Psychologists and neuroscientists have long debated the nature of memory: How many types are there? Tulving (1, 2) proposed a distinction between semantic memory (general knowledge) and episodic memory (personal experiences that carry information about time and location). Tulving noted that episodic memories are autobiographical in nature: “Most, if not all, episodic memory claims a person makes can be translated into the form ‘I did such and such, in such and such a place, and in such and such a time’” (1, p 389). He also provided examples of episodic memory (1, pp 386–387) to indicate that he had in mind both events from the laboratory (remembering words in a list encountered in a laboratory setting) and those from life (remembering meeting a sea captain who knew many jokes) as indicative of episodic memory. No distinction was made between retrieval of the two types of events. The interesting results of Patihis et al. (3) and previous papers on people with highly superior autobiographical memory (HSAM) (4, 5) call into question part of Tulving’s (1, 2) claims. Memory for laboratory events may be fundamentally different from memory for events of one’s life. A person can excel at one type of retrieval but not the other.
Specifically, Patihis et al. (3) show that people with HSAM, who demonstrate remarkable capabilities of accurate remembering of events from their past (e.g., what they had for lunch on a particular date many years ago), are average in remembering laboratory events. People with HSAM tend to recall or recognize about the same number of laboratory events (e.g., words in a list) as age- and education-matched controls (4). This study (3) builds on that work and demonstrates that in several paradigms widely used to study illusory memories (6, 7), people with HSAM are just as susceptible to false memories as are control subjects. At some level, this result is not surprising. That is, the processes that give rise to false memories include semantic elaboration and associations at encoding, accompanied by reconstruction during retrieval. These same processes give rise to accurate and false memories (8), so if HSAM subjects show normal performance in accurate memory in laboratory tasks (4), it is not too surprising that their false memory results are also normal.
Perhaps the bigger puzzle, one noted by Patihis et al., is that these findings raise a fundamental question as to “why HSAM individuals remember some trivial details, such as what they had for lunch 10 y ago, but not others, such as words on a list or photographs in a slide show” (3, p 5). The solution they pose—that HSAM individuals weave “lunch events” but not “lab events” into their daily narrative and remember this narrative—may be right, but one problem is that narrative forms are driven by schemas, and schematic processing often leads to (rather than prevents) false memories (9, 10). Why are lunch events considered worth remembering and woven into a narrative, whereas laboratory events are not? We offer a different path to understanding this puzzle.
Research on memory for artificial events in the laboratory and for events from life has historically taken separate courses. A typical task for the latter, first tried by Galton (11) but later developed by Crovitz and Schiffman (12), provides subjects with a cue (chair, strawberry, or puppy, as examples), and the task is to retrieve an event from one’s life using the cue (“I remember the morning my family adopted a Welsh Terrier named Toby”). A large literature has built up around the use of this task (and a few others), as well as around laboratory-based episodic memory tasks such as those used by Patihis et al. (see ref. 13 for a review). The standard assumption made by researchers using the Galton/Crovitz task is the same as that made by Tulving (1, 3), viz., that retrieval of the two types of events (those encoded in the laboratory and those experienced in everyday life) is similar and that the neural processes underlying these tasks are the same (or at least highly similar). Laboratory-based tasks are considered convenient proxies for understanding autobiographical memory retrieval.
