Abstract

A metal-free [3+2+1]/[2+2+1] biscyclization strategy has been developed for the stereospecific construction with concomitant derivation of biologically significant indolizin-5(1H)-ones from simple and commercial starting materials. The transformations are notable because they can yield five new sigma bonds and six stereocenters including a quaternary carbon center in a single operation.
INTRODUCTION
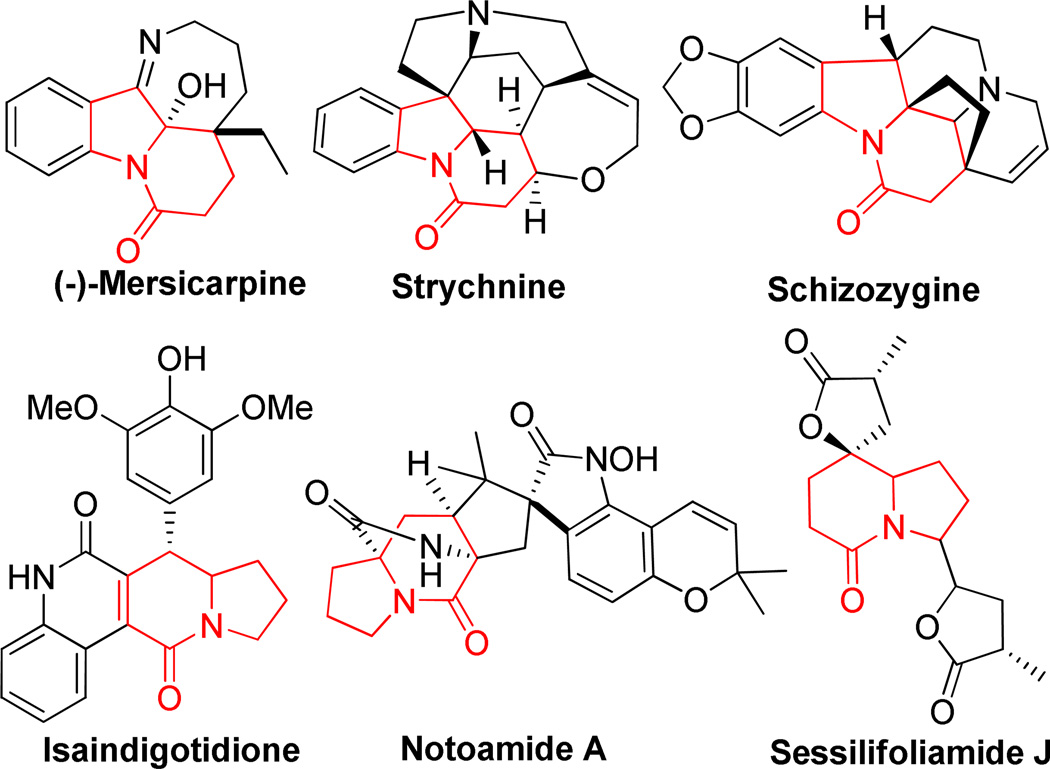
Azabicyclic ring systems are widely distributed in numerous natural products and synthetic compounds and exhibit a broad range of biological activities and pharmacological properties.1 Among these systems, indolizin-5(1H)-one as an azabicyclic framework is well-represented, and this bicyclic core is frequently found in a number of biologically active natural alkaloids (Figure 1).2 Moreover, indolizin-5(1H)-one derivatives would serve as cytotoxicity agents,3 inhibitors of α-thrombin,4 oligopeptidase inhibitors,5 dipeptide mimetics,6 integrin antagonists,7 and inhibitors of aldosterone synthase.8 Indolizin-5(1H)-ones have also been attractive synthetic targets because of their unique structures and powerful biological activities. Therefore, some research groups have devoted many efforts on the syntheses of these important products.9
Figure 1.
Representatives of natural products containing an indolizin-5(1H)-one core
In the meanwhile, indolizin-5(1H)-one bicyclic scaffolds have been proven to be key building block for the total synthesis of various indolizidine alkaloids.10 Several approaches to bicyclic motifs have been developed, including the Schmidt reaction,11 ring-closing metathesis reaction,12 metal-catalyzed cycloaddition,13 and Rh-catalyzed cyclization.14 However, most of these approaches suffer from several noticeable drawbacks, such as the use of costly and toxic metal catalysts, multi-step procedures,15 inaccessible substrates, and lack of effective derivation from the target skeleton. Thus, the development of metal-free strategies for the assembly and concomitant derivation of indolizin-5(1H)-ones from readily available starting materials with the aim of discovering new potentially interesting bioactive azabicyclic compounds would be a significant contribution to biomedical community.
In fact, creating molecular diversity and complexity from common and readily available reactants and forming various single and double bonds and rings in a single operation continue to be challenging in organic synthesis.16 Multicomponent domino reactions (MDRs) have emerged as useful tools for this purpose; such reactions present many advantages over their classical counterparts, including high atom efficiency, minimizing time-consuming isolation of steps, and required high purity of precursors, etc.17 Over the past few years, our group18 and others19 have developed various MDRs that can offer easy access to highly functionalized nitrogen-containing compounds of chemical and pharmaceutical interest. We recently discovered a novel ABC2 type MDR20 to give tricyclic pyrro[1,2-a]quinoline core of gephyrotoxin, an alkaloid isolated from the tropical frog Dendrobates histrionicus.21 Considering the open-ring reaction of 4-hydroxy-2H-chrome-2-one treated with amine,22 we hypothesized that the reactions of 4-hydroxy-2H-chromen-2-one, 3-aryl-1-(pyridin-2-yl)prop-2-en-1-one and pyridin-2-ylmethanamine or pyrazin-2-ylmethanamine will not only provide an efficient construction of bicyclic indolizin-5(1H)-one scaffolds, the parent core of Sessilifollamide J isolated from Stemona sessilifolia,2h but also provide a facile method of deriving this framework through simultaneous introduction of pyridine and pyrazine rings with biological importance.23
RESULTS AND DISCUSSION
According to the analysis described above, we began our investigation of this multi-component domino reaction by first reacting 3-(4-chlorophenyl)-1-(pyridin-2-yl)prop-2-en-1-one (1a), 4-hydroxy-2H-chromen-2-one (2a), and (pyridin-2-yl)methanamine (3a) to determine optimal conditions (Table 1). The catalyst-free model reaction was carried out at room temperature by using DMF as a solvent under inert gas protection. The desired compound 4a was obtained after chromatographic separation in 55 % yield, and its structure was confirmed by 1H NMR, 13C NMR, and HRMS (Table 1, entry 1). We then examined the solvent effect of chemical yield. Compared with DMF, EtOH, CH3CN, CH2Cl2, and THF, the use of anhydrous MeOH as a solvent provided optimal yield (Table 1, entry 3). To optimize the reaction conditions further, the optimal reaction temperature was determined. Results revealed that 35 °C is the optimal reaction temperature for the bicyclization reaction with highest yield of 79 % (Table 1, entry 8).
Table 1.
Optimization of conditions for the model reaction
 | ||||
|---|---|---|---|---|
| Entry | Solvent | T / °C | Time / h | Yield / %a |
| 1 | DMF | 25 | 16 | 55 |
| 2 | EtOH | 25 | 20 | 36 |
| 3 | MeOH | 25 | 18 | 64 |
| 4 | CH3CN | 25 | 20 | 31 |
| 5 | CH2Cl2 | 25 | 20 | 26 |
| 6 | THF | 25 | 20 | NR |
| 7 | MeOH | 30 | 18 | 73 |
| 8 | MeOH | 35 | 16 | 79 |
| 9 | MeOH | 45 | 16 | 70 |
| 10 | MeOH | 50 | 16 | 61 |
Isolated yields
We next explored the scope of present multicomponent domino reactions under the above optimized conditions (Table 2). A range of 3-aryl-1-(pyridin-2-yl)prop-2-en-1-ones were smoothly converted into their corresponding products in good yields (Table 2, entries 2–7). When 3-aryl-1-(pyrazin-2-yl)prop-2-en-1-ones were used, the corresponding indolizin-5(1H)-ones were obtained (Table 2, entries 8–10). We then replaced pyridin-2-ylmethanamine (3a) with pyrazin-2-ylmethanamine for examination, and the target compounds (Table 2, entries 11, 12) were formed in good yields of 80 % and 78 %, respectively. The use of either 4-hydroxy-6-methyl-2H-chromen-2-one or 6-bromo-4-hydroxy-2H-chromen-2-one in place of 4-hydroxy-2H-chromen-2-one (2a) also smoothly afforded the corresponding products under the same conditions (Table 2, entries 13–18). As shown in Table 2, for 3-aryl-1-(pyridin-2-yl)prop-2-en-1-ones and 4-hydroxy-2H-chromen-2-one, the electronic properties of both electron-donating groups and electron-withdrawing groups in the aryl substituent exerted very limited influence on the reactivity of reactants.
Table 2.
