Significance
This article presents our discovery that intranasal administration of oxytocin enhances activity in the brain for socially meaningful stimuli and attenuates its response to nonsocially meaningful stimuli in children with autism spectrum disorder (ASD) as measured via functional MRI. We also identified a relationship between changes in salivary oxytocin following administration and enhancements in brain function. These discoveries are particularly important given the urgent need for treatments that target the core social dysfunction in ASD. The functional neural attunement we demonstrated might facilitate social learning, thus potentially bringing about long-term change in neural systems and subsequent behavioral improvements. Our results illustrate the power of a translational neuroscience approach to facilitate the development of pharmacological interventions for neurodevelopmental disorders like ASD.
Abstract
Following intranasal administration of oxytocin (OT), we measured, via functional MRI, changes in brain activity during judgments of socially (Eyes) and nonsocially (Vehicles) meaningful pictures in 17 children with high-functioning autism spectrum disorder (ASD). OT increased activity in the striatum, the middle frontal gyrus, the medial prefrontal cortex, the right orbitofrontal cortex, and the left superior temporal sulcus. In the striatum, nucleus accumbens, left posterior superior temporal sulcus, and left premotor cortex, OT increased activity during social judgments and decreased activity during nonsocial judgments. Changes in salivary OT concentrations from baseline to 30 min postadministration were positively associated with increased activity in the right amygdala and orbitofrontal cortex during social vs. nonsocial judgments. OT may thus selectively have an impact on salience and hedonic evaluations of socially meaningful stimuli in children with ASD, and thereby facilitate social attunement. These findings further the development of a neurophysiological systems-level understanding of mechanisms by which OT may enhance social functioning in children with ASD.
Autism spectrum disorder (ASD) is a common, early-onset neurodevelopmental disorder characterized by devastating difficulties in social interaction, communication, and repetitive or restricted interests and behaviors. ASD displays great phenotypic heterogeneity and etiological diversity, but its original characterization, social dysfunction, has been its hallmark and unifying feature (1). There is no established pharmacological treatment for social impairment in ASD.
When given acutely, intranasal oxytocin (OT) leads to enhanced processing of social stimuli in typically developing adults, as evidenced by increased eye contact, in-group trust, and emotion recognition from facial expressions (2–4). At the level of neural systems, intranasal OT heightens activity in a set of neuroanatomical structures involved in processing socially meaningful stimuli in typically developing adults (5, 6). Recently, the first brain imaging study in adults with ASD examined the effects of OT administration and identified increased activation in the right amygdala during social information processing (7).
Behavioral studies demonstrate that in children and adults with ASD, a single administration of intranasal OT leads to increased willingness to interact socially (8), better comprehension of affective speech (9), reduced repetitive behaviors (10), increased understanding of others’ mental states (11), and improved social cognition (12). Despite cautionary calls regarding the use of OT in children to treat ASD before understanding the neural mechanisms underlying OT’s complex impact on behavior (13), there have been no studies on the effects of OT administration on brain activity in children. Furthermore, although there are several large-scale clinical trials currently underway (www.clinicaltrials.gov) to examine the effects of chronically administered OT in ASD, the empirical record shows that behavioral effects have been mixed at best (13–15). For example, two recent studies of the effects of repeated daily administration for a period of weeks have resulted in only modest improvements in social behavior (14, 15). The rapid movement from single administration studies in healthy adults and individuals with ASD to chronic administration to individuals with ASD has introduced a “translational hurdle” (13, 16), one that we aimed to tackle by exploring the neural basis of OT’s effects.
In a randomized, double-blind, cross-over functional MRI (fMRI) study, we sought to identify the impact of single intranasal administration of OT on brain activity in 17 children and adolescents (aged 8–16.5 y) with ASD. We studied children and adolescents because previous reports had not included children younger than 12 y of age (11). We hypothesized that during a task involving social judgments, OT vs. Placebo would heighten brain activity in the neural circuits supporting reward [dorsal and ventral striatum and nucleus accumbens (NAcc)], as well as social attention and social cognition (e.g., posterior superior temporal cortex, cingulate, precuneus), that is, the “social brain” (17).
Participants were randomized to OT and Placebo nasal sprays on two consecutive visits. Forty-five minutes following administration, brain function was assessed using the “Reading the Mind in the Eyes Test” (RMET) (18, 19), a well-validated fMRI emotion judgment task. We selected this task because performance is reliably related to autistic traits (18, 20, 21) and behavioral performance is enhanced by intranasal OT in healthy adults, as well as individuals with ASD (11, 22). We modified the original (gender attribution) control condition of the RMET to dissociate social and nonsocial processing, and thus to examine the specificity of OT effects on social processing. We asked participants either to label a mental state from pictures of the Eyes (social entities) or to label the category of automobile presented in pictures of Vehicles (nonsocial objects). Each participant practiced the tasks before the functional scan began to ensure that he or she understood and could readily perform the tasks. Within the scanner, the tasks lasted a total of 5.1 min and alternated between the social and nonsocial judgment task conditions. There were five 25-s blocks of each condition (total of 10 blocks). In each block, five different images of either Eyes or Vehicles appeared for 5 s each. Blocks were separated by 12-s rest periods (blank screen and central fixation cross). The order of the presentation of social and nonsocial blocks was quasirandomized: on the first visit, all participants started by labeling a social block, and on the second visit, all participants started by labeling a nonsocial block. Fig. S1 depicts the fMRI task design.
