1073/pnas.92.25.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nam TW, et al. The Escherichia coli glucose transporter enzyme IICBGlc recruits the global repressor Mlc. EMBO J. 2001;20(3):491–498. doi: 10.1093/emboj/20.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SJ, Boos W, Bouché JP, Plumbridge J. Signal transduction between a membrane-bound transporter, PtsG, and a soluble transcription factor, Mlc, of Escherichia coli. EMBO J. 2000;19(20):5353–5361. doi: 10.1093/emboj/19.20.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka Y, Kimata K, Aiba H. A novel regulatory role of glucose transporter of Escherichia coli: Membrane sequestration of a global repressor Mlc. EMBO J. 2000;19(20):5344–5352. doi: 10.1093/emboj/19.20.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koo B-M, et al. A novel fermentation/respiration switch protein regulated by enzyme IIAGlc in Escherichia coli. J Biol Chem. 2004;279(30):31613–31621. doi: 10.1074/jbc.M405048200. [DOI] [PubMed] [Google Scholar]
- 10.Park Y-H, Lee BR, Seok Y-J, Peterkofsky A. In vitro reconstitution of catabolite repression in Escherichia coli. J Biol Chem. 2006;281(10):6448–6454. doi: 10.1074/jbc.M512672200. [DOI] [PubMed] [Google Scholar]
- 11.Rohwer JM, Meadow ND, Roseman S, Westerhoff HV, Postma PW. Understanding glucose transport by the bacterial phosphoenolpyruvate:glycose phosphotransferase system on the basis of kinetic measurements in vitro. J Biol Chem. 2000;275(45):34909–34921. doi: 10.1074/jbc.M002461200. [DOI] [PubMed] [Google Scholar]
- 12.Seok Y-J, et al. High-affinity binding and allosteric regulation of Escherichia coli glycogen phosphorylase by the histidine phosphocarrier protein, HPr. J Biol Chem. 1997;272(42):26511–26521. doi: 10.1074/jbc.272.42.26511. [DOI] [PubMed] [Google Scholar]
- 13.Görke B, Stülke J. Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat Rev Microbiol. 2008;6(8):613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 14.Kim H-J, Lee C-R, Kim M, Peterkofsky A, Seok Y-J. Dephosphorylated NPr of the nitrogen PTS regulates lipid A biosynthesis by direct interaction with LpxD. Biochem Biophys Res Commun. 2011;409(3):556–561. doi: 10.1016/j.bbrc.2011.05.044. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y-J, et al. A mammalian insulysin homolog is regulated by enzyme IIAGlc of the glucose transport system in Vibrio vulnificus. FEBS Lett. 2010;584(22):4537–4544. doi: 10.1016/j.febslet.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 16.Lee C-R, Cho S-H, Yoon M-J, Peterkofsky A, Seok Y-J. Escherichia coli enzyme IIANtr regulates the K+ transporter TrkA. Proc Natl Acad Sci USA. 2007;104(10):4124–4129. doi: 10.1073/pnas.0609897104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jishage M, Ishihama A. A stationary phase protein in Escherichia coli with binding activity to the major sigma subunit of RNA polymerase. Proc Natl Acad Sci USA. 1998;95(9):4953–4958. doi: 10.1073/pnas.95.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piper SE, Mitchell JE, Lee DJ, Busby SJ. A global view of Escherichia coli Rsd protein and its interactions. Mol Biosyst. 2009;5(12):1943–1947. doi: 10.1039/B904955j. [DOI] [PubMed] [Google Scholar]
- 19.Westblade LF, et al. Studies of the Escherichia coli Rsd-σ70 complex. J Mol Biol. 2004;335(3):685–692. doi: 10.1016/j.jmb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann N, Wurm R, Wagner R. The E. coli anti-sigma factor Rsd: Studies on the specificity and regulation of its expression. PLoS ONE. 2011;6(5):e19235. doi: 10.1371/journal.pone.0019235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patikoglou GA, et al. Crystal structure of the Escherichia coli regulator of σ70, Rsd, in complex with σ70 domain 4. J Mol Biol. 2007;372(3):649–659. doi: 10.1016/j.jmb.2007.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma UK, Chatterji D. Differential mechanisms of binding of anti-sigma factors Escherichia coli Rsd and bacteriophage T4 AsiA to E. coli RNA polymerase lead to diverse physiological consequences. J Bacteriol. 2008;190(10):3434–3443. doi: 10.1128/JB.01792-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farewell A, Kvint K, Nyström T. Negative regulation by RpoS: A case of sigma factor competition. Mol Microbiol. 1998;29(4):1039–1051. doi: 10.1046/j.1365-2958.1998.00990.x. [DOI] [PubMed] [Google Scholar]