Synthetic results of products 4
 | |||||
|---|---|---|---|---|---|
| Entry | 4 | Ar | R | X/Z | Yield / % b |
| 1 | 4a | 4-ClC6H4 | H | CH/CH | 79 |
| 2 | 4b | 2-ClC6H4 | H | CH/CH | 76 |
| 3 | 4c | 4-CH3C6H4 | H | CH/CH | 81 |
| 4 | 4d | 4-CH3OC6H4 | H | CH/CH | 78 |
| 5 | 4e | 3,4-(CH3O)2C6H3 | H | CH/CH | 79 |
| 6 | 4f | 2,4-Cl2C6H3 | H | CH/CH | 80 |
| 7 | 4g | 2,3-(CH3O)2C6H3 | H | CH/CH | 75 |
| 8 | 4h | 4-BrC6H4 | H | CH/N | 77 |
| 9 | 4i | 4-ClC6H4 | H | CH/N | 74 |
| 10 | 4j | 2,3-(CH3O)2C6H3 | H | CH/N | 82 |
| 11 | 4k | 4-ClC6H4 | H | N/CH | 80 |
| 12 | 4l | 2,3-(CH3O)2C6H3 | H | N/CH | 78 |
| 13 | 4m | 4-ClC6H4 | Br | CH/CH | 82 |
| 14 | 4n | 4-FC6H4 | Br | CH/CH | 76 |
| 15 | 4o | 4-CH3C6H4 | Br | CH/CH | 83 |
| 16 | 4p | 4-CH3OC6H4 | Br | CH/CH | 81 |
| 17 | 4q | 4-ClC6H4 | CH3 | CH/CH | 79 |
| 18 | 4r | 4-CH3OC6H4 | CH3 | CH/CH | 77 |
Isolated yields
To confirm the stereochemistry of the indolizin-5(1H)-ones 4, the relative stereo-configuration of a single crystal of 4a was established by X-ray diffractional analysis. As shown in the crystal structure of 4a (Figure S1 in Supporting Information), six stereocenters in the molecular structure and two aryl groups in anti-configuration have been successfully formed.
According to the experimental outcomes, a mechanism hypothesis for this domino reaction is proposed as shown in Scheme 1. The first step of the mechanism is believed to be intermolecular Michael-addition between 4-hydroxy-2H-chromen-2-one (2) and 3-aryl-1-heteroarylprop-2-en-1-one (1) to generate intermediate 5. Next, the intermediate 6 is formed by intermolecular nucleophilic addition of intermediate 5 to 3, followed by an intramolecular nucleophilic addition to afford intermediate 7. Intermediate 8 was formed via a ring-opening of 7 followed by dehydration to give intermediate 9. The following step would involve intramolecular 1,4-addition or the one-step [3+2] cycloaddition to give trhe final product.
Scheme 1.
Proposed mechanism for the synthesis of 4
CONCLUSION
We have developed a metal-free [3+2+1]/[2+2+1] biscyclization strategy for the synthesis of indolizidin-5(1H)-one bicyclic scaffolds. This methodology yields indolizidin-5(1H)-ones with different substituent groups from readily accessible commercial starting materials under one-pot multi-component systems. The transformations are notable because they can yield five new sigma bonds and six stereocenters including a quaternary carbon center.
EXPERIMENTAL SECTION
General information
All reactions were carried out in an nitrogen atmosphere and solvents were dried according to established procedures. Thin layer chromatography was performed on silica gel GF254 plates. Silica gel (300–400 mesh) was used for column chromatography. Melting points are uncorrected. 1H NMR spectra were measured on 400 MHz and 13C NMR spectra were recorded on 100 MHz in CDCl3. IR spectra are reported in cm−1. HRMS were performed on TOF mass spectrometer with an ESI source. The X-ray single-crystal diffraction was performed on CCD area detector.
General procedure for synthesis of 4
A mixture of 3-aryl-1-(pyridin-2-yl)prop-2-en-1-ones or 3-aryl-1-(pyrazin-2-yl)prop-2-en-1-ones (1) (2.0 mmol) prepared according to the literature methods,24 4-hydroxy-2H-chromen-2-one or 4-hydroxy-6-methyl-2H-chromen-2-one or 6-bromo-4-hydroxy-2H-chromen-2-one (2) (1.0 mmol) and 2-(aminomethyl)pyridine or pyrazin-2-ylmethanamine (3) (1.0 mmol) was dissolved in 5 mL anhydrous methanol in a 25-mL 3-mouth flask, stirred with nitrogen incoming, heated to 35 °C progressively. The mixtures were stirred for a certain time (monitored by TLC). Then the solvent was removed in vacuum, and the residue was separated by column chromatography on silica gel (petroleum ether/ethyl acetate 4:1 v/v) to afford the producrts 4.
2,7-Bis(4-chlorophenyl)-6-(2-hydroxybenzoyl)-1-picolinoyl-3,8a-di(pyridin-2-yl)hexahydroin dolizin-5(1H)-one (4a)
White solid (583 mg, 79% yield): m.p. 278–280 °C; IR (KBr): 3049, 1696, 1671, 1585, 1492, 1435, 1348, 1232, 1160, 1091, 1033, 1012, 993, 955, 867, 776, 751, 688, 607, 571 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.90 (s, 1H), 9.51 (d, J = 8.0 Hz, 1H), 8.73 (d, J = 4.8 Hz, 1H), 8.70–8.68 (m, 1H), 8.23 (d, J = 4.8 Hz, 1H), 8.02 (td, J = 8.0, 2.0 Hz, 1H), 7.77–7.71 (m, 2H), 7.50–7.42 (m, 2H), 7.35–7.23 (m, 3H), 7.19–7.15 (m, 1H), 7.11 (d, J = 8.4 Hz, 2H), 7.04 (d, J = 8.4 Hz, 2H), 6.91–6.89 (m, 3H), 6.83–6.75 (m, 2H), 6.38 (d, J = 8.4 Hz, 2H), 5.62 (d, J = 12.8 Hz, 1H), 5.18 (d, J = 11.2 Hz, 1H), 4.50 (d, J = 11.2 Hz, 1H), 4.21–4.13 (m, 2H), 3.42 (dd, J = 14.4, 3.2 Hz, 1H), 3.28 (dd, J = 14.4, 11.2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 200.6, 196.9, 168.8, 162.7, 160.3, 157.7, 154.2, 149.3, 148.7, 148.3, 141.5, 137.3, 136.9, 136.3, 136.2, 135.4, 133.2, 132.0, 129.7, 129.5, 128.8, 128.7, 128.4, 127.3, 124.0, 123.3, 122.9, 122.7, 122.3, 120.2, 118.9, 118.6, 74.4, 69.4, 61.5, 53.1, 50.1, 44.4, 39.6; HRMS (ESI) m/z: Calcd. for C43H32Cl2N4O4 [M+Na]+ 761.1698, found: 761.1707.
2,7-Bis(2-chlorophenyl)-6-(2-hydroxybenzoyl)-1-picolinoyl-3,8a-di(pyridin-2-yl)hexahydroin dolizin-5(1H)-one (4b)
White solid (561 mg, 76% yield): m.p. 249–250 °C; IR (KBr): 3031, 1681, 1644, 1587, 1541, 1474, 1438, 1403, 1346, 1288, 1236, 1198, 1159, 1101, 1036, 995, 956, 886, 783, 749, 564 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.96 (s, 1H), 8.93 (d, J = 7.2 Hz, 1H), 8.60–8.59 (m, 2H), 8.26 (d, J = 4.0 Hz, 1H), 7.93–7.88 (m, 2H), 7.77 (td, J = 7.6, 1.6 Hz, 1H), 7.56–7.53 (m, 1H), 7.46 (td, J = 7.6, 1.6 Hz, 2H), 7.37 (ddd, J = 6.0, 4.8, 0.8 Hz, 1H), 7.32–7.27 (m, 2H), 7.21–7.10 (m, 5H), 7.07–6.98 (m, 4H), 6.79 (d, J= 7.6 Hz, 1H), 6.69–6.50 (m, 1H), 5.72 (d, J = 12.8 Hz, 1H), 5.46 (d, J = 9.6 Hz, 1H), 4.94 (dd, J = 10.8 Hz, 1H), 4.69 (dd, J = 12.4, 10.8 Hz, 1H), 3.76 (td, J = 10.8, 3.6 Hz, 1H), 2.99–2.88 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 202.3, 196.2, 168.2, 162.9, 158.9, 157.9, 155.5, 149.2, 148.1, 147.1, 138.9, 136.8, 136.6, 136.0, 135.9, 133.7, 131.5, 130.2, 130.0, 128.2, 127.9, 127.3, 127.0, 126.7, 125.8, 123.3, 122.6, 122.6, 122.4, 120.3, 118.7, 118.0, 74.3, 60.6, 56.0, 44.2, 36.9; HRMS (ESI) m/z: Calcd. for C43H32Cl2N4O4 [M+Na]+ 761.1698, found: 761.1726.
