Significance
Transfer RNAs (tRNAs) traffic in a retrograde direction from the cytoplasm to the nucleus, a process that is conserved from yeast to vertebrates. The biological significance of retrograde tRNA nuclear import is poorly understood. We report that the tRNA retrograde pathway serves as a quality control pathway that monitors 5′ and 3′ end processing and the modification status of cytoplasmic tRNAs. Aberrant tRNAs are imported into the nucleus where they are repaired or destroyed. This newly discovered quality control function of the tRNA retrograde nuclear pathway likely functions in parallel with the cytoplasmic 5′–3′ exonuclease tRNA quality control pathway.
Keywords: tRNA retrograde pathway, tRNA processing errors, quality control pathways, Mtr10, Los1
Abstract
In eukaryotes, transfer RNAs (tRNAs) are transcribed in the nucleus yet function in the cytoplasm; thus, tRNA movement within the cell was believed to be unidirectional—from the nucleus to the cytoplasm. It is now known that mature tRNAs also move in a retrograde direction from the cytoplasm to the nucleus via retrograde tRNA nuclear import, a process that is conserved from yeast to vertebrates. The biological significance of this tRNA nuclear import is not entirely clear. We hypothesized that retrograde tRNA nuclear import might function in proofreading tRNAs to ensure that only proper tRNAs reside in the cytoplasm and interact with the translational machinery. Here we identify two major types of aberrant tRNAs in yeast: a 5′, 3′ end-extended, spliced tRNA and hypomodified tRNAs. We show that both types of aberrant tRNAs accumulate in mutant cells that are defective in tRNA nuclear traffic, suggesting that they are normally imported into the nucleus and are repaired or degraded. The retrograde pathway functions in parallel with the cytoplasmic rapid tRNA decay pathway previously demonstrated to monitor tRNA quality, and cells are not viable if they lack both pathways. Our data support the hypothesis that the retrograde process provides a newly discovered level of tRNA quality control as a pathway that monitors both end processing of pre-tRNAs and the modification state of mature tRNAs.
Transfer RNAs (tRNAs) are well known for their role as adaptor molecules during the translation of mRNAs into proteins, delivering amino acids to the ribosome for incorporation into a polypeptide chain. In eukaryotes, tRNAs are transcribed in the nucleus as primary transcripts that undergo extensive processing, chemical modification, and subcellular trafficking to produce the mature tRNAs that function in cytoplasmic translation. In the yeast Saccharomyces cerevisiae, tRNA transcription in the nucleolus (1), with one exception (2), is followed by removal of the 5′ leader sequence by RNase P (3). The 3′ trailer is subsequently removed endonucleolytically, presumably by tRNase Z (4–6), and/or exonucleolytically by Rex1 (7, 8). Following removal of the 3′ trailer, the nucleotides C74C75A76, necessary for tRNA aminoacylation, are added to the 3′ terminus. Twenty percent of yeast tRNAs are transcribed with an intron (9). Splicing of tRNA introns occurs in the cytoplasm in yeast, on the outer surface of mitochondria where the tRNA splicing endonuclease complex resides (10–12). Thus, end-processed intron-containing tRNAs are exported from the nucleus to the cytoplasm before removal of the intron. This primary export of intron-containing pre-tRNA is mediated by the β-importin family member Los1 (13–15). Because LOS1 is unessential and because it is essential to have tRNAs delivered to the cytoplasm, there is at least one additional unidentified nuclear exporter for end-processed intron-containing pre-tRNA (16).
tRNAs are subject to numerous posttranscriptional chemical modifications as they mature. Among the 42 unique tRNA species in yeast, 25 different modifications are found at 36 individual nucleotide positions (17). Of these nucleotide modifications, several are added to tRNAs exclusively in the nucleus while others are added in the cytoplasm (9). Although the biological functions of some modifications are not yet known, tRNA modifications in general contribute to overall tRNA function in three major ways (17–19): (i) several modifications in the anticodon stem loop, particularly in positions 34 and 37, function in decoding and in ensuring maintenance of the translational reading frame; (ii) modifications in the main body of the tRNA affect tRNA folding; and (iii) some modifications function as identity elements for tRNA aminoacylation, ensuring that tRNAs are charged with the proper amino acid.
Because the primary function of tRNA molecules is in the cytoplasmic process of translation, tRNA subcellular transport was thought to be unidirectional—from the nucleus to the cytoplasm. However, it is now well established that mature tRNAs constitutively move back to the nucleus via retrograde tRNA nuclear import, a process that is conserved from yeast to vertebrates (15, 20–25). Although the function of this tRNA nuclear import is not yet entirely clear, it is required for the biogenesis of the wybutosine base modification of yeast tRNAPheGAA (26) and for translation of particular yeast mRNAs (27).
Finally, mature tRNAs must be re-exported to the cytoplasm where they are essential for protein synthesis. tRNA re-export is mediated in yeast by both Los1 and another β-importin family member, Msn5 (15); because msn5Δ los1Δ double mutants are viable (15, 24), there is at least one unidentified transporter involved in tRNA re-export. Because the distribution of tRNAs in the cell is responsive to nutrients (21, 28, 29) and retrograde tRNA nuclear import is constitutive (15), re-export of mature tRNAs from the nucleus by Msn5 is likely regulated.
Although much is known about the regulation of tRNA transcription and about how cells ensure accurate tRNA aminoacylation, less is known about the quality control mechanisms that cells use to ensure that the appropriate levels of properly structured, modified, and charged tRNAs reside in the cytoplasm. An initial quality control step involves discrimination of precursor tRNAs during primary tRNA export. The vertebrate homolog of Los1, Exportin-t, proofreads tRNAs during export by preferentially binding to and exporting appropriately structured tRNAs with mature 5′ and 3′ ends (30–32). Aminoacylation of mature tRNA in the nucleus also plays a role in tRNA nuclear export, providing an additional level of proofreading (33–36). In yeast, the exportin Msn5, which exports only tRNAs encoded by genes lacking introns and tRNAs previously imported from the cytoplasm, may selectively export aminoacylated tRNAs (15, 33). There also are two pathways to degrade improper tRNAs. A nuclear surveillance pathway monitors pre-tRNAs and polyadenylates and degrades initiator tRNAMeti lacking m1A58 (37, 38) as well as tRNAs with unprocessed 3′ ends (7, 8). The rapid tRNA decay (RTD) pathway monitors mature tRNAs in both the nucleus and the cytoplasm, degrading those that are destabilized in structure (39–41).