To understand how the coordination of peripheral and central OT function may have an impact on neural systems-level function (23), we measured reactivity in OT concentration in saliva from baseline to 30 min postadministration. Given recent discoveries regarding associations between peripheral levels of OT and social behavior (24–26), we explored how changes in peripheral OT are associated with changes in brain activity.
Results
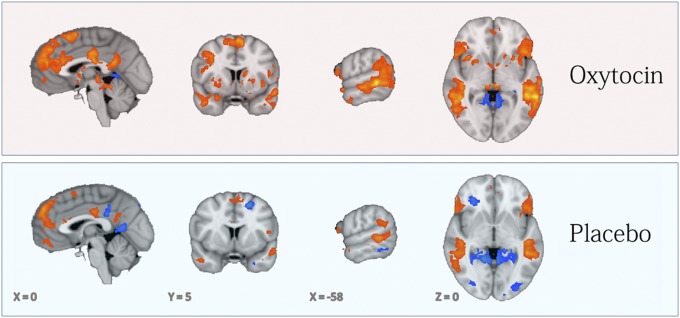
We first examined the contrast of the social (Eyes) vs. nonsocial (Vehicles) judgments across all sessions (OT or Placebo). As illustrated in Fig. 1, in a whole-brain analysis, we identified multiple regions exhibiting significant (P < 0.05, corrected; K = 729 mm3) activation in response to Eyes vs. Vehicles, including the right amygdala, bilateral superior frontal gyri, medial prefrontal cortices, superior temporal sulci, inferior frontal gyri, middle cingulate cortices, and precuneus. Notably, comparison of the OT (Fig. 1, Upper) and Placebo (Fig. 1, Lower) reveals consistently greater Eyes > Vehicles activity (orange map in Fig. 1) in these brain regions for OT relative to Placebo (Fig. S2).
Fig. 1.
In both panels, the orange map indicates regions where activity was greater during Eyes vs. Vehicles judgments and the blue map indicates the reverse (P < 0.05, corrected; K ≥ 729 mm3). (Upper) OT visits are depicted. (Lower) Placebo visits are depicted.
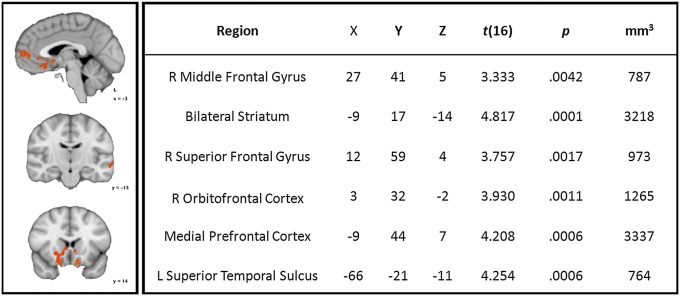
We next examined the impact of OT (the main effect: OT > Placebo) regardless of task (Eyes and Vehicles). As illustrated in Fig. 2, we identified increased activity in the ventral and dorsal striatum, including the NAcc, the medial prefrontal cortex, and the right orbitofrontal cortex, as well as the left posterior superior temporal sulcus.
Fig. 2.
(Left) Orange map illustrates regions where activity was increased by OT vs. placebo during Eyes and Vehicles judgments (P < 0.05, corrected; K ≥ 729 mm3). L, left; R, right. (Right) Descriptive statistics and localization of peak responses from regions of OT > Placebo activity across Eyes and Vehicles judgments.
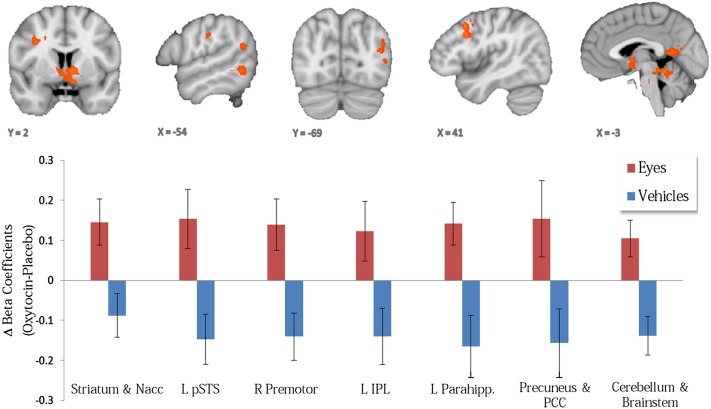
Our core hypotheses centered on identifying effects of OT specific to social judgments vs. nonsocial judgments. Therefore, we identified regions exhibiting a predicted treatment (OT > Placebo) × task (Eyes > Vehicles) interaction such that activity was enhanced for OT relative to Placebo when children with ASD make social (emotional state) but not nonsocial (vehicle) judgments. As illustrated in Fig. 3 (Upper), we identified regions exhibiting significant treatment (OT > Placebo) × task (Eyes > Vehicles) interactions in the dorsal and ventral striatum, precuneus, posterior cingulate, left inferior parietal lobule, left posterior superior temporal sulcus, left parahippocampal gyrus, and right premotor cortex (Table 1). Notably, as exemplified in Fig. 3 (Lower), in these regions, OT enhanced activity during social judgments while reducing activity during nonsocial judgments.
