Significance
FMS-like tyrosine kinase 3 (FLT3) is mutated in approximately a third of acute myeloid leukemia cases, predominantly in the forms of FLT3/internal tandem duplication mutations in the juxtamembrane domain or point mutations in the kinase domain. Although both mutations activate FLT3, they confer distinctive prognosis. To elucidate the mechanisms behind this prognostic difference, we have generated a knock-in mouse model with a FLT3/D835Y mutation that converts aspartic acid to tyrosine. Further comparisons of the FLT3/D835Y mice with the previously generated, otherwise genetically identical FLT3/ITD knock-in mice should provide an ideal platform for dissecting the molecular mechanisms that underlie the prognostic differences and for drug screening against these two different types of FLT3 mutations.
Abstract
FMS-like tyrosine kinase 3 (FLT3) is mutated in approximately one third of acute myeloid leukemia cases. The most common FLT3 mutations in acute myeloid leukemia are internal tandem duplication (ITD) mutations in the juxtamembrane domain (23%) and point mutations in the tyrosine kinase domain (10%). The mutation substituting the aspartic acid at position 838 (equivalent to the human aspartic acid residue at position 835) with a tyrosine (referred to as FLT3/D835Y hereafter) is the most frequent kinase domain mutation, converting aspartic acid to tyrosine. Although both of these mutations constitutively activate FLT3, patients with an ITD mutation have a significantly poorer prognosis. To elucidate the mechanisms behind this prognostic difference, we have generated a knock-in mouse model with a D838Y point mutation in FLT3 that corresponds to the FLT3/D835Y mutation described in humans. Compared with FLT3/ITD knock-in mice, the FLT3/D835Y knock-in mice survive significantly longer. The majority of these mice develop myeloproliferative neoplasms with a less-aggressive phenotype. In addition, FLT3/D835Y mice have distinct hematopoietic development patterns. Unlike the tremendous depletion of the hematopoietic stem cell compartment we have observed in FLT3/ITD mice, FLT3/D835Y mutant mice are not depleted in hematopoietic stem cells. Further comparisons of these FLT3/D835Y knock-in mice with FLT3/ITD mice should provide an ideal platform for dissecting the molecular mechanisms that underlie the prognostic differences between the two different types of FLT3 mutations.
FLT3 (FMS-like tyrosine kinase 3, CD135) is a type 3 receptor tyrosine kinase that plays important roles in cell survival, proliferation, and differentiation during normal hematopoiesis (1–3). It is one of the most frequently mutated genes in acute myeloid leukemia (AML), with about one-third of AML cases affected (4–6). Two predominant types of FLT3-activating mutations have been described in association with AML. The first involves internal tandem duplication (ITD) mutations mapping to the juxtamembrane domain of FLT3, found in about 25% of adult and 15% of pediatric AML cases (4–9). AML patients with FLT3/ITD mutations have a poorer prognosis than patients without these mutations. The second type is point mutations, which most frequently occur in the activation loop of the tyrosine kinase domain (KD). FLT3/KD mutations occur in ∼8–10% of both adult and pediatric AML (10). AML patients who have FLT3/KD mutations have a similar prognosis to AML patients with unmutated FLT3 (10–13). The most common KD mutation is at codon 835, converting aspartic acid to tyrosine (D835Y). Also seen are mutations D835V, D835E, and D835H, converting aspartic acid to valine, glutamic acid, and histidine at residue 835, respectively, mutations converting glycine to glutamic acid at residue 831 (G831E) and arginine to glutamine at residue 834 (R834Q), as well as the deletion of isoleucine at residue 836 (10–12).
On dimerization by FL stimulation, wild-type FLT3 activates a series of downstream signaling targets, including the p85 subunit of phosphoinositide 3-kinase (PI3K), growth factor receptor-bound protein 2 (GRB2), proto-oncogene tyrosine-protein kinase SRC, and SH2-containing inositol phosphatase (SHIP), suggesting its involvement in the PI3K and mitogen-activated protein kinases (RAS-MAPK) pathway (4–6, 14). Wild-type FLT3 activates STAT5 at a low level (15). FLT3/ITD mutations activate these same downstream targets but, in contrast, activate STAT5 at an elevated level (16, 17). Evidence from cell lines has shown that FLT3/ITD and FLT3/KD mutant signal transduction pathways have some differences, even though both mutations result in the constitutive activation of FLT3 independent of its ligand (3–5, 9, 14, 18). FLT3/KD mutations also induce activation of the RAS, ERK, and AKT pathways but demonstrate relatively weak STAT5 activity (19). The differences in signal transduction between FLT3/KD and FLT3/ITD mutations may help explain the distinctive disease progression and prognosis in leukemias harboring these mutations.
Cytogenetic, PCR, and sequencing studies show that FLT3 mutations frequently occur together with mutations/alterations of other genes, including DNA methyltransferase 3a (DNMT3a, 13.3%), nucleophosmin 1 (NPM1, 6.8%), Wilms tumor 1 (WT1, 5%), runt-related transcription factor 1 (RUNX1, 3.5%), mixed-lineage leukemia (MLL, 2.5%), CCAAT/enhancer binding protein alpha (CEBPα, 1.5%) and core-binding factor (CBF, 1.5%) (20, 21). These studies also show that both the FLT3 mutations (FLT3/ITD or FLT3/KD), as well as the collaborating mutations, can have prognostic significance. Dissecting the role of each type of FLT3 mutation in the development of leukemia in the setting of the different cooperating mutations promises to improve our understanding, and thus therapy, of patients with AML.
The prevalence and prognostic implications of FLT3 mutations make them a promising therapeutic target in AML. A number of small-molecule tyrosine kinase inhibitors (TKI) against FLT3 are currently in clinical trials, with varying degrees of clinical responses, but even those patients who respond develop resistance to monotherapy (22). One of the mechanisms of acquired resistance to several FLT3 TKIs is the selection for FLT3/KD mutations documented in relapsed patients (23, 24). Further understanding of the mechanisms involved in FLT3 activation, signaling, inhibition, and resistance will aid in understanding the complexities of both pediatric and adult AML and help develop more tailored and effective treatments. We have previously generated and characterized a FLT3/ITD knock-in mouse model that consistently develops predominantly a myeloproliferative neoplastic (MPN) phenotype (25). Here we report on the generation of a FLT3/D835Y knock-in mouse model in which an aspartic acid residue was mutated to a tyrosine at residue D838Y (corresponding to D835Y in the human gene, Fig. 1A). In both models, Flt3 is under control of the endogenous Flt3 promoter mimicking physiological expression. By comparing the phenotypic and signaling differences between FLT3/ITD and FLT3/D835Y mice, we hope to continue to explore the distinctive roles the two mutations play in leukemogenesis and why only the FLT3/ITD mutation results in poor prognosis.