Here, we report that retrograde tRNA nuclear import also functions in tRNA quality control. We show that two types of aberrant tRNAs—5′, 3′ end-extended, spliced tRNAIleUAU and m22G hypomodified tRNALysUUU and tRNATyrGUA—accumulate in mutant cells in which tRNA nuclear import is defective or when primary tRNA nuclear export is up-regulated, suggesting that the aberrant tRNAs are normally imported into the nucleus and are repaired or degraded. We show that tRNA retrograde nuclear import is a newly discovered level of tRNA quality control that functions in parallel with the RTD pathway, ensuring that only properly structured and modified tRNAs are in the cytoplasm interacting with the translational machinery.
Results
Aberrant tRNAs Accumulate in Cells in Which Retrograde tRNA Nuclear Import Is Defective.
Previous studies in yeast identified 5′, 3′ end-extended, spliced tRNAs that were thought to represent normal tRNA-processing intermediates in the nucleus (2, 42). However, it was subsequently shown that tRNAs are spliced in the cytoplasm in yeast (11, 12). Thus, these previously identified tRNA intermediates, which are spliced and therefore have accessed the cytoplasm yet retained their 5′ leader and 3′ trailer sequences, are in fact aberrant tRNAs. We hypothesized that a minor pool of aberrant tRNAs exists in the cytoplasm, including both 5′ and 3′ end-extended, spliced tRNAs as well as various hypomodified tRNAs and that, upon retrograde tRNA nuclear import, these aberrant tRNAs either might be repaired and be given a second chance to become functional tRNAs or might be degraded in the nucleus.
We first confirmed that yeast generate small pools of such aberrant 5′, 3′ end-extended, spliced tRNAs by studying tRNAIleUAU species by RT-PCR and Northern analyses (Fig. 1). In yeast, 10 tRNA families are transcribed with an intron located 3′ of nucleotide 37. tRNAIleUAU possesses the longest intron (60 nt) and therefore has the largest size difference between the nuclear tRNA transcript and the cytoplasmic mature tRNA. In addition, tRNAIleUAU lacks nucleotide modifications in the 5′ exon that block reverse transcriptase (43, 44). For these reasons, tRNAIleUAU is well suited for identification of its various intermediates by RT-PCR and Northern analyses.
Fig. 1.
The 5′, 3′ end-extended, spliced tRNAIleUAU accumulates in cells in which retrograde tRNA nuclear accumulation is defective. (A) Strategy to detect 5′, 3′ end-extended, spliced tRNAIleUAU by RT-PCR and cartoon depicting normal and aberrant (gray arrow) processing. Thin gray lines, 5′ leader and 3′ trailer; thick black lines, exons; thick gray lines, introns; slash, splice junction. Precocious nuclear export of the primary tRNA transcript followed by splicing produces the 5′, 3′ end-extended, spliced tRNA. (B) RT-PCR analysis of RNA from wild-type and mtr10∆ cells. PCR was performed with an annealing temperature of 45 °C. (C) RT-PCR analysis of RNA from wild-type, mtr10∆, and mtr10∆ cells transformed with plasmid pRSMTR10-MORF (lane 3) or MTR10End-MORF (lane 4). (D) RT-PCR analysis of RNA from wild-type, mtr10∆, dhh1∆ pat1∆, and xrn1∆ cells. (E) Northern analysis of RNA from wild-type, mtr10∆, and mtr10∆ cells transformed with plasmid MTR10End-MORF. The blot was probed with an oligonucleotide that anneals to the same position as primer 1. p, primary tRNA transcript; i, end-processed, intron-containing tRNA; a, aberrant 5′, 3′ end-extended, spliced tRNA; m, mature tRNA.
Reverse transcription using primer 1 or 2 (Fig. 1A) should produce cDNA corresponding to the three major tRNAIleUAU-processing intermediates: the primary transcript (Fig. 1A, p, ∼131 nt), the end-processed intron-containing pre-tRNA (Fig. 1A, i, 119 nt), and the mature tRNA (Fig. 1A, m, 59 nt). Based upon previous studies (42), we predicted that a minor pool of cDNA corresponding to an aberrant 5′ end-extended, spliced tRNAIleUAU (Fig. 1A, a, ∼71 nt) should exist and that we could amplify it by PCR using primers 1 or 2 and primer 4. As shown in Fig. 1B (lanes 3 and 4), RT-PCR using primers 1 and 4 amplified both the primary tRNA transcript (p) and a minor spliced tRNAIleUAU species extended at the 5′ end (a). Although primer 4 is complementary to the first three nucleotides of the tRNAIleUAU, it is specific for the 5′ leader as it does not anneal to the mature tRNA under the PCR conditions that we used because the major species amplified using this primer and primer 1 is the tRNA transcript, which normally is much less abundant in the cell than mature tRNA. As a control, we amplified cDNA with primer 3 that is complementary to the first 10 nucleotides of the 5′ exon and that therefore does anneal to the mature 5′ end. PCR using primers 1 and 3 (Fig. 1B, lanes 1 and 2) amplifies both the primary transcript (p) and the abundant mature tRNAIleUAU (m). The additional nucleotides of the 5′ leader sequence are added on during the PCR because they are contained in the sequence of primer 3; thus, primer pairs 1/3 and 1/4 generate the same-size PCR product. We conclude that the minor PCR product amplified using primers 1 and 4 (Fig. 1B, lanes 3 and 4) is not mature tRNAIleUAU, but rather a rare tRNA species that is spliced and retains the 5′ leader sequence.
We assessed the abundance of 5′ end-extended, spliced tRNAIleUAU in wild-type and mtr10∆ mutant cells in which mature tRNAs do not access the nucleus (21) presumably because retrograde tRNA nuclear import is defective. Although there is no apparent difference in the abundance of the primary tRNAIleUAU transcript, the minor 5′ end-extended, spliced tRNAIleUAU accumulates to higher levels in mtr10∆ mutants compared with wild-type cells (Fig. 1B, lanes 3 and 4). Accumulation of this aberrant species is reduced when Mtr10 is exogenously expressed in mtr10∆ cells from a low-copy (l.c.) or high-copy (h.c.) plasmid (Fig. 1C, lanes 3 and 4).