Fig. 3.
(Upper) Orange map indicates regions from a random effects, repeated measures GLM analysis exhibiting a significant (P < 0.05, corrected; K ≥ 729 mm3) treatment (OT > Placebo) by task (Eyes > Vehicles) interaction. (Lower) OT differentially affects activation supporting social (increasing the activation) vs. nonsocial information processing (decreasing the activation). IPL, inferior parietal lobule; Parahipp., parahippocampal gyrus; PCC, posterior cingulate cortex; pSTS, posterior superior temporal sulcus.
Table 1.
Centers, extent, and significance of activation for regions exhibiting a treatment × task interaction
| Region | X | Y | Z | t(16) | P | K, mm3 |
| Right precentral gyrus | 30 | 5 | 37 | 4.447 | 0.0004 | 1,877 |
| Striatum and NAcc | −6 | −1 | 1 | 4.226 | 0.0006 | 2,517 |
| Cerebellum and pons | 0 | −43 | −11 | 5.35 | 0.0000 | 3,284 |
| Posterior cingulate/precuneus | −12 | −55 | 16 | 4.85 | 0.0001 | 2,802 |
| Left parahippocampal region | −27 | −40 | −2 | 6.177 | 0.0000 | 1,096 |
| Left inferior parietal lobule | −39 | −34 | 31 | 4.087 | 0.0008 | 771 |
| Left superior temporal sulcus | −45 | −73 | 16 | 4.205 | 0.0006 | 997 |
t(16), student's t test coefficient (degrees of freedom).
We assessed whether activity in the regions exhibiting a treatment (OT > Placebo) × task (Eyes > Vehicles) interaction varied as a function of ASD severity. We used a severity score from the Social Responsiveness Scale (SRS) (27), a well-validated 65-item rating scale that measures the severity of autism spectrum symptoms as they occur in natural social settings, which was completed by the parents of each participant. We partitioned our sample into two groups according to a cutoff score of 76 [low severity (SRS t score <76) and high severity (SRS t score <76)] and conducted a series of one-way ANOVAs to compare the beta coefficients from the regions exhibiting a treatment (OT > Placebo) × task (Eyes > Vehicles) interaction for the following four conditions: OT-Eyes, OT-Vehicles, Placebo-Eyes, and Placebo-Vehicles. This analysis indicated that the effect of OT on brain activity differed as a function of severity, but only when subjects were making social judgments [F(1,16) = 6.45, P < 0.05]. When given OT, individuals with less social dysfunction exhibited higher beta coefficients while processing eyes [mean (M) = 0.08, SD = 0.12], indicative of more typical functioning, compared with more severely affected children and adolescents with ASD (M = 20.05, SD = 0.09).
Overall, behavioral accuracy and reaction times on the RMET did not differ for OT vs. Placebo visits (Fig. S3). This is consistent with the empirical record, which shows improvements only on some items from the RMET, according to difficulty level, but not overall (11, 22). Results of a repeated measures ANOVA indicated that children were faster [F(1,15) = 12.15, P < 0.05], but not more accurate [F(1,15) = 1.88, P = 0.19], when labeling Vehicles compared with Eyes. They were also faster [F(1,15) = 11.52, P < 0.05], but not more accurate [F(1,15) = 2.31, P = 0.15], for all stimuli during their second visit compared with the first (regardless of treatment condition), indicating a practice effect.