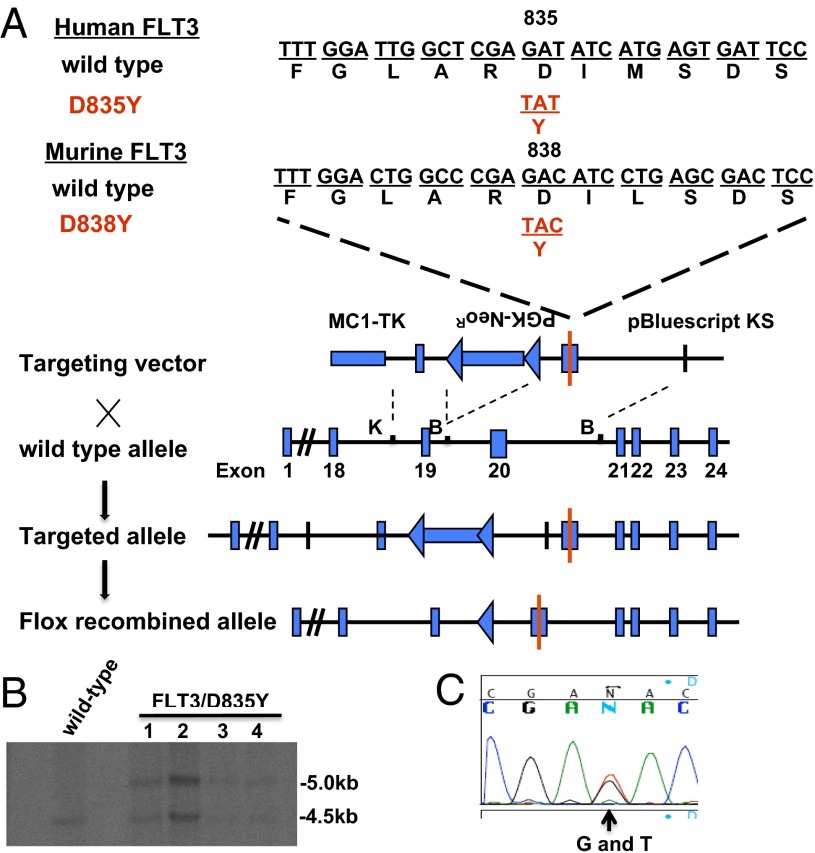
Fig. 1.
Generation of a mouse model with FLT3/D835Ymutation. (A) Strategy for targeted insertion of the D838Y point mutation into the murine Flt3 locus to generate a knock-in mouse model. K, KpnI; B, BamHI. (B) Southern blotting analysis was used to screen for ES clones with homologous recombination. (C) PCR was used to amplify the region flanking exon 20 of murine flt3 genomic DNA in screened homologously recombined ES clones followed by sequencing to confirm the presence of the G to T mutation at codon D838.
Results
Mice with a FLT3/D835Y Mutation Develop MPN That Is Less Aggressive Than That of FLT3/ITD Mutant Mice.
AML patients with FLT3/KD mutations develop less-aggressive disease compared with those with FLT3/ITD mutations, as evidenced by lower WBC and better prognosis (13). There are a number of potential reasons that could explain this prognostic difference that would be independent of the FLT3 mutations themselves, such as the different types of mutations being acquired by different cell populations or with different cooperating mutations. To determine whether the prognostic difference reported in humans would also be observed in genetically identical mouse models that differ only in the type of FLT3 mutation, we directly compared FLT3/ITD mice with the newly generated FLT3/D835Y mice. Mice were monitored for survival and killed when moribund or demonstrating obvious clinical distress. The median survival of the heterozygous FLT3/D835Y mice was 678 d and ranged from 394 to 826 d. This is prolonged survival compared with the heterozygous FLT3/ITD mice that have a median survival of 430 d (P < 0.0001; Fig. 2A).
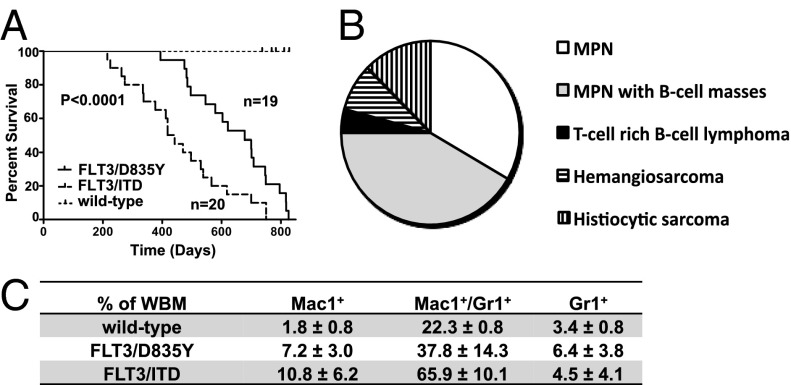
Fig. 2.
Mice with a FLT3/D835Y mutation develop less aggressive disease compared with FLT3/ITD mutant mice. (A) Kaplan-Meier survival curve of FLT3/ITD and FLT3/D835Y mice on a C57BL/6 background. P < 0.0001. (B) Disease distribution of moribund FLT3/D835Y mice (n = 24). (C) Levels of Mac1+, Mac1+/Gr1+, and Gr1+ as a percentage of whole BM in moribund FLT3/ITD (n = 20), FLT3/D835Y (n = 21; hemangiosarcoma diagnoses excluded), and wild-type (n = 11) controls.