6-(2-Hydroxybenzoyl)-1-picolinoyl-3,8a-di(pyridin-2-yl)-2,7-dip-tolylhexahydroindolizin-5(1 H)-one (4c)
White solid (565 mg, 81% yield): m.p. > 300 °C; IR (KBr): 3011, 1694, 1667, 1589, 1515, 1405, 1341, 1157, 1034, 994, 953, 884, 856, 747, 666 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.97 (s, 1H), 9.54 (d, J = 7.2 Hz, 1H), 8.72 (s, 1H), 8.67 (s, 1H), 8.24 (s, 1H), 8.03–7.99 (m, 1H), 7.70 (s, 2H), 7.43–7.29 (m, 4H), 7.23–7.13 (m, 2H), 6.98–6.94 (m, 4H), 6.88–6.86 (m, 1H), 6.79–6.72 (m, 4H), 6.33 (d, J = 6.4 Hz, 2H), 5.66 (d, J = 12.8 Hz, 1H), 5.20 (d, J = 10.8 Hz, 1H), 4.55 (d, J = 11.2 Hz, 1H), 4.16–4.12 (m, 2H), 3.43 (d, J = 12.0 Hz, 1H), 3.33–3.26 (m, 1H), 2.18 (s, 3H), 2.10 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 201.1, 197.2, 169.2, 162.59, 160.7, 158.2, 154.4, 149.1, 149.1, 148.6, 148.2, 140.2, 137.0, 136.8, 136.6, 136.0, 135.9, 135.7, 135.6, 133.9, 133.8, 129.9, 129.2, 128.9, 128.1, 127.2, 127.0, 124.0, 123.3, 122.6, 122.4, 122.3, 120.4, 118.8, 118.4, 74.5, 69.6, 61.6, 53.4, 50.4, 44.8, 40.0, 21.0, 20.8; HRMS (ESI) m/z: Calcd. for C45H38N4O4 [M+Na]+ 721.2791, found: 721.2814.
6-(2-Hydroxybenzoyl)-2,7-bis(4-methoxyphenyl)-1-picolinoyl-3,8a-di(pyridin-2-yl)hexahydro indolizin-5(1H)-one (4d)
White solid (569 mg, 78% yield): m.p. 272–273 °C; IR (KBr): 3010, 1669, 1637, 1587, 1514, 1436, 1344, 1251, 1179, 1158, 1032, 827, 744, 622 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.98 (s, 1H), 9.54 (d, J = 7.6 Hz, 1H), 8.72 (d, J = 3.6 Hz, 1H), 8.68 (d, J = 4.4 Hz, 1H), 8.26 (d, J = 3.6 Hz, 1H), 8.01 (t, J = 7.6 Hz, 1H), 7.73–7.68 (m, 2H), 7.46–7.34 (m, 2H), 7.35–7.27 (m, 2H), 7.23–7.22 (m, 1H), 7.15–7.12 (m, 1H), 7.02 (d, J = 8.8 Hz, 2H), 6.87 (d, J = 8.0 Hz, 1H), 6.80–6.74 (m, 2H), 6.66 (d, J = 8.4 Hz, 2H), 6.46 (d, J = 8.8 Hz, 2H), 6.37 (d, J = 8.8 Hz, 2H), 5.63 (d, J =13.2 Hz, 1H), 5.18 (d, J = 10.8 Hz, 1H), 4.51 (d, J = 11.2 Hz, 1H), 4.18–4.07 (m, 2H), 3.66 (s, 3H), 3.60 (s, 3H), 3.46–3.41 (m, 1H), 3.31–3.25 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 201.2, 197.3, 169.1, 162.6, 160.7, 158.7, 158.3, 157.8, 154.4, 149.2, 148.6, 148.2, 137.1, 136.70, 136.1, 136.0, 135.2, 129.9, 129.2, 128.8, 128.3, 127.0, 124.0, 123.3, 122.7, 122.5, 122.3, 120.4, 118.8, 118.4, 113.9, 113.6, 74.4, 69.6, 61.7, 55.1, 55.1, 53.513, 50.1, 44.8, 39.7; HRMS (ESI) m/z: Calcd. for C45H38N4O6 [M+Na]+ 753.2689, found: 753.2720.
2,7-Bis(3,4-dimethoxyphenyl)-6-(2-hydroxybenzoyl)-1-picolinoyl-3,8a-di(pyridin-2-yl)hexahy droindolizin-5(1H)-one (4e)
White solid (624 mg, 79% yield): m.p. 242–243 °C; IR (KBr): 3049, 1696, 1672, 1586, 1518, 1491, 1451, 1435, 1404, 1438, 1269, 1159, 1091, 1031, 1072, 933, 886, 867, 853, 824, 786, 750, 662 cm−1; 1H NMR (400 MHz, CDCl3): δ 12.00 (s, 1H), 9.55 (d, J = 8.0 Hz, 1H), 8.74–8.70 (m, 2H), 8.30 (d, J = 4.0 Hz, 1H), 8.03 (t, J = 7.6 Hz, 1H), 7.75 (d, J = 4.0 Hz, 2H), 7.49–7.42 (m, 2H), 7.32–7.28 (m, 2H), 7.25–7.22 (m, 1H), 7.17–7.14 (m, 1H), 6.93 (d, J = 7.6 Hz, 1H), 6.81–6.68 (m, 3H), 6.64 (d, J = 8.0 Hz, 1H), 6.49 (s, 1H), 6.42 (d, J = 8.4 Hz, 1H), 6.10 (s, 1H), 6.04 (d, J = 8.0 Hz, 1H), 5.68 (d, J = 12.8 Hz, 1H), 5.18 (d, J = 11.2 Hz, 1H), 4.50 (d, J = 11.2 Hz, 1H), 4.18–4.07 (m, 2H), 3.74 (s, 3H), 3.68 (s, 3H), 3.65 (s, 3H), 3.54 (s, 3H), 3.54–3.50 (m, 1H), 3.36–3.29 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 201.2, 197.3, 169.0, 162.66, 160.86, 158.28, 154.37, 149.20, 148.59, 148.47, 148.22, 148.03, 147.31, 137.11, 136.79, 136.2, 136.0, 135.6, 129.6, 129.3, 127.1, 124.3, 123.6, 122.6, 122.5, 122.4, 120.4, 119.7, 119.4, 118.8, 118.5, 111.6, 111.2, 110.9, 110.8, 74.3, 69.6, 61.4, 55.7, 50.4, 44.5, 40.4; HRMS (ESI) m/z: Calcd. for C47H42N4O8 [M+Na]+ 813.2900, found: 813.2907.
2,7-Bis(2,4-dichlorophenyl)-6-(2-hydroxybenzoyl)-1-picolinoyl-3,8a-di(pyridin-2-yl)hexahydr oindolizin-5(1H)-one (4f)
White solid (645 mg, 80% yield): m.p. 246–247 °C; IR (KBr): 3057, 1680, 1647, 1587, 1474, 1438, 1406, 1345, 1313, 1281, 1251, 1233, 1197, 1160, 1106, 1046, 995, 954, 884, 863, 780, 746, 727, 688, 662, 619 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.89 (s, 1H), 8.81 (s, 1H), 8.59 (s, 2H), 8.27 (s, 1H), 7.94–7.91 (m, 2H), 7.79 (t, J = 7.6 Hz, 1H), 7.60 (d, J = 7.6 Hz, 1H), 7.52–7.47 (m, 1H), 7.41–7.30 (m, 3H), 7.20–7.03 (m, 8H), 6.82 (d, J = 8.4 Hz, 1H), 6.74 (t, J = 8.0 Hz, 1H), 5.66 (d, J = 11.6 Hz, 1H), 5.45–5.38 (m, 1H), 4.91 (d, J = 10.8 Hz, 1H), 4.65 (t, J = 12.0 Hz, 1H), 3.77–3.72 (m, 1H), 2.93–2.77 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 201.8, 196.0, 167.9, 162.9, 158.6, 157.5, 155.3, 149.2, 148.1, 147.1, 137.5, 136.9, 136.8, 136.2, 136.1, 134.5, 134.4, 133.3, 131.5, 130.0, 129.8, 128.7, 127.7, 127.4, 126.8, 125.7, 123.4, 122.8, 122.6, 122.5, 120.2, 119.0, 118.2, 74.2, 55.6, 44.1, 36.3, 31.0; HRMS (ESI) m/z: Calcd. for C43H30Cl4N4O4 [M+Na]+ 831.0889, found: 831.0906.
2,7-Bis(2,3-dimethoxyphenyl)-6-(2-hydroxybenzoyl)-1-picolinoyl-3,8a-di(pyridin-2-yl)hexahy droindolizin-5(1H)-one (4g)
White solid (593 mg, 75% yield): m.p. 264–266 °C; IR (KBr): 3049, 1670, 1632, 1585, 1478, 1434, 1399, 1338, 1264, 1223, 1160, 1089, 1067, 995, 872, 779, 746, 667, 617, 520 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.98 (s, 1H), 9.69 (d, J = 8.0 Hz, 1H), 8.72 (s, 1H), 8.65 (s, 1H), 8.14 (s, 1H), 8.01 (t, J = 8.0 Hz, 1H), 7.65 (s, 2H), 7.50–7.26 (m, 4H), 7.18–7.11 (m, 2H), 6.87–6.78 (m, 5H), 6.68–6.67 (m, 1H), 6.53 (d, J = 7.6 Hz, 1H), 6.46–6.42 (m, 1H), 5.83 (d, J = 12.8 Hz, 1H), 5.36 (d, J = 11.2 Hz, 1H), 5.23 (d, J = 7.2 Hz, 1H), 4.70–4.66 (m, 2H), 4.47 (t, J = 12.0 Hz, 1H), 3.89 (s, 3H), 3.75 (s, 3H), 3.71 (s, 3H), 3.52 (s, 3H), 3.31 (s, 2H); 13C NMR (100 MHz, CDCl3): δ 201.1, 197.4, 169.2, 162.6, 161.0, 158.7, 154.4, 152.7, 152.6, 149.1, 148.6, 148.5, 148.1, 146.7, 136.9, 136.5, 136.0, 135.9, 130.2, 130.0, 126.9, 123.7, 123.4, 123.3, 122.4, 122.4, 122.1, 121.2, 118.8, 118.4, 117.3, 111.6, 110.2, 74.4, 68.6, 60.8, 60.6, 60.5, 55.6, 55.5, 46.1, 44.4, 32.5, 32.5; HRMS (ESI) m/z: Calcd. for C47H42N4O8 [M+Na]+ 813.2900, found: 813.2929.