Mtr10 is a member of the β-importin family of nuclear transport receptors and functions in importing mRNA export proteins as well as the RNA component of yeast telomerase to the nucleus (45–47). The precise function of Mtr10 in the retrograde process is not clear. To ensure that our results were dependent on the contribution of Mtr10 to retrograde tRNA transport and not on other Mtr10 functions, we also disrupted the retrograde pathway by a mechanism that is independent of a β-importin protein. Dhh1 and Pat1 affect retrograde nuclear accumulation, and neither is a member of the β-importin family (48). The 5′ end-extended spliced tRNAIleUAU accumulates in both mtr10Δ and dhh1Δ pat1Δ mutant strains in which retrograde tRNA nuclear accumulation is independently blocked (Fig. 1D, lanes 2 and 3). Thus, the accumulation of aberrant tRNAs in these cells is most likely due to impaired retrograde tRNA nuclear accumulation.
Using RT-PCR, we were unable to address whether the 5′ end-extended, spliced tRNAIleUAU also has a 3′ trailer. We therefore used Northern analysis to estimate the size of this tRNA species (Fig. 1E). A probe complementary to the 3′ exon detected the normal processing intermediates, the initial transcript (146–156 nt; p), and the end-processed intron-containing pre-tRNA (132 nt; i), as well as mature tRNAIleUAU (75 nt; m). In addition, in wild-type cells, there was a barely detectable RNA migrating at ∼96 nt (Fig. 1E, lane 1; a), the size predicted for a spliced tRNAIleUAU containing both the 5′ leader and 3′ trailer. This RNA accumulated to higher levels in mtr10Δ cells, and accumulation was complemented by plasmid-encoded MTR10 (Fig. 1E, lanes 2 and 3). Hybridization with a 22-nt probe complementary to the 5′ leader and 10 nucleotides of the 5′ exon demonstrated that only the aberrant RNA and the initial transcript possess the 5′ leader (Fig. S1). Thus, by two independent methods, we identified an aberrant 5′, 3′ end-extended, spliced tRNAIleUAU that accumulates to higher levels in cells with defective tRNA nuclear import.
We used real-time PCR to quantitate the relative abundance of the end-extended, spliced tRNAIleUAU in the retrograde-defective mutant strains compared with wild type. To avoid amplification of potential genomic DNA contamination, we synthesized cDNA using a primer that anneals to the splice junction (Fig. 1A, primer 5). To specifically amplify end-extended tRNAIleUAU, we used primers 4 and 5 (Fig. 1A). For a reference gene, we amplified mature tRNAGlnCUG using primers 6 and 7 (Fig. 2A). Mature tRNAGlnCUG should be present at approximately the same level in wild-type cells and in the mutant cells in which retrograde tRNA nuclear import is impaired. Aberrant end-extended, spliced tRNAIleUAU is ∼15-fold more abundant in mtr10∆ cells and approximately fivefold more abundant in dhh1∆ pat1∆ cells than in wild-type cells (Table 1). Together, the data suggest that a minor pool of aberrant end-extended, spliced tRNAIleUAU is normally imported via the retrograde pathway from the cytoplasm into the nucleus where it is repaired and/or degraded.
Fig. 2.
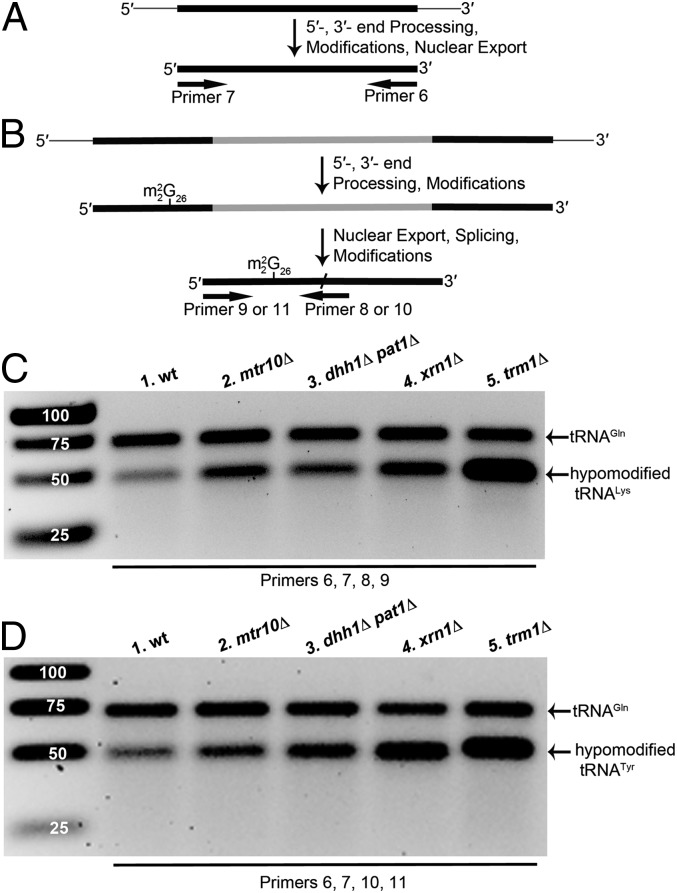
m22G26 hypomodified tRNALysUUU and tRNATyrGUA accumulate in cells in which retrograde tRNA nuclear import is defective. (A) Strategy to detect mature tRNAGlnCUG. (B) Strategy to detect mature tRNAs lacking m22G26. (A and B) Thin gray lines, 5′ leader and 3′ trailer; thick black lines, exons; thick gray line, intron; slash, splice junction. (C) RT-PCR analysis of tRNALysUUU from wild-type, mtr10∆, dhh1∆ pat1∆, xrn1∆, and trm1∆ cells. PCR was performed with primers 6, 7, 8, and 9. (D) RT-PCR analysis of tRNATyrGUA from wild-type, mtr10∆, dhh1∆ pat1∆, xrn1∆, and trm1∆ cells. PCR was performed with primers 6, 7, 10, and 11.
Table 1.
5′, 3′ end-extended, spliced tRNAIleUAU relative normalized expression
Relative to wild type and normalized to reference gene tRNAGlnCUG. Cq values for reactions without reverse transcriptase (RT) were 23- to 27-fold higher than samples with RT for amplification of tRNAIleUAU and 226- to 228-fold higher than for tRNAGlnCUG amplification.
SEM.