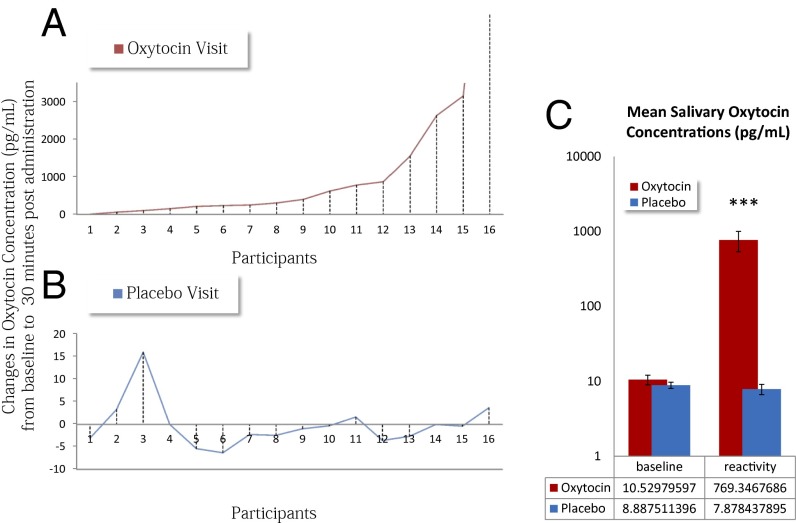
Fig. 4 depicts changes in salivary OT levels (baseline to 30 min postadministration) during OT (red line) or Placebo (blue line) visits. As expected, changes during Placebo days were essentially nonexistent. Conversely, when OT was administered, there was considerable variability in levels of peripheral OT concentration across participants. Fig. 5 presents the results of a random effects analysis of covariance in which we modeled the variability in changes in salivary OT to localize brain regions where levels of Eyes > Vehicles activity (on OT days) covaried with changes in salivary OT. A region of the right amygdala extending into Brodmann’s area 25 (BA 25, a portion of the orbitofrontal cortex extending into the subgenual anterior cingulate) was positively correlated with increases in salivary OT levels. Peak coordinates for this regions were X = 6, Y = 8, Z = −17, r(13) at the peak voxel = 0.87, and P = 0.0003. Further, we calculated the correlation between changes in salivary OT from baseline to 30 min postadministration and the average beta coefficients from the amygdala and BA 25 regions separately for the Eyes and Vehicles conditions (Fig. S4). This analysis revealed a significant positive correlation (r = 0.75, P = 0.01) for the Eyes condition and a trend-level negative correlation (r = 0.46, P = 0.08) for the Vehicles condition.
Fig. 4.
Changes in salivary OT concentrations (picograms per milliliter) for 16 of the 17 participants on the Placebo (blue line) and OT (red line) day. (A) Changes in salivary OT when OT was administered. Participant 16 had a very high rise in OT (from 3.7 to 9,879.8 pg/mL); thus, he is considered an outlier and is plotted outside of the range. (B) Changes in salivary OT when Placebo was administered. (C) Mean levels of OT before administration (baseline) and 30 min postadministration (reactivity) for OT and Placebo visits separately. Reactive OT levels were significantly higher when active OT was administered compared to baseline salivary levels and to reactive levels on placebo visits. ***p < .0001.
Fig. 5.
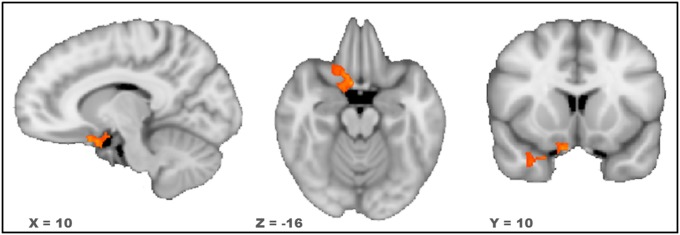
Orange to yellow map indicates regions where the level of activity during judgments of Eyes relative to judgments of Vehicles was positively associated with reactivity in salivary OT levels (P < 0.05, corrected; K ≥ 1,620 mm3).
Discussion
Our results help to elucidate the brain systems-level mechanisms by which administration of intranasal OT may serve to enhance brain activity during social information processing, and thus play a role in the treatment of social dysfunction in children with ASD. A single administration of OT enhanced activity in key nodes of some of the neural circuits that have been repeatedly implicated in the neural phenotype of ASD. Some of the enhancements were identified specifically in response to social cues rather than nonsocial cues. These neural systems-level findings indicate that OT enhances activity in key nodes of the neural circuits that have been previously implicated in reward (dorsal and ventral striatum, including the NAcc) (29, 30); social attention, perception, and cognition (premotor cortex, posterior cingulate, inferior parietal lobule, and posterior superior temporal sulcus) (31, 32); and detecting, decoding, and reasoning about mental states (medial prefrontal cortex) (33, 34). Moreover, each of the regions of heightened activation has been consistently identified as abnormal (generally hypoactive) in children, adolescents, and adults with ASD (28). We speculate that these results may imply that OT makes social stimuli more rewarding and socially salient to children with ASD. Our findings are remarkably consistent with nonhuman animal and human studies that have demonstrated influences on social reward, social attention, and salience following changes to oxytocinergic neuropathways (35–40).
In addition to enhanced activity in social brain regions during social judgments, the treatment × task interaction analysis identified several brain regions in which there was decreased activity during nonsocial judgments. This pattern of effects reveals a unique process underlying the effects of OT on brain activity: a process of attunement, in which activity in brain regions that have developed (or normally develop) to be specialized for processing the social world is enhanced for social stimuli and decreased for nonsocial stimuli. This unique mechanism can be added to other, previously described, mechanisms by which OT has an impact on the brain (5), namely, the increase of saliency of social stimuli (valence-independent) and the enhancement of the rewarding value of social stimuli (for social stimuli with positive valence). Our findings may be consistent with the recent discovery that OT administration can increase the activity of fast-spiking interneuron activity in the rodent hippocampus. This suppresses spontaneous pyramidal cell firing while simultaneously enhancing the fidelity of spike transmission, and it also sharpens spike timing (41). These two processes, one increasing activity and the other decreasing activity, effectively boost “signal-to-noise” in favor of certain stimuli and thereby serve to tune brain responses to that class of stimuli. In ASD, OT may serve to enhance responses to social stimuli in regions that should normally “prefer” socially meaningful stimuli but may also help to normalize category selectivity by suppressing the response to “nonpreferred” stimuli.