2,7-Bis(4-bromophenyl)-6-(2-hydroxybenzoyl)-8a-(pyrazin-2-yl)-1-(pyrazine-2-carbonyl)-3-(pyridin-2-yl)hexahydroindolizin-5(1H)-one (4h)
White solid (637 mg, 77% yield): m.p. > 300 °C; IR (KBr): 3046, 1697, 1632, 1591, 1574, 1488, 1437, 1395, 1338, 1302, 1245, 1206, 1157, 1073, 1055, 1010, 962, 859, 802, 748, 633, 610 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.82 (s, 1H), 10.00 (s, 1H), 9.13 (s, 1H), 8.74 (s, 1H), 8.66–8.62 (m, 3H), 8.33 (s, 1H), 7.57 (d, J = 8.0 Hz, 1H), 7.50 (t, J = 8.0 Hz, 1H), 7.37–7.26 (m, 5H), 7.16 (s, 1H), 7.07 (d, J = 8.0 Hz, 1H), 7.02 (d, J = 8.0 Hz, 2H), 6.83 (d, J = 8.0 Hz, 3H), 6.75 (t, J = 8.0 Hz, 1H), 5.32–5.26 (m, 2H), 4.71 (d, J = 10.4 Hz, 1H), 4.21 (t, J = 12.0 Hz, 1H), 3.12–3.08 (m, 1H), 2.88–2.83 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 201.5, 195.5, 167.4, 163.0, 156.8, 154.0, 149.6, 149.0, 147.8, 147.6, 144.8, 144.2, 142.9, 141.8, 140.4, 137.1, 136.4, 136.3, 132.1, 131.9, 131.8, 129.8, 128.3, 124.7, 123.0, 121.5, 121.3, 120.1, 119.1, 118.3, 72.6, 70.5, 61.5, 57.0, 48.6, 43.9, 39.5; HRMS (ESI) m/z: Calcd. for C41H30Br2N6O4 [M+Na]+ 853.0572, found: 853.0567.
2,7-Bis(4-chlorophenyl)-6-(2-hydroxybenzoyl)-8a-(pyrazin-2-yl)-1-(pyrazine-2-carbonyl)-3-(pyridin-2-yl)hexahydroindolizin-5(1H)-one (4i)
White solid (548 mg, 74% yield): m.p. 293–294 °C; IR (KBr): 3050, 1699, 1633, 1591, 1573, 1492, 1439, 1400, 1338, 1302, 1248, 1207, 1156, 1092, 1054, 962, 902, 880, 858, 824, 750, 716, 680, 665, 616 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.82 (s, 1H), 10.00 (s, 1H), 9.13 (d, J = 1.2 Hz, 1H), 8.74 (d, J = 2.4 Hz, 1H), 8.67–8.61 (m, 3H), 8.34 (s, 1H), 7.57 (d, J = 7.6 Hz, 1H), 7.51 (t, J = 8.0 Hz, 1H), 7.35 (t, J = 7.2 Hz, 1H), 7.18–7.12 (m, 5H), 7.07 (d, J = 8.0 Hz, 3H), 6.89 (d, J = 8.4 Hz, 2H), 6.82 (d, J = 8.4 Hz, 1H), 6.74 (t, J = 7.6 Hz, 1H), 5.33–5.27 (m, 2H), 4.71 (d, J = 10.0 Hz, 1H), 4.25–4.19 (m, 1H), 3.15–3.09 (m, 1H), 2.90–2.81 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 201.5, 195.5, 167.4, 163.0, 156.8, 154.1, 149.6, 149.0, 147.8, 147.7, 144.8, 144.2, 142.8, 141.8, 139.9, 137.1, 136.2, 135.9, 133.3, 133.2, 131.8, 129.4, 129.2, 129.0, 128.0, 124.7, 122.9, 120.1, 119.1, 118.3, 72.6, 70.6, 61.6, 57.1, 48.5, 44.0, 39.4; HRMS (ESI) m/z: Calcd. for C41H30Cl2N6O4 [M+Na]+ 763.1603, found: 763.1626.
2,7-Bis(2,3-dimethoxyphenyl)-6-(2-hydroxybenzoyl)-8a-(pyrazin-2-yl)-1-(pyrazine-2-carbony l)-3-(pyridin-2-yl)hexahydroindolizin-5(1H)-one (4j)
White solid (650 mg, 82% yield): m.p. 228–229 °C; IR (KBr): 3044, 1698, 1587, 1480, 1387, 1270, 1158, 1090, 1016, 957, 891, 877, 861, 834, 817, 794, 742, 670, 666, 650, 625, 605 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.93 (s, 1H), 10.15 (s, 1H), 9.11 (s, 1H), 8.65 (s, 2H), 8.59 (s, 1H), 8.51 (s, 1H), 8.32 (s, 1H), 7.71 (d, J = 8.0 Hz, 1H), 7.44 (t, J = 7.2 Hz, 1H), 7.31 (t, J = 7.6 Hz, 1H), 7.11–7.10 (m, 1H), 7.00 (d, J = 7.6 Hz, 1H), 6.92–6.84 (m, 3H), 6.79–6.65 (m, 5H), 5.48 (d, J = 12.8 Hz, 1H), 5.39 (d, J = 10.8 Hz, 1H), 5.01(d, J = 10.4 Hz, 1H), 4.52 (t, J = 11.2 Hz, 1H), 3.73 (s, 3H), 3.69 (s, 3H), 3.51–3.48 (m, 1H), 3.45 (s, 3H), 3.33 (s, 3H), 2.81 (t, J = 13.2 Hz, 1H), 2.70 (d, J = 10.8 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 202.3, 195.9, 168.0, 162.9, 158.1, 154.7, 152.8, 152.8, 149.5, 149.5, 148.4, 148.0, 147.2, 146.8, 144.8, 143.6, 142.7, 141.5, 136.6, 136.0, 134.5, 131.8, 130.8, 124.3, 123.8, 123.8, 122.5, 120.8, 120.3, 118.9, 118.8, 118.0, 111.6, 111.3, 73.0, 70.0, 61.1, 60.5, 60.3, 55.6, 55.0, 44.6, 44.5, 34.4; HRMS (ESI) m/z: Calcd. for C45H40N6O8 [M+Na]+ 815.2805, found: 815.2816.
2,7-Bis(4-chlorophenyl)-6-(2-hydroxybenzoyl)-1-picolinoyl-3-(pyrazin-2-yl)-8a-(pyridin-2-yl) hexahydroindolizin-5(1H)-one (4k)
White solid (591 mg, 80% yield): m.p. > 300 °C; IR (KBr): 3048, 1667, 1584, 1527, 1592, 1447, 1435, 1342, 1291, 1260, 1234, 1158, 1092, 1034, 1014, 909, 882, 824, 788, 696, 663, 647, 618, 572, 522 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.82 (s, 1H), 8.95 (d, J = 7.6 Hz, 1H), 8.62–8.60 (m, 2H), 8.39 (d, J = 2.4 Hz, 1H), 8.21–8.18 (m, 2H), 7.90 (td, J = 8.0, 1.6 Hz, 1H), 7.69–7.65 (m, 2H), 7.38–7.35 (m, 1H), 7.28–7.24 (m, 1H), 7.19– 7.17 (m, 2H), 7.06 (d, J = 8.8 Hz, 2H), 6.98 (d, J = 8.4 Hz, 2H), 6.83 (d, J = 8.4 Hz, 2H), 6.75 (d, J = 8.4 Hz, 1H), 6.72–6.68 (m, 1H), 6.33 (d, J = 8.4 Hz, 2H), 5.57 (d, J = 12.8 Hz, 1H), 5.21 (d, J = 11.2 Hz, 1H), 4.20 (d, J = 10.8 Hz, 1H), 4.22–4.10 (m, 2H), 3.36–3.23 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 199.5, 195.5, 168.0, 161.7, 159.0, 153.0, 152.6, 147.7, 147.5, 144.5, 142.9, 142.8, 140.1, 136.2, 135.9, 135.5, 133.5, 132.9, 131.2, 128.4, 128.4, 128.1, 127.6, 127.4, 126.4, 122.0, 121.5, 121.3, 119.0, 117.9, 117.7, 73.3, 66.0, 60.6, 51.9, 49.0, 43.2, 38.8; HRMS (ESI) m/z: Calcd. for C42H31Cl2N5O4 [M+Na]+ 762.1651, found: 762.1630.