We also investigated tRNAs lacking the modification m22G26 (Fig. 2). This modification is added by Trm1 in the nucleus before primary tRNA nuclear export (49). Thus, for intron-containing tRNA, the modification is added before the intron is removed in the cytoplasm. Yeast tRNALysUUU and tRNATyrGUA are intron-containing tRNAs modified with m22G26. Because this modification has been reported to block reverse transcription (44), we reasoned that we could identify tRNALysUUU and tRNATyrGUA lacking m22G26 by RT-PCR. Full-length cDNA is generated during reverse transcription only if G26 is unmodified; thus, PCR product using primers 8 and 9 (tRNALysUUU) or 10 and 11 (tRNATyrGUA) results only if the tRNA G26 is unmodified to m22G26 (Fig. 2B). Using a primer that anneals to the tRNA 3′ exon, the primary product was the initial tRNA transcript in the nucleus that is not yet modified at G26. To assess the G26 modification status of the spliced tRNAs, we used primers that anneal to the splice junction of tRNALysUUU or tRNATyrGUA (Fig. 2B; primer 8 or 10, respectively), thus eliminating the primary tRNA transcript from the amplified products. The expected size of both PCR products is 46 bp. In the same RT-PCR reactions, we amplified mature tRNAGlnCUG, which does not contain any reverse transcription blocking modified nucleotides (Fig. 2A), generating a 72-bp product. Each individual primer set amplified only the expected DNA (Fig. S2).
We identified a low level of m22G26 hypomodified tRNALysUUU and tRNATyrGUA (Fig. 2 C and D) in wild-type cells (lane 1). As a control, we amplified both tRNAs in trm1∆ cells in which all tRNAs lack m22G26. tRNA from this mutant generates a much greater amount of PCR product compared with tRNA from wild-type cells (Fig. 2 C and D, lanes 1 and 5 ). Although the levels of mature tRNAGlnCUG do not change, both hypomodified tRNAs accumulate to higher levels in mtr10∆ and dhh1∆ pat1∆ mutants in which retrograde tRNA nuclear import is defective (Fig. 2 C and D, lanes 2 and 3). The data support the model that a minor pool of m22G26 hypomodified tRNALysUUU and tRNATyrGUA in wild-type cells is normally imported into the nucleus via the retrograde pathway and is subsequently modified and/or degraded.
Aberrant tRNAs Accumulate When Primary tRNA Nuclear Export Is More Efficient.
tRNA exportins appear to have evolved to recognize structural features common to all tRNAs. They bind the tRNA elbow and acceptor stem (32) and thus preferentially export end-matured tRNA to the cytoplasm (30, 31), providing one level of quality control. However, this preference is not absolute as vertebrate Exportin-t can bind end-extended tRNA, albeit ∼20× less efficiently than mature tRNA, and end-extended tRNA is exported to the cytoplasm with low efficiency in Xenopus oocytes (30, 31). In addition, in yeast there is an unidentified tRNA exporter that may or may not preferentially recognize end-matured tRNA. Competition in the nucleus between tRNA processing and tRNA nuclear export may result in precocious export of a fraction of primary tRNA transcripts to the cytoplasm. Removal of introns from these transcripts would generate aberrant end-extended, spliced tRNAs as identified in Fig. 1. Moreover, Los1 is not expected to assess tRNA modification status during nuclear export unless particular hypomodified tRNAs have unstable tertiary structures. There are 42 families of tRNAs, all with individual nucleotide sequences and unique combinations of modifications. Therefore, tRNA nuclear exporters, including Los1, must recognize their substrates by common structural features. Thus, there also may be competition in the nucleus between tRNA modification and tRNA nuclear export, which results in precocious export of some hypomodified tRNAs.
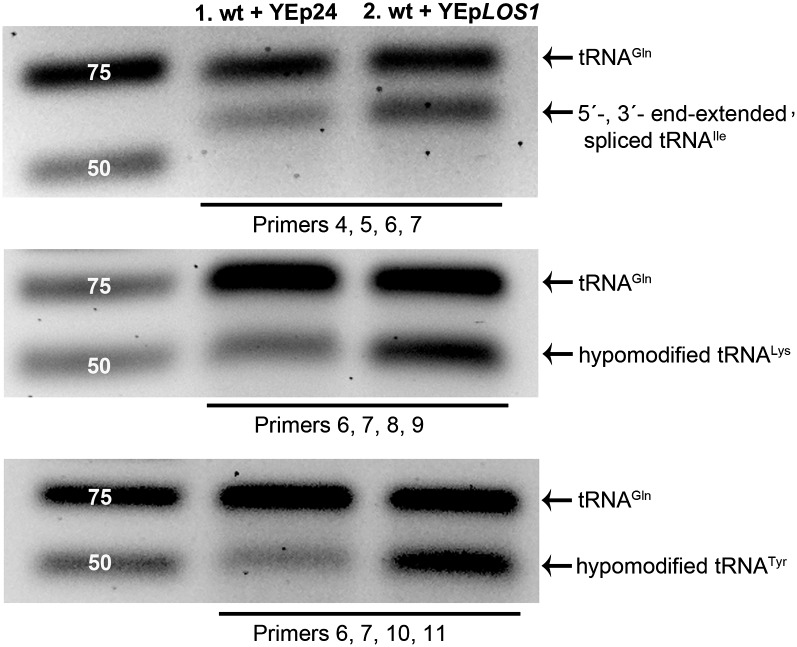
Aberrant tRNAs then may accumulate in cells in which primary tRNA nuclear export is more efficient. We overexpressed Los1 in wild-type yeast cells and assessed the levels of end-extended, spliced tRNAIleUAU and tRNALysUUU and tRNATyrGUA lacking m22G26 (Fig. 3). We amplified mature tRNAGlnCUG (Fig. 2A) in the same RT-PCR reactions. Although the levels of mature tRNAGlnCUG do not change, all three aberrant tRNAs accumulate to higher levels in wild-type cells overexpressing Los1, in which tRNA nuclear export is more efficient, compared with cells expressing vector only (Fig. 3, lanes 1 and 2). Overexpression of Los1 in mtr10Δ cells also results in higher levels of the end-extended, spliced tRNAIleUAU than vector alone (Fig. S3). Msn5 does not export intron-containing pre-tRNA to the cytoplasm (15), and its overexpression does not result in increased levels of end-extended, spliced tRNAIleUAU (Fig. S3). One interpretation of the data is that tRNA maturation competes with tRNA nuclear export, and sometimes Los1 mistakenly exports unprocessed or hypomodified tRNAs to the cytoplasm.
Fig. 3.
Aberrant tRNAs accumulate in cells in which primary tRNA nuclear export is more efficient. RT-PCR analysis of 5′, 3′ end-extended, spliced tRNAIleUAU (Top), m22G26-hypomodified tRNALysUUU (Middle), and m22G26-hypomodified tRNATyrGUA (Bottom) from wild-type cells transformed with vector (lane 1) or YEpLOS1 (lane 2). PCR was performed with primer sets 4/5 and 6/7 (Top) and with primers sets 8/9 or 10/11 and primer set 6/7 (Middle and Bottom).
m22G26 Hypomodified tRNAs Accumulate in Cells Lacking Cytoplasmic Rapid tRNA Decay.