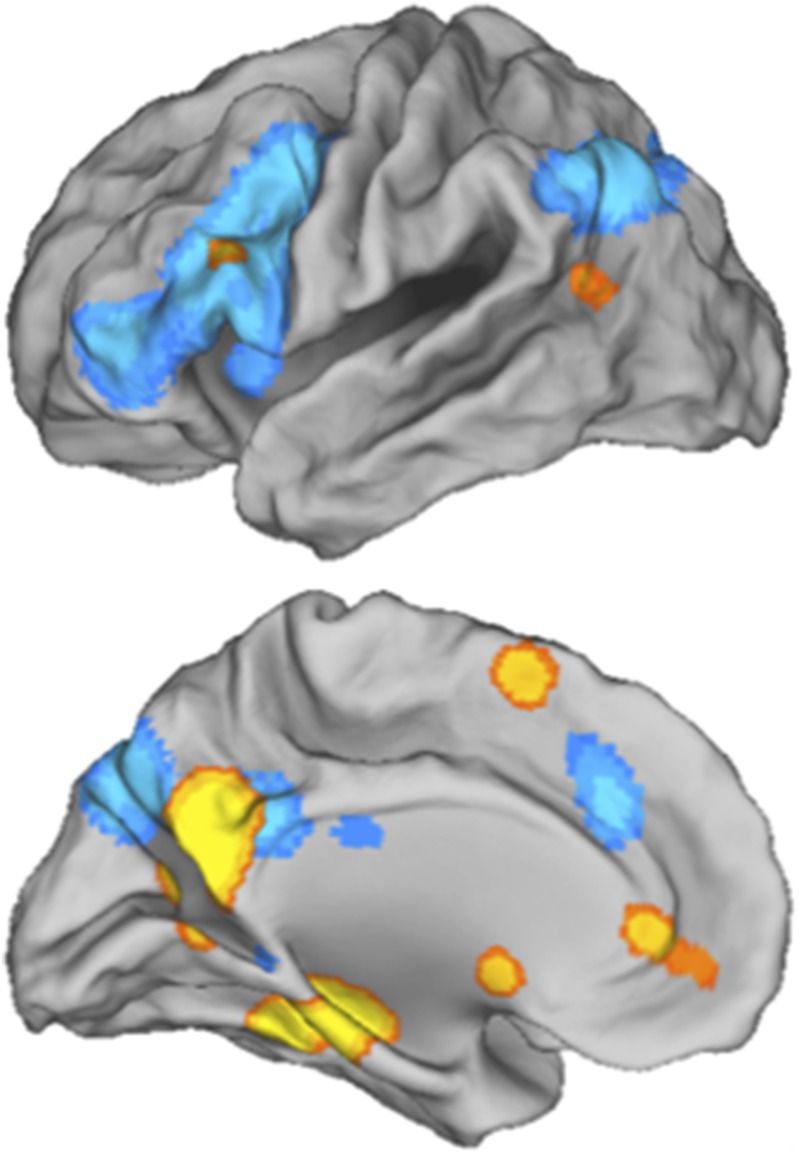
A recent meta-analysis (14) calls this assumption into question. Specifically, McDermott et al. identified regions that tend to activate during functional MRI (fMRI) studies of memory retrieval when a laboratory task (recognition memory for recently studied materials) is used (Fig. 1, blue). Using the same methodology, these researchers identified regions that tend to activate during memory retrieval when people are asked to remember extraexperimental life events using variants of the word cueing task (Fig. 1, orange). Clearly, different brain networks contribute to the two tasks. Using only one or the other approach for understanding memory retrieval would offer an incomplete picture.
Fig. 1.
The regions underlying memory retrieval critically depend on whether a laboratory-based recognition memory task is used (regions shown in blue) or whether an autobiographical memory task is used (regions in orange). Left lateral (Upper) and medial (Lower) views. Adapted from McDermott et al. (14).
That people with HSAM excel at autobiographical remembering but not at laboratory remembering suggests the differences seen in this meta-analysis are tapping two fundamentally different types of memory retrieval that might both be considered episodic in
People with HSAM excel at autobiographical remembering but not at laboratory remembering.
that they are about episodes (or events) in one’s life. That is, a dissociation exists such that people can excel at one (in this case, autobiographical memory of life events) but not the other (learning of minievents within the laboratory). Other individuals (mnemonists or memory athletes) demonstrate excellent performance in laboratory-like tasks (encode and retrieve large numbers of digits, names and faces, random words), but no evidence exists that they have abilities like HSAM individuals (15, 16). We note that we are using the terms autobiographical and laboratory to refer to the two types of task, but the key dimensions that differentiate the two and the most precise classification of these types of memory awaits further work.