- 24.Gaal T, et al. Promoter recognition and discrimination by EσS RNA polymerase. Mol Microbiol. 2001;42(4):939–954. doi: 10.1046/j.1365-2958.2001.02703.x. [DOI] [PubMed] [Google Scholar]
- 25.Waterman SR, Small PL. Transcriptional expression of Escherichia coli glutamate-dependent acid resistance genes gadA and gadBC in an hns rpoS mutant. J Bacteriol. 2003;185(15):4644–4647. doi: 10.1128/JB.185.15.4644-4647.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishihama A. Functional modulation of Escherichia coli RNA polymerase. Annu Rev Microbiol. 2000;54:499–518. doi: 10.1146/annurev.micro.54.1.499. [DOI] [PubMed] [Google Scholar]
- 27.Jishage M, Ishihama A. Transcriptional organization and in vivo role of the Escherichia coli rsd gene, encoding the regulator of RNA polymerase sigma D. J Bacteriol. 1999;181(12):3768–3776. doi: 10.1128/jb.181.12.3768-3776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell JE, et al. The Escherichia coli regulator of sigma 70 protein, Rsd, can up-regulate some stress-dependent promoters by sequestering sigma 70. J Bacteriol. 2007;189(9):3489–3495. doi: 10.1128/JB.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee C-R, et al. Potassium mediates Escherichia coli enzyme IIANtr-dependent regulation of sigma factor selectivity. Mol Microbiol. 2010;78(6):1468–1483. doi: 10.1111/j.1365-2958.2010.07419.x. [DOI] [PubMed] [Google Scholar]
- 30.Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci USA. 1998;95(10):5752–5756. doi: 10.1073/pnas.95.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SY, et al. Purification of Mlc and analysis of its effects on the pts expression in Escherichia coli. J Biol Chem. 1999;274(36):25398–25402. doi: 10.1074/jbc.274.36.25398. [DOI] [PubMed] [Google Scholar]
- 32.Hogema BM, et al. Inducer exclusion in Escherichia coli by non-PTS substrates: The role of the PEP to pyruvate ratio in determining the phosphorylation state of enzyme IIAGlc. Mol Microbiol. 1998;30(3):487–498. doi: 10.1046/j.1365-2958.1998.01053.x. [DOI] [PubMed] [Google Scholar]
- 33.Yu D, et al. An efficient recombination system for chromosome engineering in Escherichia coli. Proc Natl Acad Sci USA. 2000;97(11):5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee C-R, et al. Requirement of the dephospho-form of enzyme IIANtr for derepression of Escherichia coli K-12 ilvBN expression. Mol Microbiol. 2005;58(1):334–344. doi: 10.1111/j.1365-2958.2005.04834.x. [DOI] [PubMed] [Google Scholar]
- 35.Hultquist DE. The preparation and characterization of phosphorylated derivatives of histidine. Biochim Biophys Acta. 1968;153(2):329–340. doi: 10.1016/0005-2728(68)90078-9. [DOI] [PubMed] [Google Scholar]
- 36.Lee C-R, et al. Reciprocal regulation of the autophosphorylation of enzyme INtr by glutamine and α-ketoglutarate in Escherichia coli. Mol Microbiol. 2013;88(3):473–485. doi: 10.1111/mmi.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi H, Inada T, Postma P, Aiba H. CRP down-regulates adenylate cyclase activity by reducing the level of phosphorylated IIAGlc, the glucose-specific phosphotransferase protein, in Escherichia coli. Mol Gen Genet. 1998;259(3):317–326. doi: 10.1007/s004380050818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.