FLT3/ITD mice have been previously shown to uniformly develop a MPN that is characterized by splenomegaly, myeloid expansion in the bone marrow (BM), and mild to moderately elevated peripheral WBC counts (25, 26). In contrast, most of the FLT3/D835Y mice (18/24) developed a MPN that was less aggressive [longer survival, mildly elevated peripheral blood (PB) counts (10.9–23.4 × 103/μL; 1 with 53.4 × 103/μL], and lower levels of myeloid expansion) (Fig. 2B). Approximately half (10/18) of the FLT3/D835Y mice that developed an MPN also had extranodal B-cell masses expressing paired box 5 (Pax5). These masses were frequently adherent to or involved the gut and/or were located within the chest cavity. All mice with an MPN had varying degrees of extramedullary hematopoiesis or a myeloid infiltrate within the red pulp areas of the spleen. Many of these mice also showed expanded white pulp. The majority of the lymphocytes in the white pulp expressed Pax5, which demonstrates a B-cell expansion. This pattern of splenic involvement is often seen in B-cell neoplasms. Three mice developed a histocytic sarcoma with BM and splenic involvement. Of these mice, two had a marked thrombocytosis in the PB and increased megakaryocytes that formed clusters in the BM and spleen, consistent with a concomitant myeloproliferative neoplasm. Splenic hemangiosarcoma was found in two of 24 mice, and one of these mice also showed a concurrent B-cell expansion in the spleen. One mouse was diagnosed with a T-cell-rich B-cell lymphoma. Immunophenotyping of the BM from sick FLT3/D835Y mice demonstrated expanded single positive Mac-1+, Gr-1+ or Mac-1+/Gr-1+ fractions compared with wild-type controls, although again at lower levels than those of FLT3/ITD mice, which is consistent with a less-extensive myeloid expansion in the FLT3/D835Y mice (Fig. 2C).
To determine whether there were also differences in the age at which disease manifests, age-matched, wild-type FLT3/D835Y and FLT3/ITD mice were killed and examined for a variety of parameters. At 12 wk of age, the heterozygous FLT3/D835Y mice showed evidence of splenomegaly, although again, it was less severe compared with that seen in FLT3/ITD mice (Fig. 3A). In contrast to the 12-wk-old FLT3/ITD mice, which consistently showed elevated fractions of cells expressing Mac-1+, Gr-1+, or Mac-1+/Gr-1+ in the BM, 12-wk-old FLT3/D835Y mice only showed elevated levels of Gr-1+ cells compared with wild-type mice (Fig. 3B). Lineage negative (Lin−) cells from the BM of 12-wk-old FLT3/D835Y mice formed more colony forming unit-granulocyte/monocyte colonies than wild-type mice via in vitro colony-forming assay (Fig. S1). Histopathology of BM and spleen from mice at both 3 and 12 mo showed an intermediate level of architectural disruption and cellular expansion in the FLT3/D835Y mice compared with wild-type and FLT3/ITD mice (Fig. 3C). FLT3/D835Y mice demonstrated expansion of the white pulp by a homogenous cell population, resulting in moderate effacement of the normal splenic architecture by 3 mo, with further progression by 12 mo. In contrast, more extensive expansion and marked effacement were observed in FLT3/ITD mice at both points. The BM of FLT3/D835Y mice showed a less uniform distribution of erythroid and myeloid hematopoiesis, with some areas showing expansion of uniform cell populations in both 3- and 12-mo-old mice. In contrast, the normal BM architecture in the 3- and 12-mo-old FLT3/ITD mice is largely replaced by myeloid progenitors.
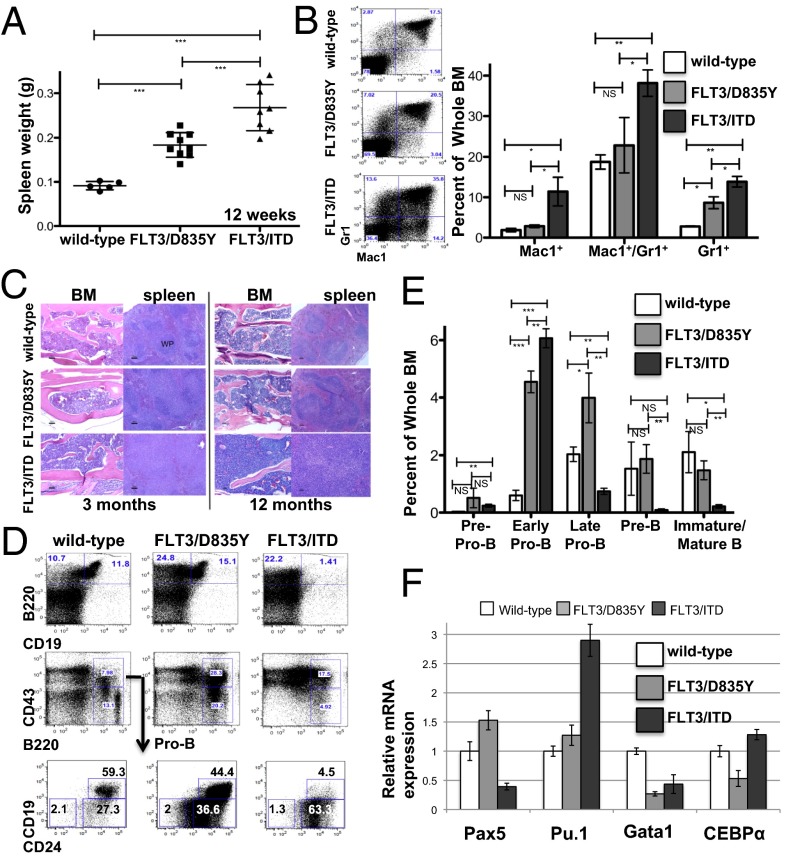
Fig. 3.
FLT3/D835Y mice develop a less aggressive MPN and less significant block in B-cell development relative to FLT3/ITD mice. (A) Twelve-week-old FLT3/D835Y mice have intermediate spleen size compared with the wild-type and FLT3/ITD mice. (B) Representative FACS analysis and graphical presentation of Mac1 and Gr1 expression in BM from 12-wk-old wild-type, FLT3/D835Y, and FLT3/ITD mice. (C) H&E staining of BM and spleen of all three genotypes at 3 and 12 mo. (Scale bar, 100 μM.) (D) Immunophenotypic analysis using B-lymphocytic-related markers of the BM of 12-wk-old mice. (E) Graphical presentation of the frequencies of cells at different stages of B-cell development, as assessed in D. Data are representative of 3 separate experiments (n = 3 for each experiment). (F) Expression levels of transcription factors in B220+CD43+CD19− cells from all of the three types of mice, evaluated by quantitative RT-PCR. Data are expressed as mean ± SEM (error bars); P values on graphs: NS, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001.