Intranasal OT can increase central (40) or peripheral (42) OT concentrations or both (43). The route by which intranasal administration induces its central effects is still unclear, but OT receptors are widely distributed throughout the brain (44–46). If OT inhalation induces OT production or activity, the effects can be extensive because OT, in its role as a neuromodulator, can quickly and broadly reach multiple brain regions and interact with multiple brain systems (47). OT is mainly produced in two hypothalamic regions: the supraoptic nuclei and paraventricular nuclei. OT fibers are present in the NAcc and the amygdala (48), both of which were found to be more active following OT administration. OT release is not limited to the synaptic cleft; hypothalamic neurons can also release OT from their entire surface area (49, 50), and OT can thus diffuse through the extracellular space and further affect a variety of brain regions. Beyond direct projections, hypothalamic neurons form collateral projections from magnocellular neurons projecting to the posterior pituitary, thus providing a potential mechanism by which OT’s release to brain and body may be coordinated (51).
The correlation between peripheral changes in OT concentrations and Eyes > Vehicles brain activity in the right amygdala, orbitofrontal cortex, and subgenual anterior cingulate represents an important clue to help understand the coordination of peripheral and central OT. A recent review pointed to the amygdala as one of the brain regions most consistently affected by the administration of OT (5). One fMRI study has used intranasal OT administration in adults with ASD, highlighting the right amygdala as a target of OT's effects (7). In animals, intranasal OT can excite neuronal populations in the amygdala (52), with different amygdala subregions mediating the variable impacts of OT on attention to affective social stimuli (53). In mothers who are synchronous while interacting with their infant (thus providing a rewarding parental context), activation in the right amygdala (when viewing one’s own infant vs. another person’s infant) is positively correlated with levels of plasma OT (54). Notably, the portion of the orbitofrontal cortex (BA 25) identified here has been implicated as a node in a socioemotional network that modulates affective evaluations and exhibits increased functional connectivity due to OT administration in typically developing adults (55–57). The association between salivary changes in OT and activation of the right amygdala and BA 25 may provide future studies with an independent peripheral biomarker with which to explore OT’s impact on neural response in ASD.
Given the universality of social deficits in the autistic phenotype, dysfunction in brain systems subserving social information processing has been a key focus in research on ASD. Our theoretical framework posits that (i) specific brain systems evolved to process information pertaining to humans and (ii) autistic dysfunction originates in these brain systems, exerting cascading, peripheral impacts throughout development. The “social motivation” hypothesis (Fig. S5) builds upon this framework and suggests that reduced social drive/motivation leads to inattention to key aspects of social information and consequent failure of developmental specialization in experience-expectant brain systems, such as the face and action perception systems (58, 59). Diminished social motivation in ASD might stem from deficits in forming representations of and categorizing the reward value of social information. Individuals with ASD show less activation in reward circuits when viewing social rewards (i.e., smiling faces) than controls (60–62). OT may well target social motivation directly, addressing the theoretical crux of social dysfunction in ASD. Our results indicate enhanced activity during social information processing, but not during nonsocial judgments, in the neural circuitry believed to support social motivation. Following the developmental predictions of the social motivation model, we observed enhanced cortical specialization for the perception of social information and decreased activity during the perception of nonsocial information, hypothesized downstream effects of changes in social motivation elicited via OT.
The fMRI results combined with the behavioral findings from this study and others highlight several important translational implications. First, the lack of behavioral improvements raises the possibility that OT may enhance and tune social brain function even in the absence of immediate changes in social behavior. Behavioral improvements may require richer, more realistic social contexts that include opportunities for social learning (4). For example, only modest improvements in social functioning were observed in adults with ASD who were treated daily, for 6 wk, with administration of intranasal OT (14). Similarly, no changes in social behavior were found when OT was given once every morning for 5 d to children with ASD, even in a context of behavioral treatment (15). It may very well be crucial for future utilization in ASD to give OT just before the setting where its impact is being tested and built upon. This emphasizes the potential for treatment approaches that use OT to enhance salience within social contexts and thereby enhance social learning. We predict that the most successful therapeutic applications of OT will be those giving the compound before evidence-based behavioral treatments that provide opportunities for feedback and learning in supportive social contexts.
Materials and Methods
Participants.
The Yale University Human Investigations Committee approved this study. Each participant’s parent(s) provided informed consent. Each child or adolescent provided verbal or written assent. Participants were recruited through the Yale Center for Translational Developmental Neuroscience. Overall, 21 children and adolescents with ASD (age range: 8–16.5 y, M = 13.2 y, SD = 2.7 y) participated in the study. ASD diagnoses were made via the autism diagnostic observation schedule (63), the autism diagnostic interview-revised (64), and expert clinical evaluation. Our sample included three girls and 18 boys. Sixteen participants were Caucasian, 2 were African-American, and 2 were of mixed ethnicity. One participant was left-handed, 1 was ambidextrous, and 19 were right-handed. The study consisted of two visits separated by at least 72 h to allow possible treatment effects of the first visit to wane completely. Otherwise, the second visit was scheduled according to participants’ availability. Visits were separated by an average of 21 d from each other (range: 3–78 d). One boy did not arrive for both visits due to major changes of health status. He was thus excluded from the study. Another participant could not perform the task because he did not understand it, and he was dropped from subsequent analysis.