2,7-Bis(2,3-dimethoxyphenyl)-6-(2-hydroxybenzoyl)-1-picolinoyl-3-(pyrazin-2-yl)-8a-(pyridi n-2-yl)hexahydroindolizin-5(1H)-one (4l)
White solid (617 mg, 78% yield): m.p. 290–291 °C; IR (KBr): 2936, 1691, 1627, 1584, 1480, 1444, 1409, 1366, 1335, 1307, 1287, 1263, 1220, 1161, 1089, 1063, 1038, 997, 966, 945, 907, 860, 825, 795, 749, 683, 618 cm−1; 1H NMR (400 MHz, CDCl3): δ 12.00 (s, 1H), 8.77 (d, J = 8.0 Hz, 1H), 8.57 (d, J = 4.0 Hz, 2H), 8.37 (d, J = 2.4 Hz, 1H), 8.29 (d, J = 4.0 Hz, 1H), 8.26 (s, 1H), 7.94–7.89 (m, 2H), 7.75 (td, J = 8.0, 1.6 Hz, 1H), 7.61 (d, J = 8.4 Hz, 1H), 7.37–7.34 (m, 1H), 7.31–7.26 (m, 1H), 7.21 (dd, J = 7.2, 4.8 Hz, 1H), 6.90–6.84 (m, 3H), 6.77 (d, J = 8.4 Hz, 1H), 6.72–6.62 (m, 4H), 5.69 (d, J = 12.8 Hz, 1H), 5.40 (d, J = 10.8 Hz, 1H), 4.99 (d, J = 10.8 Hz, 1H), 4.40 (dd, J = 12.4, 11.2 Hz, 1H), 3.72 (s, 3H), 3.67 (s, 3H), 3.56 (s, 3H), 3.50 – 3.43 (m, 1H), 3.35 (s, 3H), 2.94 – 2.85 (m, 2H); 13C NMR (100 MHz, CDCl3) : δ 202.9, 196.5, 168.7, 162.8, 159.3, 155.6, 154.7, 152.8, 148.3, 148.1, 147.1, 147.0, 145.2, 143.9, 143.2, 136.7, 136.5, 135.8, 135.0, 131.8, 130.5, 126.6, 125.4, 124.2, 124.0, 122.6, 122.4, 120.6, 120.4, 119.4, 118.8, 117.9, 111.6, 111.3, 74.4, 67.6, 60.5, 60.3, 60.2, 55.6, 56.0, 55.4, 44.4, 44.2, 35.0; HRMS (ESI) m/z: Calcd. for C46H41N5O8 [M+Na]+ 814.2853, found: 814.2825.
6-(5-Bromo-2-hydroxybenzoyl)-2,7-bis(4-chlorophenyl)-1-picolinoyl-3,8a-di(pyridin-2-yl)hex ahydroindolizin-5(1H)-one (4m)
White solid (669 mg, 82% yield): m.p. 287–289 °C; IR (KBr): 3049, 1671, 1587, 1570, 1493, 1468, 1435, 1412, 1346, 1287, 1174, 1093, 1048, 1014, 899, 857, 826, 779, 747, 697, 626 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.64 (s, 1H), 9.81 (d, J = 8.0 Hz, 1H), 8.88 (d, J = 4.0 Hz, 1H), 8.68 (d, J = 4.0 Hz, 1H), 8.27 (d, J = 4.0 Hz, 1H), 8.11–8.07 (m, 1H), 7.76–7.70 (m, 2H), 7.54–7.38 (m, 4H), 7.30–7.26 (m, 1H), 7.22–7.19 (m, 1H), 7.14 (d, J = 8.4 Hz, 2H), 7.05 (d, J = 8.4 Hz, 2H), 6.93 (d, J = 8.4 Hz, 2H), 6.81 (d, J = 8.0 Hz, 1H), 6.71 (d, J = 9.2 Hz, 1H), 6.37 (d, J = 8.4 Hz, 2H), 5.62 (d, J = 13.2 Hz, 1H), 5.18 (d, J = 8.8 Hz, 1H), 4.43 (d, J = 12.0 Hz, 1H), 4.15–4.07 (m, 2H), 3.44 (dd, J = 14.4, 3.2 Hz, 1H), 3.32–3.24 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 199.4, 196.8, 168.3, 161.2, 160.3, 157.3, 154.0, 149.7, 148.6, 148.4, 141.5, 138.7, 137.5, 136.8, 136.2, 135.1, 133.2, 132.5, 132.1, 129.5, 128.8, 128.6, 128.4, 127.3, 123.3, 123.3, 123.0, 122.7, 122.2, 121.3, 120.5, 110.6, 74.3, 69.0, 61.5, 53.6, 50.4, 44.5, 39.0; HRMS (ESI) m/z: Calcd. for C43H31BrCl2N4O4 [M+Na]+ 839.0803, found: 839.0764.
6-(5-Bromo-2-hydroxybenzoyl)-2,7-bis(4-fluorophenyl)-1-picolinoyl-3,8a-di(pyridin-2-yl)hex ahydroindolizin-5(1H)-one (4n)
White solid (596 mg, 76% yield): m.p. 247–248 °C; IR (KBr): 3046, 1670, 1585, 1510, 1348, 1231, 995, 831, 787, 745, 626 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.67 (s, 1H), 9.82 (d, J = 8.0 Hz, 1H), 8.87 (d, J = 8.0 Hz, 1H), 8.69 (d, J = 4.0 Hz, 1H), 8.28 (d, J = 4.0 Hz, 1H), 8.09 (t, J = 4.0 Hz, 1H), 7.76–7.71 (m, 2H), 7.54–7.37 (m, 4H), 7.30–7.26 (m, 1H), 7.20 (dd, J = 7.2, 5.2 Hz, 1H), 7.09 (dd, J = 8.4, 5.2 Hz, 2H), 6.87–6.79 (m, 3H), 6.72–6.64 (m, 3H), 6.43–6.40 (m, 2H), 5.63 (d, J = 12.8 Hz, 1H), 5.17 (d, J = 10.8 Hz, 1H), 4.43 (d, J = 12.0 Hz, 1H), 4.15–4.07 (m, 2H), 3.45 (dd, J = 7.2, 5.2 Hz, 1H), 3.31–3.25 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 199.6, 196.9, 168.4, 161.3, 160.4, 157.5, 154.1, 149.7, 148.7, 148.4, 138.7, 137.5, 136.9, 136.2, 132.6, 132.4, 132.3, 129.8, 129.7, 128.9, 128.8, 127.3, 123.4, 123.3, 123.0, 122.7, 122.3, 121.4, 120.5, 115.7, 115.4, 115.3, 115.0, 110.6, 74.3, 69.2, 61.6, 53.9, 50.4, 44.7, 39.0; HRMS (ESI) m/z: Calcd. for C43H31BrF2N4O4 [M+Na]+ 807.1394, found: 807.1395.
6-(5-Bromo-2-hydroxybenzoyl)-1-picolinoyl-3,8a-di(pyridin-2-yl)-2,7-dip-tolylhexahydroind olizin-5(1H)-one (4o)
White solid (644 mg, 83% yield): m.p. 275–276 °C; IR (KBr): 3012, 1696, 1669, 1587, 1570, 1515, 1468, 1435, 1404, 1344, 1287, 1175, 1046, 995, 890, 819, 780, 747, 713, 689, 627 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.73 (s, 1H), 9.84 (d, J = 8.0 Hz, 1H), 8.86 (d, J = 4.4 Hz, 1H), 8.67 (d, J = 4.8 Hz, 1H), 8.29 (d, J = 4.0 Hz, 1H), 8.09 (t, J = 8.0 Hz, 1H), 7.74–7.68 (m, 2H), 7.58 (d, J = 2.0 Hz, 1H), 7.47–7.36 (m, 3H), 7.28–7.26 (m, 1H), 7.17 (dd, J = 7.2, 5.2 Hz, 1H), 7.01 (d, J = 8.0 Hz, 2H), 6.96 (d, J = 8.0 Hz, 2H), 6.79 (t, J = 8.0 Hz, 3H), 6.69 (d, J = 8.0 Hz, 1H), 6.32 (d, J = 8.0 Hz, 2H), 5.68 (d, J = 12.8 Hz, 1H), 5.21 (d, J = 11.2 Hz, 1H), 4.49 (d, J = 12.0 Hz, 1H), 4.13–4.03 (m, 2H), 3.46 (dd, J = 14.4, 3.2 Hz, 1H), 3.33–3.26 (m, 1H), 2.20 (s, 3H), 2.13 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 200.0, 197.3, 168.9, 161.4, 160.9, 158.0, 154.5, 149.8, 148.9, 148.6, 140.4, 138.6, 137.5, 137.1, 136.9, 136.3, 136.0, 133.8, 132.9, 129.5, 129.2, 128.3, 127.4, 127.3, 123.6, 123.5, 123.0, 122.7, 122.5, 121.8, 120.6, 110.7, 74.7, 69.4, 61.8, 54.0, 51.0, 45.1, 39.6, 21.2, 21.0; HRMS (ESI) m/z: Calcd. for C45H37BrN4O4 [M+Na]+ 799.1896, found: 799.1864.