A block in B-cell development occurs in the BM of FLT3/ITD mice between the early and late Pro-B transition (27). Interestingly, BM from the FLT3/D835Y mice did not demonstrate this same block but, rather, an expansion of Pre-Pro-B, early and late Pro-B-cell populations that are able to develop further to more mature B-cell fractions (Fig. 3 D and E) (27, 28). The development of B cells, similar to all other hematopoietic cells, is strictly controlled by multiple transcription factors. We wanted to determine whether altered levels of transcription factors involved in B-cell and myeloid lineage fate contribute to the differences in B-cell development between the FLT3/D835Y and FLT3/ITD mice. Studies show that Pax5 plays a key role in promoting B-cell development through repression of essential receptors for other differentiation pathways. High levels of PU.1 expression drive cells toward myeloid differentiation, whereas high levels of PU.1/CEBPα and low levels of GATA binding protein 1 (GATA-1) are essential for differentiation into granulocytic/monocytic lineage (29–31). Thus, we focused on determining the level of expression of these transcription factors in the Pre-Pro-B and early Pro-B (B220+CD43+CD19−) fractions that were found to be expanded in both the FLT3/ITD and FLT3/D835Y mice. FLT3/ITD mice demonstrated reduced Pax5 expression, consistent with the observed block of B-cell development at this stage (Fig. 3F). These FLT3/ITD mice also had elevated levels of PU.1 and CEBPα and an extremely low level of GATA-1 expression. These levels may partly contribute to increased myeloid expansion, as seen in the BM of these mice. In contrast, the same population of sorted BM cells from FLT3/D835Y mice demonstrated increased Pax5 expression. Thus, the expansion of early B-cell progenitors (Pre-Pro-B and early-Pro-B cells) and increased expression of Pax5 may partly explain the accumulation of the more mature B-cell fractions (late pro-B and onward) in the FLT3/D835Y mice. The Pu.1 expression level in these mice is much closer to that of wild-type mice, which may help explain why most of the FLT3/D835Y mice displayed mildly increased myeloid expansion, whereas the FLT3/ITD mice demonstrated more marked myeloid expansion.
Taken together, the data demonstrate that FLT3/D835Y mutations confer a less-aggressive MPN and less-significant block in B-cell development relative to FLT3/ITD mice. The mice also develop a broader spectrum of diseases that includes lymphomas, perhaps because of the effects of FLT3/D835Y on B-cell development. Histocytic sarcomas and hemangiosarcomas were also observed, although these may be background lesions that develop in aging wild-type C57BL/6 mice and be reflective of their prolonged survival relative to FLT3/ITD mice, and thus unrelated to the genotype.
BM from FLT3/D835Y Mice Does Not Demonstrate the Hematopoietic Stem Cell Deficiency Seen in FLT3/ITD Mice.
We recently described a defect in hematopoietic stem cells (HSCs) in FLT3/ITD knock-in mice mediated by FLT3 signaling. It had been noted that Lin− or whole BM from FLT3/ITD mice has a reduced ability to engraft and recapitulate MPN in competitive transplantation and retransplantation assays (32). The BM of FLT3/ITD mice contain a reduced fraction of functional c-Kit+ Sca-1+ Lin− (KSL)-signaling lymphocyte activation molecule (defined as KSL-SLAM) cells (Lin− c-Kit+ Sca-1+ CD150+ CD48−), and this was shown to be responsible for the HSC defect. To determine whether FLT3/D835Y mice displayed a similar reduction in HSCs, we analyzed the frequencies of individual fractions of the hematopoietic compartment of 10–12-wk-old mice by flow cytometry. Both types of FLT3 mutant mice have increased levels of KSL cells compared with wild-type mice. The fractions of multipotent progenitor cells (Lin−c-Kit+Sca-1+CD135+CD34+) in the BM from FLT3/D835Y and FLT3/ITD mice were both elevated compared with that of wild-type mice (Fig. 4 A and B). Interestingly, the BM from FLT3/D835Y mice had ∼3 times the frequency of long-term HSCs (LT-HSCs, Lin−c-Kit+Sca-1+CD135−CD34−) compared with wild-type or FLT3/ITD mice (Fig. 4C). However, the fraction of KSL SLAM (Lin−c-Kit+Sca-1+CD150+CD48−) cells in the FLT3/D835Y mice was comparable to that of the wild-type mice, but much higher than that of the FLT3/ITD mice (Fig. 4D).
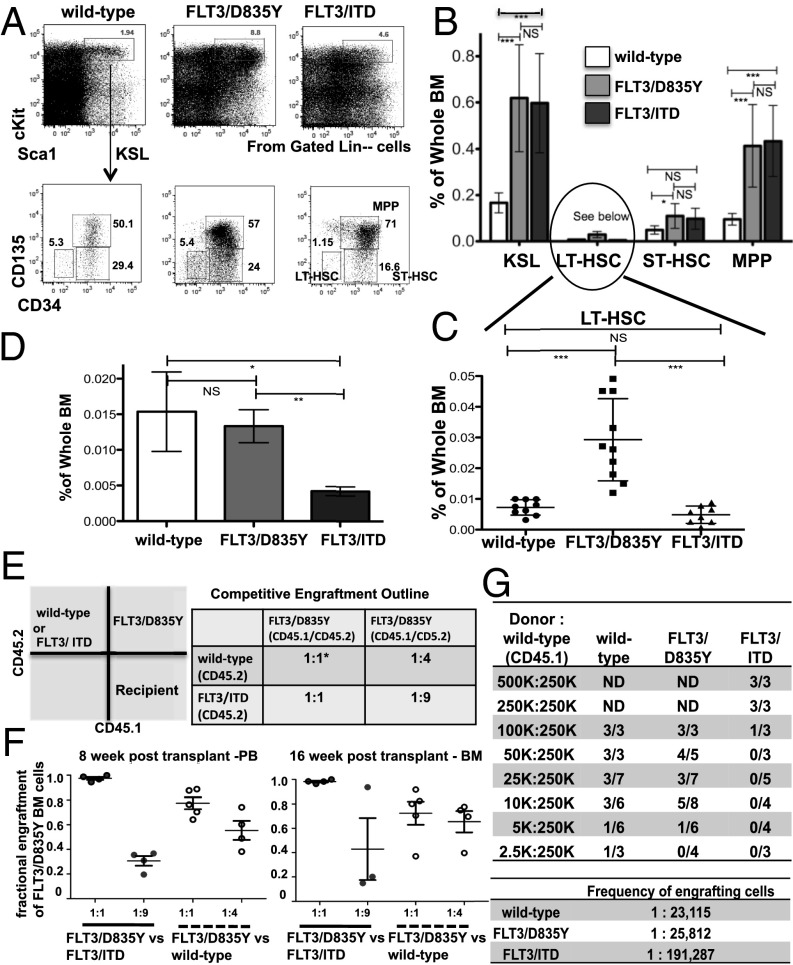
Fig. 4.