One tactic neuroscientists and psychologists have used in debating types of memory has been to study various deficiencies in memory (e.g., types of amnesia, or the deleterious effects of certain drugs or psychiatric conditions on remembering). The Patihis et al. study and others (15) suggest that another profitable avenue may be to examine groups of people with remarkably superior forms of memory, such as mnemonists who excel in memory competitions (using tests like laboratory-based memory tasks) and in general knowledge (people who excel at games like Jeopardy, Quiz Bowl, and Trivial Pursuit). Do any of these groups excel in other tasks or are their abilities limited like people with HSAM? Examining individuals with pockets of highly superior memory in certain tasks (but normal performance in other memory tasks) may provide converging evidence for theories classifying memory into various types. This enterprise is just beginning.
In sum, we suggest that brain networks and cognitive processes underlying standard laboratory memory tasks differ fundamentally from those used in remembering events from one’s life. This claim arose from a meta-analysis of functional neuroimaging studies of healthy young adults, but the Patihis et al. data (3) are fully consistent with it. The Patihis et al. paper extends this understanding by showing that HSAM people excel at one type of memory but show average performance on the other type of memory and show normal levels of errors relative to subjects without HSAM. We believe the implications of this observation are far-reaching.
Footnotes
The authors declare no conflict of interest.
See companion article on page 20947.
References
- 1.Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of Memory. New York: Academic Press; 1972. pp. 381–403. [Google Scholar]
- 2.Tulving E. Elements of Episodic Memory. New York: Oxford Univ Press; 1983. [Google Scholar]
- 3.Patihis L, et al. False memories in highly superior autobiographical memory individuals. Proc Natl Acad Sci USA. 2013;110:20947–20952. doi: 10.1073/pnas.1314373110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LePort AKR, et al. Behavioral and neuroanatomical investigation of highly superior autobiographical memory (HSAM) Neurobiol Learn Mem. 2012;98(1):78–92. doi: 10.1016/j.nlm.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker ES, Cahill L, McGaugh JL. A case of unusual autobiographical remembering. Neurocase. 2006;12(1):35–49. doi: 10.1080/13554790500473680. [DOI] [PubMed] [Google Scholar]
- 6.Roediger HL, McDermott KB. Creating false memories: Remembering words not presented in lists. J Exp Psychol Learn Mem Cogn. 1995;21(4):803–814. [Google Scholar]
- 7.Loftus EF, Miller DG, Burns HJ. Semantic integration of verbal information into a visual memory. J Exp Psychol Hum Learn. 1978;4(1):19–31. [PubMed] [Google Scholar]
- 8.Roediger HL. Memory illusions. J Mem Lang. 1996;35(2):76–100. [Google Scholar]
- 9.Sulin RA, Dooling DJ. Intrusion of a thematic idea in retention of prose. J Exp Psychol. 1974;103(2):255–262. [Google Scholar]
- 10.Owens J, Bower GH, Black JB. The “soap opera” effect in story recall. Mem Cognit. 1979;7(3):185–191. [Google Scholar]
- 11.Galton F. Psychometric experiments. Brain. 1879;2(2):149–162. [Google Scholar]
- 12.Crovitz HF, Schiffman H. Frequency of episodic memories as a function of their age. Bull Psychon Soc. 1974;4(5B):517–518. [Google Scholar]
- 13.Marsh EJ, Roediger HL. Episodic and autobiographical memory. In: Weiner IB, editor. Handbook of Psychology: Experimental Psychology. Vol 4. New York: Wiley; 2012. pp. 472–494. [Google Scholar]
- 14.McDermott KB, Szpunar KK, Christ SE. Laboratory-based and autobiographical retrieval tasks differ substantially in their neural substrates. Neuropsychologia. 2009;47(11):2290–2298. doi: 10.1016/j.neuropsychologia.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Maguire EA, Valentine ER, Wilding JM, Kapur N. Routes to remembering: the brains behind superior memory. Nat Neurosci. 2003;6(1):90–95. doi: 10.1038/nn988. [DOI] [PubMed] [Google Scholar]
- 16.Foer J. Moonwalking with Einstein: The Art and Science of Remembering Everything. New York: Penguin; 2011. p. 320. [Google Scholar]