Additional levels of tRNA quality control involve the degradation of improper tRNAs by both the nuclear surveillance pathway (37, 38) and the RTD pathway (39–41). To learn whether the aberrant tRNAs that we identified are substrates for cytoplasmic RTD by the 5′–3′ exonuclease Xrn1, we used RT-PCR studies in xrn1Δ cells. Surprisingly, end-extended, spliced tRNAIleUAU does not appear to be a substrate for cytoplasmic RTD, as this species does not accumulate in mutants lacking Xrn1 (Fig.1D, lane 4). Thus, the RTD pathway appears not to serve in quality control for tRNAs that are exported to the cytoplasm before end maturation.
The RTD pathway was discovered because tRNAs missing subsets of two modifications are subject to 5′–3′ exonucleolytic degradation in the cytoplasm by Xrn1 and/or in the nucleus by Rat1 (39, 40). Moreover, defective m22G26 modification of tRNASerCGA and tRNASerUGA in trm1Δ cells exposed to particular stress conditions results in their instability due to RTD (50). Consistent with these studies, both m22G26-hypomodified tRNALysUUU and tRNATyrGUA accumulate in xrn1∆ mutants in which there is no cytoplasmic RTD (Fig. 2 C and D, lane 4). Thus, m22G26-hypomodified tRNALysUUU and tRNATyrGUA can be degraded in the cytoplasm by RTD and can be imported into the nucleus via the retrograde pathway.
Cells Require Either Cytoplasmic RTD or Retrograde tRNA Nuclear Import for Viability.
Cells are viable if they are missing either cytoplasmic RTD (xrn1∆) or the retrograde tRNA nuclear import pathway (mtr10∆), and aberrant tRNAs accumulate in each single mutant strain (Figs. 1 and 2). Because both quality control pathways appear to assess the same substrate hypomodified tRNAs, it seemed possible that the aberrant tRNAs might accumulate to higher levels in mutant cells lacking both cytoplasmic RTD and retrograde tRNA nuclear import. To test this, we attempted to delete XRN1 from mtr10∆ cells. However, we were unsuccessful constructing the double mutant by standard procedures. Therefore, we transformed mtr10∆ cells with a URA3 plasmid that expresses MTR10 and then deleted XRN1. Yeast cells expressing a URA3 gene are selected against when grown on media containing 5-fluoroorotic acid (5-FOA), and only cells that have lost the URA3 plasmid will be able to grow on 5-FOA media (51). Interestingly, mtr10∆ xrn1∆ cells were unable to lose the MTR10-expressing plasmid by such counterselection (Fig. S4). Thus, mtr10∆ xrn1∆ mutant cells are inviable. One interpretation of the data is that it is critical for the cell to be able to deal with improper cytoplasmic tRNAs either by degradation in the cytoplasm or by retrograde import to the nucleus.
Discussion
In organisms from yeast to vertebrates, mature tRNAs are constitutively imported from the cytoplasm to the nucleus. The significance of this tRNA retrograde process is not entirely clear, although in yeast it is known to be required for modification of tRNAPheGAA G37 to wybutosine (26) and for translational regulation of a subset of mRNAs (27). Here we investigated whether the tRNA retrograde process might also function in tRNA quality control. We altered tRNA subcellular dynamics by three separate means using two different yeast strains (mtr10Δ and dhh1Δ pat1Δ), with mutations that affect tRNA retrograde nuclear import by independent mechanisms and a third yeast strain that overexpresses the tRNA exportin, Los1. Using tRNA splicing as a proxy for the presence of tRNA in the cytoplasm, we learned that each of the perturbations of tRNA nuclear-cytoplasmic dynamics causes accumulation of aberrant spliced tRNA bearing unprocessed 5′ and 3′ termini and/or spliced tRNA that is hypomodified. Although Mtr10 and Dhh1/Pat1 have functions in addition to those involved in tRNA subcellular dynamics, the cumulative data provide compelling evidence that the tRNA retrograde pathway serves as a newly discovered tRNA quality control mechanism to monitor 5′ and/or 3′ end sequences and the modification status of cytoplasmic tRNAs.
There are mechanisms dedicated to preventing improper tRNAs from escaping the nucleus and to degrading aberrant tRNAs. Why then might an additional tRNA quality control be necessary? It appears that the cell proofreads the structure of the 5′ and 3′ termini differently from the way in which it proofreads the modification status of tRNAs, and the quality control pathways involved in each process do not appear to overlap. As the retrograde pathway recognizes both end-extended and hypomodified tRNAs, conceivably it provides back-up quality control.
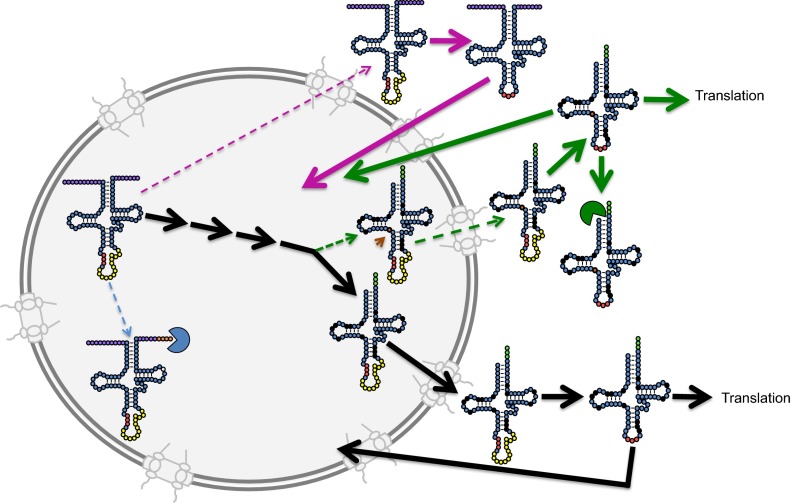
Overall, then, cells seem to use multiple quality control mechanisms that operate in parallel to maintain a cytoplasmic pool of properly structured and functional tRNAs that interact with the translation machinery (Fig. 4). As an initial quality control, primary tRNAMeti transcripts lacking m1A58 as well as incorrectly 3′ end-processed tRNAs are targeted for polyadenylation and degradation by the TRAMP complex and the nuclear exosome (7, 37, 38) (Fig. 4, blue packman). Los1 also provides initial proofreading by preferentially binding and exporting end-matured tRNAs. However, we detect low levels of improper export of primary tRNA transcripts by Los1. In addition, because export requires that Los1 recognize structures common to all 42 tRNAs that are variously modified (30–32), Los1 is not expected to monitor tRNA modifications added in the nucleus. Thus, Los1-mediated nuclear export prevents, although not completely, nuclear export of 5′, 3′ end-extended, spliced tRNAs but not likely many hypomodified tRNAs. Los1-mediated nuclear export is limiting (52), and the normal low levels of aberrant tRNAs increase when Los1 is overexpressed (Fig. 4, pink and green dotted arrows). Perhaps, this tRNA nuclear exporter is normally limiting to prevent precocious export of improper tRNAs from the nucleus.