6-(5-Bromo-2-hydroxybenzoyl)-2,7-bis(4-methoxyphenyl)-1-picolinoyl-3,8a-di(pyridin-2-yl)h exahydroindolizin-5(1H)-one (4p)
White solid (654 mg, 81% yield): m.p. 169–170 °C; IR (KBr): 3047, 1670, 1612, 1586, 1513, 1486, 1435, 1401, 1343, 1290, 1177, 1114, 1032, 995, 882, 828, 780, 746, 716, 688, 672, 618 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.75 (s, 1H), 9.85 (d, J = 8.0 Hz, 1H), 8.87 (d, J = 4.0 Hz, 1H), 8.68 (d, J = 4.0 Hz, 1H), 8.31 (d, J = 4.0 Hz, 1H), 8.10 (t, J = 8.0 Hz, 1H), 7.74–7.68 (m, 2H), 7.58 (d, J = 4.0 Hz, 1H), 7.48–7.45 (m, 1H), 7.42–7.36 (m, 2H), 7.30–7.26 (m, 1H), 7.18 (dd, J = 6.8, 4.8 Hz, 1H), 7.06 (d, J = 8.8 Hz, 2H), 6.81 (d, J = 8.0 Hz, 1H), 6.69 (dd, J = 8.8, 1.6 Hz, 3H), 6.51 (d, J = 8.8 Hz, 2H), 6.38 (d, J = 8.8 Hz, 2H), 5.65 (d, J = 13.2 Hz, 1H), 5.21 (d, J = 10.8 Hz, 1H), 4.47 (d, J = 12.0 Hz, 1H), 4.13–4.02 (m, 2H), 3.68 (s, 3H), 3.63 (s, 3H), 3.49–3.44 (m, 1H), 3.33–3.26 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 199.88, 197.18, 168.65, 161.23, 160.72, 158.71, 157.86, 157.83, 154.24, 149.58, 148.66, 148.37, 138.46, 137.37, 136.70, 136.06, 135.25, 132.65, 129.24, 128.58, 128.31, 127.09, 123.43, 123.28, 122.84, 122.51, 122.25, 121.56, 120.37, 113.96, 113.62, 110.52, 74.38, 69.19, 61.72, 55.07, 55.05, 54.03, 50.43, 44.93, 39.09. HRMS (ESI) m/z: Calcd. for C45H37BrN4O6 [M+Na]+ 831.1794, found: 831.1755.
2,7-Bis(4-chlorophenyl)-6-(2-hydroxy-5-methylbenzoyl)-1-picolinoyl-3,8a-di(pyridin-2-yl)hex ahydroindolizin-5(1H)-one (4q)
White solid (594 mg, 79% yield): m.p. 254–255 °C; IR (KBr): 3050, 1695, 1667, 1588, 1491, 1435, 1411, 1338, 1293, 1247, 1171, 1091, 1051, 1013, 825, 779, 748, 698, 670, 620 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.64 (s, 1H), 9.71 (d, J = 7.6 Hz, 1H), 8.75 (d, J = 4.0 Hz, 1H), 8.69 (d, J = 4.8 Hz, 1H), 8.25 (d, J = 3.6 Hz, 1H), 8.03 (t, J = 8.0 Hz, 1H), 7.76–7.71(m, 2H), 7.51–7.42 (m, 2H), 7.28–7.25 (m, 1H), 7.20–7.17 (m, 1H), 7.15–7.12 (m, 4H), 7.06 (d, J = 8.4 Hz, 2H), 6.93–6.88 (m, 3H), 6.72 (d, J = 9.2 Hz, 1H), 6.40 (d, J = 8.0 Hz, 2H), 5.64 (d, J = 12.8 Hz, 1H), 5.20 (d, J = 10.8 Hz, 1H), 4.50 (d, J = 11.6 Hz, 1H), 4.19–4.13 (m, 2H), 3.45 (dd, J = 14.4, 2.8 Hz, 1H), 3.32–3.25 (m, 1H), 2.29 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 200.0, 196.9, 168.9, 160.5, 160.4, 157.8, 154.1, 149.3, 148.7, 148.4, 141.7, 137.3, 137.1, 136.9, 136.3, 135.3, 133.2, 131.9, 129.8, 129.5, 128.8, 128.7, 128.4, 127.7, 127.3, 124.0, 123.3, 122.9, 122.7, 122.3, 119.7, 118.2, 74.4, 69.3, 61.5, 53.3, 50.4, 44.5, 39.2, 20.7; HRMS (ESI) m/z: Calcd. for C44H34Cl2N4O4 [M+Na]+ 775.1855, found: 775.1826.
6-(2-Hydroxy-5-methylbenzoyl)-2,7-bis(4-methoxyphenyl)-1-picolinoyl-3,8a-di(pyridin-2-yl) hexahydroindolizin-5(1H)-one (4r)
White solid (573 mg, 77% yield): m.p. 172–173 °C; IR (KBr): 2927, 1672, 1612, 1587, 1514, 1436, 1399, 1342, 1251, 1177, 1091, 1032, 995, 828, 780, 746, 688, 671, 618 cm−1; 1H NMR (400 MHz, CDCl3): δ 11.72 (s, 1H), 9.74 (d, J = 8.0 Hz, 1H), 8.74 (d, J = 4.0 Hz, 1H), 8.68 (d, J = 4.0 Hz, 1H), 8.28 (d, J = 4.0 Hz, 1H), 8.03 (t, J = 4.0 Hz, 1H), 7.73–7.68 (m, 2H), 7.48–7.44 (m, 1H), 7.42–7.39 (m, 1H), 7.26–7.24 (m, 1H), 7.17–7.11 (m, 3H), 7.04 (d, J = 8.0 Hz, 2H), 6.87 (d, J = 4.0 Hz, 1H), 6.71– 6.67 (m, 3H), 6.48 (d, J = 8.0 Hz, 2H), 6.38 (d, J = 8.0 Hz, 2H), 5.65 (d, J = 12.0 Hz, 1H), 5.20 (d, J = 8.0 Hz, 1H), 4.51 (d, J = 11.6 Hz, 1H), 4.16–4.07 (m, 2H), 3.67 (s, 3H), 3.62 (s, 3H), 3.46 (dd, J = 14.8, 3.2 Hz, 1H), 3.31–3.25 (m, 1H), 2.29 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 200.6, 197.3, 169.3, 160.9, 160.4, 158.7, 158.3, 157.8, 154.3, 149.1, 148.7, 148.3, 137.0, 137.0, 136.7, 136.1, 135.4, 129.9, 129.2, 128.7, 128.3, 127.6, 127.1, 123.9, 123.4, 122.7, 122.5, 122.3, 119.9, 118.1, 114.0, 113.6, 74.4, 69.4, 61.7, 55.1, 55.0, 53.7, 50.3, 44.9, 39.2, 20.7; HRMS (ESI) m/z: Calcd. for C46H40N4O6 [M+Na]+ 767.2846, found: 767.2870.
Supplementary Material
ACKNOWLEDGMENTS
We thank the financial support from the NSFC (Nos. 21242014, 21272095 and 21271091), the Priority Academic Program Development of Jiangsu Higher Education Institutions, Jiangsu Science and Technology Support Program (No. BE2011045), Natural Science Foundation of Xuzhou City (XM12B074), Robert A. Welch Foundation (D-1361) and NIH (4R33DA031860).
Footnotes
ASSOCIATED CONTENT
Supporting Information
Copy of 1H and 13C NMR spectra for all products. X-ray CIF files for 4a. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Shu-Jiang Tu, Email: laotu@jsnu.edu.cn.
Guigen Li, Email: guigen.li@ttu.edu.