Drug Protocol.
OT, 60 international units (IU)/mL, was prepared by the research pharmacy at Yale New Haven Hospital using OT, United States Pharmacopeia (Medisca). Placebo and OT spray containers were prepared to look identical and were counterbalanced to be randomly assigned by the pharmacy as well. Researchers, as well as participants, were blinded to the content of the spray. Doses were prescribed according to participant age. Older participants (aged 16–19 y) received a dose of 24 IU (four puffs per nostril), in accordance with most studies of intranasal OT in adults (22, 65). We used age-dependent dosing (as suggested by the scant OT inhalation studies in children (11, 15, 66), with age as a proxy for size/weight. Twelve- to 15-y-olds received 75% of the adult dose (24 IU) or 18 IU (three puffs per nostril). The youngest age group (aged 7–11 y) received 50% of the typical adult dose (12 IU or one puff per nostril).
Image Acquisition.
Images were collected on a Siemens 3T Tim Trio scanner located at the Yale University Magnetic Resonance Research Center. High-resolution, T1-weighted anatomical images were acquired using a magnetization-prepared rapid gradient echo sequence [repetition time (TR) = 1,230 ms, echo time (TE) = 1.73 ms, field of view (FOV) = 256 mm, image matrix = 2562, 1 × 1 × 1 mm]. Whole-brain functional images were acquired using a single-shot, gradient-recalled echo planar pulse sequence (TR = 2,000 ms, TE = 25 ms, flip angle = 60°, FOV = 220 mm, image matrix = 642, voxel size = 3.4 × 3.4 × 4.0 mm, 34 slices) sensitive to blood oxygenation level-dependent (BOLD) contrast. The first 10 volumes acquired were discarded.
Motion Correction.
For each participant’s functional run, any volume that exceeded 3 mm or 3° of motion relative to the first undiscarded volume was excised. Volume-wise weight was derived from condition-specific predictor values for the general linear model (GLM; described below) for that volume. For instance, a volume whose condition predictor was 0.5 would have twice the weight in determining condition proportion as a volume whose predictor value was 0.25. Two participant’s datasets were discarded from further analysis because they had not retained, proportionally, more than 0.75 of total volumes and 0.75 of weighted volumes per condition.
Of the 34 runs (17 participants × two visits) that were subjected to data scrubbing, only four had volumes removed. Following motion correction and data scrubbing, we performed paired-sample t tests to compare subject movement for the OT vs. placebo visits. The results indicated no difference in the following: (i) average absolute motion (OT: M = 0.216 mm/°, SE = 0.047 mm/°; Placebo: M = 0.196 mm/°, SE = 0.039 mm/°); (ii) maximum absolute motion (OT: M = 0.994 mm/°, SE = 0.191 mm/°; Placebo: M = 1.141 mm/°, SE = 0.212 mm/°); (iii) mean absolute volume-to-volume motion (OT: M = 0.034 mm/°, SE = 0.013 mm/°; Placebo: M = 0.042 mm/°, SE = 0.013 mm/°); (iv) maximum absolute volume-to-volume motion (OT: M = 0.816 mm/°, SE = 0.256 mm/°; Placebo: M = 0.936 mm/°, SE = 0.291 mm/°); and (v) number of removed volumes following volume correction (OT: M = 0.941, SE = 0.88; Placebo: M = 1.647, SE = 0.585).
fMRI Analyses.
Data were processed and analyzed using BrainVoyager QX 2.0.08 software (Brain Innovation). Preprocessing of functional data included sinc interpolation slice-time-correction, trilinear-sinc interpolation 3D rigid-body motion correction, spatial smoothing with an FWHM 4-mm Gaussian kernel, linear trend removal, and temporal high-pass filtering (GLM with Fourier basis set, using two cycles per time course). Functional datasets were coregistered to within-session anatomical images, which were normalized to Talairach space. For each participant, we assessed estimated motion plots and cine-loops to visualize and inspect the entire dataset for motion and other potential artifacts.
GLM-based analyses were conducted for each participant to assess task-related BOLD responses. To create predictors for Eyes and Vehicles conditions, a boxcar function with a value of 1 during the condition and 0 otherwise was convolved with a double-gamma hemodynamic response function. Predictors depicting motion in all six parameters were included as predictors of no interest. Fixations were not modeled.
All group-level analyses were limited to only voxels within the extent of the Montreal Neurological Institute brain normalized to Talairach space. We added a white matter and ventricles mask that was calculated based on the current sample. An anatomical mask was created by computing the average normalized anatomical image of experiment participants. High- and low-intensity voxels, corresponding, respectively, to conservative estimates of white matter and ventricles/cerebral spinal fluid, were identified and removed from the group analysis. Voxels outside the brain were also removed. The anatomical mask consisted of 1,321,308 structural (1-mm3) voxels.