Fig. 4.
Interactions of tRNA quality control pathways. tRNA processing/trafficking pathways are color coded. Black arrows indicate the canonical pathway leading to mature cytoplasmic tRNAs that participate in translation. Blue dotted arrow indicates aberrant 3′ processing; the aberrant transcript is destroyed by the 3′–5′ exosome quality control pathway (blue packman). Pink dotted arrow indicates precocious nuclear export before 5′ and 3′ processing; aberrant transcripts are spliced in the cytoplasm and return to the nucleus via retrograde nuclear import (long solid pink arrow). Green dotted arrows indicate precocious nuclear export before complete modification in the nucleus; solid green arrows demonstrate that hypomodified tRNAs are spliced in the cytoplasm and may participate in translation or may be destroyed by the 5′–3′ RTD exonuclease, Xrn1 (green packman), or may return to the nucleus via retrograde import (long solid green arrow), followed by repair or destruction by the nuclear RTD exonuclease. Orange arrow indicates the tRNA position normally modified by m22G26; the nucleotide missing this modification is indicated by a solid orange circle.
As Los1 does not have complete fidelity for exporting only end-matured and appropriately modified tRNAs and as there is at least one unidentified tRNA export pathway that may or may not monitor tRNA termini and/or modification, cells employ additional quality control mechanisms. The cytoplasmic RTD pathway monitors the tRNA tertiary structure, degrading tRNAs that possess destabilized acceptor and T stems, often because they lack certain modification(s) (39–41). Lack of some modifications may not decrease tRNA stability, and therefore some hypomodified tRNAs that have mistakenly been exported to the cytoplasm may be unrecognized by the cytoplasmic RTD pathway. In addition, this pathway does not appear to detect tRNAs that are extended at the 5′ and 3′ termini, presumably because these end extensions do not compromise tRNA structure. Thus, the RTD quality control pathway eliminates certain aberrant tRNAs but fails to degrade others in the cytoplasm (Fig. 4, green packman).
The 5′, 3′ end-extended tRNAs would not function in translation becuase they cannot be aminoacylated; however, hypomodified tRNAs can be aminoacylated and could be deleterious to the fidelity of translation, given the long half-life of tRNAs and the role of certain tRNA modifications in tRNA identity and translational fidelity. The retrograde tRNA nuclear import pathway recognizes both 5′, 3′ end-extended, spliced tRNAs and hypomodified tRNAs and functions, at least in part, to return such aberrant tRNAs to the nucleus (Fig. 4, long solid pink and green arrows). Thus, between degradation in the cytoplasm via RTD and import to the nucleus via the retrograde pathway, aberrant tRNAs that erroneously reach the cytoplasm are removed. Although neither single pathway is essential, cells must retain either cytoplasmic RTD or retrograde tRNA nuclear import for viability. Although the combination of mtr10Δ and xrn1Δ may cause lethality for reasons unrelated to tRNA quality control, we favor the tantalizing possibility that their essential functions are to work in parallel in tRNA quality control, underscoring the importance to the cell in maintaining functional tRNAs in the cytoplasm.
We have not addressed the fate of aberrant tRNAs upon nuclear import. Aberrant tRNAs might be repaired and given a second chance to become functional tRNAs because the enzymes necessary to fix both 5′, 3′end-extended, spliced tRNAs and hypomodified tRNAs missing nuclear-added modifications are localized in the nucleus. Alternatively, aberrant tRNAs might be degraded following nuclear import either by the TRAMP complex and nuclear exosome or by the nuclear RTD. These possibilities are not mutually exclusive, and there may be competition in the nucleus between repairing and degrading imported aberrant tRNAs as well.
Materials and Methods
Yeast Strains and Plasmids.
The following yeast strains derived from BY4741 were used in this study: mtr10∆ (21), dhh1∆ pat1∆ (48), and xrn1∆ (Open Biosystems). XRN1 was deleted from strain mtr10∆ by gene replacement with a LEU2 gene (53) to construct strain mtr10∆ xrn1∆. YEpLOS1 was previously described (54). Multicopy plasmid MTR10End-MORF was constructed by replacing the SacII/XhoI fragment of plasmid MTR10-MORF (55) with a PCR-amplified fragment from BY4741 genomic DNA that includes 300 bp upstream of MTR10 to the XhoI site within MTR10. The SacII/KpnI fragment of plasmid MTR10End-MORF was subcloned into low-copy plasmid pRS416 to construct plasmid pRSMTR10-MORF.
Oligonucleotides.
The sequences of the oligonucleotides used are provided in SI Materials and Methods.
RT-PCR.
Small RNAs were extracted from yeast cultures grown at 23 °C to an OD600 of 0.4–0.6 as described (29). First-strand cDNA synthesis was carried out using 5 µg of RNA and SuperScript II reverse transcriptase (Invitrogen), according to the manufacturer’s protocol. PCR conditions are provided in SI Materials and Methods. For experiments in Figs. 2 and 3 in which different tRNAs were simultaneously assessed, the concentrations of each of the primer sets was optimized to allow for visualization of DNA resulting from amplifications of high- and low-abundance tRNAs on a single gel.
Real-Time RT-PCR.
RNA extracts were treated with Turbo DNase (Ambion) according to the manufacturer’s protocol. SuperScript II RT was used to generate cDNAs from RNA. PCR was performed using SsoFast Eva Green Supermix (Bio-Rad) and the CFX96 instrument (Bio-Rad). PCR conditions, validation experiments, controls, and analysis are described in SI Materials and Methods.
Northern Analysis.
Twenty-five micrograms of small RNA extract was separated by electrophoresis and transferred onto a Hybond N+ membrane (Amersham) as described (29). tRNAs were detected with a γ32P-labeled probe complementary to the 3′-exon of tRNAIleUAU (primer 1, Fig. 1A). The blot was exposed to a phosphor screen (Amersham Pharmacia), and the screen was scanned for radioactivity using a Typhoon Trio imager.