REFERENCES
- 1.(a) Daly JW, Garraffo HM, Spande TF. J. Nat. Prod. 2005;68:1556–1575. doi: 10.1021/np0580560. [DOI] [PubMed] [Google Scholar]; (b) Pollini GP, Benetti S, De Risi C, Zanirato V. Chem. Rev. 2006;106:2434–2454. doi: 10.1021/cr050995+. [DOI] [PubMed] [Google Scholar]; (c) Michael JP. Nat. Prod. Rep. 2007;24:191–222. doi: 10.1039/b509525p. [DOI] [PubMed] [Google Scholar]; (d) Rayabarapu DK, Cheng CH. Acc. Chem. Res. 2007;40:971–983. doi: 10.1021/ar600021z. [DOI] [PubMed] [Google Scholar]; (e) Michael JP. Nat. Prod. Rep. 2008;25:139–165. doi: 10.1039/b612166g. [DOI] [PubMed] [Google Scholar]; (f) Greger H, Schinnerl J, Vajrodaya S, Brecker L, Hofer O. J. Nat. Prod. 2009;72:1708–1711. doi: 10.1021/np900294c. [DOI] [PubMed] [Google Scholar]; (g) Nicolai S, Piemontesi C, Waser J. Angew. Chem. Int. Ed. 2011;50:4680–4683. doi: 10.1002/anie.201100718. [DOI] [PubMed] [Google Scholar]; (h) Fadeyi OO, Senter TJ, Hahn K, Lindsley CW. Chem. Eur. J. 2012;18:5826–5831. doi: 10.1002/chem.201200629. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) José B, Carlos M, Fernando A, Carlos V. J. Org. Chem. 2003;69:7114–7122. [Google Scholar]; (j) Szostak M, Aubé J. Chem. Rev. 2013;113:5701–5765. doi: 10.1021/cr4000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Kam TS, Subramaniam G, Lim KH, Choo YM. Tetrahedron Lett. 2004;45:5995–5998. [Google Scholar]; (b) Padwa A, Flick AC, Ik Lee H. Org. Lett. 2005;7:2925–2928. doi: 10.1021/ol0508779. [DOI] [PubMed] [Google Scholar]; (c) Greshock T, Grubbs JAW, Jiao P, Wicklow DT, Gloer JB, Williams RM. Angew. Chem. Int. Ed. 2008;47:3573–3577. doi: 10.1002/anie.200800106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhao N, Li L, Liu J, Zhuang P, Yu S, Ma S, Qu J, Chen N, Wu L. Tetrahedron. 2012;68:3288–3294. [Google Scholar]; (e) Wu X, Qin G, Cheng KK, Cheng KF. Tetrahedron. 1997;53:13323–13328. [Google Scholar]; (f) Hollis Showalter HD. J. Nat. Prod. 2013;76:455–467. doi: 10.1021/np300753z. [DOI] [PubMed] [Google Scholar]; (g) Hitotsuyanagi Y, Takeda E, Fukaya H, Takeya K. Tetrahedron Lett. 2008;49:7376–7379. [Google Scholar]
- 3.(a) Kimball FS, Turunen BJ, Ellis KC, Himesb RH, Georga GI. Bioorg. Med. Chem. 2008;16:4367–4377. doi: 10.1016/j.bmc.2008.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sharma VM, Seshu KVA, Krishna CV, Prasanna P, Sekhar VC, Venkateswarlu V, Rajagopal S, Ajaykumar R, Deevi DS, Mamidic NVSR, Rajagopalan R. Bioorg. Med. Chem. Lett. 2003;13:1679–1682. doi: 10.1016/s0960-894x(03)00263-4. [DOI] [PubMed] [Google Scholar]; (c) Boto A, Miguélez J, Marín R, Díaz M. Bioorg. Med. Chem. Lett. 2012;22:3402–3407. doi: 10.1016/j.bmcl.2012.03.109. [DOI] [PubMed] [Google Scholar]; (d) Kimball FS, Tunoori AR, Victory SF, Dutta D, White JM, Himes RH, Georg GI. Bioorg. Med. Chem. Lett. 2007;17:4703–4707. doi: 10.1016/j.bmcl.2007.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Geng X, Geney R, Pera P, Bernackib RJ, Ojima I. Bioorg. Med. Chem. Lett. 2004;14:3491–3494. doi: 10.1016/j.bmcl.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 4.Hanessian S, Balaux E, Musil D, Olsson L, Nilsson I. Bioorg. Med. Chem. Lett. 2000;10:243–247. doi: 10.1016/s0960-894x(99)00669-1. [DOI] [PubMed] [Google Scholar]
- 5.Haffner CD, Diaz CJ, Miller AB, Reid RA, Madauss KP, Hassell A, Hanlon MH, Porter DJT, Becherer JD, Carter LH. Bioorg. Med. Chem. Lett. 2008;18:4360–4363. doi: 10.1016/j.bmcl.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 6.(a) Vartak AP, Skoblenick K, Thomas N, Mishra RK, Johnson RL. J. Med. Chem. 2007;50:6725–6729. doi: 10.1021/jm070895r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hanessian S, Smith GM, Lombart HG, Lubell WD. Tetrahedron. 1997;53:12789–12854. [Google Scholar]; (c) Wessig P. Tetrahedron Lett. 1999;40:5987–5988. [Google Scholar]; (d) Hanessian S, Auzzas L. Acc. Chem. Res. 2008;41:1241–1251. doi: 10.1021/ar8000052. [DOI] [PubMed] [Google Scholar]
- 7.(a) Belvisi L, Bernardi A, Checchia A, Manzoni L, Potenza D, Scolastico C, Castorina M, Cupelli A, Giannini G, Carminati P, Pisano C. Org. Lett. 2001;3:1001–1004. [PubMed] [Google Scholar]; (b) Manzoni L, Bassanini M, Belvisi L, Motto I, Scolastico C, Castorina M, Pisano C. Eur. J. Org. Chem. 2007:1309–1317. [Google Scholar]
- 8.Lucas S, Negri M, Heim R, Zimmer C, Hartmann RW. J. Med. Chem. 2011;54:2307–2319. doi: 10.1021/jm101470k. [DOI] [PubMed] [Google Scholar]
- 9.(a) Satyanarayana G, Maichle-Mössmer C, Maier ME. Chem. Commun. 2009:1571–1573. doi: 10.1039/b820636h. [DOI] [PubMed] [Google Scholar]; (b) Amat M, Ramos C, Pérez M, Molins E, Florindo P, Santosc MMM, Boscha J. Chem. Commun. 2013;49:1954–1956. doi: 10.1039/c2cc38540f. [DOI] [PubMed] [Google Scholar]; (c) Huang H, Ji X, Wu Q, Jiang H. Chem. Commun. 2013;49:3351–3353. doi: 10.1039/c3cc40643a. [DOI] [PubMed] [Google Scholar]; (d) Mmutlane EM, Harris JM, Padwa A. Eur. J. Org. Chem. 2002:3304–3314. [Google Scholar]; (e) Vartak AP, Johnson RL. Org. Lett. 2006;8:983–986. doi: 10.1021/ol0600335. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhang Y, Xiong C, Wang W, Ying J, Hruby VJ. Org. Lett. 2002;4:4029–4032. doi: 10.1021/ol020160a. [DOI] [PubMed] [Google Scholar]; (g) Amat M, Santos MMM, Gómez AM, Jokic D, Molins E, Bosch J. Org. Lett. 2007;9:2907–2910. doi: 10.1021/ol0712327. [DOI] [PubMed] [Google Scholar]; (h) Sugimoto K, Toyoshima K, Nonaka S, Kotaki K, Ueda H, Tokuyama H. Angew. Chem. Int. Ed. 2013;52:7168–7171. doi: 10.1002/anie.201303067. [DOI] [PubMed] [Google Scholar]; (i) Alcaide B, Almendros P, Redondo MC, Ruiz MP. J. Org. Chem. 2005;70:8890–8889. doi: 10.1021/jo051402y. [DOI] [PubMed] [Google Scholar]; (j) Wu X, Liu Q, Fang H, Chen J, Cao W, Zhao G. Chem. Eur. J. 2012;18:12196–12201. doi: 10.1002/chem.201202240. [DOI] [PubMed] [Google Scholar]; (k) Iwama Y, Okano K, Sugimoto K, Tokuyama H. Chem. Eur. J. 2013;19:9325–9334. doi: 10.1002/chem.201301040. [DOI] [PubMed] [Google Scholar]; (l) Bodwell GJ, Li J. Angew. Chem. Int. Ed. 2002;41:3261–3262. doi: 10.1002/1521-3773(20020902)41:17<3261::AID-ANIE3261>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.(a) Sudau A, Münch W, Bats JW, Nubbemeyer U. Eur. J. Org. Chem. 2002:3304–3314. doi: 10.1021/jo991484o. [DOI] [PubMed] [Google Scholar]; (b) Jiang XP, Cheng Y, Shi GF, Kang ZM. J. Org. Chem. 2007;72:2212–2215. doi: 10.1021/jo0624290. [DOI] [PubMed] [Google Scholar]; (c) Kuhakarn C, Seehasombat P, Jaipetch T, Pohmakotr M, Reutrakul V. Tetrahedron. 2008;64:1663–1670. [Google Scholar]; (d) Lesma G, Colombo A, Sacchetti A, Silvani A. J. Org. Chem. 2009;74:590–596. doi: 10.1021/jo801638u. [DOI] [PubMed] [Google Scholar]; (e) Kuhakarn C, Seehasombat P, Jaipetch T, Pohmakotr M, Reutrakul V. Tetrahedron. 2008;64:1663–1670. [Google Scholar]; (f) Park SH, Kang HJ, Ko S, Park S, Chang S. Tetrahedron:. Asymmetry. 2001;12:2621–2624. [Google Scholar]; (g) Wang B, Fang K, Lin GQ. Tetrahedron Lett. 2003;44:7981–7984. [Google Scholar]; (h) Yun H, Kim J, Sim J, Lee S, Han YT, Chang D, Kim D, Suh Y. J. Org. Chem. 2012;77:5389–5393. doi: 10.1021/jo300309z. [DOI] [PubMed] [Google Scholar]; (i) Jiang X, Cheng Y, Shi G, Kang Z. J. Org. Chem. 2007;72:2212–2215. doi: 10.1021/jo0624290. [DOI] [PubMed] [Google Scholar]