Whole-brain analyses were conducted using random effects GLM-based analyses. Analyses were assessed at an uncorrected threshold of P < 0.05 and were then corrected for multiple comparisons using cluster thresholds determined by the Brain Voyager QX Cluster-Level Statistical Threshold Estimator plug-in (67, 68). After 5,000 iterations of a Monte Carlo simulation, the cluster size corresponding to a whole-brain–corrected threshold of α < 0.05 was determined as 27 voxels (729 mm3), and this cluster size was used to correct for multiple comparisons in the fMRI analyses.
Collection of Saliva Samples.
Saliva samples were collected using Salivettes (Sarstedt) twice during each visit. The first sample was taken as a baseline immediately after consent and before OT or Placebo administration. The second sample was taken 30 min following OT or Placebo administration, before participants were taken for the fMRI scan. Participants were asked to chew a roll of cotton for 1 min until it was saturated with saliva. Salivettes were kept ice-chilled for up to 2 h before being stored at −20 °C. Salivettes were then shipped overnight via Federal Express on dry ice to the laboratory of Ruth Feldman at the Bar-Ilan Gonda Brain Research Center, where they were concentrated three or four times. Liquid samples were lyophilized overnight and kept at −20 °C until assayed. The dry samples were reconstructed in the assay buffer immediately before analysis by an OT enzyme immunoassay commercial kit, consistent with previous research.
Determination of Salivary OT.
Determination of OT was performed using a commercial OT ELISA kit (Assay Design) consistent with previous research (24, 69). Measurements were performed in duplicate, and the concentrations of samples were calculated using MATLAB 7 (MathWorks) according to relevant standard curves. The intraassay and interassay coefficient are 12.4% and 14.5%, respectively.
Supplementary Material
Acknowledgments
We are grateful to the children and families who participated in this study. We thank Osama Abdelghany from the Yale New Haven Hospital Investigational Drug Services. We also thank Allison Jack for her technical help. A Harris Family Professorship (to K.A.P.), a Lee Foundation Postdoctoral Award (to I.G.), and a grant from the Binational Science Foundation (to R.F., K.A.P., I.G., and J.F.L.) supported this research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312857110/-/DCSupplemental.
References
- 1.Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2(3):217–250. [PubMed] [Google Scholar]
- 2.Striepens N, Kendrick KM, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol. 2011;32(4):426–450. doi: 10.1016/j.yfrne.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Van IJzendoorn MH, Bakermans-Kranenburg MJ. A sniff of trust: Meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology. 2012;37(3):438–443. doi: 10.1016/j.psyneuen.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: Context and person matter. Trends Cogn Sci. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Bethlehem RA, van Honk J, Auyeung B, Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: A review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2013;38(7):962–974. doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Zink CF, Meyer-Lindenberg A. Human neuroimaging of oxytocin and vasopressin in social cognition. Horm Behav. 2012;61(3):400–409. doi: 10.1016/j.yhbeh.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domes G, et al. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiatry. 2013;74(3):164–171. doi: 10.1016/j.biopsych.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Andari E, et al. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA. 2010;107(9):4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollander E, et al. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61(4):498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Hollander E, et al. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology. 2003;28(1):193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- 11.Guastella AJ, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67(7):692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Bartz JA, Hollander E. Oxytocin and experimental therapeutics in autism spectrum disorders. Prog Brain Res. 2008;170:451–462. doi: 10.1016/S0079-6123(08)00435-4. [DOI] [PubMed] [Google Scholar]
- 13.Miller G. Neuroscience. The promise and perils of oxytocin. Science. 2013;339(6117):267–269. doi: 10.1126/science.339.6117.267. [DOI] [PubMed] [Google Scholar]
- 14.Anagnostou E, et al. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: A randomized controlled trial. Mol Autism. 2012;3(1):16. doi: 10.1186/2040-2392-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dadds MR, et al. Nasal oxytocin for social deficits in childhood autism: A randomized controlled trial. J Autism Dev Disord. 2013;43(7):1–11. doi: 10.1007/s10803-013-1899-3. [DOI] [PubMed] [Google Scholar]
- 16.Insel TR. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65(6):768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brothers L. The neural basis of primate social communication. Motiv Emot. 1990;14(2):81–91. [Google Scholar]
- 18.Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42(2):241–251. [PubMed] [Google Scholar]
- 19.Baron-Cohen S, Jolliffe T, Mortimore C, Robertson M. Another advanced test of theory of mind: Evidence from very high functioning adults with autism or asperger syndrome. J Child Psychol Psychiatry. 1997;38(7):813–822. doi: 10.1111/j.1469-7610.1997.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 20.Senju A, Tojo Y, Konno M, Dairoku H, Hasegawa T. [Reading mind from pictures of eyes: Theory of mind, language ability, general intellectual ability, and autism] Shinrigaku Kenkyu. 2002;73(1):64–70. doi: 10.4992/jjpsy.73.64. Japanese. [DOI] [PubMed] [Google Scholar]
- 21.Baron-Cohen S. Theory of mind and autism: A review. Int Rev Res Ment Retard. 2001;23(23):169–184. [Google Scholar]