Supplementary Material
Acknowledgments
We thank all members of the A.K.H. laboratory for excellent scientific discussions. This work was supported by National Institutes of Health Grant GM-27930 (to A.K.H.) and National Cancer Institute T32 Postdoctoral Training Grant CA106196 (to E.B.K.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316579110/-/DCSupplemental.
References
- 1.Thompson M, Haeusler RA, Good PD, Engelke DR. Nucleolar clustering of dispersed tRNA genes. Science. 2003;302(5649):1399–1401. doi: 10.1126/science.1089814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kufel J, Tollervey D. 3′-Processing of yeast tRNATrp precedes 5′-processing. RNA. 2003;9(2):202–208. doi: 10.1261/rna.2145103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertrand E, Houser-Scott F, Kendall A, Singer RH, Engelke DR. Nucleolar localization of early tRNA processing. Genes Dev. 1998;12(16):2463–2468. doi: 10.1101/gad.12.16.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takaku H, Minagawa A, Takagi M, Nashimoto M. A candidate prostate cancer susceptibility gene encodes tRNA 3′ processing endoribonuclease. Nucleic Acids Res. 2003;31(9):2272–2278. doi: 10.1093/nar/gkg337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, et al. Characterization of TRZ1, a yeast homolog of the human candidate prostate cancer susceptibility gene ELAC2 encoding tRNase Z. BMC Mol Biol. 2005;6:12. doi: 10.1186/1471-2199-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel A, Schilling O, Späth B, Marchfelder A. The tRNase Z family of proteins: Physiological functions, substrate specificity and structural properties. Biol Chem. 2005;386(12):1253–1264. doi: 10.1515/BC.2005.142. [DOI] [PubMed] [Google Scholar]
- 7.Copela LA, Fernandez CF, Sherrer RL, Wolin SL. Competition between the Rex1 exonuclease and the La protein affects both Trf4p-mediated RNA quality control and pre-tRNA maturation. RNA. 2008;14(6):1214–1227. doi: 10.1261/rna.1050408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozanick SG, et al. Rex1p deficiency leads to accumulation of precursor initiator tRNAMet and polyadenylation of substrate RNAs in Saccharomyces cerevisiae. Nucleic Acids Res. 2009;37(1):298–308. doi: 10.1093/nar/gkn925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopper AK. Transfer RNA post-transcriptional processing, turnover, and subcellular dynamics in the yeast Saccharomyces cerevisiae. Genetics. 2013;194(1):43–67. doi: 10.1534/genetics.112.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhungel N, Hopper AK. Beyond tRNA cleavage: Novel essential function for yeast tRNA splicing endonuclease unrelated to tRNA processing. Genes Dev. 2012;26(5):503–514. doi: 10.1101/gad.183004.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshihisa T, Yunoki-Esaki K, Ohshima C, Tanaka N, Endo T. Possibility of cytoplasmic pre-tRNA splicing: The yeast tRNA splicing endonuclease mainly localizes on the mitochondria. Mol Biol Cell. 2003;14(8):3266–3279. doi: 10.1091/mbc.E02-11-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshihisa T, Ohshima C, Yunoki-Esaki K, Endo T. Cytoplasmic splicing of tRNA in Saccharomyces cerevisiae. Genes Cells. 2007;12(3):285–297. doi: 10.1111/j.1365-2443.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar S, Hopper AK. tRNA nuclear export in Saccharomyces cerevisiae: in situ hybridization analysis. Mol Biol Cell. 1998;9(11):3041–3055. doi: 10.1091/mbc.9.11.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hellmuth K, et al. Yeast Los1p has properties of an exportin-like nucleocytoplasmic transport factor for tRNA. Mol Cell Biol. 1998;18(11):6374–6386. doi: 10.1128/mcb.18.11.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murthi A, et al. Regulation of tRNA bidirectional nuclear-cytoplasmic trafficking in Saccharomyces cerevisiae. Mol Biol Cell. 2010;21(4):639–649. doi: 10.1091/mbc.E09-07-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurt DJ, Wang SS, Lin YH, Hopper AK. Cloning and characterization of LOS1, a Saccharomyces cerevisiae gene that affects tRNA splicing. Mol Cell Biol. 1987;7(3):1208–1216. doi: 10.1128/mcb.7.3.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phizicky EM, Hopper AK. tRNA biology charges to the front. Genes Dev. 2010;24(17):1832–1860. doi: 10.1101/gad.1956510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackman JE, Alfonzo JD. Transfer RNA modifications: Nature’s combinatorial chemistry playground. Wiley Interdiscip Rev RNA. 2013;4(1):35–48. doi: 10.1002/wrna.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Yacoubi B, Bailly M, de Crécy-Lagard V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu Rev Genet. 2012;46:69–95. doi: 10.1146/annurev-genet-110711-155641. [DOI] [PubMed] [Google Scholar]
- 20.Barhoom S, et al. Quantitative single cell monitoring of protein synthesis at subcellular resolution using fluorescently labeled tRNA. Nucleic Acids Res. 2011;39(19):e129. doi: 10.1093/nar/gkr601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaheen HH, Hopper AK. Retrograde movement of tRNAs from the cytoplasm to the nucleus in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2005;102(32):11290–11295. doi: 10.1073/pnas.0503836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaheen HH, et al. Retrograde nuclear accumulation of cytoplasmic tRNA in rat hepatoma cells in response to amino acid deprivation. Proc Natl Acad Sci USA. 2007;104(21):8845–8850. doi: 10.1073/pnas.0700765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaitseva L, Myers R, Fassati A. tRNAs promote nuclear import of HIV-1 intracellular reverse transcription complexes. PLoS Biol. 2006;4(10):e332. doi: 10.1371/journal.pbio.0040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takano A, Endo T, Yoshihisa T. tRNA actively shuttles between the nucleus and cytosol in yeast. Science. 2005;309(5731):140–142. doi: 10.1126/science.1113346. [DOI] [PubMed] [Google Scholar]
- 25.Miyagawa R, Mizuno R, Watanabe K, Ijiri K. Formation of tRNA granules in the nucleus of heat-induced human cells. Biochem Biophys Res Commun. 2012;418(1):149–155. doi: 10.1016/j.bbrc.2011.12.150. [DOI] [PubMed] [Google Scholar]