BM from FLT3/D835Y mice does not demonstrate the hematopoietic stem cell deficiency seen in FLT3/ITD mice. (A–C) Flow cytometry analysis and graphical presentation of the frequencies of KSL compartments from the BM of 3-mo-old mice. Data are combined from 3 independent experiments (FLT3/ITD, n = 8; FLT3/D835Y, n = 10; wild-type, n = 9). (D) KSL SLAM cells are not depleted in the BM from FLT3/D835Y mice. Data are combined from four independent experiments with n = 3 in each experiment. (E) Outline for competitive engraftment experiment. Lin− BM from donor mice was injected into lethally irradiated CD45.1+ recipient mice. *1 unit is equal to 75,000 Lin− BM cells. (F) Engraftment of BM as measured in the PB at 8 wk and in the BM at 16 wk in recipients of the competitive repopulation assays. (G) Wild-type and FLT3/D835Y mice have a higher frequency of engrafting cells compared with FLT3/ITD mice, as demonstrated by a limiting dilution assay. Data are combined from 3 independent experiments. Engraftment was defined as >1% CD45.2 positive by FACS analysis. BM from recipient mice was harvested and analyzed at 15–16 wk posttransplant. Frequency of engrafting cells was calculated using l-calc (Stemcell Technologies). Data are expressed as mean ± SEM (error bars). ND, not determined. P values on graphs: NS, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001.
To determine whether the differences in the fraction of the immunophenotypic LT-HSC compartments between FLT3/D835Y and FLT3/ITD mice would result in a functional difference in HSCs, we performed a competitive repopulation experiment using increasing ratios of FLT3/ITD and wild-type cells versus FLT3/D835Y cells (Fig. 4E). Transplanted at a 1:1 ratio, the FLT3/D835Y Lin− BM cells engrafted at a higher level vs both FLT3/ITD and wild-type cells, as measured at both 8 and 16 wk. (Fig. 4F).
The higher-level engraftment of FLT3/D835Y cells in the recipients could be a result of a proliferative advantage of the FLT3/D835Y progenitors or to increased numbers of functional LT-HSCs in the FLT3/D835Y BM. To separate the issue of increased proliferative capacity from HSC numbers, a limiting dilution transplantation assay experiment was conducted. A decreasing number of CD45.2+ whole BM cells from mice of each genotype (wild-type, FLT3/D835Y, and FLT3/ITD), along with a constant number of wild-type recipient CD45.1+ whole BM cells, were transplanted into CD45.1+ recipients, as outlined (Fig. 4G). Engraftment levels were measured from BM samples 15–16 wk posttransplant. Positive engraftment was defined as >1% donor cells. After calculating a frequency for engrafting cells (l-calc; StemCell Technologies) the FLT3/D835Y point mutant mice had approximately a 7.4-fold higher frequency of engrafting cells compared with the FLT3/ITD mice, and a similar frequency to that of wild-type mice. These data suggest that, unlike the deficits in HSCs from FLT3/ITD mice, the HSC compartment in FLT3/D835Y mice appears less affected, with KSL SLAM populations and functional engraftment capacity closer to that of wild-type mice.
BM Cells from FLT3/D835Y Mice Activate Targets Different from Those of the FLT3/ITD Mice.
Both FLT3/ITD and FLT3/KD mutations constitutively activate the tyrosine kinase activity of FLT3. Accumulating evidence suggests there are some differences in the downstream targets activated by FLT3 ligand (FL)-stimulated wild-type FLT3 versus FLT3/ITD mutants versus FLT3/KD mutants. For example, AKT, MAPK, and STAT5 activation have been well-documented as downstream targets of FLT3/ITD signaling, but there is little activation of STAT5 by wild-type FLT3 (18, 33). This lack of STAT5 activation has also been proposed to be one of the differences in signaling between FLT3/ITD and FLT3/KD mutants. However, most of this evidence comes from forced overexpression of these forms of FLT3 in cell lines, and there are few data from their physiologically controlled expression in primary hematopoietic cells. To investigate the downstream signaling pathways activated by FLT3/KD mutations, we compared pathways activated by FLT3/D835Y or FLT3/ITD mutations in the BM cells from both knock-in mice.