- 11.(a) Aubé J, Milligan GL. J. Am. Chem. Soc. 1991;113:8966–8967. [Google Scholar]; (b) Ghosh P, Judd WR, Ribelin T, Aubé J. Org. Lett. 2009;11:4140–4142. doi: 10.1021/ol901645j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Huh CW, Somal GK, Katz CE, Pei H, Zeng Y, Douglas JT, Aubé J. J. Org. Chem. 2009;74:7618–7626. doi: 10.1021/jo901843w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Lesma G, Colombo A, Sacchetti A, Silvani A. J. Org. Chem. 2009;74:590–596. doi: 10.1021/jo801638u. [DOI] [PubMed] [Google Scholar]; (b) Bu1chert M, Meinke S, Prenzel AHGP, Deppermann N, Maison W. Org. Lett. 2006;8:5553–5556. doi: 10.1021/ol062219+. [DOI] [PubMed] [Google Scholar]
- 13.(a) Perreault S, Rovis T. Chem. Soc. Rev. 2009;38:3149–3159. doi: 10.1039/b816702h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chiou WH, Lin GH, Hsu CC, Chaterpaul SJ, Ojima I. Org. Lett. 2009;11:2659–2662. doi: 10.1021/ol900702t. [DOI] [PubMed] [Google Scholar]; (c) Dalton DM, Rovis T. Org. Lett. 2013;15:2346–2349. doi: 10.1021/ol400529k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yu RT, Rovis T. J. Am. Chem. Soc. 2006;128:12370–12371. doi: 10.1021/ja064868m. [DOI] [PubMed] [Google Scholar]; (e) Dalton DM, Oberg KM, Yu RT, Lee EE, Perreault S, Oinen ME, Pease ML, Malik G, Rovis T. J. Am. Chem. Soc. 2009;131:15717–15728. doi: 10.1021/ja905065j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Chiou WH, Lin YH, Chen GT, Gao YK, Tseng YC, Kao CL, Tsai JC. Chem. Commun. 2011;47:3562–3564. doi: 10.1039/c0cc05646d. [DOI] [PubMed] [Google Scholar]; (b) Chiou WH, Mizutani N, Ojima I. J. Org. Chem. 2007;72:1871–1882. doi: 10.1021/jo061692y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Varga TR, Nemes P, Mucsi Z, Scheiber P. Tetrahedron Lett. 2007;48:1159–1161. [Google Scholar]; (b) Liu KX, Ye JL, Ruan YP, Li YX, Huang PQ. J. Org. Chem. 2013;78:35–41. doi: 10.1021/jo3014484. [DOI] [PubMed] [Google Scholar]
- 16.(a) Liéby-Muller F, Constantieux T, Rodriguez J. J. Am. Chem. Soc. 2005;127:17176–17177. doi: 10.1021/ja055885z. [DOI] [PubMed] [Google Scholar]; (b) de Graaff C, Ruijter E, Orru RVA. Chem. Soc. Rev. 2012;41:3969–4009. doi: 10.1039/c2cs15361k. [DOI] [PubMed] [Google Scholar]
- 17.(a) Tietze LF. Chem. Rev. 1996;96:115–136. doi: 10.1021/cr950027e. [DOI] [PubMed] [Google Scholar]; (b) Tietze LF, Brasche G, Gerike K. Domino Reactions in Organic Chemistry. Wiley-VCH: Weinheim; 2006. [Google Scholar]; (c) Touré BB, Hall DG. Chem. Rev. 2009;109:4439–4486. doi: 10.1021/cr800296p. [DOI] [PubMed] [Google Scholar]; (d) Dömling A, Ugi I. Angew. Chem. Int. Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]; (d) Zhu JP, Bienayme H. Multicomponent Reactions. Wiley-VCH: 2004. [Google Scholar]; (e) Estévez V, Villacampa M, Menéndez JC. Chem. Soc. Rev. 2010;39:4402–4421. doi: 10.1039/b917644f. [DOI] [PubMed] [Google Scholar]; (f) Tejedor D, González-Cruz D, Santos-Expósito A, Marrero-Tellado JJ, Armas P, García-Tellado F. Chem. Eur. J. 2005;11:3502–3510. doi: 10.1002/chem.200401267. [DOI] [PubMed] [Google Scholar]
- 18.(a) Jiang B, Li C, Shi F, Tu S, Kaur P, Wever W, Li G. J. Org. Chem. 2010;75:2962–2965. doi: 10.1021/jo1002278. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jiang B, Tu S, Kaur P, Wever W, Li G. J. Am. Chem. Soc. 2009;131:11660–11661. doi: 10.1021/ja904011s. [DOI] [PubMed] [Google Scholar]; (c) Jiang B, Yi M, Shi F, Tu S, Pindi S, McDowell P, Li G. Chem. Commun. 2012:808–810. doi: 10.1039/c1cc15913e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Jiang B, Feng B, Wang S, Tu S, Li G. Chem. Eur. J. 2012;18:9823–9826. doi: 10.1002/chem.201201109. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Fan W, Ye Q, Xu H, Jiang B, Wang S, Tu S. Org. Lett. 2013;15:2258–2261. doi: 10.1021/ol4008266. [DOI] [PubMed] [Google Scholar]
- 19.(a) Snyder SA, Breazzano SP, Ross AG, Lin Y, Zografos AL. J. Am. Chem. Soc. 2009;131:1753–1765. doi: 10.1021/ja806183r. [DOI] [PubMed] [Google Scholar]; (b) Xu Z, De Moliner F, Cappelli AP, Hulme C. Org. Lett. 2013;15:2738–2741. doi: 10.1021/ol401068u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sharma SK, Mandadapu AK, Kumar B, Kundu B. J. Org. Chem. 2011;76:6798–6805. doi: 10.1021/jo201228t. [DOI] [PubMed] [Google Scholar]; (d) Feng X, Wang Q, Lin W, Dou G, Huang Z, Shi D. Org. Lett. 2013;15:2542–2545. doi: 10.1021/ol4010382. [DOI] [PubMed] [Google Scholar]; (e) Shiri M. Chem. Rev. 2012;112:3508–3549. doi: 10.1021/cr2003954. [DOI] [PubMed] [Google Scholar]; (g) Dömling A, Wang W, Wang K. Chem. Rev. 2012;112:3083–3135. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Sun J, Zhang L, Xia E, Yan C. J. Org. Chem. 2009;7:3398–3401. doi: 10.1021/jo900215a. [DOI] [PubMed] [Google Scholar]; (g) Marigo M, Schulte T, Franzén J, Jørgensen KA. J. Am. Chem. Soc. 2005;127:15710–15711. doi: 10.1021/ja055291w. [DOI] [PubMed] [Google Scholar]; (h) Presset M, Coquerel Y, Rodriguez J. Org. Lett. 2009;11:5706–5709. doi: 10.1021/ol9024056. [DOI] [PubMed] [Google Scholar]; (i) Chataigner I, Piettre SR. Org. Lett. 2007;9:4159–4162. doi: 10.1021/ol701608b. [DOI] [PubMed] [Google Scholar]; (j) Xu Z, De Moliner F, Cappelli AP, Hulme C. Org. Lett. 2013;15:2738–2741. doi: 10.1021/ol401068u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Liéby-Muller F, Constantieux T, Rodriguez J. J. Am. Chem. Soc. 2005;127:17176–17177. doi: 10.1021/ja055885z. [DOI] [PubMed] [Google Scholar]; (l) Tietze LF, Kinzel T, Brazel CC. Acc. Chem. Res. 2009;42:367–378. doi: 10.1021/ar800170y. [DOI] [PubMed] [Google Scholar]
- 20.Li TJ, Yin HM, Yao CS, Wang XS, Jiang B, Tu SJ, Li G. Chem. Commun. 2012;48:11966–11968. doi: 10.1039/c2cc37066b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Daly JW, Witkop B, Tokuyama T, Nishikawa T, Karle IL. Helv. Chim. Acta. 1977;60:1128–1140. doi: 10.1002/hlca.19770600336. [DOI] [PubMed] [Google Scholar]; (b) Fujimoto R, Kishi Y. Tetrahedron Lett. 1981:4197–4198. [Google Scholar]
- 22.Tu SJ, Li CM, Shi F, Zhou DX, Shao QQ, Cao LJ, Bo J. Synthesis. 2008:369–376. [Google Scholar]
- 23.(a) Santos VAFFM, Regasini LO, Nogueira CR, Passerini GD, Martinez I, Bolzani VS, Graminha MAS, Cicarelli RMB, Furlan M. J. Nat. Prod. 2012;75:991–995. doi: 10.1021/np300077r. [DOI] [PubMed] [Google Scholar]; (b) Rajagopalan R, Neumann WL, Poreddy AR, Fitch RM, Freskos JN, Asmelash B, Gaston KR, Galen KP, Shieh J, Dorshow RB. J. Med. Chem. 2011;54:5048–5058. doi: 10.1021/jm200257k. [DOI] [PubMed] [Google Scholar]; (c) Henry GD. Tetrahedron. 2004;60:6043–6061. [Google Scholar]; (d) Niculescu-Duvaz I, Roman E, Whittaker SR, Friedlos F, Kirk R, Scanlon IJ, Davies LC, Niculescu-Duvaz D, Marais R, Springer CJ. J. Med. Chem. 2006;49:407–416. doi: 10.1021/jm050983g. [DOI] [PubMed] [Google Scholar]
- 24.Wang G, Dong Y, Wu P, Yuan T, Shen Y. J. Org. Chem. 2005;73:7088–7095. doi: 10.1021/jo800870z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.