- 22.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61(6):731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Leckman JF. Variations in maternal behavior—Oxytocin and reward pathways—Peripheral measures matter?! Neuropsychopharmacology. 2011;36(13):2587–2588. doi: 10.1038/npp.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: Considering stress and affiliation components of human bonding. Dev Sci. 2011;14(4):752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- 25.Feldman R, Gordon I, Zagoory-Sharon O. The cross-generation transmission of oxytocin in humans. Horm Behav. 2010;58(4):669–676. doi: 10.1016/j.yhbeh.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology. 2010;35(8):1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Constantino JN, et al. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser MD, et al. Neural signatures of autism. Proc Natl Acad Sci USA. 2010;107(49):21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: Microcircuitry. Neuropsychopharmacology. 2010;35(1):27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7(12):942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- 32.Seghier ML. The angular gyrus: Multiple functions and multiple subdivisions. Neuroscientist. 2013;19(1):43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabbagh MA. Understanding orbitofrontal contributions to theory-of-mind reasoning: Implications for autism. Brain Cogn. 2004;55(1):209–219. doi: 10.1016/j.bandc.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 35.Higashida H, et al. Oxytocin signal and social behaviour: Comparison among adult and infant oxytocin, oxytocin receptor and CD38 gene knockout mice. J Neuroendocrinol. 2010;22(5):373–379. doi: 10.1111/j.1365-2826.2010.01976.x. [DOI] [PubMed] [Google Scholar]
- 36.Gordon I, Martin C, Feldman R, Leckman JF. Oxytocin and social motivation. Dev Cogn Neurosci. 2011;1(4):471–493. doi: 10.1016/j.dcn.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohls G, Chevallier C, Troiani V, Schultz RT. Social ‘wanting’ dysfunction in autism: Neurobiological underpinnings and treatment implications. J Neurodev Disord. 2012;4(1):10. doi: 10.1186/1866-1955-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Averbeck BB. Oxytocin and the salience of social cues. Proc Natl Acad Sci USA. 2010;107(20):9033–9034. doi: 10.1073/pnas.1004892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parr LA, Modi M, Siebert E, Young LJ. Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions. Psychoneuroendocrinology. 2013;38(9):1748–1756. doi: 10.1016/j.psyneuen.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci USA. 2012;109(3):959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owen SF, et al. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500(7463):458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weisman O, Zagoory-Sharon O, Feldman R. Intranasal oxytocin administration is reflected in human saliva. Psychoneuroendocrinology. 2012;37(9):1582–1586. doi: 10.1016/j.psyneuen.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 43.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38(10):1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322(5903):900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 45.Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr Opin Neurobiol. 2010;20(6):784–794. doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 46.Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 47.Carter CS. Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol. 2013 doi: 10.1146/annurev-psych-010213-115110. in press. [DOI] [PubMed] [Google Scholar]
- 48.Sofroniew MV. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res. 1983;60:101–114. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- 49.Pow DV, Morris JF. Differential distribution of acetylcholinesterase activity among vasopressin- and oxytocin-containing supraoptic magnocellular neurons. Neuroscience. 1989;28(1):109–119. doi: 10.1016/0306-4522(89)90236-4. [DOI] [PubMed] [Google Scholar]
- 50.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7(2):126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 51.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30(4):534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 53.Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci USA. 2010;107(20):9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atzil S, Hendler T, Zagoory-Sharon O, Winetraub Y, Feldman R. Synchrony and specificity in the maternal and the paternal brain: Relations to oxytocin and vasopressin. J Am Acad Child Adolesc Psychiatry. 2012;51(8):798–811. doi: 10.1016/j.jaac.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Bos PA, Panksepp J, Bluthé RM, van Honk J. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: A review of single administration studies. Front Neuroendocrinol. 2012;33(1):17–35. doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28(26):6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riem MM, et al. No laughing matter: Intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology. 2012;37(5):1257–1266. doi: 10.1038/npp.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Dev Neuropsychol. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- 59.Schultz RT. Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23(2-3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 60.Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Res. 2010;3(2):53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dichter G, Adolphs R. Reward processing in autism: A thematic series. J Neurodev Disord. 2012;4(1):20. doi: 10.1186/1866-1955-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McPartland JC, et al. Preserved reward outcome processing in ASD as revealed by event-related potentials. J Neurodev Disord. 2012;4(1):16. doi: 10.1186/1866-1955-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lord C, et al. Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19(2):185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- 64.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 65.Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63(1):3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 66.Guastella AJ, MacLeod C. 2012 A critical review of the influence of oxytocin nasal spray on social cognition in humans: Evidence and future directions. Horm Behav 61(3):410–418 and erratum (2012) 61(5):773. [Google Scholar]
- 67.Forman SD, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 68.Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27(5):392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carter CS. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behav Brain Res. 2007;176(1):170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.