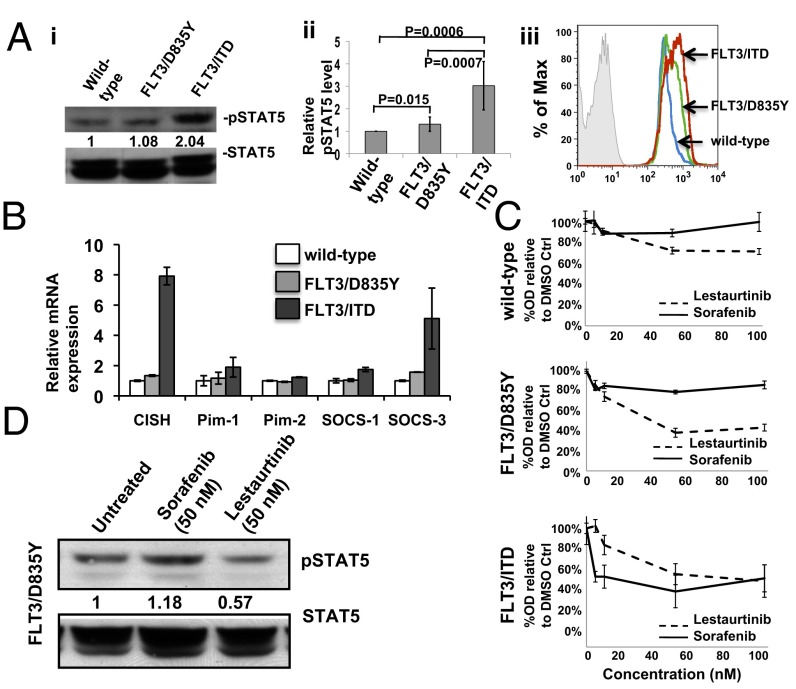
Phospho-STAT5 levels were higher in Lin− BM from FLT3/ITD mice compared with those in wild-type mice. Lin− BM from FLT3/D835Y mice demonstrated moderately increased STAT5 phosphorylation that was higher than that of the wild-type mice but significantly lower than that of the FLT3/ITD mice (P = 0.015 and 0.0007, respectively; Fig. 5A, i and ii). Consistent with the Western blot findings, phospho-flow analysis also displayed intermediately elevated phospho-STAT5 levels in sorted KSL cells from the FLT3/D835Y mice, which is significantly lower than that from the FLT3/ITD mice but slightly higher than the wild-type control (Fig. 5Aiii). This important data from genetically engineered primary hematopoietic cells is further evidence that differences in the level of activation of STAT5 may account for some of the prognostic differences attributable to FLT3/ITD and FLT3/KD mutations. These data are consistent with what has been observed previously in primary AML blasts and cell lines with FLT3 mutations. To further investigate whether the FLT3/D835Y mutation activates STAT5 at a higher level than in wild-type cells, we examined expression of downstream targets of STAT5 signaling, including cytokine-inducible SH2-containing protein (CISH), Pim1 and 2, and suppressor of cytokine signalling (Socs)1 and 3, by RT-PCR of Lin− BM cells. FLT3/ITD cells demonstrated higher levels of STAT5 downstream target expression compared with wild-type cells, consistent with the stronger STAT5 phosphorylation of FLT3/ITD cells shown by Western analysis. In contrast, expression of STAT5 downstream targets in cells from FLT3/D835Y mice are lower than those from the FLT3/ITD mice, with most of the genes expressed at levels not significantly different from that of wild-type cells (Fig. 5B).
Fig. 5.
BM cells from FLT3/D835Y mice activate targets different from those of the FLT3/ITD mice. (A, i) Representative Western blot of phospho- and total Stat5 levels from Lin− BM. Ratios below the blot indicate quantization of relative level of phosphorylation normalized to total protein levels, as determined by densitometry, using QuantityOne densitometry analysis software. (A, ii) Graphical presentation of the relative phosph-STAT5 levels in Lin− BM cells shown by Western blot, as determined by densitometry (n = 7 independent mice and Western blots for each group). (A, iii) Representative flow cytometry analysis showing moderately elevated pSTAT5 level in sorted KSL cells from FLT3/D835Y mice. (B) Relative expression of some of the downstream targets of Stat5 signaling in Lin− BM cells, as determined by quantitative RT-PCR. Data are expressed as mean ± SEM (error bars). (C) Viability assay using whole BM from 6-mo-old mice. (D) Western blot of pStat5 and total Stat5 from Lin− BM cells from a 6-mo-old FLT3/D835Y mouse incubated for 1 h with either Sorafenib or Lestaurtinib, illustrating that pStat5 levels from FLT3/D835Y cells only respond to Lestaurtinib.
Cells with different types of FLT3 mutations may respond to TKIs differently, depending on the types of the mutations they harbor (34). For example, Lestaurtinib and Sorafenib are two TKIs effective against FLT3/ITD mutations, but only Lestaurtinib is effective against FLT3/KD mutations. To explore the efficacy of these two FLT3 TKIs on whole BM cells from the FLT3/ITD and FLT3/D835Y mice, they were treated in vitro with Sorafenib or Lestaurtinib and assayed for proliferation by cell counting. After 48 h of treatment, the FLT3/D835Y BM responded to Lestaurtinib but not Sorafenib, whereas the FLT3/ITD BM was sensitive to both drugs. There was little response of BM from wild-type mice to either drug (Fig. 5C). Western blot analysis demonstrated phospho-STAT5 levels from Lin− BM cells from FLT3/D835Y mice only decreased after incubation with Lestaurtinib, and not Sorafenib (Fig. 5D). These data indicate that cells from FLT3/D835Y mice have distinctive responses to different TKIs.
In summary, FLT3/D835Y knock-in mice develop less aggressive disease compared with FLT3/ITD mice. The FLT3/KD mice are characterized by increased survival, less severe myeloproliferation, no significant block in B-cell differentiation, no appreciable HSC defect, and a broader spectrum of disease. Thus, we can conclude that the mutations themselves confer the degree of aggressiveness of disease. These mice model the difference seen in AML patients with the two types of mutations. Hematopoietic cells from FLT3/D835Y mice activate STAT5 to a lower extent than seen in the FLT3/ITD mice. This signaling difference might explain at least part of the disease differences noted between these mice.
Discussion
The reasons for the prognostic differences between patients harboring a FLT3/ITD versus a FLT3/KD mutation are not fully understood. They could be explained by a number of reasons, including differences in the type of hematopoietic cells in which the mutations occur, a tendency to occur with different cooperating mutations such that the prognosis is actually the result of the cooperating mutation and not FLT3, or differences in downstream signaling that have been previously observed. In this study, we compare and contrast the aggressiveness of disease mediated by the two types of FLT3-activating mutations most frequently identified in human AML. We investigated these mutations by generating mouse models in which each of these mutations has been “knocked in” to the FLT3 locus through homologous recombination. Using this technique ensures both mutations are expressed under control of the endogenous FLT3 promoter, and thus are expressed at physiological levels and in the appropriate hematopoietic populations. Thus, it is a better model than those in which FLT3 mutant constructs are transfected into cell lines or primary hematopoietic cells, where control is that of an exogenous promoter. Thus, if the first or second reasons given here were the explanation for the differences in prognosis, we would expect to see identical phenotypes and severity of disease in the knock-in mice.