- 26.Ohira T, Suzuki T. Retrograde nuclear import of tRNA precursors is required for modified base biogenesis in yeast. Proc Natl Acad Sci USA. 2011;108(26):10502–10507. doi: 10.1073/pnas.1105645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu HY, Hopper AK. Genome-wide investigation of the role of the tRNA nuclear-cytoplasmic trafficking pathway in regulation of the yeast Saccharomyces cerevisiae transcriptome and proteome. Mol Cell Biol. 2013;33(21):4241–4254. doi: 10.1128/MCB.00785-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurto RL, Tong AH, Boone C, Hopper AK. Inorganic phosphate deprivation causes tRNA nuclear accumulation via retrograde transport in Saccharomyces cerevisiae. Genetics. 2007;176(2):841–852. doi: 10.1534/genetics.106.069732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitney ML, Hurto RL, Shaheen HH, Hopper AK. Rapid and reversible nuclear accumulation of cytoplasmic tRNA in response to nutrient availability. Mol Biol Cell. 2007;18(7):2678–2686. doi: 10.1091/mbc.E07-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arts GJ, Kuersten S, Romby P, Ehresmann B, Mattaj IW. The role of exportin-t in selective nuclear export of mature tRNAs. EMBO J. 1998;17(24):7430–7441. doi: 10.1093/emboj/17.24.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipowsky G, et al. Coordination of tRNA nuclear export with processing of tRNA. RNA. 1999;5(4):539–549. doi: 10.1017/s1355838299982134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook AG, Fukuhara N, Jinek M, Conti E. Structures of the tRNA export factor in the nuclear and cytosolic states. Nature. 2009;461(7260):60–65. doi: 10.1038/nature08394. [DOI] [PubMed] [Google Scholar]
- 33.Feng W, Hopper AK. A Los1p-independent pathway for nuclear export of intronless tRNAs in Saccharomycescerevisiae. Proc Natl Acad Sci USA. 2002;99(8):5412–5417. doi: 10.1073/pnas.082682699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grosshans H, Hurt E, Simos G. An aminoacylation-dependent nuclear tRNA export pathway in yeast. Genes Dev. 2000;14(7):830–840. [PMC free article] [PubMed] [Google Scholar]
- 35.Sarkar S, Azad AK, Hopper AK. Nuclear tRNA aminoacylation and its role in nuclear export of endogenous tRNAs in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1999;96(25):14366–14371. doi: 10.1073/pnas.96.25.14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lund E, Dahlberg JE. Proofreading and aminoacylation of tRNAs before export from the nucleus. Science. 1998;282(5396):2082–2085. doi: 10.1126/science.282.5396.2082. [DOI] [PubMed] [Google Scholar]
- 37.Kadaba S, et al. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev. 2004;18(11):1227–1240. doi: 10.1101/gad.1183804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadaba S, Wang X, Anderson JT. Nuclear RNA surveillance in Saccharomyces cerevisiae: Trf4p-dependent polyadenylation of nascent hypomethylated tRNA and an aberrant form of 5S rRNA. RNA. 2006;12(3):508–521. doi: 10.1261/rna.2305406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexandrov A, et al. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell. 2006;21(1):87–96. doi: 10.1016/j.molcel.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 40.Chernyakov I, Whipple JM, Kotelawala L, Grayhack EJ, Phizicky EM. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′-3′ exonucleases Rat1 and Xrn1. Genes Dev. 2008;22(10):1369–1380. doi: 10.1101/gad.1654308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whipple JM, Lane EA, Chernyakov I, D’Silva S, Phizicky EM. The yeast rapid tRNA decay pathway primarily monitors the structural integrity of the acceptor and T-stems of mature tRNA. Genes Dev. 2011;25(11):1173–1184. doi: 10.1101/gad.2050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Connor JP, Peebles CL. In vivo pre-tRNA processing in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11(1):425–439. doi: 10.1128/mcb.11.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machnicka MA, et al. MODOMICS: A database of RNA modification pathways—2013 update. Nucleic Acids Res. 2013;41(Database issue):D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motorin Y, Muller S, Behm-Ansmant I, Branlant C. Identification of modified residues in RNAs by reverse transcription-based methods. Methods Enzymol. 2007;425:21–53. doi: 10.1016/S0076-6879(07)25002-5. [DOI] [PubMed] [Google Scholar]
- 45.Pemberton LF, Rosenblum JS, Blobel G. A distinct and parallel pathway for the nuclear import of an mRNA-binding protein. J Cell Biol. 1997;139(7):1645–1653. doi: 10.1083/jcb.139.7.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Senger B, et al. Mtr10p functions as a nuclear import receptor for the mRNA-binding protein Npl3p. EMBO J. 1998;17(8):2196–2207. doi: 10.1093/emboj/17.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrezuelo F, Steiner B, Aldea M, Futcher B. Biogenesis of yeast telomerase depends on the importin mtr10. Mol Cell Biol. 2002;22(17):6046–6055. doi: 10.1128/MCB.22.17.6046-6055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hurto RL, Hopper AK. P-body components, Dhh1 and Pat1, are involved in tRNA nuclear-cytoplasmic dynamics. RNA. 2011;17(5):912–924. doi: 10.1261/rna.2558511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li JM, Hopper AK, Martin NC. N2,N2-dimethylguanosine-specific tRNA methyltransferase contains both nuclear and mitochondrial targeting signals in Saccharomyces cerevisiae. J Cell Biol. 1989;109(4 Pt 1):1411–1419. doi: 10.1083/jcb.109.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dewe JM, Whipple JM, Chernyakov I, Jaramillo LN, Phizicky EM. The yeast rapid tRNA decay pathway competes with elongation factor 1A for substrate tRNAs and acts on tRNAs lacking one or more of several modifications. RNA. 2012;18(10):1886–1896. doi: 10.1261/rna.033654.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boeke JD, LaCroute F, Fink GR. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-Fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 52.Ghavidel A, et al. Impaired tRNA nuclear export links DNA damage and cell-cycle checkpoint. Cell. 2007;131(5):915–926. doi: 10.1016/j.cell.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 53.Gueldener U, Heinisch J, Koehler GJ, Voss D, Hegemann JH. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res. 2002;30(6):e23. doi: 10.1093/nar/30.6.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen WC, Selvakumar D, Stanford DR, Hopper AK. The Saccharomyces cerevisiae LOS1 gene involved in pre-tRNA splicing encodes a nuclear protein that behaves as a component of the nuclear matrix. J Biol Chem. 1993;268(26):19436–19444. [PubMed] [Google Scholar]
- 55.Gelperin DM, et al. Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes Dev. 2005;19(23):2816–2826. doi: 10.1101/gad.1362105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.