FLT3/D835Y mice showed much less aggressive disease than FLT3/ITD mice in terms of both disease severity and overall survival. The phenotypes mediated by the two types of FLT3 mutations parallel the disease aggressiveness observed in AML patients with FLT3/KD mutations (average prognosis and WBC levels) compared with FLT3/ITD mutations (poor prognosis and elevated WBC levels). It appears that constitutive activation of FLT3 by KD mutations provides the FLT3-expressing cells with enhanced proliferation and survival. However, as described previously for the FLT3/ITD mice, a FLT3/D835Y mutation alone is insufficient to cause leukemia. Given the broad spectrum of the hematological diseases the FLT3/D835Ymice developed, it is likely that both the developmental stages at which the cooperative mutations occur and the types of the cooperative mutations determine the type of disease that develops.
We recently observed that the FLT3/ITD mutation expands the myeloid progenitor populations at the cost of the mobilization of KSL SLAM cells into the cell cycle, thus depleting the quiescent HSC compartment. Similar to the FLT3/ITD mice, the FLT3/D835Y mice also displayed a mild expansion of progenitor fractions, including short-term HSCs, multipotent progenitors, common lymphoid progenitors, and common myeloid progenitors. However, the frequency of KSL SLAM cells in FLT3/D835Y mice is comparable to that in wild-type mice. Functionally, FLT3/D835Y BM showed an equivalent or slightly higher engraftment ability compared with wild-type mice, and both are higher than that of the FLT3/ITD mice. These results suggest that the more quiescent HSC pool is not affected by signaling through the FLT3/D835Y mutations and that the FLT3/D835Y mutation does not mobilize the functional HSCs capable of long-term engraftment into cell cycle.
Although both FLT3/ITD and FLT3/KD mutations confer constitutive activation to the FLT3 receptor, accumulating evidence suggests there are also some differences in the downstream pathways they activate, and thus in the transcription profiles of each (18, 19). It has been proposed that the altered juxtamemebrane domain (JM) domain of the FLT3/ITD mutant destabilizes the autoinhibited kinase conformation of FLT3, resulting in a high level of autophosphorylation of the receptor and its subsequent retention in the perinuclear region with prolonged exposure to perinuclear substrates such as STAT5. In contrast, the normal JM domain of FLT3/KD mutants is more efficiently processed and trafficked to the plasma membrane, and thus would activate downstream signals more similar to those of wild-type FLT3 (33). Consistent with this, we observed significantly higher levels of STAT5 phosphorylation, as well as increased expression of STAT5 downstream targets, in the BM of FLT3/ITD mice compared with FLT3/D835Y mice, supporting the idea that the two mutations have at least this one difference in the activation of downstream pathways. STAT5 is one of the pathways frequently involved in leukemic transformation. Constitutive activation of STAT5 and its downstream targets have been shown to promote cell proliferation and prolong cell survival. The weak STAT5 activation by FLT3/KD mutation may account for the differences between the effects of FLT3/D835Y and FLT3/ITD mutations on HSC biology.
Overall, the FLT3/D835Y mice develop less aggressive hematopoietic disease, with a broader range of diseases compared with the FLT3/ITD mice. Similar to the FLT3/ITD mutation, the FLT3/D835Y mutation alone is insufficient to cause leukemia in the absence of cooperative mutational events. Functional HSCs are not disturbed by signaling of the FLT3/D835Y mutation, in contrast to the significant HSC depletion seen in the FLT3/ITD mice. This FLT3/D835Y mouse model, in parallel with the FLT3/ITD mouse model, should provide a useful platform for further understanding the molecular mechanisms of leukemogenesis in which FLT3 mutations participate. Most important, it should enable us to further explore why poor prognosis in AML patients is only associated with FLT3/ITD mutations.
Materials and Methods
Generation of a Mouse Model Containing a Knock-in D838Y (D835Y Equivalent) Point Mutation in the Mouse Flt3 Gene.
A 10-kb fragment spanning exons 18–24 of mouse Flt3 genomic DNA (obtained from the RPCI-22 mouse 129S6/SvEvTac bacterial artificial chromosome library; Roswell Park Cancer Institute) was isolated to construct the targeting vector. PCR site-directed mutagenesis was used to generate the point mutation, substituting the aspartic acid at position 838 (equivalent to the human aspartic acid residue at position 835) with a tyrosine (referred to as FLT3/D835Y hereafter). (Fig. 1A). Southern blot and sequencing were used to verify successful homologous recombination and the presence of the D838Y mutation (Fig. 1 B and C). Additional details including primers for embryonic stem cell clone screening and genotyping are listed in the SI Materials and Methods. All mice in this report were backcrossed to >98% C57BL/6 background, using speed congenics, and housed in a pathogen-free environment in microisolator cages at the Johns Hopkins School of Medicine. Procedures and experiments were approved by the university’s institutional animal care and use committee.
Histopathology, Cytology, and Immunohistochemistry Assays.
Murine tissues were harvested, preserved, and analyzed as described previously (25, 35). Immunohistochemistry on tissues from the moribund cohort was performed using myeloperoxidase, CD3, and Pax5 using a sodium citrate buffer (pH 6) and high heat for antigen retrieval. Please see SI Materials and Methods for expanded details on flow cytometry analysis, Western blot, RT-PCR, and viability assay.
BM Transplantation and Engraftment Assays.
Ly5.2 mice (CD45.1+; National Cancer Institute) were lethally irradiated (10 Gy) and injected with whole BM or sorted fractions of BM cells 4–6 h after irradiation. PB was collected via facial vein to monitor engraftment every 4 wk. Engraftment levels were analyzed by comparing CD45.1+/CD45.2+ levels in the BM via FACS.
P values were preformed using an unpaired t test with a 95% confidence interval for each grouping of samples. For the survival curve, P value was calculated using a Log-rank (Mantel-Cox) test.
Supplementary Material
Acknowledgments
We thank Ursula L. Harper (Genome Technology Branch of the National Human Genome Research Institute) for performing the speed congenics work and the Johns Hopkins transgenic core for their assistance in generating the chimeric founder mice. This work was supported by the National Institutes of Health Research Project Grant CA 090668 and Program Project Grant CA 070970, the Leukemia & Lymphoma Society, and the Giant Food Pediatric Cancer Research Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.S. is a guest editor invited by the Editorial Board.
See Commentary on page 20860.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310559110/-/DCSupplemental.
References
- 1.Matthews W, Jordan CT, Wiegand GW, Pardoll D, Lemischka IR. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell. 1991;65(7):1143–1152. doi: 10.1016/0092-8674(91)90010-v. [DOI] [PubMed] [Google Scholar]
- 2.Rosnet O, Matteï MG, Marchetto S, Birnbaum D. Isolation and chromosomal localization of a novel FMS-like tyrosine kinase gene. Genomics. 1991;9(2):380–385. doi: 10.1016/0888-7543(91)90270-o. [DOI] [PubMed] [Google Scholar]
- 3.Small D, et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci USA. 1994;91(2):459–463. doi: 10.1073/pnas.91.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 5.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 6.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3(9):650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 7.Schnittger S, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: Correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100(1):59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 8.Kottaridis PD, Gale RE, Linch DC. Prognostic implications of the presence of FLT3 mutations in patients with acute myeloid leukemia. Leuk Lymphoma. 2003;44(6):905–913. doi: 10.1080/1042819031000067503. [DOI] [PubMed] [Google Scholar]
- 9.Meshinchi S, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97(1):89–94. doi: 10.1182/blood.v97.1.89. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97(8):2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 11.Kottaridis PD, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 12.Fröhling S, et al. AML Study Group Ulm. Acute myeloid leukemia Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: A study of the AML Study Group Ulm. Blood. 2002;100(13):4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 13.Mead AJ, et al. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110(4):1262–1270. doi: 10.1182/blood-2006-04-015826. [DOI] [PubMed] [Google Scholar]
- 14.Leischner H, et al. SRC is a signaling mediator in FLT3-ITD- but not in FLT3-TKD-positive AML. Blood. 2012;119(17):4026–4033. doi: 10.1182/blood-2011-07-365726. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, et al. Essential role of signal transducer and activator of transcription (Stat)5a but not Stat5b for Flt3-dependent signaling. J Exp Med. 2000;192(5):719–728. doi: 10.1084/jem.192.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tse KF, Mukherjee G, Small D. Constitutive activation of FLT3 stimulates multiple intracellular signal transducers and results in transformation. Leukemia. 2000;14(10):1766–1776. doi: 10.1038/sj.leu.2401905. [DOI] [PubMed] [Google Scholar]
- 17.Hayakawa F, et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene. 2000;19(5):624–631. doi: 10.1038/sj.onc.1203354. [DOI] [PubMed] [Google Scholar]
- 18.Choudhary C, et al. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol Cell. 2009;36(2):326–339. doi: 10.1016/j.molcel.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Meshinchi S, Appelbaum FR. Structural and functional alterations of FLT3 in acute myeloid leukemia. Clin Cancer Res. 2009;15(13):4263–4269. doi: 10.1158/1078-0432.CCR-08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cancer Genome Atlas Research Network Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel JP, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu SH, Small D. Mechanisms of resistance to FLT3 inhibitors. Drug Resist Updat. 2009;12(1-2):8–16. doi: 10.1016/j.drup.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heidel F, et al. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006;107(1):293–300. doi: 10.1182/blood-2005-06-2469. [DOI] [PubMed] [Google Scholar]
- 24.Moore AS, et al. Selective FLT3 inhibition of FLT3-ITD+ acute myeloid leukaemia resulting in secondary D835Y mutation: A model for emerging clinical resistance patterns. Leukemia. 2012;26(7):1462–1470. doi: 10.1038/leu.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, et al. Knock-in of an internal tandem duplication mutation into murine FLT3 confers myeloproliferative disease in a mouse model. Blood. 2008;111(7):3849–3858. doi: 10.1182/blood-2007-08-109942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee BH, et al. FLT3 mutations confer enhanced proliferation and survival properties to multipotent progenitors in a murine model of chronic myelomonocytic leukemia. Cancer Cell. 2007;12(4):367–380. doi: 10.1016/j.ccr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, et al. Defective nonhomologous end joining blocks B-cell development in FLT3/ITD mice. Blood. 2011;117(11):3131–3139. doi: 10.1182/blood-2010-05-286070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardy RR, Kincade PW, Dorshkind K. The protean nature of cells in the B lymphocyte lineage. Immunity. 2007;26(6):703–714. doi: 10.1016/j.immuni.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Revilla-I-Domingo R, et al. The B-cell identity factor Pax5 regulates distinct transcriptional programmes in early and late B lymphopoiesis. EMBO J. 2012;31(14):3130–3146. doi: 10.1038/emboj.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeKoter RP, Singh H. Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science. 2000;288(5470):1439–1441. doi: 10.1126/science.288.5470.1439. [DOI] [PubMed] [Google Scholar]
- 31.Anguita E, et al. Globin gene activation during haemopoiesis is driven by protein complexes nucleated by GATA-1 and GATA-2. EMBO J. 2004;23(14):2841–2852. doi: 10.1038/sj.emboj.7600274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu SH, et al. FLT3-ITD knockin impairs hematopoietic stem cell quiescence/homeostasis, leading to myeloproliferative neoplasm. Cell Stem Cell. 2012;11(3):346–358. doi: 10.1016/j.stem.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan PM. Differential signaling of Flt3 activating mutations in acute myeloid leukemia: A working model. Protein Cell. 2011;2(2):108–115. doi: 10.1007/s13238-011-1020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Small D. Targeting FLT3 for treatment of leukemia. Semin Hematol. 2008;45(3 Suppl 2):S17–S21. doi: 10.1053/j.seminhematol.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L, Bailey E, Greenblatt S, Huso D, Small D. Loss of the wild-type allele contributes to myeloid expansion and disease aggressiveness in FLT3/ITD knockin mice. Blood. 2011;118(18):4935–4945. doi: 10.1182/blood-2011-01-328096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.